Abstract
Background
Emerging evidence suggests survival benefit from resection beyond all MRI abnormalities present on T1-enhanced and T2‒fluid attenuated inversion recovery (FLAIR) modalities in glioma (supratotal resection); however, the quality of evidence is unclear. We addressed this question via systematic review of the literature.
Methods
EMBASE, MEDLINE, Scopus, and Web of Science databases were queried. Case studies, reviews or editorials, non-English, abstract-only, brain metastases, and descriptive works were excluded. All others were included.
Results
Three hundred and nine unique references yielded 41 studies for full-text review, with 7 included in the final analysis. Studies were mostly of Oxford Center for Evidence-Based Medicine Level 4 quality. A total of 88 patients underwent supratotal resection in a combined cohort of 492 patients (214 males and 278 females, age 18 to 82 years). Fifty-one supratotal resections were conducted on high-grade gliomas, and 37 on low-grade gliomas. Karnofsky performance status, overall survival, progression-free survival, neurological deficits postoperatively, and anaplastic transformation were the main measured outcomes. No randomized controlled trials were identified. Preliminary low-quality support was found for supratotal resection in increasing overall survival and progression-free survival for both low-grade and high-grade glioma.
Conclusion
The literature suggests insufficient evidence for carte blanche application of supratotal resection, particularly in lower-grade gliomas where neurological deficits can result in long-term disability. While the preliminary studies discussed here, containing data from only a few centers, have reported increased progression-free and overall survival, these claims require validation in prospective research studies involving larger patient populations with clearly defined appropriate outcome metrics in order to reduce potential bias.
Keywords: FLAIR, glioma, resection, supratotal, surgery
Key Points
New studies are advocating for supratotal resection in both low- and high-grade glioma.
A systematic review identified 51 high- and 37 low-grade patients undergoing supratotal resection.
Evidence to support supratotal resection is low quality, but shows some promise.
Importance of the study
Glioma tumor cells are found at low frequency in areas distant from imaging abnormalities. These cells are likely involved in recurrence and progression, following resection of the tumor mass as defined on MRI. An emerging concept in neurosurgical oncology is to continue resection, when safe, beyond MRI abnormalities observed on T1-enhanced and T2-FLAIR modalities (ie, “supratotal resection”). However, the quality and level of evidence to support the widespread application of the supratotal resection approach has not been determined. In this study, we performed the first systematic review of all the literature on supratotal resection, and we further subdivided the studies to evaluate those performed in low-grade glioma, where the risk of neurological deficits could result in long-term quality of life consequences, and those performed in high-grade glioma, where extension of survival benefit is desperately needed. We identified low quality evidence in support of the supratotal resection approach, necessitating further research with larger cohorts and clearly defined outcome metrics.
Gliomas constitute the largest group of adult primary brain tumors.1 While surgical treatment alone of some grade I gliomas may result in a cure, there is currently no cure for higher-grade tumors. Grades II and III gliomas will eventually undergo transformation to higher grades, and thus surgical removal combined with chemo/radiotherapy is not curative, but will significantly delay progression and extend survival. The timeframe for progression of low-grade diffuse (ie, grade II) gliomas is approximately 5–15 years.2 On the other end of the scale, grade IV gliomas (glioblastoma [GBM]) have a markedly short median overall survival (OS) of merely ~15 months.3 GBM is not only the most aggressive brain tumor, but also the most common primary malignant adult brain tumor. As advances in immunotherapies, virus or gene therapy, and other small molecule therapeutics are pursued,4,5 the best approach presently remains to excise as much of the tumor as possible, as indicated by greater extent of resection (EOR) and improvements in survival.6–14 However, research has demonstrated that tumor cells can be found, albeit at lower frequency, at distant sites from the primary lesion15–18 and even as far as the opposite hemisphere,19 indicating that merely “complete” resection of the tumor may not be enough. Despite this, it has been shown that a near complete EOR (>98%), resulting in less than 1 or 2 cc of residual bulk (enhancing) tumor, could significantly prolong life in patients suffering from these tumors.10,20 Similarly, aggressive resection in low-grade tumors may also further delay progression to anaplastic transformation and result in improved overall survival.7,21
Extent of resection has served as a metric by which to judge the success of surgical tumor removal and with which to correlate improved long-term outcomes, such as progression-free survival (PFS) and OS. While many studies have supported this metric thus far8,9 and EOR is intrinsically linked to residual tumor volume, there are now indications that the residual tumor volume may be a more valuable and accurate metric in determining outcomes.10 In order to achieve greater EOR, stepwise improvements in survival have been achieved with the use of fluid attenuated inversion recovery (FLAIR) imaging,22–24 ultrasound-guided resections,25 intraoperative MRI (iMRI),11,26,27 or 5-aminolevulinic acid (5-ALA),11,28 a precursor in porphyrin biosynthesis which results in preferential fluorescence accumulation in tumor cells. Jenkinson et al reviewed the use of intraoperative imaging improvements to maximize EOR, and found 4 randomized controlled trials in support of iMRI, 5-ALA, and neuronavigation, but stated that evidence of improvements in OS and PFS was lacking.29 Recently, studies in primarily low-grade glioma have suggested that more aggressive resection using intraoperative electrostimulation to identify functional or eloquent brain can result in supratotal resection, which may improve PFS and OS.30–34 This modality is not without concern, as patients could suffer permanent neurological deficits for a decade or longer. Therefore, we sought out the evidence to support supratotal resection, in both low-grade and high-grade glioma.
Previously, some studies have defined supratotal resection as resection beyond T1 enhancement but within the boundaries of FLAIR abnormalities.35 Here, we defined true supratotal resection as resection beyond any visible MRI abnormalities, including FLAIR boundaries, which may be achieved via 5-ALA guided removal of tumor tissue, the use of iMRI for non-enhancing residual tumor, or resection until eloquent or functional tissue is reached.
