Abstract
Glioblastoma (GBM) is the most common primary malignant brain tumor, with a universally poor prognosis. The emergence of molecular biomarkers has had a significant impact on histological typing and diagnosis, as well as predicting patient survival and response to treatment. The methylation status of the O6-methylguanine-DNA methyl-transferase (MGMT) gene promoter is one such molecular biomarker. Despite the strong evidence supporting the role of MGMT methylation status in prognostication, its routine implementation in clinical practice has been challenging. The methods and optimal cutoff definitions for MGMT status determination remain controversial. Variation in detection methods between laboratories presents a major challenge for consensus. Moreover, consideration of other clinical and genetic/epigenetic factors must also be incorporated into treatment decision making. In this review, we distill the available evidence to summarize our position on the optimal use of available assays, and propose strategies for resolving cases with equivocal methylation status and a framework for incorporating this important assay into research and clinical practice.
Keywords: diagnostic, glioma, methylation, MGMT, molecular markers, prognostic
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults.1 Due to the variability of patient response to the current standard of care, there is a need for better prognostication and individualization of treatment regimens. The methylation status of the O6-methylguanine-DNA methyl-transferase (MGMT) gene promoter has been demonstrated as an important biomarker of tumor response to temozolomide (TMZ) chemotherapy.2–4 However, despite the positive prognostic role of MGMT promoter methylation and the pressing need for the identification of prognostic biomarkers for patients with high-grade gliomas, the routine implementation of this biomarker in clinical practice has been challenging.
In this review, we describe the assays used to determine MGMT methylation status and discuss some of the potential reasons for the variability of applying this biomarker in clinical practice. Based on an overview of the latest evidence, we propose a possible approach to MGMT promoter methylation testing and subsequent therapeutic decision making.
The Role of MGMT Promoter Methylation in GBMs
The MGMT gene resides on chromosome 10 (10q26), and because in the majority of GBMs one allele of this chromosome is commonly lost, the remaining gene copy drives function.5 Alkylating chemotherapeutic agents such as TMZ induce cytotoxic cell death in tumor cells by alkylating DNA at multiple sites. Repair of the most toxic event, alkylation of the O6 group of guanine, is dependent on MGMT.
The MGMT gene encodes a repair protein (MGMT; formerly also termed alkyl guanine alkyltransferase6) that reverses this alkylation process. In this process, the methyl moiety is transferred onto the MGMT protein, which is thus consumed.2,7 Epigenetic modification of the cytosine-phosphate-guanine (CpG) island at specific CpG sites within the MGMT promoter silences the gene, leading to inefficient repair of DNA alkylation and enhanced response to TMZ.8–10
Original evidence demonstrating the role of MGMT methylation status in response to TMZ emerged from the Stupp trial.4 The predictive/prognostic role of MGMT promoter methylation has since been demonstrated in several other studies as well. Select examples of these studies are illustrated in Table 1.2,11–17
Table 1.
Landmark trials establishing the predictive and prognostic role of MGMT promoter methylation
| Study | Design | MGMT Testing Assay | MGMT Status | Prognostic Value |
|---|---|---|---|---|
| Hegi et al, 2005 (EORTC 26981/22981- NCIC CE.3)2 | Adults with WHO grade IV glioma Randomized to RT vs RT + TMZ (phase III) 573 patient enrolled → 307 evaluable samples→ methylation status determined in 206 (67% of evaluable samples) |
MS-PCR | Methylated: 92/206 patients (44.7%)Unmethylated: 114/206 patients (55.3%) |
Median OS: - RT = 15.3 months - RT + TMZ = 21.7 months OS HR (RT vs RT + TMZ): - 0.51 (95% CI 0.31–0.84) Median PFS: - RT = 5.9 months - RT + TMZ = 10.3 months PFS HR (RT vs RT + TMZ): - 0.48 (95% CI 0.31–0.75)Median OS - RT = 12.7 months - RT + TMZ = 11.8 months OS HR (RT vs RT + TMZ): - 0.69 (95% CI 0.47–1.02) Median PFS - RT = 4.4 months - RT + TMZ = 5.3 months PFS HR (RT vs RT + TMZ): - 0.62 (95% CI 0.42–0.92) |
| Wick et al, 2009 (NOA-04)13 |
Adults with WHO grade III glioma Randomized to RT vs TMZ vs procarbazine/lomustine/ vincristine (phase III) Only samples with 80% + tumor content were evaluated 274 patients enrolled → 202 evaluable samples → methylation status determined in 202 (100% of evaluable samples) |
MS-PCR | Methylated: 123/202 patients (60.9%) Unmethylated: 79/202 patients (39.1%) |
PFS HR (methylated vs unmethylated): - 0.59 (95% CI 0.37–1.1) TTF HR (methylated vs unmethylated): - 0.52 (95% CI 0.29–0.91) |
| Gilbert et al, 2013 (RTOG 0525)11 |
Adults with primary glioblastoma Randomized to RT + TMZ (standard) vs RT + TMZ (dose-dense) 1173 patients registered → 833 patients randomized → methylation status determined in 762 (91% of evaluable samples) |
qMS-PCR | Methylated: 245/762 patients (32.1%) Unmethylated: 517/762 patients (67.9%) |
Median OS (methylated): - 21.2 months (95% CI 17.9- 24.8) Median OS (unmethylated): - 14.0 months (95% CI 12.9- 14.7) OS HR (methylated vs unmethylated): - 0.58 (95% CI 0.48–0.69) Median PFS (methylated): - 8.7 months (95% CI 6.6–11.2) Median PFS (unmethylated): - 5.7 months (95% CI 5.1 to 6.1) PFS HR (methylated vs unmethylated): - 0.61 (95% CI 0.52–0.73) |
