Abstract
Background
Although glioblastomas are heterogeneous brain-infiltrating tumors, their treatment is mostly focused on the contrast-enhancing tumor mass. In this study, we combined conventional MRI, diffusion-weighted imaging (DWI), and amino acid PET to explore imaging-defined glioblastoma subregions and evaluate their potential prognostic value.
Methods
Contrast-enhanced T1, T2/fluid attenuated inversion recovery (FLAIR) MR images, apparent diffusion coefficient (ADC) maps from DWI, and alpha-[11C]-methyl-L-tryptophan (AMT)-PET images were analyzed in 30 patients with newly diagnosed glioblastoma. Five tumor subregions were identified based on a combination of MRI contrast enhancement, T2/FLAIR signal abnormalities, and AMT uptake on PET. ADC and AMT uptake tumor/contralateral normal cortex (T/N) ratios in these tumor subregions were correlated, and their prognostic value was determined.
Results
A total of 115 MRI/PET-defined subregions were analyzed. Most tumors showed not only a high-AMT uptake (T/N ratio > 1.65, N = 27) but also a low-uptake subregion (N = 21) within the contrast-enhancing tumor mass. High AMT uptake extending beyond contrast enhancement was also common (N = 25) and was associated with low ADC (r = −0.40, P = 0.05). Higher AMT uptake in the contrast-enhancing tumor subregions was strongly prognostic for overall survival (hazard ratio: 7.83; 95% CI: 1.98–31.02, P = 0.003), independent of clinical and molecular genetic prognostic variables. Nonresected high-AMT uptake subregions predicted the sites of tumor progression on posttreatment PET performed in 10 patients.
Conclusions
Glioblastomas show heterogeneous amino acid uptake with high-uptake regions often extending into non-enhancing brain with high cellularity; nonresection of these predict the site of posttreatment progression. High tryptophan uptake values in MRI contrast-enhancing tumor subregions are a strong, independent imaging marker for longer overall survival.
Keywords: glioblastoma, diffusion-weighted imaging, positron emission tomography, amino acid, survival
Key Points
1. Regions with high tryptophan uptake in peritumoral brain show high cellularity on diffusion MRI.
2. Nonresection of such regions predicts the site of posttreatment tumor progression.
3. High tryptophan uptake in contrast-enhancing tumor regions is prognostic for longer survival.
Importance of the Study
Multimodal imaging, including diffusion-weighted MRI and amino acid PET, can capture glioblastoma heterogeneity, detect viable tumor parts beyond contrast enhancement, and define tumor subregions with a potential effect on prognosis. In the present study, we combined MRI and amino acid PET features (using the radiotracer AMT) to study 5 tumor subregions in 30 patients with newly diagnosed glioblastoma. High AMT uptake extended beyond the contrast-enhancing mass in most tumors into non-enhancing regions with low ADC indicating high cellularity. Surprisingly, higher AMT uptake in the contrast-enhancing tumor region was strongly prognostic for longer overall survival, independent of several clinical and molecular genetic prognostic factors. Nonresection of high-AMT subregions predicted sites of posttreatment tumor progression. The results identify glioblastoma subregions critical for treatment targeting and prognosis.
Despite aggressive multimodal treatment with surgery and chemoradiation therapy, glioblastomas continue to have extremely poor prognosis, with a median overall survival of 15 months.1,2 Clinical prognostic factors for glioblastoma include age, performance status, tumor radiologic features, and extent of initial tumor resection.3,4 Among molecular features, high Ki-67 nuclear labeling index carries unfavorable prognosis,5 whereas isocitrate dehydrogenase 1 (IDH1) mutation is associated with prolonged survival.6,7 O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation is associated with a favorable response to the alkylating chemotherapeutic agent temozolomide.8,9
In clinical practice, conventional MRI, including T1-weighted gadolinium-enhanced (T1-Gad), T2-weighted, and fluid attenuated inversion recovery (FLAIR) sequences are commonly used for diagnosis, treatment planning, and follow-up of high-grade gliomas.10 Advanced MRI techniques, including diffusion-weighted imaging (DWI; providing information about tumor cellularity by measuring the apparent diffusion coefficient [ADC]), and PET can provide additional diagnostic information.11 Amino acid PET has been accurate to distinguish tumor tissue from normal brain and nontumoral lesions,12,13 as well as to detect glioma-infiltrated brain beyond the MRI contrast-enhancing regions.14,15 This capability may explain the reported cost-effectiveness of PET-guided surgery in high-grade gliomas.16 Furthermore, amino acid PET features may have prognostic value. For example, an O-2-[18F]-fluoroethyl-L-tyrosine PET study showed that PET-defined biological tumor volume in newly diagnosed glioblastomas was a prognostic imaging biomarker for survival, independent of MGMT promoter methylation status.17 Another study did not find a difference in MGMT promoter methylation in relation to tumor 1-[methyl-11C]-methionine (MET) uptake, but methylated MGMT promoter and lower standardized uptake values (SUVs) were prognostic for prolonged progression-free survival.18
Glioblastomas are extremely heterogeneous tumors, and imaging features may capture this heterogeneity. High-resolution MRI can identify distinct glioblastoma subregions with different histopathological and molecular genetic features with a potential effect on prognosis.19–21 Amino acid PET studies revealed different histopathological characteristics of diverse glioma subregions defined by discordant amino acid PET and MRI features.22–24 Our recent study with alpha-[11C]-methyl-L-tryptophan (AMT)-PET demonstrated that combination of conventional MRI and AMT-PET can identify quantitative imaging variables associated with prognostic molecular markers in primary glioblastomas.25 While AMT is transported from the blood to tumor by the same transport systems as other commonly utilized amino acid tracers, its tumoral metabolism is unique, as it can be converted to kynurenine metabolites in an immunosuppressive tumor microenvironment.13 However, whether MRI and/or AMT-PET–derived imaging variables from glioblastoma subregions have prognostic value for survival has not been previously established.
In the present study, we utilized a combined MRI/PET approach to define glioblastoma subregions and analyze their structural and metabolic abnormalities. We determined whether AMT-PET can identify metabolic heterogeneity within this contrast-enhancing tumor mass, the main target of clinical treatment. We also hypothesized that tumor regions with high amino acid uptake will show high cellularity, as opposed to subregions showing imaging characteristics indicative of peritumoral vasogenic edema or intratumoral necrosis. In addition, we evaluated the potential prognostic value of ADC and tryptophan uptake values in the imaging-defined tumor subregions while taking other prognostic clinical, histologic, molecular, and imaging characteristics into consideration.