For this systematic review, we searched the published literature for all studies that consisted of patients undergoing supratotal resection. We also searched for registered clinical trials that have assessed this parameter. The focus of this systematic review is on the impact of supratotal resection on measures of survival.
Methods
As a template for the methodology, we utilized the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews. This review was not registered as a systematic review protocol in the Cochrane database.
Search Strategy
Queries were completed in 4 databases: EMBASE, MEDLINE, Scopus, and Web of Science. In Scopus and Web of Science, 2 different strategies were employed to obtain articles relating to “supratotal resection”: (i) searching for papers related to glioma resection and FLAIR [glioma* AND flair AND resect*], and (ii) glioma with supra- or super-total, -marginal, -maximal, or –complete resection [glioma* AND (sup*total OR sup*maximal OR sup*marginal OR sup*complete)]. EMBASE and MEDLINE required more extensive search parameterization as detailed in Supplemental Information 1 & 2. Non-English publications were eliminated from the final analysis. Additional studies were included based on the reference list of the original search results, as appropriate. The search period ended on December 27, 2017 (Fig. 1).
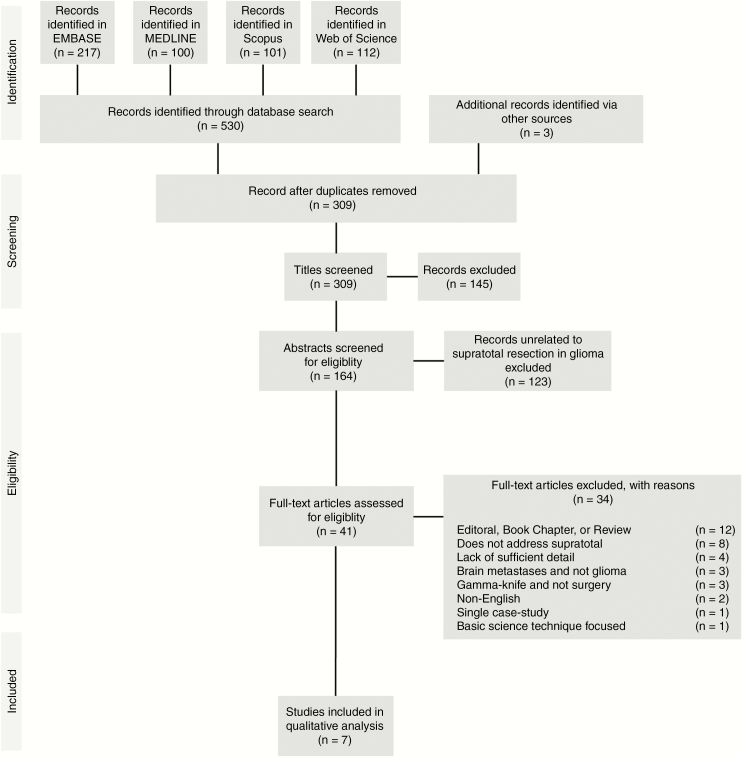
Fig. 1.
PRISMA flow diagram. EMBASE, MEDLINE, Scopus, and Web of Science were exhaustively searched for articles concerning supratotal resection in glioma, with reference lists also used as article sources. A total of 309 unique entries were considered for inclusion; 41 articles proceeded to full-text analysis, with 7 satisfying criteria for qualitative review, and 0 satisfying criteria for quantitative analysis.
Inclusion and Exclusion Criteria
Studies were included if supratotal resection was mentioned or implied in the title or the abstract, as defined in the “Search Strategy” section. Included studies were those which contained patients undergoing resection beyond the T1 ± gadolinium (Gd) and T2 MRI imaging modalities, including FLAIR. Only primary gliomas were considered. Studies were excluded if they mentioned supratotal resection but did not contain any such patients, or if the surgical treatment was for CNS metastases. Reviews, editorials, and descriptive works without survival-related outcomes were eliminated.
Data Extraction
Duplicates were automatically eliminated from the articles of the initial database searches using the EndNote software package first, and then additional duplicate entries manually eliminated by comparing authors, publication dates, and titles. The titles of the remaining unique publications were then reviewed independently by the authors to select articles relevant to supratotal, supramaximal, supracomplete, or supramarginal resection of glioma (hereafter simply referred to as supratotal). Subsequently, the abstracts of the selected articles were reviewed for eligibility within this study. Full text was further evaluated for reporting data on supratotal resection. Data extraction was performed using a standardized template. The final list of articles was assessed using the Oxford Center for Evidence Based Medicine (OCEBM; http://www.cebm.net/index.aspx?o=5653; accessed September 13 2018 v2.1) for level of evidence.
Quality Assessment and Risk of Bias
Studies were examined for quality based on whether they were retrospective or prospective, included control groups, performed appropriate statistical analyses, and contained single-center or multicenter data. OCEBM criteria were applied.
Results
Search Results
The initial database search returned 530 total records, of which 214 duplicates were removed by automatic and manual screening in EndNote X8, yielding 306 records for title screening published between 1965 and 2017 (Fig. 1). An additional 3 records were considered based upon reviewing references. A further 145 records were excluded as being unrelated during screening of titles. Abstract review of the remaining 164 records resulted in the exclusion of 123 records, with 41 considered in the full-text analysis. Of these, 7 were suitable for inclusion in qualitative analysis, and 0 were suitable for quantitative meta-analysis. Publication dates of included articles ranged from 2011 to 2017. Study characteristics are reported in Table 1.
Table 1.