| Wick et al, 2012 (NOA-08)12 |
Adults >65 years old with grades III and IV glioma Randomized to RT vs TMZ (phase III) Only samples with 80% + tumor content were evaluated 412 patients enrolled → 209 evaluable samples → methylation status determined in 209 (100% of evaluable samples) |
qMS-PCR on 182 samples MS-PCR on all samples |
Methylated: 73/209 patients (35%)Unmethylated 136/209 patients (65%) |
OS HR (TMZ vs RT): - 0.69 (95% CI 0.35–1.16) EFS HR (TMZ vs RT): - 0.53 (95% CI 0.33–0.86)OS HR (TMZ vs RT): - 1.34 (95% CI 0.92–1.95) EFS HR (TMZ vs RT): - 1.95 (95% CI 1.41–2.69) |
| Malmstrom et al, 2012 (Nordic)14 | Adults >60 years old with WHO grade IV astrocytoma (changed to >65 years after October 15, 2004) Randomized to TMZ vs hypofractionated RT vs standard RT (phase III) 343 patients enrolled → 291 patients randomized → methylation status determined in 203 (70% of evaluable samples) |
qMS-PCR | Methylated: 91/203 patients (45%) |
OS HR (TMZ vs any RT): - 0.64 (95% CI 0.39–1.04) |
| Unmethylated: 102/203 patients (55%) |
OS HR (TMZ vs any RT): - 1.16 (95% CI 0.78–1.72) |
|||
| Gilbert et al, 2014 (RTOG 0825)15 |
Adults with primary glioblastoma Randomized to RT + TMZ + bevacizumab vs RT + TMZ (phase III) 978 patients enrolled → 637 patients randomized (65% of evaluable samples) |
qMS-PCR | N/A | Median OS (methylated): - 23.2 months (95% CI 20.1–28.3) Median OS (unmethylated): - 14.3 months (95% CI 13.6–15.3) OS HR (methylated vs unmethylated) - 2.10 (95% CI 1.65–2.68) Median PFS (methylated): - 14.1 months (95% CI 10.5–16.1) Median PFS (unmethylated): - 8.2 months (95% CI 7.5–9.2) PFS HR (methylated vs unmethylated) - 1.67 (95% CI 1.36–2.05) |
| Wick et al, 201716 | Adults with recurrent glioblastoma Randomized to lomustine + bevacizumab vs lomustine (phase III) 437 patients randomized → 270 evaluable samples → methylation status determined in 270 (100% of evaluable samples) |
450k array | Methylated: 124/270 patients (45.9%) Unmethylated: 146/270 patients (54.1%) Undetermined: 97 patients |
Median PFS - 5.7 months (95% CI 4.4–6.9) Median PFS - 2.8 months (95% CI 2.6- 2.9) Median PFS - 3.0 months (95% CI 2.8–4.2) |
| Perry et al. 2017 (CE.6 trial)17 |
Adults ≥65 years old with primary glioblastoma Randomized to short-course RT + TMZ vs short- course RT (phase III) 562 patients randomized → 462 evaluable samples → methylation status determined in 354 (77% of evaluable samples) |
MS-PCR | Methylated: 165/354 patients (46.6%)Unmethylated: 189/354 patients (53.4%) |
OS HR (RT + TMZ vs RT): - 0.53 (95% CI 0.38–0.73)OS HR (RT + TMZ vs RT): - 0.75 (95% CI 0.56–1.01) |
EFS: event-free survival; HR: hazard ratio; TTF: time to treatment failure.
Withholding TMZ in unmethylated patients and exclusive treatment with TMZ for methylated tumors have been investigated in prospective randomized trials in elderly patients.12,14,18 The NOA-08 and Nordic Elderly trials found that among patients ≥65 years and >60 years, respectively, who received TMZ alone, MGMT promoter methylation was associated with significantly longer survival than “unmethylated” tumors, while TMZ therapy was detrimental in patients with an unmethylated tumor.12,14 Similarly, trials replacing TMZ by investigational agents in unmethylated tumors have demonstrated that omission of the alkylating agent chemotherapy is not detrimental.19–21
Although these trials support withholding TMZ in unmethylated tumors, in current clinical practice this is reserved for clinical trials or for the treatment of elderly patients (in an effort to limit toxicity and burden from combined modality therapy). A survey of specialists caring for neuro-oncology patients showed that 77% would consider assessment of MGMT promoter methylation status in the management of elderly patients with GBM.22 Further, the 2017 European Association for Neuro-Oncology (EANO) guidelines for the treatment of malignant gliomas recommended that MGMT testing be considered as standard practice in elderly patients (>65–70 y).23 These guidelines suggest that MGMT methylated patients should receive TMZ as part of their regimen, whereas patients with unmethylated tumors should receive hypofractionated radiotherapy alone. Nevertheless, the definition of ‘elderly’ varies in clinical trials and practice.12,14,24 The recent Canadian Cancer Trials Group CE.6 clinical trial (CE.6 study) reported by Perry et al, comparing a short course of radiation therapy (RT) alone versus a short course of RT combined with TMZ in adults ≥65 years old with GBM demonstrated a notable increase in survival in elderly patients with an unmethylated MGMT status, though this difference was not statistically significant (10.0 vs 7.9 mo, P = 0.08).17 This was the first study to examine the benefit of a combined chemo-radiation strategy in elderly patients, whereas the NOA-08 and Nordic trials directly compared radiation-based regimens with TMZ, and the pivotal European Organisation for Research and Treatment of Cancer (EORTC) 26981/National Cancer Institute of Canada (NCIC) CE.3 trial had an upper age limit of 70 years. We conclude that in fit elderly patients, hypofractionated radiotherapy with TMZ remains the treatment of first choice; however, in more fragile or very old patients, exclusive therapy with TMZ may be better for tumors that are MGMT methylated, while for patients with an unmethylated promoter, RT should be considered.