Materials and Methods
Subjects
Thirty patients (20 males, mean age: 59 y; Supplementary Table) with newly diagnosed glioblastoma underwent presurgical MRI and AMT-PET. Twenty of the 30 patients underwent a gross total tumor resection, and 8 had subtotal resection at initial surgery. One patient (#12) with a tumor extending in the basal ganglia underwent a stereotactic biopsy followed by chemoradiation. Another patient (#26) completed presurgical imaging but died from pulmonary embolism prior to tumor resection; glioblastoma diagnosis was confirmed at autopsy. Tissue analysis indicated that 28 of the 30 tumors were IDH1 wild-type, while 12 specimens had a methylated MGMT promoter. Twenty-nine patients (except #26) were treated with standard postsurgical chemoradiation.1 Additional therapy after posttreatment tumor progression is listed in the Supplementary Table. The study was approved by the Wayne State University institutional review board with written informed consent obtained from all participants.
MRI Acquisition
Diagnostic MRIs were performed on a Philips Achieva TX 3.0 Tesla scanner. In all patients, T1, T2, FLAIR, and DW images were acquired followed by T1-Gad sequence. The mean interval between the preoperative MRI and AMT-PET was 3.3 ± 3.4 days. DWI data were acquired at repetition time: 3391 ms; echo time: 73 ms; field of view: 250 cm; 124 × 106 acquisition matrix; 3 mm slice thickness; 0 gap (voxel size: 2.0 mm × 2.4 mm × 3.0 mm); and b-values of 0 and 1000 s/mm2, with an acquisition time of 71 seconds. ADC maps were generated from the DWI data using the MRI scanner software.
AMT-PET
PET studies were performed using a GE Discovery STE PET/CT scanner at the PET Center, Children’s Hospital of Michigan, Detroit Medical Center. The PET image in-plane resolution was 7.5 ± 0.4 mm at full-width half-maximum (FWHM) and 7.0 ± 0.5 mm FWHM in the axial direction, with a slice thickness of 3 mm. The procedure for AMT-PET scanning has been described previously.25–29 Briefly, after 6-hour fasting, a slow bolus of AMT (37 MBq/kg) was injected via a venous line. At 25 minutes after AMT injection, a dynamic emission scan of the brain (7 × 5 min) was acquired. Measured attenuation correction, scatter, and decay correction were applied to all images. For visualization of AMT uptake, averaged activity images 30–55 minutes post-injection were created and converted to an AMT SUV image.
Multimodal Image Analysis
First, the preoperative axial AMT-PET, T2/FLAIR images, and ADC maps were registered to the axial T1-Gad images using the semiautomatic registration tool of AMIDE (A Medical Image Data Examiner version 1.0.4).30 Tumor size was determined by measuring the 2 longest perpendicular diameters of the contrast enhancement or T2/FLAIR hyperintense area (in non-enhancing tumors). For multimodal image analysis, circular or oval tumoral regions of interest (ROIs) were selected on the coregistered MRI/PET images based on the combination of Gad enhancement (positive vs negative) and AMT uptake (high vs low, see criteria below), with a mean ROI volume of 280 mm3. In addition, oval ROIs of similar size were placed in the contralateral cortex homologous to the tumor locations in order to calculate AMT uptake (SUV) and ADC tumor/normal cortex (T/N) ratios. High AMT uptake (ie, “high-AMT”) regions were defined in ROIs exceeding a 1.65 T/N ratio; this cutoff value was found to be optimal to differentiate active tumor from nontumorous tissue abnormalities in our previous studies of patients with glioblastoma.28,29 ROIs on Gad-positive tumor portions were placed both on high-AMT regions, consistent with metabolically active tumor mass and, where clearly present, on low-AMT regions. In addition, 3 types of Gad-negative subregions were analyzed (where present): (i) Gad-negative/high-AMT uptake subregion, consistent with tumor-infiltrated brain, as shown by image-guided stereotactic biopsy sampling in our previous study24; (ii) Gad-negative/low-AMT uptake subregion in the tumor core, consistent with central necrosis; and (iii) Gad-negative/hyperintense T2/FLAIR subregion with low-AMT uptake, consistent with vasogenic edema. Thus, up to 5 types of tumor subregions were defined in each tumor based on Gad enhancement, T2/FLAIR abnormalities, and AMT uptake characteristics (Fig. 1). These ROIs were then applied on coregistered ADC maps and AMT SUV images, and ADC and AMT T/N ratios were calculated for each tumor subregion.
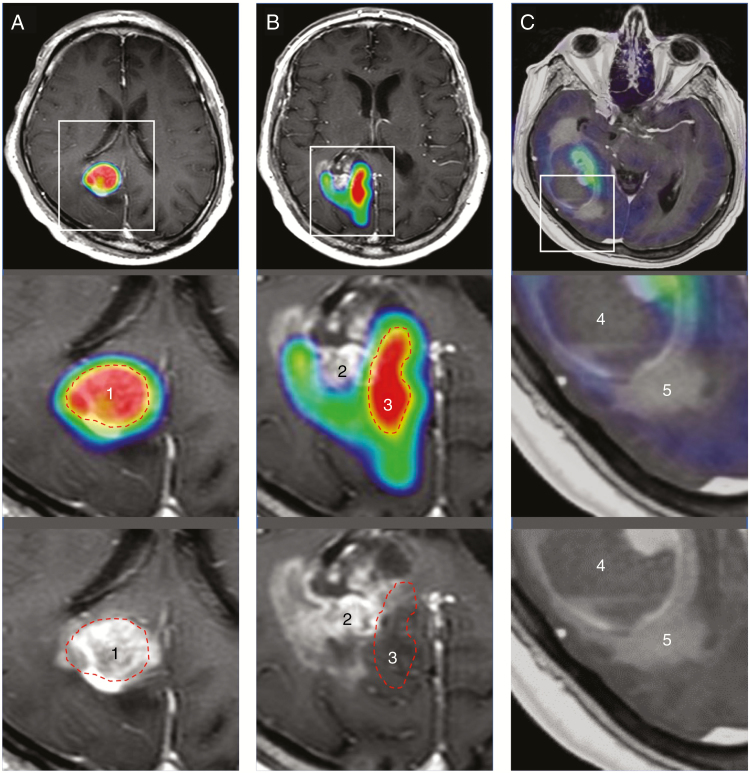
Fig. 1.