Overview of included studies
| First Author | Number of Patients (N) | Type of Study | OCEBM Level of Evidence | Disease Classification(s) | Supratotal Resection (n/N) | Population Demographics | Sex | Location | Time Period |
|---|---|---|---|---|---|---|---|---|---|
| Age, y | |||||||||
| Duffau et al (2012)31 | 11 | Prospective case series | 4 | WHO grade II glioma | 3 / 11 | 18–50 (mean = 31.5) | 3 M, 8 F | Montpellier, France | Dec 1998– Dec 2010 |
| Duffau et al (2016)30 | 16* (new: 10) | Retrospective case series | 4 | Diffuse low- grade glioma | 16 / 16* (new 10/10) | 26–62 (mean = 41.3) | 7 M, 9 F* (new 5 M, 5 F) | Montpellier, France | Nov 1998– Jun 2007 |
| Eyüpoglu et al (2016)36 | 105 | Parallel-group single-center trial | 3 | Primary glioblastoma | 30 / 105 | 37–81 (mean = 62) | 60 M, 45 F | Erlangen, Germany | Dec 2001– Feb 2013 |
| Lima et al (2015)38 | 21 | Prospective case series | 4 | Incidental low- grade glioma | 4 / 21 | 18–57 (mean = 34.9) | 6 M, 15 F | Montpellier, France | Dec 1998– Sep 2012 |
| Lima et al (2017)37 | 19 | Retrospective case series | 4 | Incidental diffuse low-grade glioma | 5 / 19 | 18–51 (mean = 31.2) | 8 M, 11 F | Montpellier & Paris, France | Dec 1998– Dec 2013 |
| Pessina et al (2017)39 | 282 | Retrospective case series | 4 | Glioblastoma multiforme | 21 / 282 | 21–82 (mean = 61) | 105 M, 177 F | Rozzano, Italy | Dec 2003– Nov 2015 |
| Yordanova et al (2011)34 | 44 | Retrospective case series | 4 | WHO grade II glioma | 15 / 44 | 24–59 (mean = 36.4) | 8 M, 7 F | Montpellier, France | Dec 1998– Feb 2010 |
| All studies | 492 | 88 | Range = 18–82 | 214 M, 278 F | Nov 1998– Nov 2015 |
* Six patients from Yordanova (2011)34 were included based on a longer follow-up.
Overall Findings
The 7 included studies consisted of 88 unique patients undergoing supratotal resection in a combined cohort of 492 patients. The age range for the entire cohort was 18 to 82 years, consisting of 214 males and 278 females. None of the studies were conducted in the United States or Canada. Patient data were utilized from nearly 2 decades, spanning November 1998 to November 2015. All studies consisted of a subset of patients wherein resection was beyond the margins denoted by FLAIR imaging. The highest level of evidence (OCEBM) was 3, in a single study by Eyüpoglu et al.36 All others were graded as level 4. Of particular note is a study by Li et al35 (excluded) which assessed maximal surgical resection in 1229 glioblastoma patients. This study defined supratotal resection as beyond the T1-enhancing regions with a small portion of patients (n = 39) who also had resection of 90–100% of the FLAIR abnormalities. Thus, there may be a subset of these 39 patients who qualify as supratotal as defined here; however, the data presented do not indicate which patients had 100% removal of FLAIR and thus could not be included for consideration in this study.
Five publications from a single research group based in France conducted studies on supratotal resection of low-grade glioma. Of these 5 studies, 2 included statistical analyses. Two of the low-grade studies were prospective, whereas 3 were retrospective. There was a total of 37 unique supratotal resection patients and 68 non-supratotal. Two studies contained a partially overlapping cohort of patients (n = 6 overlapping): Yordanova (2011) and Duffau (2016) (Table 1).
Two separate research groups each conducted research on supratotal resection of high-grade glioma, both with statistical assessments. One study consisted of a parallel-group single-center trial, whereas the second was retrospective in nature. A total of 51 patients had undergone supratotal resection, and 336 had not.
Low-Grade Gliomas
The first study of supratotal resection in World Health Organization (WHO) grade II glioma was published by Yordanova et al34 in 2011 (Table 2). Fifteen patients underwent resection beyond FLAIR boundaries using an “asleep-awake-asleep” protocol of intraoperative functional electrostimulation. Recurrence rate, anaplastic transformation, and salvage chemotherapy administration were compared between this group and 29 control patients with complete resection (ie, gross total resection). Postoperative follow-up duration of supratotal patients had a mean of 35.7 months (range, 6–135 mo). Recurrence rate was 41% in controls and 26% in supratotal resections, but did not reach statistical significance. No instances of anaplastic transformation occurred in those patients undergoing supratotal resection (P = 0.037), in contrast to 24.1% of patients with complete resection. Salvage therapy (radiotherapy or chemotherapy) was used in only 1 supratotal resection patient, compared with 10 in the control group (6.7% vs 34.5%, P = 0.043).
Table 2.