MGMT methylation status alone is not the sole predictor of response to treatment. Hegi et al observed that even among unmethylated patients treated with radiation and TMZ, progression-free survival (PFS) was significantly improved, while increase in overall survival (OS) approached significance.2 In all trials there are occasional patients who appear to benefit from TMZ chemotherapy despite the absence of MGMT promoter methylation (Table 1). This is due in part to the fact that trials are reported as intent to treat and may thus include misclassified tumors or other histologies, as, for example, revealed in the report on the central pathology review of EORTC 26981/NCIC CE.3 trial.25 Other factors such as assay variability, cutoff definitions, or other molecular aberrations should also be considered. The median age in the Hegi et al study was 57 years, and as expected a notable proportion (7%; 9/130 analyzed) of patients had been isocitrate dehydrogenase 1 (IDH1) mutated,25 portending favorable prognostic factors not accounted for by the study.2 In addition, almost all patients with the CpG island methylator phenotype (G-CIMP) also had MGMT promoter methylation.26 Posttranscriptional modifications of MGMT mRNA affect the expression of the gene product as well and these have been comprehensively reviewed elsewhere.24 Other molecular changes may also correlate with MGMT promoter methylation. In anaplastic oligodendrogliomas, van den Bent et al have shown a strong correlation between MGMT promoter methylation and 1p/19q codeletion, though this may in part be confounded by the high correlation between IDH1 mutation status.27 This has been supported recently by the observation that almost all high-risk low-grade glioma patients with IDH mutation, with or without 1p/19q codeletion, have a methylated MGMT promoter.28
The MGMT Promoter
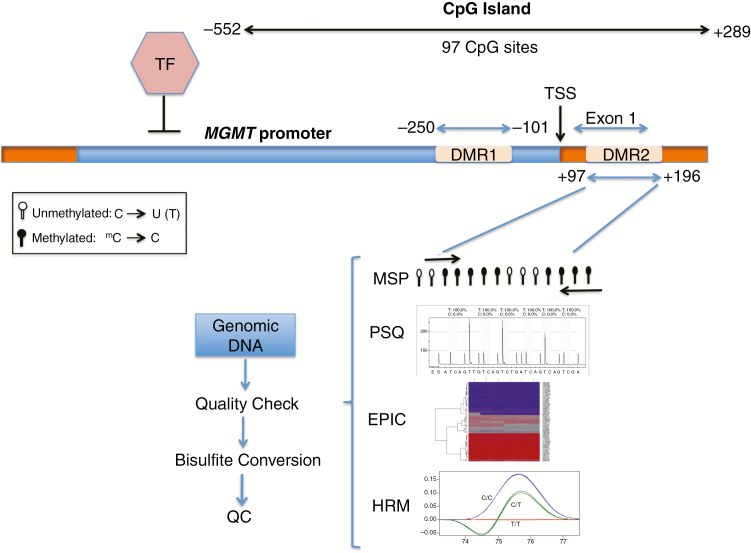
The promoter of the MGMT gene contains a CpG island that is 777 bp long and contains 97 CpG dinucleotides (Fig. 1).
Fig. 1.
MGMT promoter region and commonly used methylation assays. The promoter region and exon 1 of the MGMT gene contain the CpG island which spans 97 CpG sites. Methylation of CpG sites within 2 regions, DMR1 and DMR2, has been shown to negatively influence transcription. Five commonly methylated CpG sites within DMR2 are shown. The assays require isolation of DNA, followed by quality check (QC) for purity and integrity of the DNA. The efficiency of bisulfite conversion should also be tested to avoid false negative results. PSQ: pyrosequencing; TF: transcription factor; TSS: transcription start site.
To determine methylation at which specific CpG site(s) influence transcription, Malley et al correlated the MGMT mRNA expression level with methylation status of each CpG dinucleotide within the CpG island in both GBM cell lines and xenografts.29 Ninety-six CpG dinucleotides were tested and the authors demonstrated that the island can be segmented into distinct, nonrandom methylated blocks. Among these, 2 distinct regions (differentially methylated region 1 [DMR1] and DMR2) (Fig. 1) had the greatest impact on mRNA expression. Through selective site-directed mutagenesis, mutation of any CpG dinucleotide within DMR2 altered the expression of MGMT. Furthermore, it was shown that DMR2 was always methylated when DMR1 was methylated, making DMR2 the critical region for methylation testing. This finding has been further validated by Bady et al26; using results from the patient cohort in Hegi et al for classification of methylation for a training set, the authors showed that DMR1 and DMR2 not only were found to correlate inversely with MGMT expression when methylated but also were associated with better OS when exposed to alkylating chemotherapy.26
Methylation Detection Assays
A number of assays are currently available to measure MGMT promoter methylation status, and categorical variability between institutions and tests exists (Table 2). For all assays, except for the Illumina Infinium MethylationEPIC BeadChip, the bulk of the sample preparation is conducted locally while sequencing is performed in core labs. This difference is reflected in the costs as well. In the USA, the reimbursement for pathologists is governed primarily by CPT code 88363, which outlines reimbursement for facility and non-facility fees. When sample preparation from archival tissue is needed, CPT code 88380 or 88381 is used and bundled with 88363.
Table 2.
Summary of assays used for analysis of MGMT promoter methylation
| Assay | Turnaround Time | Benefits | Disadvantages | Estimated cost per Sample (USD) |
|---|---|---|---|---|
| Simple MSP | 2 days | - Shown to have predictive and prognostic value - Relatively inexpensive |
- Unreliable results - Poor reliability in FFPE tissues |
$5/sample |
| Quantitative MSP | 2 days | - Cutoff point validated in clinical trials | - Unreliable results with mosaic methylation patterns - Poor reliability in FFPE tissues |
$10–20/sample |
| Pyrosequencing | 5 days | - Quantitative | - High cost - Longer time to results - Cutoff threshold not validated in clinical trials - High-throughput core facility required |
$10–30/sample |
| High resolution melt | 2 days | - Quantitative | - Cutoff not validated in clinical trials | $20–30 /sample |
| EPIC | 5 days | - Offers testing of other biomarkers (1p/19q, G-CIMP) - Compatible with different sample preparations |
- Longer time to results - High cost - High-throughput core facility required - Cutoff not validated in clinical trials |
$500–700/sample |
| IHC | 2 days | - Low cost | - High interobserver variability - Inconsistent correlation with clinical outcomes |
$10–30/sample |
| MLPA | 2 days | - No need for bisulfite conversion of samples - Low cost |
- Predictive utility is not validated | $10–30/sample |
‘Turnaround time’ is estimated from DNA isolation point. The timelines presented are the minimum amount of time required for sample processing and may increase depending on availability of equipment and core facility.
There is currently no consensus regarding the best assay.30 Lassman et al analyzed concordance of MGMT analyses between local and central laboratories from tissue specimens obtained as part of a recently completed randomized phase III trial (Radiation Therapy Oncology Group [RTOG] 3508/AbbVie).31 The authors compared local versus central biomarker results among patients screened for M12-356 (a prior trial) or RTOG 3508 at Columbia University Medical Center. MGMT promoter methylation was analyzed by methylation-specific PCR (MSP). One hundred and nine GBMs were molecularly profiled from 73 patients who underwent 1–4 resection(s) and MGMT promoter methylation was observed in 27% of tumors tested locally versus 43% centrally; MGMT methylation interlaboratory concordance was only 61%.31 Considering the impact of this biomarker on treatment allocation in clinical trials and practice, this underscores the importance of using a validated and quality-controlled assay.