Five glioblastoma subregions defined by combined MRI and PET features demonstrated on 3 glioblastomas. Post-contrast MRIs with (upper and middle panels) and without (lower panels) PET fusion of glioblastomas; the lower 2 panels show enlarged regions outlined on the upper images. PET images are thresholded so that red areas are consistent with >65% increase, as compared with normal contralateral cortex. (A) 1: Gadolinium (Gad) enhancement with high alpha-[11C]-methyl-L-tryptophan (AMT) uptake, consistent with metabolically active tumor mass. (B) 2: Gad-enhancing mass with low-AMT uptake, suggesting blood–brain barrier damage with low tumor burden. 3: No enhancement outside the MRI-defined tumor mass, with high-AMT uptake, suggesting tumor-infiltrated brain. (C) 4: No enhancement, low-AMT uptake in the tumor core, consistent with central necrosis. 5: Non-enhancing, hyperintense T2/FLAIR, low-AMT subregion, consistent with vasogenic edema. Red dashed lines indicate the outline of the high PET uptake areas (1 and 3) on MRI with or without fused PET images (A and B).
T1-Gad images obtained shortly (mean, 3 days) after the surgical resection were available in 27 patients and coregistered to the preoperative T1-Gad and PET images to determine the resection/persistence of the above-mentioned subregions.
Evaluation of Tumor Recurrence by Posttreatment AMT-PET
For a subgroup of 10 patients, a second, posttreatment AMT-PET was performed when MRI suggested tumor recurrence31 (mean interval between surgery and posttreatment AMT-PET: 225 ± 134 days). Posttreatment T1-Gad and AMT-PET images were coregistered to the preoperative T1-Gad images, ROIs were placed on the contrast-enhancing region, and AMT SUVs were measured in these ROIs. Regions exceeding the 1.65 T/N SUV ratio threshold on PET were defined as tumor progression.28,29 These PET-defined locations were compared with the original MRI/PET-defined subregions to determine if the progression was colocalized with unresected high-AMT areas.
Histopathology and Prognostic Glioma Markers
In all 30 patients, routine histopathological analysis confirmed the diagnosis of glioblastoma. Glioma prognostic molecular markers were analyzed as described previously.25
Statistical Analysis
First, ADC and AMT T/N ratios were compared among the tumor subregions using pair-wise Wilcoxon signed rank tests in those patients where all 5 types of subregions were present (N = 14), and P-values were Bonferroni corrected. To evaluate the relation between AMT uptake and ADC values, corresponding T/N ratios in the same tumor subregion were correlated using Spearman rank correlation first in the whole set of regions (N = 115), then in the 5 types of tumor subregions separately. In addition, 28 patients were included in a survival analysis (2 subjects were excluded because of death before surgery [patient #26] or very short [<6 mo] postsurgical follow-up [patient #30]). The effect of binary prognostic clinical variables (resection type [total vs subtotal], resection of the MRI/PET-defined subregions, MGMT methylation status, history of second surgery for tumor resection, postoperative tumor-treating fields therapy) on overall survival was analyzed using Cox regression analysis. For other clinical and imaging prognostic variables (age, Karnofsky performance status [KPS] score, tumor size, Ki-67 labeling index, and ADC and AMT T/N ratios in tumor subregions), a receiver operating characteristic (ROC) analysis was first performed to identify an optimal threshold for differentiating patients who were alive at 1-year follow-up from those who had died. For variables where the area under the curve (AUC) was significant or showed a strong statistical trend (P < 0.1) at the defined cutoff threshold, uni- and multivariate Cox regression analyses were performed to obtain a hazard ratio (HR) for overall survival. To determine the value of pretreatment AMT-PET to predict the site(s) of tumor progression/recurrence, the positive and negative predictive values (PPV, NPV, respectively) of unresected high-AMT uptake area were calculated in comparison to posttreatment PET abnormalities. Statistical analysis was carried out using SPSS Statistics 24.0 software (IBM). A P-value < 0.05 was considered to be significant.
Results
Glioblastoma Subregions Defined by MRI and PET Characteristics
In the 30 patients, a total of 115 tumor subregions were analyzed (Table 1). Contrast-enhancing (Gad-positive) tumor was present in 28 patients, and all but 1 had a high-AMT subregion, consistent with metabolically active tumor mass. On the other hand, 21 of the contrast-enhancing tumors also had a distinct, low-AMT subregion (T/N ratio range: 0.85–1.65; see examples in Fig. 1B and Fig. 2A). Altogether, 67 Gad-negative tumor subregions were analyzed, including 25 high-AMT subregions consistent with tumor-infiltrated brain (Fig. 2B); in 22 patients, high-AMT uptake subregions were detected in both contrast-enhancing and non-enhancing tumor portions, while high AMT was confined to the enhancing mass in 5 cases. The 42 low-AMT subregions included 18 with central necrosis and 24 subregions consistent with vasogenic edema.
Table 1.
Comparison of AMT and ADC T/N ratios in the 5 types of tumor subregions
| Gad-Positive | Gad-Negative | ||||
|---|---|---|---|---|---|
| High-AMT | Low-AMT | High-AMT | Hyperintense T2/FLAIR/ Low-AMT |
Low-AMT* | |
| AMT T/N ratio | 2.83 ± 0.69 | 1.26 ± 0.24 | 2.76 ± 0.65 | 0.94 ± 0.27 | 0.94 ± 0.44 |
| ADC T/N ratio | 1.24 ± 0.25 | 1.34 ± 0.35 | 1.12 ± 0.28 | 1.64 ± 0.46 | 2.03 ± 0.81 |
| Subregions | N = 27 | N = 21 | N = 25 | N = 24 | N = 18 |
*In central necrosis.
