Abstract
The European Society of Gastrointestinal Endoscopy (ESGE) and United European Gastroenterology present a list of key performance measures for endoscopy services. We recommend that these performance measures be adopted by all endoscopy services across Europe. The measures include those related to the leadership, organization, and delivery of the service, as well as those associated with the patient journey. Each measure includes a recommendation for a minimum and target standard for endoscopy services to achieve. We recommend that all stakeholders in endoscopy take note of these ESGE endoscopy services performance measures to accelerate their adoption and implementation. Stakeholders include patients and their advocacy groups; service leaders; staff, including endoscopists; professional societies; payers; and regulators.
Keywords: Endoscopy, quality improvement, quality and safety, patient experience, leadership
Introduction
The quality of gastrointestinal endoscopy is important to patients. It is known that there is considerable variation in the quality and safety of endoscopy, indicating significant room for improvement.1–3 Historically, the focus of quality and safety has been on the performance of individual endoscopists, with the definition and measurement of performance metrics, and the use of these to target interventions designed to improve performance. There has been less focus on the environment within which endoscopists work, and the role or responsibility of an endoscopy service in the quality improvement cycle.
An endoscopy is part of a patient’s diagnostic or therapeutic journey. What happens before and after the procedure impacts on his or her experience and safety. An endoscopist performs the procedure, but he or she is dependent on a team to perform the procedure well and safely. Thus, the quality and safety of endoscopy depends on the environment within which endoscopists work (including the facilities and equipment) and the staff who work in that environment. Individual endoscopists and their staff have to be aware that there is room for improvement, believe that improvement will make a difference, be motivated to improve, participate in further development, and finally audit that improvement to ensure the required level has been achieved.
The endoscopy service has a key role to play in providing high-quality, safe, and patient-centred endoscopy. Collecting performance data and feeding it back to endoscopists provides metrics that may be used to target interventions designed to improve performance; for example, by motivating endoscopists to change their practice; providing time and opportunity to improve, possibly by in-house training; and finally by applying restrictions if the individual does not achieve the required levels of performance. Such ongoing monitoring, in the context of an understanding of the roles and responsibilities that an endoscopy service has in the quality improvement cycle, should lead to continuing improvement.
Within this context, the purpose of this guideline is to provide recommendations on what an endoscopy unit should have in place to meet these requirements. It is recognized that the recommendations may require new roles and information-gathering systems, and that there will be implications for the types of staff and staffing levels. Therefore, to achieve the recommendations, there will need to be extra resource allocation. Moreover, we appreciate that an excellent patient experience, and high-quality and safe endoscopy brings potential savings. This guideline provides explicit recommendations about what is required to deliver a modern endoscopy service. We recognize that payers, the organizations within which endoscopy services sit, and those who allocate resources, as well as those who work within the service, already understand the importance of quality.
Methodology
The group followed the European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Committee (QIC) performance measures processes, as outlined in an earlier paper.4 The literature base that informs the requirements for endoscopy services is potentially huge, but there is paradoxically relatively little high-quality evidence on which to base recommendations. Moreover, technical and safety requirements are usually defined by current regulations and legislation in force in each country. A comparative analysis of national safety regulations in European Union (EU) countries is beyond the aims of this project. Therefore, on the basis that legislative requirements and regulations will be addressed within the organizational context, the group decided to focus on a review of general guiding principles that should be considered when organizing endoscopy services and evaluating their performance.
It was decided that the approach to the potentially vast literature was to focus the search on recently published guidelines or recommendations (details of the search strategy and results can be viewed in the Supporting Information, available online). Two consensus documents discussing quality and safety indicators common to endoscopy services,5,6 as well as the EU quality assurance guidelines focused on colorectal cancer (CRC) screening activity,7 were retrieved and assessed using the AGREE II checklist. We performed a comparative analysis of these documents considering the domains addressed, the quality indicators (and eventually performance targets) proposed, including the rationale for the choice of the indicator, and finally a judgement of the quality of the evidence reported by each document.
In addition, the working group agreed to take note of a fourth document, the UK Endoscopy Global Rating Scale (GRS) that, while not published in a peer-reviewed journal and not based on literature directly related to health services, was nevertheless founded on a wide consensus established within a nation, over a 10-year period. The GRS framework8 has been used to plan the organization of the endoscopy services involved in the National Health Service (NHS) bowel cancer screening programme. The GRS framework is now integrated into the Joint Advisory Group on Gastrointestinal Endoscopy (JAG) endoscopy service accreditation standards.9 The working group agreed it would act as a ‘sense check’ on the other guidance. Another reason for including this approach was because it was the starting point for two of the published guidelines and has also been tested in experimental studies evaluating the impact of quality-promoting interventions on endoscopy outcomes.10–12 These studies support an association between procedural requirements indicated in the GRS recommendations and clinically relevant outcomes.
