Abstract
Low-grade serous ovarian cancer represents a minority of ovarian cancers and has distinctive features from high grade epithelial ovarian cancer. While less aggressive, in advanced stage they can be poorly chemo-responsive and incur a treatment challenge. Next generation sequencing of tumors has allowed for the potential for targeted therapy in cancer treatment, which can allow for avoidance of traditional cytotoxic chemotherapy. We present a case of a 56 year old female with advanced recurrent low grade serous ovarian cancer found to have NRAS mutation who underwent targeted therapy with trametinib with immediate and sustained disease response. We review the response and toxicity experienced by the patient, as well as treatment for her toxicity.
Keywords: Low-grade serous ovarian cancer, Targeted therapy, MEK-inhibitor
Highlights
-
•
Trametinib was effective in low grade ovarian cancer with NRAS mutation.
-
•
Next generation sequencing of tumors can provide new options.
-
•
Common side effects of trametinib can be managed with dose reduction.
1. Introduction
Low-grade serous ovarian cancer represents a minority of ovarian cancers. However, it is a distinct entity with unique challenges, and separate treatment guidelines have not yet been developed. Low-grade tumors generally have a better survival rate and are less aggressive than high-grade tumors, but they can result in multiple recurrences and be less responsive to chemotherapeutics (Diaz-Padilla et al., 2012; Plaxe, 2008). Exploring the use of new targeted therapies in these patients could be beneficial toward meeting the treatment challenges of these and other gynecologic cancers.
FoundationOne™ offers next-generation massively parallel DNA sequencing of tumors to provide the opportunity to find unique mutations which may be amenable to treatment with various newer targeted therapies instead of or in addition to traditional cytotoxic chemotherapeutics (Frampton et al., 2013). FoundationOne CDx™ sequencing for solid tumors was approved in November 2017.
We present a case of a patient with advanced-stage low grade ovarian cancer who recurred after her initial standard treatment regimen. She went on to several different chemotherapies and hormonal therapy. An enlarging perirectal mass was noted while she was on treatment. Next generation sequencing with Foundation medicine allowed us to focus on her treatment to the use of a MEK inhibitor with great response.
2. Case
The patient is a 56-year-old para 1 Chinese woman with low grade stage IIIC serous ovarian cancer, who initially underwent debulking surgery consisting of a total abdominal hysterectomy, bilateral salpingo-oophorectomy, pelvic and paraaortic lymph node sampling, omentectomy, and appendectomy. This was followed by three cycles of intravenous/intraperitoneal carboplatinum and paclitaxel which was discontinued due to toxicity, at which point she was transitioned to intravenous carboplatinum and paclitaxel for an additional three cycles. The patient was diagnosed with recurrence via laparoscopic biopsy 3 years later where extensive disease was noted with a vaginal cuff mass, anterior abdominal wall and bowel studding, and nodule near the falciform ligament. The patient then received a course of pegylated doxorubicin and carboplatin. This was complicated by doxorubicin Hand-Foot Syndrome. She was subsequently placed on anastrazole. Hormonal therapy provided her with sustained response. Unfortunately, the patient was again found to have a recurrence in one year later with a rising CA-125 level and growing perirectal mass (Fig. 1).
Fig. 1.
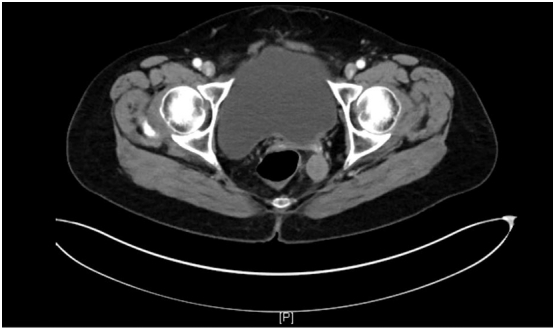
CT imaging of the pelvis showing a perirectal mass prior to treatment with trametinib.
A tumor specimen from her second surgery was sent for FoundationOne™ testing. This testing revealed NRAS Q61K, DNMT3A R882C, and KMT2C (MLL3) P821L mutations. The NRAS (Neuroblastoma RAS viral oncogene homolog) gene codes for a GTPase that is involved in regulating cell division and has been identified as an oncogene. In ovarian cancer specifically, NRAS mutations have been identified as a distinguishing feature found in low grade serous ovarian cancer such as in this patient, but it was not found in borderline tumors (Emmanuel et al., 2014; Hunter et al., 2015). The NRAS mutation identified in our patient's Foundation report was of particular clinical interest since MEK (mitogen activated protein kinase kinase) inhibitors have been identified as being potentially effective against tumors of this type (Miller et al., 2014).