Methylation-Specific Polymerase Chain Reaction
The use of the MSP method for detection of MGMT methylation status is supported by evidence from multiple randomized trials.2,32,33 First described by Herman et al,34 the technique is dependent on bisulfite conversion (which converts unmethylated cytosines to uracils), followed by use of primers designed to measure multiple CpG dinucleotides within the MGMT promoter to specifically amplify alleles with either unconverted or converted cytosines, representing methylated and unmethylated sequences, respectively (Fig. 1).2,17,35 The amplified sequences are then assessed via gel electrophoresis, and a qualitative interpretation of methylation signal is made. While distinctly positive and negative signals are simple to interpret, faint signals are typically referred to as “equivocal.”36 When samples are run in replicates, performed infrequently, a variation noted may be termed “inconsistently” methylated. Retrospectively assessing 465 GBM samples in which MGMT status was determined using MSP, Xia et al demonstrated an inconsistency rate of 12% among their MSP replicates.32 The survival of patients with inconsistent results paralleled that of patients with unmethylated samples. The authors noted a “dose-response” trend, wherein the ratio of methylated:total number of replicates correlated with survival and this trended toward significance. Others have argued that even patients with low levels of methylation may benefit from alkylating agents such as TMZ, based on evidence suggesting that these tumors could have glioma-initiating cells that are enriched for methylated MGMT.37
Quantitative MSP (qMSP) uses quantitative PCR technology and normalizes the copy number of methylated MGMT to the copy number of an unmethylated gene, such as beta-actin (ACTB), controlled by standard curves. The technical cutoff, where the probability of being methylated/unmethylated is 50%, is usually applied to dichotomize the test result.14,33 A respective certified commercial qMSP assay has been used for central prospective testing in most recent phase III trials for GBM for patient selection or stratification.15,38,39 The term “equivocal” or “gray zone” has also been applied to the qMSP method, as the uncertainty in the vicinity of the cutoff is high. Therefore, trials selecting for MGMT unmethylated patients and omitting TMZ in the treatments have used the lower bound of the 95% confidence interval as a safety margin in order not to deny patients a potentially effective therapy.20,21 The technical cutoff has shown to be a good predictor of outcome in GBM trials where patients have been treated with TMZ.12,14,15 However, for strategies aiming at enriching patient populations with only methylated or unmethylated tumors, respectively, this technical cutoff may need to be further optimized for a “clinical” cutoff.
The principle of MSP aims at amplification of fully methylated MGMT alleles that are considered to be biologically most relevant for gene silencing. This increases the specificity but reduces the sensitivity, as heterogeneous methylation may not be detected. This applies for any qualitative or quantitative MSP assay. Despite limitations, the large body of data, including from large clinical trials, supports the clinical utility of qMSP. In our opinion, qMSP provides a good and reproducible balance between reliability, availability, and cost.
Pyrosequencing
Pyrosequencing also relies on bisulfite conversion of DNA and PCR amplification. However, through sequencing DNA by synthesis, this technique also enables quantification of methylated DNA within each CpG site tested, which is displayed as a “pyrogram” (Fig. 1). Pyrosequencing allows for better detection of heterogeneous patterns of methylation than MSP-based technology, as it yields quantitative methylation values for individual CpG dinucleotides. The technology is robust and cutoffs for specific sets of CpGs have been validated in independent, although small, datasets.40 In a large prospective study, utilizing both MSP and pyrosequencing, Reifenberger et al demonstrated a strong concordance between both assays when a cutoff of <8% vs ≥8% methylated alleles was used for the pyrosequencing method. Furthermore, a significantly better outcome was achieved in response to alkylating chemotherapy when a cutoff of >25% methylated alleles was used.41 However, while quantitatively more methylated alleles may provide prognostic value for survival, there is uncertainty regarding the cutoff to define MGMT “methylated” versus “unmethylated” status for stratifying patients into treatment groups.42 Moreover, “partial methylation,” wherein not all CpG sites are methylated, is a scenario that arises with pyrosequencing that is difficult to interpret in terms of clinical relevance.40 As pyrosequencing is significantly more expensive than MSP, it may be reserved for high-volume settings such as clinical trials.43
High-Resolution Melt
Quantitative real-time PCR high-resolution melt (PCR-HRM) has been shown to have high reproducibility in assessing methylation.44 This method also involves bisulfite conversion of DNA followed by PCR amplification and analysis of melting profiles of the PCR products. In one study, PCR-HRM was found to be more accurate than MSP for predicting PFS and OS of high-grade glioma patients treated with radiation and TMZ, and was comparable in detection ability to pyrosequencing.45 Quillien et al, on the other hand, compared HRM with MSP and pyrosequencing and found that HRM had a weaker predictive value, and a higher percentage of patients with heterogeneous methylation as observed by pyrosequencing were defined as “unmethylated” using HRM.46 Therefore, the current evidence for HRM is not robust.
Multiplex Ligation-Dependent Probe Amplification
Methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) is a semi-quantitative method that does not require the DNA-modifying bisulfite treatment step and, therefore, avoids additional damage to the DNA.35 MS-MLPA can detect changes in both CpG methylation and copy number of approximately 40 chromosomal regions per reaction.47 In MS-MLPA, the ligation of probes is combined with digestion of template DNA-probe complex with the methylation-sensitive endonuclease HhaI. Probe–DNA complex is first treated with HhaI and then subjected to PCR. The limitation is that the methylation-specific probes can only be designed for sequences that contain the HhaI restriction site GCGC. If the CpG locus is methylated, the HhaI restriction site is protected from restriction enzyme digestion and the PCR product is not generated. An additional limitation of the MS-MLPA method is the need for dedicated equipment and costly reagents.
Compared with other well-established assays, the utility of MS-MLPA in detection of methylation status has been assessed in a limited number of studies.48,49 Although the semi-quantitative aspect of MS-MLPA may prove to be of great value, additional studies will need to be conducted to evaluate the prognostic ability and clinical utility of this method.