Endoscopy Global Rating Scale
The GRS8 arose from a need to have a measure of performance of endoscopy services within England in 2004 and, ultimately, for all the countries of the United Kingdom. While the initial intention was to use the GRS as a performance measure, its main function has been to act as a service improvement tool – a roadmap for services to follow to improve the quality of care. It currently consists of a series of statements in 19 domains: quality and safety (6); patient experience (7); staffing (3); and endoscopist training (3). The statements within each domain are layered to provide a measure of performance from D to A. All endoscopy units in England are required to self-assess against the GRS at least once a year, and the output from this self-assessment forms part of an accreditation process.
The initial versions of the GRS grew from a consensus view of what an endoscopy service should have in place. It has been subject to constant challenge from the service and has undergone several reviews, the latest in 2016. Thus, as feedback from the entire endoscopy service has formed part of its evolution, one might consider the GRS has evolved with the ultimate consensus process. However, its weakness is that at no stage did the evolution involve a review of the relevant literature.
EU guideline on quality assurance of CRC screening
Chapter 5 of the EU guideline published in 201013 was devoted to recommendations for quality assurance of endoscopy services involved in CRC screening. The first step in the process was to agree fundamental principles on which the guidance would be based. The second step was to create a series of PICO (population; intervention; comparator; outcome) questions that were subject to evidence search and review. Finally, the authors of the chapter developed a set of recommendations (in line with the methodology of the other chapters of the guideline) that were graded in two domains: strength of the evidence and strength of the recommendation.
The recommendations were reached by consensus of the group without a Delphi process. The chapter was subject to formal review and feedback from external experts. In keeping with the paucity of high-quality literature in this area, many of the recommendations did not have a sound evidence base, but nevertheless came with strong recommendation.
Canadian consensus guideline
The Canadian guideline process6 used the GRS as a starting point to generate a series of questions, with a subsequent search for and review of the evidence. Almost 2500 publications were identified. A working group was presented with summary evidence and required to vote on a series of recommendations using a Delphi process. The eventual output of this methodology was similar to the EU guideline: overall strong recommendations but weak evidence.
Quality Indicators for Gastrointestinal Endoscopy Units – ASGE Endoscopy Unit Quality Indicator Taskforce
The American Society of Gastrointestinal Endoscopy (ASGE)5 created a taskforce with subgroups charged with identifying and reviewing the literature in five domains: patient experience; employee experience; efficiency and operations; procedure-related unit issues; and safety and infection control. The process followed three stages: systematic literature review; generation of potential endoscopy unit quality indicators; and rating of these potential indicators on several parameters by invited participants (beyond the taskforce) in two rounds of voting using a modified Delphi process. The taskforce reached consensus on a final set of endoscopy unit quality indicators. The outcome of this approach was similar to the EU and Canadian guidelines: strong recommendations based on weak evidence.
Development process
A critical appraisal of the four documents indicated that three of them were based on systematic extensive searches of the literature, which found relatively little substantive evidence. Furthermore, all four documents had used consensus methods to make recommendations. The ESGE Endoscopy Service Working Group, in consultation with the parent ESGE QIC, agreed that three of the four documents should form the basis of the initial draft of consensus statements, being the three that were based on systematic reviews of the literature. It was agreed that areas/domains deemed to be relevant by the working group but not covered by these three documents should be subject to further literature review. Four areas of interest were identified:
Determining the importance of leadership in an endoscopy service;
The impact of programmes of monitoring and reviewing adverse events;
The effect of recognition and reward systems for endoscopy staff;
The effectiveness of objective setting and plans for improvements.
Clinical questions, structured using the PICO framework, were formulated/defined to inform searches for available evidence to support the performance measures related to these areas of interest (see Supporting Information, available online).
Summary position and a way forward
It is clear that there is a substantial literature relating to guidelines for endoscopy services, but that this literature is unable to answer key questions as there are few high-quality intervention studies that provide evidence on which to base recommendations for practice. This is perhaps not surprising, because the recommendations in these various guidelines are largely recommendations about process. Demonstrating that process impacts on quality and safety is difficult because patient outcomes are usually dependent on a variety of correctly applied processes. Teasing out the relative contributions is difficult and, in some situations, impossible. We searched for intervention studies, in addition to evidence found in previous guideline publications.