The decision was made to attempt treatment with trametinib (Mekinist), which, although not yet approved for treatment of ovarian cancer, has been used to treat several other cancers and has promise to be effective against ovarian cancer with NRAS mutations, such as that found in this patient (Blumenschein Jr et al., 2015; Lugowska et al., 2015). After approval from the manufacturer for compassionate use, the patient started treatment with trametinib 2 mg daily.
Approximately 10 days later, the patient's cancer antigen 125 (CA-125) had decreased from 91.4 U/mL (on 9/28/2017) to 56.7. After three weeks of treatment, however, the patient was admitted for workup of a fever and rash and trametinib was stopped. The rash was described as pruritic, papulopustular over the face, and a pink papular rash over the trunk and extremities. The patient was evaluated by her gynecologic oncologist and consultation from dermatologist. She was treated with doxycycline and steroids. The rash was thought most likely to be an adverse effect of trametinib and less likely a viral exanthem. The patient was noted to have symptomatic relief after initiation of treatment and was discharged on hospital day four.
After three weeks without treatment, CA-125 had decreased further to 23.2. The patient was restarted on trametinib at a decreased dose of 1.5 mg, 3 weeks following resolution of the rash. The patient's CA-125 was also found to have normalized at that time. The patient did have a recurrence of the rash, and trametinib was once again stopped after another month on the medication. However, the rash was noted to be much milder than at the time of admission. The patient was instructed to resume taking trametinib at a dose of 1 mg daily, but she did not initially take it because she was concerned about side effects. A CT scan on one month later showed a significant decrease in the size of the patient's perirectal mass from 2.3 cm to 1.1 cm. However, there was also an 8.7 × 5.1 cm new loculated fluid collection noted in the upper abdomen. This collection was sampled and was positive for malignant cells. She agreed to begin trametinib 2 months later, after the resolution of her dermatologic symptoms, at 1 mg daily. A repeat CT scan on 3 months later showed near resolution of the perirectal nodule, and the fluid collection was noted to be stable (Fig. 2). The patient was last seen in the office 2 months later, was doing well, and a plan was made to continue treatment with trametinib. She also continues to follow with dermatology and uses topical treatments for her now mild papulopustular skin lesions.
Fig. 2.
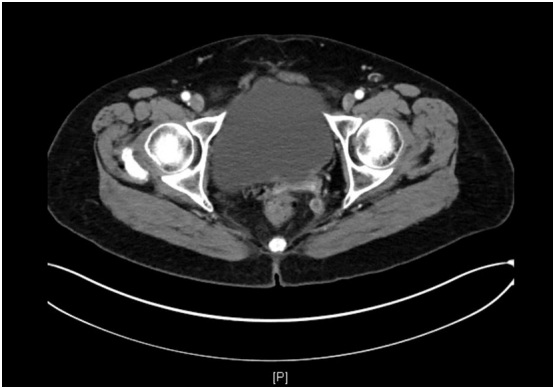
CT imaging of the pelvis showing near complete resolution of the perirectal mass.
3. Comment
We describe the case of a patient with low-grade endometrial cancer that recurred despite initial treatments with surgical cytoreduction and multiple courses of chemotherapeutics. The patient subsequently underwent tumor genetic sequencing and then showed an impressive response to targeted therapy with trametinib. This case report provides further evidence of the potential usefulness of trametinib for patients with low-grade ovarian cancer and the usefulness of tumor sequencing and targeted therapy in general. Other case reports and in vitro studies have shown success in treatment of ovarian cancer with trametinib, some using both trametinib and metformin with synergistic effect for patients with RAS mutations (Mert et al., 2017; Pejovic et al., 2015). A randomized phase II/III clinical trial is currently underway to further study the response to trametinib for patients with recurrent low-grade ovarian cancer (NCT02101788).
Although targeted therapies are generally thought of as having less adverse effects than traditional chemotherapies, it is worthwhile to note the significant dermatologic reaction this patient had, which was most likely a side effect of her treatment with trametinib. Known common side effects of trametinib include diarrhea, serous central retinopathy, and rash including the following types dermatologic findings as reported in a phase 1 dose-escalation trial: erythematous rash, follicular rash, generalized rash, macular rash, maculopapular rash, pruritic rash, pustular rash, seborrhoeic dermatitis, and skin exfoliation (Infante et al., 2012). Dermatitis acneiform involving the face and trunk was noted to be among the most common findings, often developing within 4 weeks of treatment as in our patient. Additionally, the dermatologic side effects were noted to be dose-related. For our patient, her dermatologic side effects indeed seemed to subside after a reduction in trametinib dose.