Immunohistochemistry
Assessment of MGMT status at the protein level through immunohistochemistry (IHC) has also demonstrated variable accuracy across studies.46,50,51 The advantage of IHC is that it enables focused interpretation of signal in regions of high tumor purity, avoiding a potentially erroneous “signal” from samples mixed with nontumor tissue. However, conventional IHC is also associated with poor reproducibility and high interobserver variability, along with variations in the detection capabilities of different antibodies used across laboratories.52 Furthermore, individual MGMT proteins are sacrificed upon interaction with the appropriate substrate and, therefore, the amount of protein detected may not necessarily be a reliable indicator of expression.53
The accuracy of IHC may be increased when expression levels are assessed in combination with assessment of MGMT promoter methylation.54,55 Quantitative methods may overcome some of the major shortcomings of conventional IHC. However, IHC is substantially limited by false positives, MGMT expression by nontumor cells, or false negatives as expression of MGMT is induced upon treatment in unmethylated tumors.56 These and other considerations are discussed comprehensively elsewhere.57 In conclusion, despite its simplicity, MGMT determination by IHC is not sufficiently reliable for basing management decisions upon.
Infinium Methylation EPIC BeadChip Array (27k, 450k, or 850k)
Genome-wide analysis of DNA methylation patterns has improved our understanding of glioma biology and has contributed to the advancement of tumor classification.58–60 The analysis of DNA methylation using BeadChip arrays allows interrogation of 850000 CpG sites in its most recent version (EPIC, 850k). The platform shows good performance also for DNA isolated from formalin-fixed paraffin-embedded (FFPE) tissue samples and is therefore suitable for analysis of clinical samples,61 which lends itself well to the possibility of application for centralized testing. Furthermore, the array technology also enables inference of genome-wide copy number changes26,59,61 and assessment sample purity.62
Of interest for the present review, the MGMT gene is also well covered. A BeadChip-based classifier for MGMT methylation status has been developed and validated using 2 CpG probes located in the MGMT promoter (one in DMR1, the other in DMR2), whose methylations were identified to be most influential for MGMT expression, as well as for outcome in TMZ-treated GBM patients (MGMT-STP27).26 The assay was shown to be also valid for predicting the MGMT methylation status in other tumor types and demonstrated similarity in methylation score regardless of DNA sources (frozen versus FFPE).5,26 The usefulness of the MGMT-STP27 classifier for outcome prediction was shown in several clinical trials.16,63,64
A binary classification of methylated versus unmethylated carries the risk of erroneous misclassification of patients close to the technical cutoff, where uncertainty is high. In the original model, Bady et al established upper and lower confidence intervals around the cutoff, which defines a “gray zone” to minimize the margin of error in identifying “unmethylated” and “methylated” patients.16 It is also of note that there may be a difference between the technical cutoff (50% probability to be methylated) and the clinically relevant cutoff.
The array technology has yet to be accredited by the College of American Pathologists (CAP) and certified by Clinical Laboratory Improvement Amendments (CLIA). Though this is on the horizon, it will likely increase cost. Furthermore, the technology is not available at all sites and a minimum number of samples are necessary to conduct the assay.24 However, DNA methylation-based diagnosis of tumors is likely the future of pathology, redefining histological classifications in up to 12% of cases.60 The added costs of the array technology will therefore likely be offset in the long run by the increase in diagnostic accuracy and the ability to assess a broad range of molecular prognostic markers. Hence, we trust that there is great utility for this assay in both clinical and research settings, particularly for centralized assessment of equivocal cases.
The Need for Quality Control and Establishing Standards
The caveat for the evaluation of MGMT tests is that the large body of publications that interrogate different sets of CpGs in heterogeneous patient populations rarely validate their assays for technical reproducibility and most often lack validation in an independent dataset. Rigorous quality control necessitates the collaborative effort of neuro-oncological surgeons, oncologists, neuropathologists, molecular pathologists, and other team members involved in the processing and assessment of data. The adequacy of the sample provided, including amount of tumor tissue and sampling from geographically independent regions of the tumor tissue, would contribute to decreasing heterogeneity. Sampling of nontumor cells (eg, macrophages and neighboring brain parenchyma) and nonviable tumor regions is a major source of inconsistencies. During histological determination of tumor subtype, selection of regions of high tumor cell purity within a hematoxylin and eosin–stained slide for processing and methylation assessment is critical. This emphasizes the importance of input from an expert neuropathologist.35
Aside from MLPA, all of these assays are limited by the efficiency of bisulfite reaction32 resulting in false positive results.35 This should be addressed in the quality control of the assays. Based on these and other factors, the debate regarding the cutoffs to be applied—hence the amount of methylation that should be considered significant—persists for quantitative methods. This certainly also depends on the clinical question (eg, patient stratification to balance trials versus selection for therapy excluding TMZ).19,20,65 Everhard et al reported that a minimum of at least 9% mean methylation, as determined by pyrosequencing, over 52 CpGs associated with expression was associated with low MGMT RNA expression.66 Brigliadori et al, on the other hand, suggested that a cutoff of >30% of 10 CpG dinucleotides within DMR2 through the pyrosequencing method was most predictive of patient survival.6 Simple qualitative methods (simple MSP) are likely inferior to quantitative methods, as they preclude setting safety margins or evaluating the extent of methylation. Furthermore, the dichotomous classification of methylated versus unmethylated may need to be reevaluated. To this end, we believe that the consideration of 95% confidence intervals around a signal threshold status may be prudent.
A Decision-Making Framework Based on MGMT Promoter Methylation Testing
The utilization of MGMT methylation testing outside of trial settings may be limited. Assessing the National Cancer Database, Lee et al observed that in ~87% of patients with a diagnosis of GBM, the methylation status was unknown or not coded.67 The survival of patients with unknown methylation status was similar to unmethylated patients. With the era of precision medicine upon us, ignoring the strongest prognostic and predictive factor is unacceptable. Standardized algorithms for assessment of methylation status in a much higher proportion of patients will not only lead to better care for methylated patients but also enable the possibility of new therapeutics in unmethylated patients.
Given the universally poor prognosis of high-grade gliomas (histological diagnosis of World Health Organization [WHO] grades III/IV glioma, IDH wildtype, 1p/19q retained), we cannot afford to miss the opportunity to identify the subgroups of patients who may benefit from adjuvant therapy while also minimizing toxicity and developing alternative treatment strategies for others.