Supratotal resection in low-grade gliomas
| First Author | Comparison Made | Surgical Technique | Definition of Supratotal | Volume Assessment | Adjuvant Therapy | Follow-up Time | Outcomes Measured |
|---|---|---|---|---|---|---|---|
| Duffau et al (2012)31 | None | Intraoperative functional electrostimulation “asleep- awake-asleep” protocol. No margins left around eloquent areas. | Beyond the tumor limits visible on preoperative MRI (and intraoperative ultrasound) | MRI: FLAIR and T2; postop at 3 mo. | None after surgery | up to 150 mo (mean: 40 mo) | Adjuvant treatment; KPS; OS; postop seizures |
| Duffau et al (2016)30 | None | Goal of maximal resection using intraoperative functional mapping (cortical and subcortical electrical stimulation) | Complete removal of signal abnormalities with postoperative cavity larger than preoperative tumor volume | MRI: T1 ± Gd; T2/ FLAIR; postop at 3 mo by FLAIR. | Radiotherapy, chemotherapy, or none | 97–198 mo (mean: 132 mo) | Adjuvant treatment; KPS; malignant transformation; postop seizures; relapse time |
| Lima et al (2015)38 | None | Asleep-awake-asleep technique with intraoperative direct electrical mapping of cortical and subcortical eloquent structures. | Supratotal when a margin of parenchyma was removed around the preoperative FLAIR signal abnormality (ie, larger volume of surgical cavity compared with presurgical tumor volume). | MRI: T1 ± Gd, T2, FLAIR; postop at 3 mo using FLAIR. | Radiotherapy (n = 1) and chemotherapy (n = 6) | 20–181 mo (med: 49.5 mo) | Adjuvant treatment; KPS; postop seizures; tumor regrowth |
| Lima et al (2017)37 | Supratotal, total, subtotal, partial resection. Median PFS: Supratotal + Total vs Subtotal + Partial. | Maximal surgical resection according to intraoperative functional boundaries w/ asleep-awake-asleep protocol. Cortical and subcortical direct electrostimulation mapping utilized. | Supratotal when a margin of parenchyma around FLAIR/T2 tumor volume removed and cavity is larger postoperatively, evaluated at 3 mo post-op. | MRI: FLAIR or T2; postop at 3 mo. | Radiotherapy (n = 2) or chemotherapy (n = 5) | 22–231 mo (mean: 62.6 mo) | Adjuvant treatment; KPS; OS; PFS; postop seizures |
| Yordanova et al (2011)34 | Control group of 29 patients who had only complete resection | Intraoperative functional electrostimulation using “asleep-awake-asleep” protocol. Alternating resection and subcortical stimulation. Resection extended beyond tumor limits on preop MRI, and all resections pursued until reaching eloquent structures. | Resection extended beyond the MRI abnormalities. Postop cavity greater than preop tumor volume. | MRI: Preop using 3 largest diameters on FLAIR or T2. postop at 3 mo. | None after surgery, except 1 patient radiotherapy at relapse. | 6–135 mo (mean: 35.7 mo) | Adjuvant treatment; Anaplastic transformation; KPS; OS; postop seizures; recurrence rate |
Similarly, Lima et al37 evaluated outcomes of EOR in incidental diffuse low-grade glioma in 19 patients. Supratotal resection was achieved in 5 patients. The combined PFS of supratotal (n = 5) and total resection patients (n = 5) had not reached the median versus 65 months (P = 0.006) for the combined group of subtotal (n = 7) and partially resected patients (n = 2). Subtotal resection was defined as <10 cm3 residual tumor, whereas partial was ≥10 cm3. Salvage radio/chemotherapy was administered in 75% of subtotal and partially resected patients upon tumor progression, whereas none of the supratotal and total patients received salvage therapy (P < 0.001). None of the patients in the study suffered neurological deficits, and 18 patients (94.7%) had a postoperative KPS of 100. The average follow-up for supratotal and total resection was 38.7 months (median: 37.5 mo, range: 25–41), and in subtotal and partial 89.2 months (median: 61.0 mo, range: 29–231). By homoscedastic Student’s t-test comparison, there was a significant difference in follow-up time (P = 0.041, our analysis). Patients undergoing supratotal/total resection had an average age at surgery of 35.3 years (median: 36 y), whereas those undergoing subtotal/partial had an average age of 26.7 years (median: 26 y) (P = 0.051, homoscedastic Student’s t-test; our analysis).
The 3 remaining studies of low-grade glioma did not include statistical comparisons.30,31,38 Duffau et al31 reported on 11 patients with WHO grade II glioma incidentally found in the left hemisphere in or near eloquent areas. Supratotal resection was achieved in 3 patients using the “asleep-awake-asleep” protocol. KPS score was 100 at >3 months postoperatively, and there was no administration of anti-epileptic drugs (AEDs), and no seizures reported with a total follow-up of 5–8 months for supratotal resection patients. In 2015, four supratotal resections were achieved in a series of incidental low-grade glioma published by Lima et al.38 Follow-up ranged from 22 to 40 months postsurgically. None of the patients had early or delayed postoperative seizures, long-term use of AEDs (which were utilized in the early postoperative period, <3 months), or adjuvant therapy. All patients were still alive, had a KPS score of 100, and had returned to normal social and professional life. In a subsequent publication by Duffau and colleagues,30 a set of 16 supratotal resection patients with diffuse low-grade glioma were evaluated for long-term outcomes. Six of these patients were previously reported on (all left hemisphere tumors),34 with 10 new patients (all right hemisphere tumors). Patients were 26–63 years of age and presented preoperatively with a KPS score between 90 and 100 and a tumor volume at surgery of 2–55 mL (mean, 25.5 mL). Fifteen of the 16 patients had presented with seizures. The volume of the surgical cavity ranged from 6 to 63 mL (mean, 35.6 mL). Four patients were confirmed histologically to have astrocytoma and 12 confirmed as oligodendroglioma. Postoperative follow-up was 97–198 months (mean, 132 months). Eight patients had relapsed between 32 to 105 months, five of whom had received adjuvant treatment thereafter (surgery, chemotherapy, and/or radiation). There was no long-term epilepsy in any of the 16 patients, but 4 patients were taking AEDs. None had undergone transformation to higher-grade gliomas at last follow-up, and KPS scores ranged 80–100.
High-Grade Gliomas
Two studies from separate research groups evaluated supratotal resection in high-grade gliomas (primary glioblastoma) in a total of 51 patients and 336 non-supratotal controls between December 2001 and November 2015 (Tables 1, 3).36,39
Table 3.
