Abstract
Phage L encodes a trimeric 43 kDa decoration protein (Dec) that noncovalently binds and stabilizes the capsids of the homologous phages L and P22 in vitro. At physiological pH Dec was unsuitable for NMR. We were able to obtain samples amenable for NMR spectroscopy by unfolding Dec to pH 2 and refolding it to pH 4. Our unfolding/refolding protocol con verted trimeric Dec to a folded 14.4 kDa monomer. We verified that the acid-unfolding protocol did not perturb the secondary structure, or the capsid-binding function of refolded Dec. We were able to obtain complete 1H, 15N, and 13C assignments for the Dec monomer, as well as information on its secondary structure and dynamics based on chemical shift assignments. The assigned NMR spectrum is being used to determine the three-dimensional structure of Dec, which is important for understanding how the trimer binds phage capsids and for the use of the protein as a platform for phage-display nanotechnology.
Keywords: Bacteriophage, Viral assembly, Procapsid, Nanomaterials, Protein stabilization
Biological context
Icosahedral viruses are generally stable but sometimes require additional proteins to further protect their proteinaceous capsid shells from environmental insults. One strategy, is for the viruses to encode ‘cementing’ or ‘decoration’ proteins that non-covalently bind and confer extra mechanical stability to capsids (Casjens and Hendrix 1988; Gilcrease et al. 2005). Cementing proteins encoded by different phages and viruses such as gpD (lambda phage) and SCP (Kaposi’s sarcoma-associated herpesvirus), have different sequences and different folding motifs but share the function of increasing the stability of viruses by non-covalently binding to their icosahedral capsids, thus allowing them to withstand the extreme internal pressures they experience when they become filled with their genomes (Dai et al. 2010; Gilcrease et al. 2005; Hernando-Perez et al. 2014; Iwai et al. 2005; Qin et al. 2010).
The subject of the present work, the decoration protein ‘Dec’, is encoded by bacteriophage L. Dec trimers bind and stabilize capsids of both phages L and P22 in vitro, as the phages have nearly identical coat protein sequences (Casjens and Hendrix 1988; Gilcrease et al. 2005; Parent et al. 2012; Schwarz et al. 2015; Tang et al. 2006). The Dec trimers only bind to mature forms of the viruses, primarily at a specific subset of quasi-threefold axes, with a KD of 9.2 ± 0.5 nM (Parent et al. 2012; Schwarz et al. 2015; Tang et al. 2006). Interaction at the true icosahedral threefold axes occurs with much weaker affinity (Schwarz et al. 2015). In cryo-EM reconstructions of P22 capsids bound by Dec, the trimer has a tripod structure (Parent et al. 2012; Tang et al. 2006). The three legs of the Dec tripod are formed by globular domains that interact with the P22 capsid, while the C-terminal tail forms a spike that extends from the virus surface. We initiated NMR studies of the structure and dynamics of Dec to better understand its capsid binding function. A second motivation for structural studies on Dec is that the protein can be used as a platform for molecular display on the surfaces of phages (Parent et al. 2012; Schwarz et al. 2015). Here we report NMR assignments for monomeric Dec, paving the way for studies aimed at determining the structure of the protein in its free and capsid-bound states.
Methods and experiments
Protein expression and purification
The phage L Dec gene encoding residues M1-S134 was cloned into a pET-15b plasmid (Invitrogen, Carlsbad, CA). For purification, the recombinant gene was inserted after an engineered N-terminal His6-tag and a thrombin cut site. As a result of the thrombin site, the Dec protein used for NMR studies has the extra three N-terminal residues Gly(−2)-Ser(−1)-His(0). The construct was verified through DNA sequencing by Genewiz (South Plainfield, New Jersey). The vector encoding Dec was transformed into Escherichia coli BL21 (DE3) cells, and cultures were grown at 30 °C in M9 media containing 100 μg/mL ampicillin. To prepare perdeuterated samples, cells were acclimatized for growth in D2O. A starter culture grown to mid-log phase, was diluted tenfold into fresh M9 medium composed of 30% (v/v) D2O/H2O. This process was repeated, serially increasing the fraction of D2O, until the final D2O concentration reached ~ 100%. For isotopic labeling, the 100% D2O M9 medium was supplemented with 1 g/L 15NH4Cl and 3 g/L D-glucose 13C6 (99%; 1,2,3,4,5,6,6-d7, 97–98%). Expression was induced by the addition of 1 mM isopropyl thio-β-D-galactoside, and the cells were grown for a further 16 h.