Terminology
The various guidance documents use different language to describe similar concepts: recommendations; indicators; metrics; measures; or facilities, services, and units. This guideline will use the words ‘services’ and ‘recommendation’ or ‘suggestion’. The recommendations are intended to help endoscopy services become better organized and more effective: a vehicle for service improvement. The word ‘suggestion’ is used when the strength of the recommendation is weaker but nevertheless positive.
Some jurisdictions may want to use the recommendations as part of a process of quality assurance, such as the JAG accreditation process in the UK.9 If used for this purpose, it is suggested that the word ‘requirement’ be used in place of ‘recommendation’ for things that must be in place for the assurance process – whatever they might be. It is not expected that all the recommendations would become requirements. The ESGE is not in a position, and neither is it appropriate, to mandate requirements because different jurisdictions will have different challenges, varied demands on their services, and varied resources to meet them.
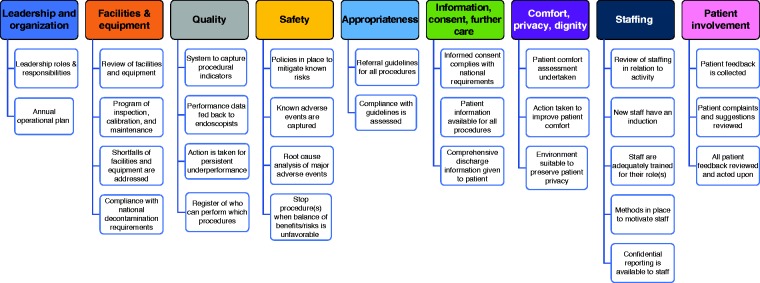
The performance measures are laid out in tabular form with the rationale and proposed minimum and target standards. Unlike the performance measures for endoscopic procedures, it has not been possible to recommend a target percentage. In most circumstances, the standard refers to a process or structure that needs to be in place: it is either in place or not in place. The 30 performance measures identified across nine different domains are summarized in Figure 1.
Figure 1.
Overview of endoscopy services performance measures.
For performance measures referring to the four areas identified for further exploration, the results of the review of the literature and the evidence appraisal are discussed below the measure. For the other measures, we have indicated whether they were mentioned/considered by the reference documents and the eventual reported judgement about the quality of available evidence.
1 Domain: Leadership and organization
1.1 Leadership roles and responsibilities
| Performance measure | We recommend endoscopy services have a competent leadership team with defined roles and responsibilities, including a description of accountability | |||
| Domain | Leadership and organization | |||
| Category | Process | |||
| Rationale | There are a variety of leadership competency frameworks against which endoscopy leaders can be assessed Accountability here refers to who the team is accountable to for governance (essentially quality and safety): in a hospital, there will usually be well-defined pathways for governance; in stand-alone units, it may not be so clear – but is important A leadership team should create a culture of high quality and safety, and one that is patient centred | |||
| Standards | Minimum standard: a description of the leadership roles and responsibilities for the service (clinical lead, nurse lead, training lead, management leadership, and support), including lines of accountability; members of the leadership team have defined time allocated to their leadership roles Target standard: the leadership team makes it clear to staff what is meant by patient-centred, safe, and high-quality care in the service, and what is expected of staff to achieve this | |||
| Consensus agreement | 100% | |||
| PICO | Population: Any healthcare organization/unit/department, or any healthcare provider Intervention: Introduction of leadership team, with defined roles and responsibilities and accountability Control: No defined leadership team Outcome: Continued improvements in technique, quality, and safety of services/care provided | |||
| Concordance with other guidelines | ASGE Not assessed | Canada Not assessed | EU Not assessed | GRS/JAG accreditation Yes |
| Evidence grading | Quality of the evidence according to the GRADE approach was not assessed because the retrieved literature was too heterogeneous (different study design, many of the reviews and primary studies reported results only in a narrative way) | |||
Seven systematic reviews were included.14–20 A detailed description of their characteristics and of the relevant studies included is available in Supporting Information, available online.