Although low-grade serous epithelial ovarian cancer represents less than 10% of all ovarian cancers, it can be a challenging entity to treat. This case shows an excellent response to a MEK-inhibitor for an advanced-stage recurrent low-grade cancer that recurred after traditional chemotherapy. We feel that this case highlights a promising treatment for appropriate low-grade lesions and the effectiveness and rising importance of personalized targeted therapy in medicine.
Conflict of interest statement
The authors report no conflict of interest relevant to this manuscript
Authorship contribution statement
Miriam Champer contributed the majority of original writing to the introduction and case. Devin Miller contributed the majority of the discussion, edits, and figures. Dennis Kuo contributed significantly to the editing and preparation of the manuscript as well as mentor for the paper.
References
- Blumenschein G.R., Jr., Smit E.F., Planchard D., Kim D.-W., Cadranel J., De Pas T. A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC)†. Ann. Oncol. 2015;26(5):894–901. doi: 10.1093/annonc/mdv072. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Padilla I., Malpica A.L., Minig L., Chiva L.M., Gershenson D.M., Gonzalez-Martin A. Ovarian low-grade serous carcinoma: a comprehensive update. Gynecol. Oncol. 2012;126(2):279–285. doi: 10.1016/j.ygyno.2012.04.029. Aug. [DOI] [PubMed] [Google Scholar]
- Emmanuel C., Chiew Y.-E., George J., Etemadmoghadam D., Anglesio M.S., Sharma R. Genomic classification of serous ovarian cancer with adjacent borderline differentiates RAS pathway and TP53-mutant tumors and identifies NRAS as an oncogenic driver. Clin. Cancer Res. 2014;20(24):6618–6630. doi: 10.1158/1078-0432.CCR-14-1292. Dec 15. [DOI] [PubMed] [Google Scholar]
- Frampton G.M., Fichtenholtz A., Otto G.A., Wang K., Downing S.R., He J. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol. 2013;31(11):1023–1031. doi: 10.1038/nbt.2696. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S.M., Anglesio M.S., Ryland G.L., Sharma R., Chiew Y.-E., Rowley S.M. Molecular profiling of low grade serous ovarian tumours identifies novel candidate driver genes. Oncotarget. 2015;6(35):37663–37677. doi: 10.18632/oncotarget.5438. Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante J.R., Fecher L.A., Falchook G.S., Nallapareddy S., Gordon M.S., Becerra C. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet. Oncol. 2012;13(8):773–781. doi: 10.1016/S1470-2045(12)70270-X. Aug. [DOI] [PubMed] [Google Scholar]
- Lugowska I., Koseła-Paterczyk H., Kozak K., Rutkowski P. Trametinib: a MEK inhibitor for management of metastatic melanoma. Onco. Targets. Ther. 2015;8:2251–2259. doi: 10.2147/OTT.S72951. Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mert I., Chhina J., Allo G., Dai J., Seward S., Carey M.S. Synergistic effect of MEK inhibitor and metformin combination in low grade serous ovarian cancer. Gynecol. Oncol. 2017;146(2):319–326. doi: 10.1016/j.ygyno.2017.05.019. Aug. [DOI] [PubMed] [Google Scholar]
- Miller C.R., Oliver K.E., Farley J.H. MEK1/2 inhibitors in the treatment of gynecologic malignancies. Gynecol. Oncol. 2014;133(1):128–137. doi: 10.1016/j.ygyno.2014.01.008. Apr. [DOI] [PubMed] [Google Scholar]
- Pejovic T., Corless C.L., Taylor M., Allen A.J., Fung A., Beer T.M. Case report significant response to trametinib in a woman with recurrent KRAS-mutated low-grade serous carcinoma of the ovary-a case report. Am. J. Clin. Exp. Obstet. Gynecol. 2015;2(3):140–143. [Google Scholar]
- Plaxe S.C. Epidemiology of low-grade serous ovarian cancer. Am. J. Obstet. Gynecol. 2008;198(4) doi: 10.1016/j.ajog.2008.01.035. Apr. 459.e1–8; discussion 459.e8–9. [DOI] [PubMed] [Google Scholar]