Cells were sedimented at 3,470 g in a Sorvall Lynx 6000 fixed angle rotor and re-suspended in 20 mM sodium phosphate, pH 7.6 containing a 1:100 dilution of protease inhibitor cocktail (Sigma), 0.15% w/v Triton, 0.2 mg/mL lysozyme, 7 mM MgSO4, 0.7 mM CaCl2, and 0.15 mg/mL DNase and RNase. Cells were lysed on a sonicator (Misonix) for 3 min (15 s pulses interspersed with 30 s delays), using an amplitude of 35. To remove cellular debris, the crude extract was sedimented at 28,928×g in a Thermo Scientific F20–12×50 LEX rotor. The supernatant was further centrifuged at 115,630×g in a Sorvall T-865 fixed-angle rotor, to remove cellular membranes. The resulting supernatant was run over at TALON (Clontech) column using a 1 mL/min flow rate. Fractions containing pure Dec were identified by 15% SDS-PAGE. To minimize aggregation, the pooled fractions were dialyzed against 20 mM sodium phosphate buffer, 300 mM NaCl, pH 7.6, before concentration in an Amicon Ultra 10K centricon. Removal of the N-terminal His6-tag was accomplished by treatment of the sample with thrombin (GE Healthcare, Pittsburgh, PA) at a concentration of 1:100 U/μg for 16 h at 4 °C. For the final purification step, samples were run over a Superdex 10/300 GL sizing column, and Dec fractions were collected in 20 mM sodium acetate, 50 mM NaCl, 1 mM EDTA, pH 4, before concentrating the samples to 0.5 mM Dec monomer using an Amicon Ultra 30K filter.
NMR spectroscopy
NMR experiments were done at a temperature of 33 °C on a Varian INOVA 800 MHz spectrometer equipped with a cryogenic probe. NMR samples consisted of 0.3–0.5 mM Dec monomer in 20 mM sodium acetate, 50 mM sodium chloride, and 1 mM EDTA. Initial 1D spectra of Dec at physiological pH gave rather broad NMR lines. We noticed that the spectrum could be significantly improved by acidifying the sample to pH 4 (Fig. 1a). Similar albeit smaller improvements in NMR linewidths were obtained by increasing the salt concentration of the sample up to 1 M at neutral pH, albeit high ionic strength abated the NMR sensitivity of the cryogenic probe. The observation that the spectrum was improved at high salt and at pH 4.0, a pH value near the pKa values of acidic Asp and Glu residues, suggests that aggregation of Dec at neutral pH occurs due to electrostatic interactions. Adjusting Dec samples to pH 4, however, did not give reproducibly good spectra. In some cases, only about ~ 5 crosspeaks from the N and C-termini of the molecule could be observed due to aggregation (Fig. 1b). We were able to obtain reproducibly good NMR samples by unfolding the protein to pH 2 for 20 min, followed by refolding to pH 4 (Fig. 1b). After refolding to pH 4.0, samples monitored by 1H–15N HSQC spectra remained stable for sufficiently long to collect 3D NMR spectra, although some aggregation was evident as a broadening of NMR lines for periods longer than about a week. Circular dichroism (CD) spectra of refolded Dec were indistinguishable from Dec that had not undergone refolding (Fig. 1c), indicating that the secondary structure of the protein is retained following refolding. Upon completion of NMR assignments, 15N relaxation experiments indicated that amide nitrogens from the folded portion of Dec between residues 12–89 had average T2 values of ~ 75 ms. These T2 values are more consistent with the presence of a 14.4 kDa Dec monomer after our refolding protocol, rather than a 43 kDa Dec trimer for which T2 values should be around 20 ms. Native gel electrophoresis confirmed that the acid-unfolding/refolding protocol converted the Dec trimer to a monomer (Fig. 1d). To test that Dec remains functional after refolding, we did an experiment where fresh Dec, as well as Dec that had been unfolded at pH 2 and refolded at pH 4, were incubated with P22 phage. The phages were separated from unbound Dec by a CsCl gradient, the samples were TCA precipitated and run on a 15% SDS gel. Refolded Dec was thus shown to retain its functional ability to bind P22 phage (Fig. 1d), presumably by reforming a trimeric oligomerization state in the presence of capsid.