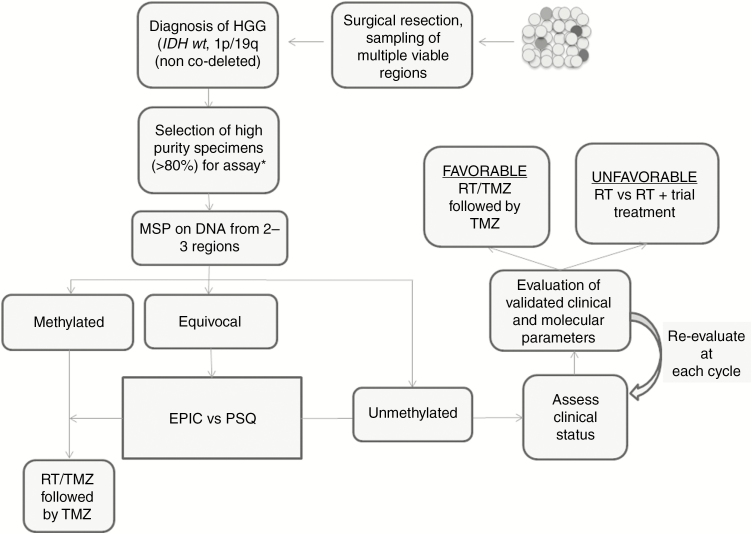
Through distilling the evidence on the available assays, we propose a decision-making algorithm outlined in Fig. 2. In this algorithm, the surgical neuro-oncologist samples multiple regions of the tumor for viable tumor cells. In addition to establishing a histological and molecular diagnosis, the neuropathologist is tasked with selecting slides with the highest tumor purity. qMSP is a good initial method to assess MGMT methylation status, based on its wide availability, ease of interpretation, and low cost (Table 2). The 2 distinctly methylated regions (DMR1 and DMR2) identified by Malley et al and validated by Bady et al should be the area of focus for probe-based assays.26,29 We recommend that patients with methylated status based on MSP testing undergo RT/TMZ followed by TMZ therapy if patients meet clinically appropriate criteria.
Fig. 2.
Proposed management algorithm for patients with glioblastomas, based on methylation status and other clinical parameters. PSQ: pyrosequencing; KPS: Karnofsky performance status; ECOG: Eastern Cooperative Oncology Group.
Equivocal cases based on MSP testing would need further refinement. In high-throughput settings (such as clinical trials or batch testing of clinical samples), either pyrosequencing or Infinium MethylationEPIC BeadChip array can be of great value. The array method has the added value of providing methylation-based tumor classification, including assessing copy number variations and tumor purity. Emerging computer algorithms have the potential to establish the DNA methylation profile of tumors with high fidelity. Head-to-head comparisons, providing correlations with patient outcomes and analysis of cost-effectiveness, would be necessary to select the ideal assay moving forward.
In patients categorized as “unmethylated,” particularly those >65 years old, while strong consideration should be given to withholding TMZ, the results of trials such as the CE.6 study must also be taken into account. An open conversation regarding the risk-benefit profile of adjuvant therapy is also necessary with the patient and/or caregivers. Ideally, recently published prognostic categorization of patients based on clinical and molecular parameters would be implemented, which would add objectivity and help facilitate the discussion between the physician and the patient.55,60 In current practice, the assessment of the various parameters is based on the overall impression of the clinical team and patient preference, which is not captured objectively in clinical trials. Not all elderly patients are homogeneously in a poor clinical condition and thus we suggest the age variable as only part of the decision-making paradigm. Consideration of comorbidities and performance status must be made in allocating patients with “unmethylated” status to different treatment regimens. The efficacy of novel agents should be assessed in the setting of clinical trials. Assignment of patients to a particular intervention is certainly not rigid and we recommend patient reevaluation at each adjuvant treatment cycle in order to maximize safety and efficacy.
Future Directions
Current studies assessing the predictive value of the various assays are likely underpowered.43,51 The performance of the most promising assays can potentially be compared head-to-head prospectively in observational or randomized studies. The yield, feasibility, and cost-effectiveness of “double testing” to refine equivocal cases has to be explored. The objective of this approach would be either to randomize patients with equivocal MSP results to MGMT determination using different assays or to simply compare single testing versus double testing, using a consensus assay for the latter. Focusing on equivocal cases can reduce the required sample size; however, the overall low prevalence of these cases may prolong the study. Still, ultimately correlating the test result with OS will be required.
The array method of assessing methylation status would likely impose not only upfront capital cost but also ongoing material and maintenance costs as well. However, considering that the average per-patient cost in a phase III clinical trial can be as high as $42000, the added cost of this diagnostic assay would only be marginal, considering its many benefits.68
In non-GBM tumors, the clinical impact of MGMT methylation on benefit from TMZ or other alkylating agents remains to be established. GBMs generally have only one MGMT allele, suggesting that methylation of the second allele completely blocks MGMT mediated DNA repair—conferring sensitivity to TMZ treatment. In contrast, other tumor types, including IDH mutant low-grade gliomas, retain both alleles. Hence, a higher extent of methylation may be indicative of inactivation of both alleles, as suggested by the predictive value of a high MGMT methylation score in IDH mutant low-grade glioma patients treated with TMZ in EORTC 22033.62 The assessment of methylation status based on Bady et al’s algorithm using a higher cutoff may be helpful in predicting benefit from TMZ providing a tool for stratified therapy.62 Therefore, relevant cutoffs for IDH mutant tumors need to be established and validated for quantitative assays.
The selection of a valid endpoint would be critical for study design and sample size calculations. Assessment of the accuracy of each assay in the traditional sense (eg, sensitivity and specificity) may not be feasible for MGMT, particularly given that there is currently no gold standard confirmatory test.69 Correlation with response to TMZ therapy and survival is likely more clinically relevant. Furthermore, such an endeavor would require standardization of methods across laboratories. Given the increasing constraints of health care resources, health economic evaluations of the utility of these proposed strategies are necessary as well.
While in the setting of research trials stratification of patients based purely on methylation status would be helpful in choosing therapeutic options, implementation of such important biomarkers in clinical practice has its own challenges, not the least being the lack of a better alternative for patients with an unmethylated MGMT promoter. Within the limitations of the evidence to date, the clinical management of patients based on the MGMT promoter methylation status should be considered together with other molecular and clinical factors that contribute to patient outcome. The merits of this approach need to be examined systematically.
Funding
None
Conflict of interest statement. No conflicts to declare.