Supratotal resection in high-grade gliomas
| First Author | Comparison Made | Surgical Technique | Definition of Supratotal | Volume Assessment | Adjuvant Therapy | Follow-up Time | Outcomes Measured |
|---|---|---|---|---|---|---|---|
| Eyüpoglu et al (2016)36 | DiVA vs Control for supra-complete resection | Dual intraoperative Visualization Approach (DiVA): iMRI with integrated functional navigation and 5-ALA, using an iterative approach. | Beyond both vague and distinct 5-ALA borders and confirmed with iMRI | MRI: T1-weighted MPRAGE 3D, T2, DWI, and BOLD functional with iPlan Cranial Software. Postop assessment at time of operation end. | Radio- chemotherapy with temozolomide. | Up to 44 mo (med: 18.5 mo) | KPS; OS; Neurological deficits |
| Pessina et al (2017)39 | Supratotal vs gross total vs subtotal vs biopsy-only | Maximal removal of the tumor mass according to functional boundaries. Use of intraoperative neuro-navigation and ultrasound. Resection extended in select cases only if no new neurological deficits identified by cortical and subcortical stimulation. | 100% of enhanced and FLAIR resection. | MRI: T1, Enhanced, FLAIR; postop <48 h after surgery. | Concurrent and adjuvant chemo- radiotherapy. | 4.0–86.5 mo (med: 13.8 mo) | Adjuvant treatment; KPS; PFS; OS; postop morbidity and toxicity. |
Abbreviations: BOLD, blood oxygenation level dependent; DWI, diffusion-weighted imaging; MPRAGE, magnetization-prepared rapid acquisition with gradient echo.
In the Dual intraoperative Visualization Approach (DiVA) study by Eyüpoglo et al,36 gross total resection (n = 75, retrospectively identified control group) was compared with supratotal resection using a surgical strategy consisting of 5-ALA administration and iMRI (n = 30, prospectively selected). The following comorbidities were similarly distributed in both groups: diabetes mellitus, hypertension, hypercholesterolemia, bronchial asthma, cardiovascular diseases, obesity, and gastrointestinal disease. Tumor volume and median age were similar in both groups. Supra-complete (ie, supratotal) resection was defined as including 100% resection of both T1 ± Gd and T2/FLAIR abnormalities (Table 3). The DiVA protocol consisted of iterative use of 5-ALA and neuronavigation with iMRI until no more residual tumor could be detected (ie, no distinct or vague 5-ALA fluorescence). EOR in the DiVA arm ranged from 104–364%, with an average of 170 ± 75% (mean ± SD). EOR was set to 100% by definition in the gross total resection control arm. No significant differences existed for KPS scores either pre- or postoperatively (control: KPS preop 77 ± 10%, postop 76 ± 10%; DiVA: KPS preop 77 ± 15%, postop 77 ± 14%). However, survival was significantly increased in the DiVA group compared with control (control: 13 ± 6 months; DiVA: 19 ± 11 months; P < 0.004 by log-rank and P < 0.0081 by Gehan-Breslow-Wilcoxon). Specifically, survival was increased for both tumors in non-eloquent (Functional Grading System [FGS] I; P < 0.012 by log-rank) and eloquent-adjacent areas (FGS II; P < 0.010 by log-rank). FGS III, or tumors within eloquent brain, was not included in this study. There were no significant differences in motor deficits, visual field deficits, speech impairment, cognitive deficits, or seizures either pre- or postoperatively. In the control group only, there was a significant inverse correlation between patient age and OS (P = 0.035, Spearman r), but not in the DiVA group (P = 0.306, Spearman r). There was no correlation between initial tumor volume and OS for either group. Although assignment of the control group was not done at the same time as the experimental group, the authors noted no differences between initial mean tumor volume (28 ± 21 cm3 in control vs 30 ± 24 cm3 in DiVA group, P = 0.932) in all patients, or O6-methylguanine-DNA methyltransferase (MGMT) methylation in a subset of randomly tested samples (P = 0.1344). Furthermore, there was no correlation between MGMT methylation and OS (P = 0.325 for control group, P = 0.280 for DiVA group). Isocitrate dehydrogenase (IDH) mutation status was not assessed.
A second retrospective study, by Pessina and colleagues, evaluated 282 newly diagnosed GBM patients undergoing surgery and concurrent chemo- and radiation therapy, with a goal of maximal resection according to functional boundaries utilizing imaging and brain mapping.39 Median preoperative tumor volume was 59.1 cm3, and 7.1% of tumors were located in eloquent brain, 70.2% in near eloquent areas, and 22.7% in non-eloquent regions. Residual tumor volume was assessed at 24 h post-surgery (3T MRI with contrast-enhanced T1 and FLAIR, and diffusion-weighted imaging to exclude ischemic injury). Supratotal resection was achieved via resection of 100% of the enhanced and FLAIR abnormalities in 21 patients only, when it was safe to do so and not contraindicated by important cortical and subcortical structures after stimulation. Median follow-up time for the entire cohort was 13.8 months (range, 4.0–86.5 mo). Recurrence occurred in 73.0% of cases. Several factors were found to influence PFS and OS after multivariate correction: KPS and PFS (P < 0.001), KPS and OS (P < 0.001), age and PFS (P = 0.03), age and OS (P = 0.004), MGMT methylation status and PFS (P = 0.02), MGMT methylation status and OS (P = 0.02), EOR and PFS (P = 0.001), EOR and OS (P = 0.001), amount of FLAIR removal and PFS (P = 0.03), and amount of FLAIR removal and OS (P = 0.001). Specifically, the median PFS for supratotal resection was 24.5 ± 2.4 months and median OS was 28.6 ± 5.2 months, compared with gross total resection PFS of 11.9 ± 0.6 months and OS of 16.2 ± 1.2 months. Decreases in PFS and OS were observed with subtotal and biopsy cases. The authors identified a cutoff of 45% removal of the FLAIR residual tumor volume as having an impact on 2-year OS: 54% survival with lower residual tumor volume, compared with 12% with higher residual tumor volume. Fewer patients had worsened or developed new neurological deficits with supratotal resection (n = 1 of 21; 4.8%) compared with gross total resection (n = 8 of 60; 13.3%) or subtotal resection (n = 16 of 143; 11.2%), but not biopsy (n = 2 of 58; 3.4%). Tumor location was not utilized as a predetermined randomized variable and may not have been balanced between resection groups. IDH mutation status was assessed in all patients, with only 9 (3.2%) having a mutant allele.