Fig. 1.
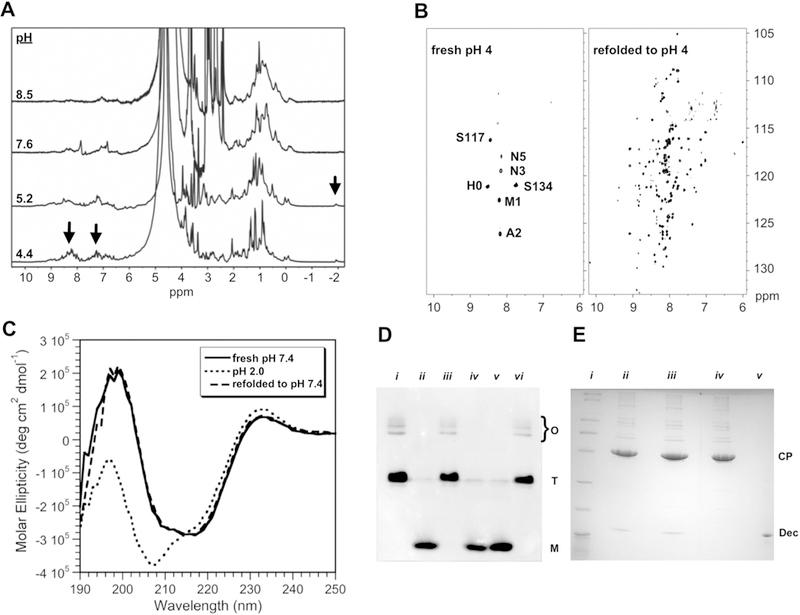
Optimization of NMR conditions. a 1D 1H NMR spectra showing narrowing of signals (arrows) at low pH. b Comparison of 600 MHz 1H–15N TROSY spectra of freshly prepared Dec at pH 4, with Dec that was unfolded at pH 2 for 20 min before refolding to pH 4. c CD experiments to verify that the refolding protocol does not change the secondary structure of Dec. These spectra are compared with Dec at pH 2, which has a CD spectrum typical of an unfolded protein. CD spectra were performed as previously described (Parent et al. 2012), using 0.45 mg/ml Dec in 10 mM NaPO4 at 33 °C. d Native (15% polyacrylamide) gel electrophoresis showing that unfolding of Dec to pH 2 followed by refolding to higher pH, converts it from a trimer to a monomer. Monomer (M), trimer (T), and bands from higher-order oligomers (O) are indicated. Lanes: (i) Dec at pH 7.6; (ii) Dec unfolded at pH 2, followed by refolding to pH 7.6.; (iii) Dec at pH 4; (iv) Dec at pH 4 after unfolding to pH 2 and refolding; (v) Dec in its acid-unfolded state at pH 2; (vi) Lowering the pH from 7.6 to 4 does not convert Dec from a trimer to a monomer (the protein needs to be unfolded to pH 2 to obtain the monomer). e Experiment to show Dec remains competent to bind P22 capsids after unfolding at pH 2, followed by refolding to pH 7.4. Dec samples were mixed with P22 phage and the complexes were separated from unbound Dec by a CsCl gradient, followed by TCA precipitation and analysis by 15% SDS-PAGE. The positions of the Dec and CP (phage coat protein) bands are indicated. Lanes: (i) MW markers (BioRad broad-range SDS-PAGE standards #161–0737). (ii) Native Dec bound to P22 phages at pH 7.4. (iii) Dec retains its ability to bind P22 after refolding from pH 2 to pH 7.4. (iv) P22 phages alone. (v) Dec protein marker
Backbone assignments for the Dec monomer were obtained from the suite of 3D experiments HNCACB, HNCA, HN(CO)CA, HNCO, and HN(CA)CO, recorded in TROSY mode with deuterium-decoupling on a perdeuterated 2H/15N/13C-Dec sample. Side-chain assignments were obtained using 3D HCCH-TOCSY, CCH-TOCSY, and 15N TOCSY-HSQC experiments recorded on 15N/13C-Dec samples. 15N NOESY-HSQC spectra proved additionally useful for sequential and side-chain assignments. These were recorded on both 15N/13C-labled protonated and 50% fractionally deuterated samples of Dec, since the former sample gave predominantly weak long-range NOE contacts. The latter 50% fractionally deuterated sample was prepared by inducing Dec expression in E. coli BL21 (DE3) cells grown in 100% D2O M9 media supplemented with 15NH4Cl and 13C6-glucose (whereas the media was supplemented with 2H7,13C6-glucose to make perdeuterated 2H/15N/13C Dec samples).