Overall, the evidence about the impact of the introduction of a leadership team, with defined roles and responsibilities, and accountability on continued improvements in technique, quality, and safety of services/care provided, is sparse, heterogeneous, and of low quality (many of the studies are uncontrolled studies). The most reliable evidence is about the association of nursing leadership and patient outcomes, showing that relational leadership practices are positively associated with some categories of patient outcomes and nurse satisfaction. Walk rounds* seem to give promising results in increasing a climate of safety.
Finally, there is a randomized controlled trial that shows the positive impact on colonoscopy performance of targeting leaders of endoscopy services with an educational intervention aimed at both leadership and endoscopic skills.21
1.2 Annual operational plan
| Performance measure | We recommend endoscopy services be organized to acquire the necessary resources to deliver the service and to maximize utilization of these resources while maintaining high patient satisfaction, quality, and safety | |||
| Domain | Leadership and organization | |||
| Category | Structure and process | |||
| Rationale | An endoscopy service should first of all determine the demand it expects and what level of service provision it is required to deliver, as indicated by European and national regulation and guidance; it can then define the resources it needs – resources in this context include human as well as physical resources (see Domain 8 on staffing) Many nations will have referral guidelines but most will not have target intervention rates per head of population for common endoscopic procedures on which to base demand; however, it should be possible to estimate future demand of a service based on past activity (both actual and trends), size of backlog, and length of waiting lists, while new screening programmes will usually have accurate predictions of the extra demand on the service, making it possible for endoscopy services to plan for this There is intense pressure on endoscopic capacity in most countries and resources are constrained everywhere, so it is important to maximize use of resources (many services will be under intense pressure to do more for less, which could put patients at risk and affect quality and patient experience) This recommendation recognizes the tension and that achieving it will expose resource constraints that will impact on patient care | |||
| Standards | Minimum standard: an annual operational plan to meet the demands on the service that includes the necessary facilities, kit, information technology, safety equipment, workforce, and endoscopy list capacity Target standard: an annual assessment of the effectiveness of the plan; a medium- to long-term plan for future investment based on expected changes in demand | |||
| Consensus agreement | 96.3% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Not assessed | Canada Not assessed | EU Not assessed | GRS/JAG accreditation Yes |
| Evidence grading | Not applicable | |||
2 Domain: Facilities and equipment
2.1 Review of facilities and equipment
| Performance measure | We recommend that the endoscopy service carry out an assessment of the facilities and equipment required to deliver the service at least annually | |||
| Domain | Facilities and equipment | |||
| Category | Process | |||
| Rationale | An endoscopy unit cannot function without the necessary facilities and equipment | |||
| Standards | Minimum standard: an annual review of the endoscopy facility and kit requirement that informs the annual operating plan (see Performance measure 1.2) identifying: ▪ shortfalls of existing facilities and equipment ▪ necessary replacement of existing facilities and equipment ▪ facilities and equipment required for future demand Target standard: facility and kit requirements match the recommendations of the annual review, plus ad hoc reviews undertaken when there are significant changes in service provision, such as a move to new premises, adoption of new procedures, or when review of adverse events identifies inadequate facilities or kit | |||
| Consensus agreement | 100% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Low/very low | |||
2.2 Programme of inspection, calibration, and maintenance
| Performance measure | We recommend that the endoscopy service has a planned programme of inspection, calibration, and maintenance of its clinical equipment according to the manufacturers’ advice and relevant national regulations | |||
| Domain | Facilities and equipment | |||
| Category | Process | |||
| Rationale | This is a basic requirement to minimize the risk of equipment failure | |||
| Standards | Minimum standard: a planned annual programme of inspection, calibration, and maintenance of its clinical equipment, with clinical equipment not meeting planned inspection and calibration requirements being withdrawn from use Target standard: none, the minimum standard is a safety and regulatory requirement | |||
| Consensus agreement | 96.3% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Low/very low | |||
2.3 Shortfalls of facilities and equipment are addressed
| Performance measure | We recommend that the endoscopy service has a plan to address shortfalls, plus replacement and purchase of facilities and equipment | |||
| Domain | Facilities and equipment | |||
| Category | Process | |||
| Rationale | Planning equipment replacement is a basic requirement as it ensures safe continuity of the service | |||
| Standards | Minimum standard: systems in place to ensure that all facilities and equipment replacement identified in Performance measures 2.1 and 2.2 is planned, including a rolling programme of replacement of endoscopic equipment Target standard: acquisition of necessary facilities and equipment is not constrained by business planning, purchasing, or tendering processes | |||
| Consensus agreement | 96.3% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Not assessed | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Low/very low | |||