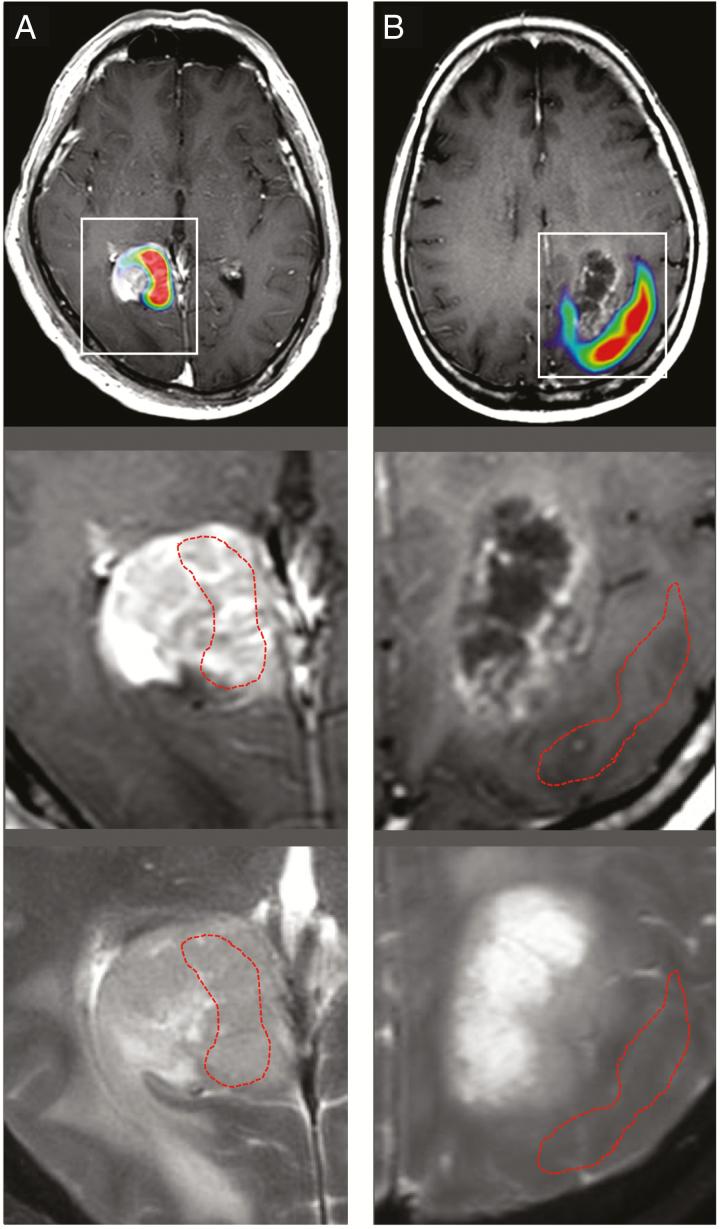
Fig. 2.
Examples showing a mismatch between MRI abnormalities and high alpha-[11C]-methyl-L-tryptophan (AMT) uptake on PET in 2 glioblastomas. (A) Heterogeneous AMT uptake in a Gad-positive glioblastoma, with both high- and low-AMT uptake subregions. (B) Increased AMT uptake localized outside the cystic, contrast-enhancing low-AMT uptake tumor mass. On the middle and lower panels, the tumor region is enlarged, and red dashed lines indicate the outline of high-AMT uptake areas (red on the upper panels, based on a 1.65 tumor/normal cortex ratio) on both T1-Gad (middle) and T2 (lower) MR images.
Comparison of ADC and AMT T/N Ratios Across Tumor Subregions
When the ADC T/N ratios were compared among the 5 types of tumor subregions, the lowest mean values (1.12 ± 0.28), consistent with high cellularity, were found in the Gad-negative/high-AMT subregions (ie, tumor-infiltrated brain), while the highest ADC ratios (2.03 ± 0.81, indicating the lowest cellularity) were observed in areas with central necrosis (Table 1; statistical comparisons in Table 2). Gad-negative/hyperintense T2/FLAIR/low-AMT regions (vasogenic edema) showed the second highest ADC T/N ratios (1.64 ± 0.46). AMT T/N ratios were similar high in the metabolically active (contrast-enhancing) tumor mass and tumor-infiltrated (non-enhancing) brain (P > 0.1; Table 2). Neither the AMT nor the ADC T/N ratios were different in MGMT methylated and MGMT unmethylated tumors in any subregions (P > 0.1).
Table 2.
Pairwise statistical differences between AMT (italic) and ADC (bold) T/N ratios in the 5 tumor subregions. P-values after Bonferroni correction are shown.
| Gad-Positive/ High-AMT | Gad-Positive/ Low-AMT |
Gad-Negative/ High-AMT |
Gad-Negative/ Hyperintense T2/ FLAIR/ Low-AMT |
Gad-Negative/ Low-AMT* | |
|---|---|---|---|---|---|
| Gad-positive/high-AMT | – | <0.01 | >0.1 | <0.01 | <0.01 |
| Gad-positive/low-AMT | >0.1 | – | <0.01 | 0.02 | >0.1 |
| Gad-negative/high-AMT | 0.06 | >0.1 | – | <0.01 | 0.01 |
|
Gad-negative/
hyperintense T2/FLAIR/low-AMT |
0.01 | 0.09 | <0.01 | – | >0.1 |
| Gad-negative/low-AMT* | 0.04 | 0.05 | 0.07 | >0.1 | – |
*In central necrosis.
Correlations Between ADC and PET Uptake Values
In the whole set of the MRI- and PET-defined tumor subregions (N = 115), there was a strong negative correlation between AMT and ADC T/N ratios (r = −0.52, P < 0.0001) (Fig. 3A). Analyzed in the 5 tumor subregions separately, a negative correlation between AMT and ADC T/N ratios was found in the Gad-negative/high-AMT subregions, consistent with infiltrating tumor (r = −0.40, P = 0.05) (Fig. 3B) and in the Gad-negative/hyperintense T2/FLAIR/low-AMT subregions (r = −0.41, P = 0.047). In the 3 other subregions, the correlations between the 2 imaging variables were not significant (P > 0.1).