Assignments and data deposition
The assigned 1H–15N HSQC spectrum of monomeric Dec is shown in Fig. 2. Backbone assignments were obtained for all residues except Thr130, and are in total 98% complete. Side-chain assignments were obtained for 99% of carbons, 85% of hydrogens, and 94% of nitrogens. The chemical shifts have been deposited in the Biological Magnetic Resonance Bank (http://www.bmrb.wisc.edu/) with the accession number 27435.
Fig. 2.
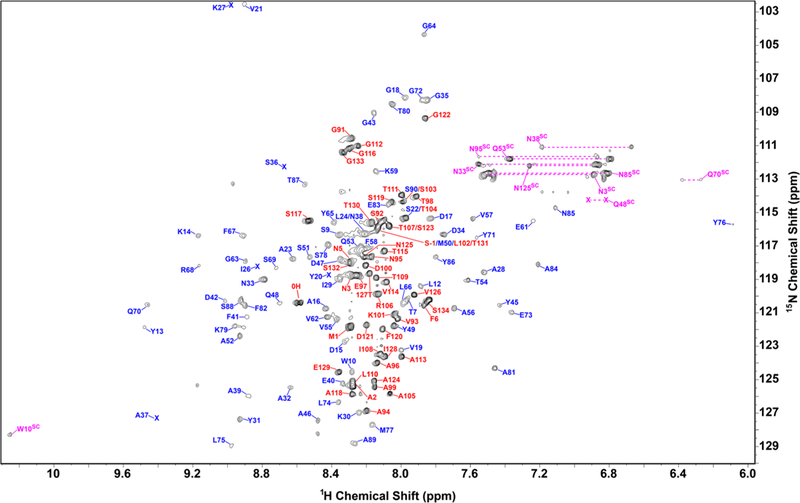
Assigned 1H–15N HSQC fingerprint spectrum of monomeric Dec at 800 MHz. The Dec sample was refolded to pH 4 from pH 2, and contained 50 mM NaCl, 20 mM sodium acetate at a temperature of 33 °C. Backbone resonances from the folded core of Dec (residues 12–90) are indicated with blue labels, those from the disordered N- and C-termini with red labels, side-chain resonances with purple labels. The shifts of V21 and K27 (upper left corner) are aliased in the 15N dimension
Figure 3 shows the secondary structure of Dec calculated from the assigned chemical shifts with the program TALOS-N (Shen and Bax 2015). The program predicts the Dec monomer has a secondary structure composed of six β-strands and two α-helices. The CS-ROSETTA (Shen et al. 2009) server (http://spin.niddk.nih.gov/bax/software/CSROSETTA) predicts based on the assigned NMR chemical shifts of monomeric Dec, that the secondary structure elements β2–β6 (residues 18–77) adopt an OB-fold topology (Murzin 1993), flanked by strand β1 and helix α2. S2 order parameter prediction based on the assigned chemical shifts using the program TALOS-N (Shen and Bax 2015), indicate the first ~ 10 and last 40 residues of the Dec monomers are unfolded (Fig. 3). Consistently, these residues have chemical shifts in the random coil region of the 1H–15N HSQC spectrum (indicated with red labels in Fig. 2). Differences from the secondary structure predicted directly from the sequence, include that strands β4 and β7 are identifi as α-helices based on their chemical shifts. Another important difference is that the sequence-based prediction suggests considerable β-sheet secondary structure for the C-terminus of Dec, downstream of residue 90. Based on NMR data the C-terminus is disordered in Dec monomers. We note, however, that the C-terminus becomes structured when the Dec trimer binds to P22 capsids, as it gives detectable cryo-EM density in the complex (Parent et al. 2012; Tang et al. 2006). Work is underway to determine the 3D-structure of the folded core of the Dec monomers by NMR, and in conjunction with cryo-EM to establish how Dec trimers bind capsids.
Fig. 3.