2.4 Compliance with national decontamination requirements
| Performance measure | We recommend that decontamination facilities, equipment, and processes meet national and/or European standards | |||
| Domain | Facilities and equipment | |||
| Category | Structure and process | |||
| Rationale | This is a basic requirement and services should follow ESGE guidance if there is no national guidance It is suggested that there be a named person responsible for overseeing compliance of decontamination | |||
| Standards | Minimum standard: decontamination procedures and processes that comply with national or European regulatory requirements Target standard: none, the minimum standard is a safety and regulatory requirement | |||
| Consensus agreement | 100% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Low/very low | |||
3 Domain: Quality
3.1 System to capture procedural indicators
| Performance measure | We recommended endoscopy services have systems in place for capturing and presenting key endoscopy performance indicators for all procedures undertaken by the service | |||
| Domain | Quality | |||
| Category | Structure | |||
| Rationale | Capturing and presenting performance data is essential for a unit to be able to demonstrate its endoscopists reach required standards and to monitor improvements if they are required The ESGE and some national bodies recommend the minimum key performance indicators that should be captured | |||
| Standards | Minimum standard: a list of procedural indicators based on ESGE and/or national body guidelines, and an endoscopy reporting system (ERS), or equivalent, to capture procedural indicators continuously Target standard: a system for collating and presenting individual and summary performance data for all procedures performed by the service | |||
| Consensus agreement | 100% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Low/very low | |||
3.2 Performance data fed back to endoscopists
| Performance measure | We recommend key performance indicators are fed back to and discussed with endoscopists on a regular basis, and that corrective action for improvement, when indicated, with objectives are agreed with the individuals | |||
| Domain | Quality | |||
| Category | Process | |||
| Rationale | Systematic reviews indicate that when healthcare professionals are given data on their performance they will, in most circumstances, improve; there is evidence that this is the case in endoscopy | |||
| Improvement in response to feedback is, however, highly variable because some may not consider it necessary to improve and others may not know how to get better; not all endoscopists will automatically get better when presented with performance data, so a discussion and plan, with agreed objectives, are necessary if all endoscopists are to improve It is expected that the endoscopist member of the leadership team will conduct this discussion; objectives may include further training that may have to be sourced elsewhere The frequency of feedback and discussion depends on the metrics for the procedure and the sample size required to know whether performance is below acceptable levels, but it is recommended that feedback occurs at least annually, more frequently if concerns have been raised about performance by patients, staff, or other endoscopists An open discussion of performance (all endoscopists knowing each other’s data) is to be recommended to foster an open and quality-focused culture; however, it is important that within the discussion of improving performance it is made clear what factors about the service (particularly the team) can be improved and what factors the individual is responsible for | ||||
| Standards | Minimum standard: procedural performance data is fed back to individual endoscopists at least annually and there is guidance on what to do if recommended performance levels are not achieved and/or maintained Target standard: objectives are agreed with individuals to improve performance and all endoscopists are made aware of each other’s performance data | |||
| Consensus agreement | 96.3% | |||
| PICO | Population: Any healthcare organization/unit/department, or any healthcare provider Intervention: Audit and feedback programmes Control: No audit and feedback programmes Outcome: Continued improvements in technique, quality, and safety of services/care provided | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | The overall quality of evidence was judged as low for inconsistency and indirectness | |||
Two Cochrane reviews22,23 and three systematic reviews1,24,25 providing evidence about the effects of audit and feedback interventions, as compared with usual care, were retrieved. Most studies considered in these reviews were focused on the assessment of the effects of audit and feedback on the practice of healthcare professionals, but some of them also assessed outcomes related to patients’ health. The available evidence suggests that audit and feedback generally lead to small, but potentially important, improvements in professional practice. The effects are generally small to moderate, and vary based on the way the intervention is designed and delivered.
Provider assessment and feedback strategies may be effective in increasing breast, cervical, and colorectal faecal occult blood test screening uptake, with positive effects observed from studies using both continuous and dichotomous outcome measures, while results were not consistent when assessing other patient-related outcomes, such as immunization rates or hypertension control.
There is a recent publication26 that outlines a pragmatic approach to identifying and supporting underperformance of endoscopists. The proposed approach recognizes that performance may be influenced by several factors pertaining to individuals or to their departments. The strategies aimed to address underperformance should adopt a stratified approach, modulating the intensity of the interventions (ranging from feedback and audit to specific re-training) based on the level of risk to patient safety.