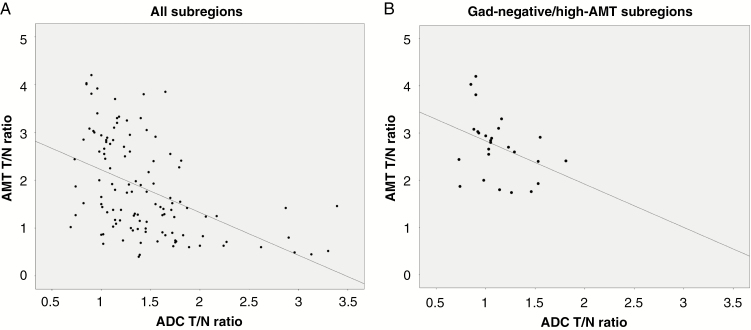
Fig. 3.
Correlation between alpha-[11C]-methyl-L-tryptophan (AMT) uptake and apparent diffusion coefficient (ADC) tumor/normal (T/N) ratios. (A) Negative correlation in the whole set of tumor subregions (N = 115; Spearman’s rho [r] = −0.52, P < 0.0001). (B) A similar trend was detected in Gad-negative/high-AMT subregions, consistent with tumor-infiltrated brain (N = 25, r = −0.40, P = 0.05).
Survival Analysis
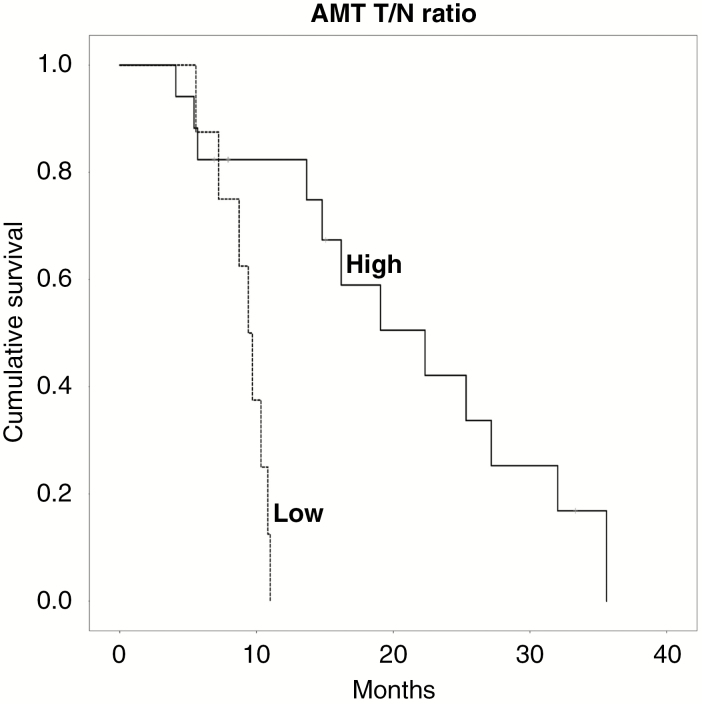
The median overall survival time of the 28 patients was 16.3 months, and 14 patients (50%) had more than 1 year of survival (Supplementary Table). None of the binary clinical predictors showed a significant association with survival in Cox regression analyses. For the other predictors, the ROC analysis showed a significant AUC for AMT T/N ratios measured in the Gad-positive/high-AMT subregions (AUC = 0.84, P = 0.008) and was not significant in the other subregions. When using a cutoff value of 2.38, the AMT T/N ratios measured in the Gad-positive/high-AMT subregion correctly predicted 1-year survival with 100% sensitivity, 73% specificity, 79% PPV, and 100% NPV. In addition, the ROC analysis found a strong trend for the KPS scores (AUC = 0.73, P = 0.052, with an optimal cutoff threshold of 80). Cox regression analysis showed that AMT T/N ratios above the 2.38 threshold were strongly prognostic for longer survival (HR, 7.8; 95% CI: 2.0–31.0; P = 0.003). Estimated mean overall survival was 21.0 (SEM, 2.8) months versus 9.1 (SEM, 0.7) months in patients with above- versus those with below-threshold AMT uptake ratios, respectively (Fig. 4). In Cox regression analysis, KPS scores above 80 were prognostic for longer survival (HR, 4.0; 95% CI: 1.5–10.7; P = 0.005). When both variables (KPS and AMT T/N ratio) were included in a multivariate regression analysis, only the AMT T/N ratio in Gad-positive/high-AMT subregions remained prognostic for longer survival (HR, 5.4; 95% CI: 1.3–23.0; P = 0.02). No other imaging, clinical, or molecular variables showed an association with overall survival.
Fig. 4 .
Kaplan–Meier survival curves for alpha-[11C]-methyl-L-tryptophan (AMT) uptake tumor/normal (T/N) ratios in the gadolinium-positive/high-AMT subregions. Patients with high AMT T/N ratios (based on a cutoff threshold of 2.38; solid line) had a substantially longer cumulative survival than those with low-AMT uptake (dashed line) (HR: 7.8, 95% CI: 2.0–31.0, P = 0.003).
The immediate postoperative MRIs (available in 27 cases) showed that out of 24 Gad-positive/high-AMT uptake subregions, 21 (88%) were completely resected. On the other hand, out of 23 Gad-negative/high-AMT uptake subregions, only 13 (56%) were completely resected, including 2 with only non-enhancing tumor. Gad-positive/low-AMT uptake subregions (N = 20) were resected in 85% of cases. The resection versus nonresection of any of these subregions showed no significant effect on overall survival in Cox regression analysis.
Tumor Progression Evaluated by Posttreatment AMT-PET
Among 10 patients with posttreatment AMT-PET, 7 had high-AMT (above-threshold) uptake in the posttreatment contrast-enhancing lesion, suggestive of tumor recurrence, while 4 lesions showed low-AMT uptake, suggesting radiation injury (patient #29 had 2 separate resected lesions close to each other, 1 had high and 1 low uptake on AMT-PET). Tumor progressed from an unresected Gad-negative/high-AMT uptake subregion in 3 cases and from an unresected or partially resected Gad-positive/high-AMT uptake subregion in 2; moreover, tumor recurrence originated from next to an initially resected Gad-positive/high-AMT uptake subregion in 2 patients. In the 4 tumors without signs of progression on PET, total resection of all subregions had been performed. The PPV of resection of high-AMT uptake regions was 67%, while the NPV was 100% in this subgroup.