Discussion
Supratotal resection for both high- and low-grade glioma is an emerging concept in neuro-oncology that is being pursued with the hope that it will lead to improved PFS and OS. This aggressive approach is not without significant risk of loss of neurological functions, however, due to potential compromise of essential cortical or subcortical tissues. As stated in a recent review of surgical oncology for glioma: “the desire to achieve optimal oncological outcomes must be tempered by the possibility of loss of neurological function, including motor deficits, language dysfunction, and neurocognitive impairment, after radical excision of brain tissues.”40
We identified 7 studies that specifically evaluated tumor resection beyond all currently detectable MRI capabilities. The majority of these studies focused on retrospective analyses and incorporated intraoperative cortical and subcortical electrostimulation as a means to resect until the boundary of functional nervous system tissue had been reached. Currently, there are only 3 groups that have published series focused on supratotal resection: 2 in high-grade glioma, and only 1 in low-grade glioma. These studies provide no evidence beyond OCEBM level 3 that supports this methodology. A further concern is the mere sparsity of patients who have been reported to have undergone this type of treatment—only 37 in low-grade glioma and only 51 in high-grade glioma, with a distinct lack of extensive follow-up. Most of these studies either lack controls entirely or have included limited control groups: 3 low-grade glioma studies had none, 1 low-grade glioma study combined supratotal and total resection and compared it with a combined group of subtotal and partial, and 1 low-grade glioma study compared results with a typical control group. In high-grade glioma, 1 study compared outcomes with a retrospectively identified control group, and another study also combined supratotal with gross total resection and compared it with a combined group of subtotal and biopsy only.
Findings from these studies which may support supratotal resection include: in low-grade gliomas, a significant increase in PFS (median not reached vs 65 mo),37 decreased incidence of anaplastic transformation (0 vs 24.1%),34 and reduced use of postoperative adjuvant treatment (6.7% vs 34.5%)34 for supratotal compared with partial and subtotal resection; and in high-grade glioma, supratotal resection prolonged PFS time by ~6 months in one study,36 and both PFS and OS by ~12 months in another.39 Notably, it has previously been shown that a survival benefit is realized in grade III anaplastic astrocytomas and grade IV glioblastoma tumors when the resection includes the FLAIR-visible disease only in conjunction with an IDH1 mutation.22 Furthermore, IDH1 mutant tumors were more likely to be completely resected.22,41 Recently, one study also questioned whether additional FLAIR resection beyond complete removal of the contrast-enhancing volume has any benefit in GBM, finding that postoperative FLAIR volume was not associated with either survival or recurrence.42 Thus, despite these preliminary findings, it is essential to recognize that rigorous comparisons to appropriate controls in the majority of the reports included in our present literature review is lacking, and tumors that are biologically less malignant may also be those that are more amenable to a complete (or supratotal) resection. Furthermore, tumor location in relation to eloquent brain is a likely confounder in resectability. Lastly, there is the potential for significant patient selection bias without a clear understanding or evaluation of the factors that enable supratotal resection in the select cases of these reports.
It will be challenging to obtain more robust data to objectively assess the true value of supratotal resection while simultaneously balancing its risks. These data are particularly needed in low-grade glioma where patients may be at risk of having to live with neurological deficits for a decade or even longer as a consequence of surgery. The conventionally applied approach for generating high quality evidence is a randomized controlled trial in which currently accepted standard of care consisting of complete resection of enhancing (high-grade glioma) or non-enhancing (low-grade glioma) should be directly compared with supratotal resection. Unblinded randomized trials for surgical treatment have not been feasible in neurosurgical oncology and it is unlikely that there will be sufficient equipoise to engage in a prospective randomized trial for supratotal resection. While there are presently no published randomized controlled trials addressing supratotal resection, there is one registered trial on ClinicalTrials.gov: NCT02676687, which aims to assess supratotal resection of gliomas in non-eloquent areas, as defined by resection of at least 1 cm beyond FLAIR or enhanced MRI abnormalities, and comparing it with total resection in a double-blind randomized trial. This study, based in China, is currently recruiting a total of 120 patients, looking at a primary outcome of PFS, and secondary outcomes of volume of resection and KPS.