3.3 Action is taken for persistent underperformance
| Performance measure | We recommend that the endoscopy service ensures that, if corrective actions for improvement have been ineffective, new actions are agreed and implemented, and/or that the host organization quality and risk committee is informed of the continued underperformance | |||
| Domain | Quality | |||
| Category | Process | |||
| Rationale | To protect patients, an endoscopy service must check that its corrective actions have been effective and, if not, that something further is being done – the way to show a corrective action has been effective is to set some measurable objectives for the improvement plan and then ensure those objectives have been achieved within a set timescale Clearly, it is unacceptable if the objectives are not achieved; in this case there should be a review of why they have not been achieved and, if the reason is beyond the control of the endoscopy team, the problem should be escalated ‘up’ to someone who has the influence and control to do something about it For example, if an endoscopist refuses to improve his/her performance, or shows unacceptably bad behaviour when in the unit and refuses to or cannot change, the endoscopy unit may have little power to deal with the problem if they do not directly employ this endoscopist; in these circumstances, the problem needs to be escalated to someone who does have the power to deal with them The organization, at the very least, should have a governance structure to deal with such problems and it would be completely unacceptable to allow the problem to continue unchecked | |||
| Standards | Minimum standard: when objectives agreed with an endoscopist to improve performance have not been achieved within agreed timescales, new actions are agreed and implemented, and/or the host organization quality and risk committee is informed of continued underperformance Target standard: when an individual has not met the required level of performance despite repeated efforts to support and re-train them, their rights to perform that procedure are withdrawn | |||
| Consensus agreement | 92.59% (1 disagree vote) | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Low/very low | |||
3.4 Register of who can perform which procedures
| Performance measure | We recommend that it is made clear which diagnostic and therapeutic procedures endoscopists are competent in and allowed to perform in the service | |||
| Domain | Quality | |||
| Category | Structure | |||
| Rationale | An endoscopist performing a procedure he/she is not trained and competent to perform will put patients at risk and is therefore a major governance issue, so we suggest a register is kept of who is allowed to do what in the endoscopy unit, which will empower nursing staff and other endoscopists, ideally through the leadership team, to challenge endoscopists who perform procedures for which they do not have permission This raises issues of who is responsible for governance, such as local services, professional bodies, national health services, or health insurance companies, and also how competence is defined, which will be the subject of future ESGE guidance There is also the issue of how many procedures an individual should be expected to do during a given time period and what cover there should be for emergency endoscopy (e.g. for upper gastrointestinal [GI] bleeding and endoscopic retrograde cholangiopancreatography [ERCP]), two points that are beyond the remit of this guideline | |||
| Standards | Minimum standard: an up-to-date register is kept of who is allowed to perform which endoscopic procedures Target standard: review of the register of who is allowed to perform procedures in the department (based on performance; see Performance measures 3.1 and 3.2) at least annually | |||
| Consensus agreement | 88.89% (3 disagree votes) | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Low/very low | |||
4 Domain: Safety
4.1 Policies in place to mitigate known risks
| Performance measure | We recommend endoscopy services identify potential risks to patients and staff and implement policies and procedures to mitigate them | |||
| Domain | Safety | |||
| Category | Process | |||
| Rationale | The best way to avoid risks is to prevent them, and the best way to prevent risks is to know what they are and put in place processes to avoid them –examples of this would be protocols for patients on anticoagulants and in-room checklists (‘time out’), which are risk-mitigation processes While there will be some risks common to all patients, different services will also have different risks – risks here include the risks associated with infrastructure and equipment, such as air quality; disposal of effluents; and ensuring the use of disposable equipment complies with national guidance Services are referred to other guidance on safety, such as antibiotic and anticoagulation guidelines | |||
| Standards | Minimum standard: a list of principle known risks with policies, protocols, and/or checklists in place to mitigate these Target standard: regular (at least annual) review of the risk-mitigation processes to ensure that they are effective | |||
| Consensus agreement | 96.3% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Low/very low | |||
4.2 Known adverse events are captured
| Performance measure | We recommend there be a process for capturing and reviewing adverse events to determine whether further improvements are required | |||
| Domain | Safety | |||
| Category | Process | |||
| Rationale | Safety is the primary concern of the airline industry, which is obsessional about identifying, reporting, and reviewing safety-related events, both the expected (as per Performance measure 4.1) and the unexpected; this measure requires there to be methods in place to do exactly the same The review process should be a formal and defined exercise as per Performance measure 4.3, which this measure also leads into: it is a basic requirement not just to identify and review adverse events but to know that what has been put in place has been successful – if you don’t measure you don’t know | |||
| Standards | Minimum standard: adherence to the system for capturing and reviewing adverse events within the host organization (if there is no such system then the service should create one specific to the service) Target standard: none, the minimum standard is safety related and will be a requirement in most organizations | |||
| Consensus agreement | 96.3% | |||
| PICO | Population: Any healthcare organization/unit/department or any healthcare provider Intervention: Programmes of monitoring and revision of adverse events Control: No defined programmes of monitoring and revision of adverse events Outcome: Continued improvements in technique, quality, and safety of services/care provided | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | The overall quality of evidence was judged as low for risk of bias and indirectness | |||
Three Cochrane reviews27–29 and one systematic review,30 including randomized controlled trials and observational studies using a before/after, or interrupted-time-series design, were retrieved.