Discussion
Previous amino acid PET studies of gliomas mostly analyzed mean or maximum uptake in the whole tumor mass. However, glioblastomas are highly heterogeneous tumors with distinct subregions that can possess different histopathologic and imaging characteristics.19,20 To address this heterogeneity, we classified 115 tumor subregions into 5 types by combined MRI and PET characteristics in patients with newly diagnosed glioblastoma. As expected, the contrast-enhancing tumor portions had a high-AMT uptake subregion in all but one case. However, in about two-thirds of the tumors we also observed a distinct contrast-enhancing subregion with low-AMT uptake, suggesting low tumor burden despite MRI enhancement. In addition, high-AMT uptake often extended to adjacent non-enhancing areas, and these regions showed low ADC values, consistent with high cellularity likely due to tumor cell infiltration. Nonresection of high-AMT uptake regions was associated with subsequent tumor recurrence in a subgroup with a second, posttreatment AMT-PET evaluation. While resection versus nonresection of any of the subregions did not predict overall survival, high-AMT uptake values in the contrast-enhancing tumor regions were associated with prolonged survival. Although the exact reason of this apparently paradoxical finding remains to be determined, the results indicate that very high tryptophan uptake in the contrast-enhancing glioblastoma region defines an imaging phenotype associated with better prognosis, regardless of other prognostic variables.
Glioblastoma Subregions Defined by MRI and PET Characteristics
Since the clinical diagnosis, treatment planning, and follow-up of high-grade gliomas are based on conventional MRI features, particularly contrast enhancement, tumor-infiltrated regions outside the contrast-enhancing tumor mass may be missed or undertreated. High uptake of amino acid PET radiotracers beyond the Gad-enhancing glioma areas has been described, and several reports (including ours with AMT-PET) provided histopathological evidence that such regions have tumor cell infiltration.14,15,22–24 Our previous preliminary study also suggested that detection of such high-AMT uptake areas may improve the accuracy of radiation targeting.32 Interestingly, contrast-enhancing tumor areas showing low amino acid uptake were also common in our study. In posttreatment cases, such combination is consistent with radiation injury.12,27 Some prior amino acid PET studies have looked for such regions in newly diagnosed glioblastomas but showed no data for their presence.22,23 The underlying pathology of contrast-enhancing glioblastoma parts with low amino acid uptake remains to be determined. One plausible explanation is that these areas represent partly necrotic regions with a cellular component in a transitional zone between the core (necrotic) lesion and the tumor brain infiltration. This is consistent with findings of a histopathological analysis of MRI-guided stereotactic biopsies where 60% of the contrast-enhancing regions had “viable tumor cells,” but 31% showed “necrosis with cellular component” undistinguishable on conventional T1-Gad MRI.33 Currently, this remains speculative, as the underlying histopathology in such subregions can only be determined by image-guided stereotactic tumor biopsy.
Relation Between ADC and Amino Acid Uptake
While DWI can provide information about tumor cellularity,34 a previous study found only limited anatomic overlap between regions with low ADC and those with high amino acid uptake (using 3,4-dihydroxy-6-[18F]-fluoro-L-phenyl-alanine [FDOPA] SUV ratios), with a poor correlation between the 2 modalities in newly diagnosed high-grade gliomas.35 Our findings are more in line with a subsequent study that reported a negative correlation between amino acid uptake ratios and normalized minimum ADC values measured in the whole tumor area; although in a voxel-based analysis, these parameters did not correlate well within the tumor.36 In the present study, the correlation between the ADC and AMT uptake ratios was present in non-enhancing, high-AMT tumor subregions which indicate tumor-infiltrated brain shown in our previous study.24 High amino acid uptake with low ADC values can also indicate an area with high tumor proliferative index as reported in both newly diagnosed and recurrent high-grade gliomas.37,38 The other tumor region where these 2 imaging variables correlated was the non-enhancing, T2/FLAIR hyperintense area with low-AMT uptake, supporting that this region has low cellularity and metabolism, consistent with peritumoral vasogenic edema.
Prognostic Value of Amino Acid Uptake
In previous studies, high tumoral amino acid uptake on PET imaging was generally associated with poor prognosis in patients with malignant glioma.18,39,40 Different amino acid PET studies also demonstrated that high metabolic tumor volume was associated with shorter progression-free or overall survival.17,41,42 Our data extend the results of our previous study where high-AMT uptake in the whole tumor mass predicted longer overall survival in newly diagnosed IDH1 wild-type glioblastomas.25 In the present study with more patients and regional analyses, we have identified a specific tumor region (the contrast-enhancing, high-AMT uptake subregion) that appears to drive this prognostic value, independent of several key clinical and tissue prognostic markers. The key clinical and molecular characteristics of our patient group (age, median survival, presence of IDH1 mutation and MGMT promoter methylation) were similar to most other glioblastoma cohorts; the lack of other survival predictors (except the KPS score) may be explained by the limited sample size. Interestingly, resection or nonresection of any of the PET/MRI-defined subregions (including non-enhancing high-AMT uptake regions) also did not affect survival. One plausible explanation is that high-AMT uptake was often seen in the vicinity of the contrast-enhancing mass and, therefore, likely received effective postoperative radiation doses. This is consistent with our previous study demonstrating that targeting of such non-enhancing, high-AMT regions may affect subsequent tumor progression.32 Nevertheless, in the subgroup of 10 patients with a second, posttreatment AMT-PET, PET-defined tumor progression coincided with unresected high-AMT tumor subregions in most cases, while complete resection was followed by negative posttreatment PET findings, suggesting that the observed MRI changes were more likely due to radiation injury. These results support the notion that high uptake regions outside the contrast-enhancing mass are to be resected to prevent/delay posttreatment tumor progression.
Good prognosis of glioblastomas with high initial tryptophan uptake appears paradoxical. While the exact mechanism for this remains to be determined, one might speculate about potential explanations. For example, this finding may be related to the unique characteristics of tumoral tryptophan metabolism. AMT, similar to other common amino acid PET radiotracers, is transported by the same L-type transport system.13 Our recent study also demonstrated that high uptake is largely independent of regional variations of tumoral blood flow measures,43 and this may also explain why mean AMT uptake values were very similar in Gad-enhancing versus non-enhancing regions (with high-AMT uptake), as shown in Tables 1 and 2. However, unlike other PET tracers used in glioma imaging, AMT can undergo at least partial tumoral metabolism via the immunosuppressive kynurenine pathway.44,45 Rate-limiting enzymes of this pathway, such as indoleamine-2,3-dioxygenase (IDO) 1 and 2, as well as tryptophan-2,3-dioxygenase (TDO) can be upregulated in various cancers, including brain tumors,46,47 and their high activity has been associated with poor survival in several cancer types, including malignant gliomas.48,49 As a result of high IDO/TDO activity in the metabolically active tumor mass, tryptophan and its metabolites may undergo breakdown and elimination from tumor tissue, and this may result in lower AMT SUV measured beyond 30 minutes after tracer injection. This theory can be tested in future clinical trials with IDO/TDO inhibitors combined with tryptophan PET imaging in the treatment of glioblastomas.50 Regional tumor analysis could also clarify if our prognostic findings are indeed specific for tryptophan or could be replicated with any other amino acid PET tracers.