This systematic review is limited by the number of independent centers reporting supratotal resection in either low-grade glioma or high-grade glioma. It is further limited by the total number of patients (less than 100 combined), the lack of similar outcome measurements and patient stratification variables that would otherwise enable meta-analytic approaches, and in some instances, short follow-up times of supratotal resected patients. These limitations are, in themselves, instructive and should make clear to the neurosurgical community that the concept of supratotal resection remains an interesting hypothesis, but one that bears more rigorous evaluation. Despite these limitations, it is clear that other groups are demonstrating interest in cautiously pursuing this surgical technique.43,44 Given this interest, it further stresses the need for more objective data. Even in GBM, where deficits may be more acceptable to the patient, it has been proposed that prospective studies are necessary to assess survival and quality of life.45 Patients undergoing planned supratotal resection should be evaluated by a multidisciplinary neuro-oncology team, which should include neurocognitive studies and neuropsychological assessment.33
Our current recommendation is that additional rigorous studies, in particular by multiple independent research centers, are required before supratotal resection can be recommended as a standard in neurosurgical oncology. Further improvements in intraoperative visualization of tumor tissue, advances in microsurgical technique, and better evaluation of functional pathways, such as intraoperative stimulation mapping, could also greatly add to our ability to localize tumor cells and accomplish safer and more extensive resection.40 Lastly, given the importance of establishing both the advantages and disadvantages of supratotal resection in a rigorous fashion, a feasible alternative to a randomized controlled trial may be a registry approach whereby neurocognitive assessments and centralized volumetric analysis with tumor location are recorded and a standardized set of outcomes objectively reported. These kinds of approaches are gaining traction as we move to health care reimbursement based on quality metrics and are already being employed in neurosurgical spine studies via the National Neurosurgery Quality and Outcomes Database (N2QOD) and the NeuroPoint Alliance.46,47
Funding
None.
Conflict of interest statement
The authors have no conflicts of interest relating to this topic. MAV has an unrelated equity interest in Johnson & Johnson and in Infuseon Therapeutics, Inc.
Authorship statement
This study was conceptualized and overseen by MAV. CNdL collected data and drafted the manuscript. Both MAV and CNdL assessed records for inclusion and finalized the manuscript.
Supplementary Material
References
- 1. Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY. Primary brain tumours in adults. Lancet. 2012;379(9830):1984–1996. [DOI] [PubMed] [Google Scholar]
- 2. Mandonnet E, Delattre JY, Tanguy ML, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. 2003;53(4):524–528. [DOI] [PubMed] [Google Scholar]
- 3. Brandes AA, Tosoni A, Franceschi E, Reni M, Gatta G, Vecht C. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67(2):139–152. [DOI] [PubMed] [Google Scholar]
- 4. Miranda A, Blanco-Prieto M, Sousa J, Pais A, Vitorino C. Breaching barriers in glioblastoma. Part I: molecular pathways and novel treatment approaches. Int J Pharm. 2017;531(1):372–388. [DOI] [PubMed] [Google Scholar]
- 5. Bush NA, Chang SM, Berger MS. Current and future strategies for treatment of glioma. Neurosurg Rev. 2017;40(1):1–14. [DOI] [PubMed] [Google Scholar]
- 6. Berger MS. Surgical resection strategies for optimizing glioma removal. Neuro Oncol. 2009;11(6):879–880. [Google Scholar]
- 7. Berger MS, Deliganis AV, Dobbins J, Keles GE. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74(6):1784–1791. [DOI] [PubMed] [Google Scholar]
- 8. Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D’Amico RS, Englander ZK, Canoll P, Bruce JN. Extent of resection in glioma-a review of the cutting edge. World Neurosurg. 2017;103:538–549. [DOI] [PubMed] [Google Scholar]
- 10. Grabowski MM, Recinos PF, Nowacki AS, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121(5):1115–1123. [DOI] [PubMed] [Google Scholar]
- 11. Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016;130(2):269–282. [DOI] [PubMed] [Google Scholar]
- 12. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–764; discussion 264–266. [DOI] [PubMed] [Google Scholar]
- 13. Snyder LA, Wolf A, Porter R, Smith K, Spetzler R, Sanai N. The impact of extent of resection on malignant transformation of pure oligodendrogliomas. J Neurosurg. 2012;117(2):A429. [DOI] [PubMed] [Google Scholar]
- 14. Yan JL, van der Hoorn A, Larkin TJ, Boonzaier NR, Matys T, Price SJ. Extent of resection of peritumoral diffusion tensor imaging-detected abnormality as a predictor of survival in adult glioblastoma patients. J Neurosurg. 2017;126(1):234–241. [DOI] [PubMed] [Google Scholar]
- 15. Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66(6):865–874. [DOI] [PubMed] [Google Scholar]
- 16. Silbergeld DL, Chicoine MR. Isolation and characterization of human malignant glioma cells from histologically normal brain. J Neurosurg. 1997;86(3):525–531. [DOI] [PubMed] [Google Scholar]
- 17. Matsukado Y, Maccarty CS, Kernohan JW. The growth of glioblastoma multiforme (astrocytomas, grades 3 and 4) in neurosurgical practice. J Neurosurg. 1961;18:636–644. [DOI] [PubMed] [Google Scholar]
- 18. Pallud J, Varlet P, Devaux B, et al. Diffuse low-grade oligodendrogliomas extend beyond MRI-defined abnormalities. Neurology. 2010;74(21):1724–1731. [DOI] [PubMed] [Google Scholar]