Evidence for effectiveness has been documented for approaches based on technique or personnel changes, for educational interventions, for interventions involving structured process changes (including triage protocols, feedback steps, addition of a checklist, and quality improvement processes), the adoption of technology-based system interventions (including computerized decision-support systems and alerting systems), or the introduction of additional review methods (i.e. an additional review step, usually by a separate reader).
Overall, all the processes and methods assessed seemed to be beneficial in reducing diagnostic errors. The evidence seemed strongest for approaches using technology-based systems (for example, text message alerting) and specific techniques (for example, testing equipment adaptations), which could be implemented in endoscopy settings. However, the studies were very heterogeneous for study design, settings, and outcomes.
4.3 Root cause analysis of major adverse events
| Performance measure | We recommend endoscopy services perform a root cause analysis of major events, such as missed cancers, unplanned admissions, and unexpected deaths following endoscopic procedures, and use the learning from the analysis to improve the service | |||
| Domain | Safety | |||
| Category | Process | |||
| Rationale | As adverse events are so rare in endoscopy, it is reasonable to review them to determine whether anything could have been done, with the benefit of hindsight, to prevent them, this being a basic safety behaviour: learn from things that happen to avoid them recurring Root cause analysis is a specific process whereby every aspect of the event is reviewed to extract maximum learning There is a question of what ‘major’ means in this context, with various publications having categorized degrees of harm, but no equivalent publications on quality; in addition, there are some indicators that are not clearly quality or safety issues: for example, endoscopists would regard delayed diagnosis of cancer as a major quality indicator but for the patient it is a major adverse event and, because of this importance to the patient, it has been included here as a safety measure Services might consider using a critical incident reporting system (CIRS); a process of learning from adverse events such as this is how the airline industry reduces the risk of planes crashing | |||
| Standards | Minimum standard: a list of known major adverse events relevant to the service, with a reporting and review process that systematically identifies these major adverse events and learns from them Target standard: actions required in response to learning from major events are implemented within 3 months of being reported | |||
| Consensus agreement | 88.89% (3 undecided) | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Not assessed | GRS/JAG accreditation Yes |
| Evidence grading | Low/very low | |||
4.4 Stop procedures when balance of benefits/risk is unfavourable
| Performance measure | We recommend that if there is insufficient resource to reduce the risks of a procedure to recommended levels, the service should review whether it should, on the balance of benefits and risks, continue to perform that procedure | |||
| Domain | Safety | |||
| Category | Structure | |||
| Rationale | The first step, if there is insufficient resource to reduce the risk, is to decide whether the service should continue performing that procedure Ultimately it may be decided that there are some risks that have to be accepted even if there is insufficient resource to reduce them – for example, a service may not be able to stock all the available devices | |||
| to arrest bleeding following a polypectomy Declaring that there is an outstanding risk (for example, on a risk register, which may be called something different in different countries) raises awareness that there is still a potential problem and increases the likelihood that the necessary resources will be found | ||||
| Standards | Minimum standard: decisions to continue to provide or withdraw procedures (when there is insufficient resource to mitigate the risks associated with them) are based on a formal written review of the balance of benefits and risks Target standard: continuous monitoring of risks associated with inadequate resource and at least annual re-assessment of the balance of risks and benefits identified in the minimum standard | |||
| Consensus agreement | 96.3% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Not assessed | Canada Not assessed | EU Not assessed | GRS/JAG accreditation Yes |
| Evidence grading | Not applicable | |||
5 Domain: Appropriateness
5.1 Referral guidelines for all procedures
| Performance measure | We recommend endoscopy services have available, in written and electronic form, referral guidelines for all endoscopic procedures performed within the service that are based on regional and/or national guidelines | |||
| Domain | Appropriateness | |||
| Category | Process | |||
| Rationale | Most jurisdictions accept that there should be criteria for performing an invasive and potentially dangerous procedure, and having these criteria available makes it more likely they will be used We recommend endoscopy services make accessible to all endoscopists their referral guidelines (based on regional and/or national guidelines) for all endoscopic procedures performed within the service | |||