Conclusions
Amino acid uptake can differentiate metabolically active glioblastoma subregions (often showing dense cellularity) from necrotic or edematous areas, in both enhancing and non-enhancing areas within the tumor. High tryptophan uptake in MRI contrast-enhancing tumor subregions appears to be a strong, independent imaging biomarker for longer survival in patients with newly diagnosed glioblastomas. Future studies should evaluate the histopathologic and molecular characteristics of multimodal imaging-defined tumor subregions by applying image-targeted tumor sampling.
Supplementary Material
Supplementary data are available at Neuro-Oncology online.
Funding
This study was supported by a grant from the National Cancer Institute (R01 CA123451) and a grant from the Fund for Medical Research and Education of Wayne State University. The Biobanking and Correlative Sciences Core is supported, in part, by National Cancer Institute grant P30 CA022453.
Acknowledgments
We thank William J. Kupsky, MD for the clinical histopathology evaluation of the tumor specimens. We are grateful to the staff at the PET Center, Detroit Medical Center, who provided invaluable technical help in patient scheduling and performing the PET scans.
Conflict of interest statement. None of the authors report any conflict of interest or disclosure.
Authorship statement. Study conception and design: SM and CJ.
Patient recruitment and follow-up: GRB, KDS, and SM.
MRI image acquisition and processing: NLR and AJA-Y.
Image post-processing, analysis and interpretation: FJ and EB.
Design and performance of molecular genetic tissue analyses: SKM and NVK.
Statistical analysis and preparation of illustrations: FJ.
Initial draft of the manuscript: FJ.
Revision of the manuscript: EB, NLR, AJA-Y, GRB, KDS, SKM, NVK, SM, and CJ.
Supervision of data collection and analysis, finalization of the manuscript: CJ.
References
- 1. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2. Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. [DOI] [PubMed] [Google Scholar]
- 3. Delgado-López PD, Corrales-García EM. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol. 2016;18(11):1062–1071. [DOI] [PubMed] [Google Scholar]
- 4. Gittleman H, Lim D, Kattan MW, et al. . An independently validated nomogram for individualized estimation of survival among patients with newly diagnosed glioblastoma: NRG Oncology RTOG 0525 and 0825. Neuro Oncol. 2017;19(5):669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen WJ, He DS, Tang RX, Ren FH, Chen G. Ki-67 is a valuable prognostic factor in gliomas: evidence from a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2015;16(2):411–420. [DOI] [PubMed] [Google Scholar]
- 6. Xie Q, Mittal S, Berens ME. Targeting adaptive glioblastoma: an overview of proliferation and invasion. Neuro Oncol. 2014;16(12):1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng HB, Yue W, Xie C, Zhang RY, Hu SS, Wang Z. IDH1 mutation is associated with improved overall survival in patients with glioblastoma: a meta-analysis. Tumour Biol. 2013;34(6):3555–3559. [DOI] [PubMed] [Google Scholar]
- 8. Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 9. Lalezari S, Chou AP, Tran A, et al. . Combined analysis of O6-methylguanine-DNA methyltransferase protein expression and promoter methylation provides optimized prognostication of glioblastoma outcome. Neuro Oncol. 2013;15(3):370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weller M, van den Bent M, Hopkins K, et al. ; European Association for Neuro-Oncology (EANO) Task Force on Malignant Glioma EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15(9):e395–e403. [DOI] [PubMed] [Google Scholar]
- 11. Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9(9):906–920. [DOI] [PubMed] [Google Scholar]
- 12. la Fougère C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC. Molecular imaging of gliomas with PET: opportunities and limitations. Neuro Oncol. 2011;13(8):806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Juhasz C, Dwivedi S, Kamson DO, Michelhaugh SK, Mittal S. Comparison of amino acid positron emission tomographic radiotracers for molecular imaging of primary and metastatic brain tumors. Mol Imaging. 2014;13. doi:10.2310/7290.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nariai T, Tanaka Y, Wakimoto H, et al. . Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J Neurosurg. 2005;103(3):498–507. [DOI] [PubMed] [Google Scholar]
- 15. Grosu AL, Astner ST, Riedel E, et al. . An interindividual comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET)- and L-[methyl-11C]methionine (MET)-PET in patients with brain gliomas and metastases. Int J Radiat Oncol Biol Phys. 2011;81(4):1049–1058. [DOI] [PubMed] [Google Scholar]
- 16. Heinzel A, Stock S, Langen KJ, Müller D. Cost-effectiveness analysis of amino acid PET-guided surgery for supratentorial high-grade gliomas. J Nucl Med. 2012;53(4):552–558. [DOI] [PubMed] [Google Scholar]
- 17. Suchorska B, Jansen NL, Linn J, et al. ; German Glioma Network Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology. 2015;84(7):710–719. [DOI] [PubMed] [Google Scholar]
- 18. Lopci E, Riva M, Olivari L, et al. . Prognostic value of molecular and imaging biomarkers in patients with supratentorial glioma. Eur J Nucl Med Mol Imaging. 2017;44(7):1155–1164. [DOI] [PubMed] [Google Scholar]
- 19. Barajas RF Jr, Phillips JJ, Parvataneni R, et al. . Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro Oncol. 2012;14(7):942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ElBanan MG, Amer AM, Zinn PO, Colen RR. Imaging genomics of Glioblastoma: state of the art bridge between genomics and neuroradiology. Neuroimaging Clin N Am. 2015;25(1):141–153. [DOI] [PubMed] [Google Scholar]
- 21. Kolakshyapati M, Adhikari RB, Karlowee V, et al. . Nonenhancing peritumoral hyperintense lesion on diffusion-weighted imaging in glioblastoma: a novel diagnostic and specific prognostic indicator. J Neurosurg. 2018;128(3):667–678. [DOI] [PubMed] [Google Scholar]