References
- 1. Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4(4):278–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 4. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 5. Bady P, Delorenzi M, Hegi ME. Sensitivity analysis of the MGMT-STP27 model and impact of genetic and epigenetic context to predict the MGMT methylation status in gliomas and other tumors. J Mol Diagn. 2016;18(3):350–361. [DOI] [PubMed] [Google Scholar]
- 6. Brigliadori G, Foca F, Dall’Agata M, et al. Defining the cutoff value of MGMT gene promoter methylation and its predictive capacity in glioblastoma. J Neurooncol. 2016;128(2):333–339. [DOI] [PubMed] [Google Scholar]
- 7. Kaina B, Christmann M, Naumann S, Roos WP. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst). 2007;6(8):1079–1099. [DOI] [PubMed] [Google Scholar]
- 8. Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59(4):793–797. [PubMed] [Google Scholar]
- 9. Qian XC, Brent TP. Methylation hot spots in the 5ʹ flanking region denote silencing of the O6-methylguanine-DNA methyltransferase gene. Cancer Res. 1997;57(17):3672–3677. [PubMed] [Google Scholar]
- 10. Hegi ME, Liu L, Herman JG, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26(25):4189–4199. [DOI] [PubMed] [Google Scholar]
- 11. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wick W, Platten M, Meisner C, et al. ; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 13. Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. [DOI] [PubMed] [Google Scholar]
- 14. Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 15. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 17. Perry JR, Laperriere N, O’Callaghan CJ, et al. ; Trial Investigators Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 18. Gállego Pérez-Larraya J, Ducray F, Chinot O, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2011;29(22):3050–3055. [DOI] [PubMed] [Google Scholar]
- 19. Hegi ME, Stupp R. Withholding temozolomide in glioblastoma patients with unmethylated MGMT promoter—still a dilemma?Neuro Oncol. 2015;17(11):1425–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wick W, Gorlia T, Bady P, et al. Phase II study of radiotherapy and temsirolimus versus radiochemotherapy with temozolomide in patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation (EORTC 26082). Clin Cancer Res. 2016;22(19):4797–4806. [DOI] [PubMed] [Google Scholar]
- 21. Herrlinger U, Schäfer N, Steinbach JP, et al. Bevacizumab plus irinotecan versus temozolomide in newly diagnosed o6-methylguanine-DNA methyltransferase nonmethylated glioblastoma: the randomized GLARIUS trial. J Clin Oncol. 2016;34(14):1611–1619. [DOI] [PubMed] [Google Scholar]
- 22. Aldape K, Nejad R, Louis DN, Zadeh G. Integrating molecular markers into the World Health Organization classification of CNS tumors: a survey of the neuro-oncology community. Neuro Oncol. 2017;19(3):336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weller M, van den Bent M, Tonn JC, et al. ; European Association for Neuro-Oncology (EANO) Task Force on Gliomas European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329. [DOI] [PubMed] [Google Scholar]
- 24. Wick W, Weller M, van den Bent M, et al. MGMT testing—the challenges for biomarker-based glioma treatment. Nat Rev Neurol. 2014;10(7):372–385. [DOI] [PubMed] [Google Scholar]
- 25. Hegi ME, Janzer RC, Lambiv WL, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Presence of an oligodendroglioma-like component in newly diagnosed glioblastoma identifies a pathogenetically heterogeneous subgroup and lacks prognostic value: central pathology review of the EORTC_26981/NCIC_CE.3 trial. Acta Neuropathol. 2012;123(6):841–852. [DOI] [PubMed] [Google Scholar]
- 26. Bady P, Sciuscio D, Diserens AC, et al. MGMT methylation analysis of glioblastoma on the infinium methylation BeadChip identifies two distinct CpG regions associated with gene silencing and outcome, yielding a prediction model for comparisons across datasets, tumor grades, and CIMP-status. Acta Neuropathol. 2012;124(4):547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van den Bent MJ, Dubbink HJ, Sanson M, et al. MGMT promoter methylation is prognostic but not predictive for outcome to adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors: a report from EORTC Brain Tumor Group Study 26951. J Clin Oncol. 2009;27(35):5881–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malley DS, Hamoudi RA, Kocialkowski S, Pearson DM, Collins VP, Ichimura K. A distinct region of the MGMT CpG island critical for transcriptional regulation is preferentially methylated in glioblastoma cells and xenografts. Acta Neuropathol. 2011;121(5):651–661. [DOI] [PubMed] [Google Scholar]
- 30. Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. PLoS Med. 2012;9(5):e1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lassman AB, Dimino C, Mansukhani MM, et al. Concordance of EGFR and MGMT analyses between local and central laboratories: implications for clinical trial design and precision medicine for depatuxizumab-mafodotin (ABT-414) in glioblastoma (GBM). NeuroOncol 2017; 19(S6):vi15. [Google Scholar]
- 32. Xia D, Reardon DA, Bruce JL, Lindeman NI. The clinical implications of inconsistently methylated results from glioblastoma MGMT testing by replicate methylation-specific PCR. J Mol Diagn. 2016;18(6):864–871. [DOI] [PubMed] [Google Scholar]
- 33. Vlassenbroeck I, Califice S, Diserens AC, et al. Validation of real-time methylation-specific PCR to determine O6-methylguanine-DNA methyltransferase gene promoter methylation in glioma. J Mol Diagn. 2008;10(4):332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93(18):9821–9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cankovic M, Nikiforova MN, Snuderl M, et al. The role of MGMT testing in clinical practice: a report of the association for molecular pathology. J Mol Diagn. 2013;15(5):539–555. [DOI] [PubMed] [Google Scholar]
- 36. Hsu CY, Ho HL, Lin SC, et al. Prognosis of glioblastoma with faint MGMT methylation-specific PCR product. J Neurooncol. 2015;122(1):179–188. [DOI] [PubMed] [Google Scholar]
- 37. Sciuscio D, Diserens AC, van Dommelen K, et al. Extent and patterns of MGMT promoter methylation in glioblastoma and respective glioblastoma-derived spheres. Clin Cancer Res. 2011;17(2):255–266. [DOI] [PubMed] [Google Scholar]