- 19. Kageji T, Nagahiro S, Uyama S, et al. Histopathological findings in autopsied glioblastoma patients treated by mixed neutron beam BNCT. J Neurooncol. 2004;68(1):25–32. [DOI] [PubMed] [Google Scholar]
- 20. Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. [DOI] [PubMed] [Google Scholar]
- 21. Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. [DOI] [PubMed] [Google Scholar]
- 22. Cahill DP, Beiko J, Cheung V, et al. IDH1 status determines the survival benefit of surgical resection for malignant astrocytomas. J Neurosurg. 2012;117(2):A388. [Google Scholar]
- 23. Bette S, Kaesmacher J, Huber T, et al. Value of early postoperative FLAIR volume dynamic in glioma with no or minimal enhancement. World Neurosurg. 2016;91:548–559.e541. [DOI] [PubMed] [Google Scholar]
- 24. Quan GM, Zheng YL, Yuan T, Lei JM. Increasing FLAIR signal intensity in the postoperative cavity predicts progression in gross-total resected high-grade gliomas. J Neurooncol. 2018;137(3):631–638. [DOI] [PubMed] [Google Scholar]
- 25. Hervey-Jumper SL, Berger MS. Role of surgical resection in low- and high-grade gliomas. Curr Treat Options Neurol. 2014;16(4):284. [DOI] [PubMed] [Google Scholar]
- 26. Mohammadi AM, Sullivan TB, Barnett GH, et al. Use of high-field intraoperative magnetic resonance imaging to enhance the extent of resection of enhancing and nonenhancing gliomas. Neurosurgery. 2014;74(4):339–348; discussion 349; quiz 349–350. [DOI] [PubMed] [Google Scholar]
- 27. Leroy HA, Delmaire C, Le Rhun E, Drumez E, Lejeune JP, Reyns N. High-field intraoperative MRI in glioma surgery: a prospective study with volumetric analysis of extent of resection and functional outcome. Neurochirurgie. 2018;64(3):155–160. [DOI] [PubMed] [Google Scholar]
- 28. Mansouri A, Mansouri S, Hachem LD, et al. The role of 5-aminolevulinic acid in enhancing surgery for high-grade glioma, its current boundaries, and future perspectives: a systematic review. Cancer. 2016;122(16):2469–2478. [DOI] [PubMed] [Google Scholar]
- 29. Jenkinson MD, Barone DG, Bryant A, et al. Intraoperative imaging technology to maximise extent of resection for glioma. Cochrane Database Syst Rev. 2018;1:CD012788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duffau H. Long-term outcomes after supratotal resection of diffuse low-grade gliomas: a consecutive series with 11-year follow-up. Acta Neurochir (Wien). 2016;158(1):51–58. [DOI] [PubMed] [Google Scholar]
- 31. Duffau H. Awake surgery for incidental WHO grade II gliomas involving eloquent areas. Acta Neurochir (Wien). 2012;154(4):575–584; discussion 584. [DOI] [PubMed] [Google Scholar]
- 32. Duffau H. Surgery for diffuse low-grade gliomas (DLGG) oncological considerations. In: Hugues Duffau, ed. Diffuse Low-Grade Gliomas in Adults. Vol 9781447122135: London, UK: Springer-Verlag London Ltd; 2013:359–374. [Google Scholar]
- 33. Yordanova YN, Duffau H. Supratotal resection of diffuse gliomas—an overview of its multifaceted implications. Neurochirurgie. 2017;63(3):243–249. [DOI] [PubMed] [Google Scholar]
- 34. Yordanova YN, Moritz-Gasser S, Duffau H. Awake surgery for WHO grade II gliomas within “noneloquent” areas in the left dominant hemisphere: toward a “supratotal” resection. Clinical article. J Neurosurg. 2011;115(2):232–239. [DOI] [PubMed] [Google Scholar]
- 35. Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection?J Neurosurg. 2016;124(4):977–988. [DOI] [PubMed] [Google Scholar]
- 36. Eyüpoglu IY, Hore N, Merkel A, Buslei R, Buchfelder M, Savaskan N. Supra-complete surgery via Dual intraoperative Visualization Approach (DiVA) prolongs patient survival in glioblastoma. Oncotarget. 2016;7(18):25755–25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lima GLO, Dezamis E, Corns R, et al. Surgical resection of incidental diffuse gliomas involving eloquent brain areas. Rationale, functional, epileptological and oncological outcomes. Neurochirurgie. 2017;63(3):250–258. [DOI] [PubMed] [Google Scholar]
- 38. Lima GL, Duffau H. Is there a risk of seizures in “preventive” awake surgery for incidental diffuse low-grade gliomas?J Neurosurg. 2015;122(6):1397–1405. [DOI] [PubMed] [Google Scholar]
- 39. Pessina F, Navarria P, Cozzi L, et al. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: is it useful and safe? A single institution retrospective experience. J Neurooncol. 2017;135(1):129–139. [DOI] [PubMed] [Google Scholar]
- 40. Sanai N, Berger MS. Surgical oncology for gliomas: the state of the art. Nat Rev Clin Oncol. 2018;15(2):112–125. [DOI] [PubMed] [Google Scholar]
- 41. Beiko J, Suki D, Hess KR, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mampre D, Ehresman J, Pinilla-Monsalve G, et al. Extending the resection beyond the contrast-enhancement for glioblastoma: feasibility, efficacy, and outcomes. Br J Neurosurg. 2018;1–8. [DOI] [PubMed] [Google Scholar]
- 43. Motomura K, Chalise L, Ohka F, Aoki K, Tanahashi K, Hirano M, Nishikawa T, Wakabayashi T, Natsume A. World Neurosurg. 2018 Jul 31. pii: S1878-8750(18)31671-1. doi: 10.1016/j.wneu.2018.07.193. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 44. Rapp M, Baernreuther J, Turowski B, Steiger HJ, Sabel M, Kamp MA. Recurrence pattern analysis of primary glioblastoma. World Neurosurg. 2017;103:733–740. [DOI] [PubMed] [Google Scholar]
- 45. Duffau H. Is supratotal resection of glioblastoma in noneloquent areas possible?World Neurosurg. 2014;82(1-2):e101–e103. [DOI] [PubMed] [Google Scholar]
- 46. Asher AL, McCormick PC, Selden NR, Ghogawala Z, McGirt MJ. The National Neurosurgery Quality and Outcomes Database and NeuroPoint Alliance: rationale, development, and implementation. Neurosurg Focus. 2013;34(1):E2. [DOI] [PubMed] [Google Scholar]
- 47. McGirt MJ, Speroff T, Dittus RS, Harrell FE Jr, Asher AL. The National Neurosurgery Quality and Outcomes Database (N2QOD): general overview and pilot-year project description. Neurosurg Focus. 2013;34(1):E6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.