- 22. Ewelt C, Floeth FW, Felsberg J, et al. . Finding the anaplastic focus in diffuse gliomas: the value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin Neurol Neurosurg. 2011;113(7):541–547. [DOI] [PubMed] [Google Scholar]
- 23. Pafundi DH, Laack NN, Youland RS, et al. . Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro Oncol. 2013;15(8):1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kamson DO, Juhász C, Buth A, et al. . Tryptophan PET in pretreatment delineation of newly-diagnosed gliomas: MRI and histopathologic correlates. J Neurooncol. 2013;112(1):121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bosnyák E, Michelhaugh SK, Klinger NV, et al. . Prognostic molecular and imaging biomarkers in primary glioblastoma. Clin Nucl Med. 2017;42(5):341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Juhász C, Chugani DC, Muzik O, et al. . In vivo uptake and metabolism of alpha-[11C]methyl-L-tryptophan in human brain tumors. J Cereb Blood Flow Metab. 2006;26(3):345–357. [DOI] [PubMed] [Google Scholar]
- 27. Alkonyi B, Barger GR, Mittal S, et al. . Accurate differentiation of recurrent gliomas from radiation injury by kinetic analysis of α-11C-methyl-L-tryptophan PET. J Nucl Med. 2012;53(7):1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamson DO, Mittal S, Robinette NL, et al. . Increased tryptophan uptake on PET has strong independent prognostic value in patients with a previously treated high-grade glioma. Neuro Oncol. 2014;16(10):1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bosnyák E, Kamson DO, Robinette NL, Barger GR, Mittal S, Juhász C. Tryptophan PET predicts spatial and temporal patterns of post-treatment glioblastoma progression detected by contrast-enhanced MRI. J Neurooncol. 2016;126(2):317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging. 2003;2(3):131–137. [DOI] [PubMed] [Google Scholar]
- 31. Wen PY, Macdonald DR, Reardon DA, et al. . Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 32. Christensen M, Kamson DO, Snyder M, et al. . Tryptophan PET-defined gross tumor volume offers better coverage of initial progression than standard MRI-based planning in glioblastoma patients. J Radiat Oncol. 2014;3(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eidel O, Burth S, Neumann JO, et al. . Tumor Infiltration in Enhancing and Non-Enhancing Parts of Glioblastoma: A Correlation with Histopathology. PLoS One. 2017;12(1):e0169292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen L, Liu M, Bao J, et al. . The correlation between apparent diffusion coefficient and tumor cellularity in patients: a meta-analysis. PLoS One. 2013;8(11):e79008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rose S, Fay M, Thomas P, et al. . Correlation of MRI-derived apparent diffusion coefficients in newly diagnosed gliomas with [18F]-fluoro-L-dopa PET: what are we really measuring with minimum ADC?AJNR Am J Neuroradiol. 2013;34(4):758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choi H, Paeng JC, Cheon GJ, et al. . Correlation of 11C-methionine PET and diffusion-weighted MRI: is there a complementary diagnostic role for gliomas?Nucl Med Commun. 2014;35(7):720–726. [DOI] [PubMed] [Google Scholar]
- 37. Karavaeva E, Harris RJ, Leu K, et al. . Relationship between [18F]FDOPA PET uptake, apparent diffusion coefficient (ADC), and proliferation rate in recurrent malignant gliomas. Mol Imaging Biol. 2015;17(3):434–442. [DOI] [PubMed] [Google Scholar]
- 38. Jeong JW, Juhász C, Mittal S, et al. . Multi-modal imaging of tumor cellularity and Tryptophan metabolism in human Gliomas. Cancer Imaging. 2015;15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim S, Chung JK, Im SH, et al. . 11C-methionine PET as a prognostic marker in patients with glioma: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2005;32(1):52–59. [DOI] [PubMed] [Google Scholar]
- 40. Patel CB, Fazzari E, Chakhoyan A, et al. . 18F-FDOPA PET and MRI characteristics correlate with degree of malignancy and predict survival in treatment-naïve gliomas: a cross-sectional study. J Neurooncol. 2018;139(2):399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Galldiks N, Dunkl V, Kracht LW, et al. . Volumetry of [¹¹C]-methionine positron emission tomographic uptake as a prognostic marker before treatment of patients with malignant glioma. Mol Imaging. 2012;11(6):516–527. [PubMed] [Google Scholar]
- 42. Kobayashi K, Hirata K, Yamaguchi S, et al. . Prognostic value of volume-based measurements on (11)C-methionine PET in glioma patients. Eur J Nucl Med Mol Imaging. 2015;42(7):1071–1080. [DOI] [PubMed] [Google Scholar]
- 43. Bosnyák E, John F, Robinette NL, et al. . Amino acid PET and perfusion MRI in contrast-enhancing and non-enhancing regions of glioblastomas [abstract]. Neuro Oncol. 2017;19(suppl 6):vi161. [Google Scholar]
- 44. Chugani DC, Muzik O. Alpha[C-11]methyl-L-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J Cereb Blood Flow Metab. 2000;20(1):2–9. [DOI] [PubMed] [Google Scholar]
- 45. Bosnyák E, Kamson DO, Guastella AR, et al. . Molecular imaging correlates of tryptophan metabolism via the kynurenine pathway in human meningiomas. Neuro Oncol. 2015;17(9):1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Uyttenhove C, Pilotte L, Théate I, et al. . Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–1274. [DOI] [PubMed] [Google Scholar]
- 47. Adams S, Teo C, McDonald KL, et al. . Involvement of the kynurenine pathway in human glioma pathophysiology. PLoS One. 2014;9(11):e112945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Opitz CA, Litzenburger UM, Sahm F, et al. . An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. [DOI] [PubMed] [Google Scholar]
- 49. Wainwright DA, Balyasnikova IV, Chang AL, et al. . IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012;18(22):6110–6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. De Felice F, Musio D, Cassese R, Gravina GL, Tombolini V. New approaches in glioblastoma multiforme: the potential role of immune- check point inhibitors. Curr Cancer Drug Targets. 2017;17(3):282–289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.