- 38. Stupp R, Hegi ME, Gorlia T, et al. ; European Organisation for Research and Treatment of Cancer (EORTC); Canadian Brain Tumor Consortium; CENTRIC study team Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 39. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quillien V, Lavenu A, Ducray F, et al. Validation of the high-performance of pyrosequencing for clinical MGMT testing on a cohort of glioblastoma patients from a prospective dedicated multicentric trial. Oncotarget. 2016;7(38):61916–61929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reifenberger G, Hentschel B, Felsberg J, et al. ; German Glioma Network Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer. 2012;131(6):1342–1350. [DOI] [PubMed] [Google Scholar]
- 42. Bienkowski M, Berghoff AS, Marosi C, et al. Clinical neuropathology practice guide 5-2015: MGMT methylation pyrosequencing in glioblastoma: unresolved issues and open questions. Clin Neuropathol. 2015;34(5):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Christians A, Hartmann C, Benner A, et al. Prognostic value of three different methods of MGMT promoter methylation analysis in a prospective trial on newly diagnosed glioblastoma. PLoS One. 2012;7(3):e33449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wojdacz TK, Dobrovic A. Methylation-sensitive high resolution melting (MS-HRM): a new approach for sensitive and high-throughput assessment of methylation. Nucleic Acids Res. 2007;35(6):e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Switzeny OJ, Christmann M, Renovanz M, Giese A, Sommer C, Kaina B. MGMT promoter methylation determined by HRM in comparison to MSP and pyrosequencing for predicting high-grade glioma response. Clin Epigenetics. 2016;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Quillien V, Lavenu A, Karayan-Tapon L, et al. Comparative assessment of 5 methods (methylation-specific polymerase chain reaction, MethyLight, pyrosequencing, methylation-sensitive high-resolution melting, and immunohistochemistry) to analyze O6-methylguanine-DNA-methyltranferase in a series of 100 glioblastoma patients. Cancer. 2012;118(17):4201–4211. [DOI] [PubMed] [Google Scholar]
- 47. Nygren AO, Ameziane N, Duarte HM, et al. Methylation-specific MLPA (MS-MLPA): simultaneous detection of CpG methylation and copy number changes of up to 40 sequences. Nucleic Acids Res. 2005;33(14):e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jeuken JW, Cornelissen SJ, Vriezen M, et al. MS-MLPA: an attractive alternative laboratory assay for robust, reliable, and semiquantitative detection of MGMT promoter hypermethylation in gliomas. Lab Invest. 2007;87(10):1055–1065. [DOI] [PubMed] [Google Scholar]
- 49. Trabelsi S, Mama N, Ladib M, et al. MGMT methylation assessment in glioblastoma: MS-MLPA versus human methylation 450K beadchip array and immunohistochemistry. Clin Transl Oncol. 2016;18(4):391–397. [DOI] [PubMed] [Google Scholar]
- 50. Kristensen LS, Michaelsen SR, Dyrbye H, et al. Assessment of quantitative and allelic MGMT methylation patterns as a prognostic marker in glioblastoma. J Neuropathol Exp Neurol. 2016;75(3):246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Karayan-Tapon L, Quillien V, Guilhot J, et al. Prognostic value of O6-methylguanine-DNA methyltransferase status in glioblastoma patients, assessed by five different methods. J Neurooncol. 2010;97(3):311–322. [DOI] [PubMed] [Google Scholar]
- 52. Mason S, McDonald K. MGMT testing for glioma in clinical laboratories: discordance with methylation analyses prevents the implementation of routine immunohistochemistry. J Cancer Res Clin Oncol. 2012;138(11):1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pieper RO. Understanding and manipulating O6-methylguanine-DNA methyltransferase expression. Pharmacol Ther. 1997;74(3):285–297. [DOI] [PubMed] [Google Scholar]
- 54. Lalezari S, Chou AP, Tran A, et al. Combined analysis of O6-methylguanine-DNA methyltransferase protein expression and promoter methylation provides optimized prognostication of glioblastoma outcome. Neuro Oncol. 2013;15(3):370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bell EH, Pugh SL, McElroy JP, et al. Molecular-based recursive partitioning analysis model for glioblastoma in the temozolomide era: a correlative analysis based on NRG oncology RTOG 0525. JAMA Oncol. 2017;3(6):784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kitange GJ, Carlson BL, Schroeder MA, et al. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro Oncol. 2009;11(3):281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu CC, Smith DR, Chin C, Wang TJC. The success of NRG-GBM-RPA: biomarker-based classification, where to next?Translationa Cancer Res. 2017;6(S3):S541–S543. [Google Scholar]
- 58. Noushmehr H, Weisenberger DJ, Diefes K, et al. ; Cancer Genome Atlas Research Network Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. [DOI] [PubMed] [Google Scholar]
- 60. Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hovestadt V, Remke M, Kool M, et al. Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol. 2013;125(6):913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bady P, Kurscheid S, Delorenzi M, et al. The DNA methylome of DDR genes and benefit from RT or TMZ in IDH mutant low-grade glioma treated in EORTC 22033. Acta Neuropathol. 2018;135(4):601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513–5522. [DOI] [PubMed] [Google Scholar]
- 64. Wiestler B, Capper D, Sill M, et al. Integrated DNA methylation and copy-number profiling identify three clinically and biologically relevant groups of anaplastic glioma. Acta Neuropathol. 2014;128(4):561–571. [DOI] [PubMed] [Google Scholar]
- 65. Shah N, Lin B, Sibenaller Z, et al. Comprehensive analysis of MGMT promoter methylation: correlation with MGMT expression and clinical response in GBM. PLoS One. 2011;6(1):e16146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Everhard S, Tost J, El Abdalaoui H, et al. Identification of regions correlating MGMT promoter methylation and gene expression in glioblastomas. Neuro Oncol. 2009;11(4):348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee A, Youssef I, Osborn VW, Safdieh J, Becker DJ, Schreiber D. The utilization of MGMT promoter methylation testing in United States hospitals for glioblastoma and its impact on prognosis. J Clin Neurosci. 2018;51:85–90. [DOI] [PubMed] [Google Scholar]
- 68. Information CE. Clinical Development and Trial Operations—Protocol Design and Cost per Patient Benchmarks. 2013. [Google Scholar]
- 69. Janes H, Pepe MS, McShane LM, Sargent DJ, Heagerty PJ. The fundamental difficulty with evaluating the accuracy of biomarkers for guiding treatment. J Natl Cancer Inst. 2015;107(8):157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]