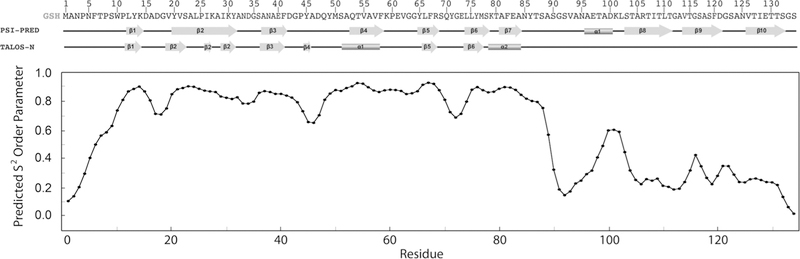
Secondary structure of Dec. From top to bottom: sequence of Dec, secondary structure predicted from sequence with the Psi-Pred server (http://bioinf.cs.ucl.ac.uk/psipred/), secondary structure and backbone dynamics predicted from the assigned NMR chemical shifts with the program Talos-N (Shen and Bax 2015)
Acknowledgements
This work was supported by NIH Grant R01 GM076661 and a Grant from the UConn Research Excellence Program. We thank Prof. Dmitry Korzhnev (UConn Health) for help in setting up deuterium-decoupled experiments, Prof. Angela Gronenborn (U. Pittsburgh School of Medicine) for useful discussion, and Prof. Kristin Parent for providing protocols and assistance for the Dec purification and CsCl gradient experiments.
Footnotes
Conflict of interest The authors declare no conflict of interest.
Ethical standards All experiments complied with all laws of the United States of America.
References
- Casjens S, Hendrix R (1988) Control mechanisms in dsDNA bacteriophage assembly. In: Caendar R (ed) The bacteriophages Plemum Press, New York, pp 15–91 [Google Scholar]
- Dai W, Hodes A, Hui WH, Gingery M, Miller JF, Zhou ZH (2010) Three-dimensional structure of tropism-switching Bordetella bacteriophage. Proc Natl Acad Sci USA 107:4347–4352. 10.1073/pnas.0915008107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilcrease EB, Winn-Stapley DA, Hewitt FC, Joss L, Casjens SR (2005) Nucleotide sequence of the head assembly gene cluster of bacteriophage L and decoration protein characterization. J Bacteriol 187:2050–2057. 10.1128/JB.187.6.2050-2057.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando-Perez M, Lambert S, Nakatani-Webster E, Catalano CE, de Pablo PJ (2014) Cementing proteins provide extra mechanical stabilization to viral cages. Nat Commun 5:4520 10.1038/ncomms5520 [DOI] [PubMed] [Google Scholar]
- Iwai H, Forrer P, Pluckthun A, Guntert P (2005) NMR solution structure of the monomeric form of the bacteriophage lambda capsid stabilizing protein gpD. J Biomol NMR 31:351–356. 10.1007/s10858-005-0945-7 [DOI] [PubMed] [Google Scholar]
- Murzin AG (1993) OB(oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. EMBO J 12:861–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent KN, Deedas CT, Egelman EH, Casjens SR, Baker TS, Teschke CM (2012) Stepwise molecular display utilizing icosahedral and helical complexes of phage coat and decoration proteins in the development of robust nanoscale display vehicles. Biomaterials 33:5628–5637. 10.1016/j.biomaterials.2012.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Fokine A, O’Donnell E, Rao VB, Rossmann MG (2010) Structure of the small outer capsid protein, Soc: a clamp for stabilizing capsids of T4-like phages. J Mol Biol 395:728–741. 10.1016/j.jmb.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz B et al. (2015) Symmetry controlled, genetic presentation of bioactive proteins on the P22 virus-like particle using an external decoration protein. ACS Nano 9:9134–9147. 10.1021/acsnano.5b03360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Bax A (2015) Protein structural information derived from NMR chemical shift with the neural network program TALOS-N. Methods Mol Biol 1260:17–32. 10.1007/978-1-4939-2239-0_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Vernon R, Baker D, Bax A (2009) De novo protein structure generation from incomplete chemical shift assignments. J Biomol NMR 43:63–78. 10.1007/s10858-008-9288-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Gilcrease EB, Casjens SR, Johnson JE (2006) Highly discriminatory binding of capsid-cementing proteins in bacteriophage L. Structure 14:837–845. 10.1016/j.str.2006.03.010 [DOI] [PubMed] [Google Scholar]