| Standards | Minimum standard: local referral guidelines based on regional, national, or European guidelines are available for all procedures performed by the service Target standard: guidelines are accessible in the department and to all endoscopists | |||
| Consensus agreement | 96.3% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Not assessed | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Very low | |||
5.2 Compliance with guidelines is assessed
| Performance measure | We recommend endoscopy services have policies and processes in place to assess the appropriateness of procedures against guidelines and take action when endoscopic procedures have been performed inappropriately | |||
| Domain | Appropriateness | |||
| Category | Process | |||
| Rationale | Having methods in place to check compliance with guidelines reduces risks to patients and ensures resources are used appropriately; at the very least, referrals from non-GI specialists should be | |||
| reviewed, and some services may choose to review referrals from GI specialists to reassure payers that their resources are being used appropriately as there is considerable evidence that GI specialists fail to follow either upper or lower endoscopy surveillance guidelines, meaning a strong case can be made for always reviewing surveillance decisions It is noted that there are sometimes very good reasons to perform procedures outside of published guidelines, in which case the reasons should be made explicit in the patient record – if for no other reason than to protect the referrer in the event something goes wrong – and any review of referrals outside of guidelines should take exceptional circumstances into account For some situations, such as intervals to next surveillance procedure, decisions should only rarely fall outside the guidelines; however, failure to comply with guidelines in this situation is more likely to have resource implications than put patients at risk, whereas failure to adhere to guidelines for high-risk procedures, or for patients at high risk, may put patients in jeopardy Auditing adherence to guidelines is a time-consuming process and services should prioritize this activity based on impact on resources and risk | ||||
| Standards | Minimum standard: defined criteria and processes on how compliance with guidelines is assessed, including prioritization of the assessment of compliance that is based on risk to patients and resources Target standard: compliance with guidelines is assessed according to processes defined in the minimum standard of Performance measure 5.2 | |||
| Consensus agreement | Consensus: 81.48% (1 disagree, 4 undecided) | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Moderate to very low | |||
6 Domain: Information, consent, and further care
6.1 Informed consent complies with national requirements
| Performance measure | We recommend endoscopy services have policies and procedures in place that are aligned with national and organizational requirements to ensure patients provide informed consent prior to having an endoscopic procedure | |||
| Domain | Information, consent, and further care | |||
| Category | Process | |||
| Rationale | This is a basic requirement in most countries, and good quality consent starts well in advance of the procedure | |||
| Standards | Minimum standard: a policy for informed consent compliant with national and organizational requirements Target standard: procedures and processes to ensure guidance is adhered to | |||
| Consensus agreement | 100% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Low/very low | |||
6.2 Patient information available for all procedures
| Performance measure | We recommend endoscopy services provide patients with information about their procedure that is sufficiently understandable to them to enable them to provide informed consent | |||
| Domain | Information, consent, and further care | |||
| Category | Process | |||
| Rationale | This is a basic right for patients, so the endoscopy service should ideally provide basic information and give patients an opportunity to ask further questions, as well as regularly ask patients what amount and detail of information is appropriate | |||
| Standards | Minimum standard: patient information is available for all procedures (diagnostic and therapeutic) performed by the service Target standard: an assessment of whether the information for endoscopic procedures is understandable to most patients has been undertaken | |||
| Consensus agreement | 100% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Low/very low | |||
6.3 Comprehensive discharge information given to patient
| Performance measure | We recommend endoscopy services provide patients, prior to leaving the service, with the results of the procedure, the timing and mode of communication of pathology results, a plan of the next steps, and an explanation of what delayed complications can occur and what to do about them | |||
| Domain | Information, consent, and further care | |||
| Category | Process | |||
| Rationale | This is a basic right for patients – what any patient would want – and the information should also be made available to other healthcare professionals involved in the management of the patient, such as referring physician and ward personnel | |||
| Standards | Minimum standard: a process to provide all patients and relevant healthcare professionals with the recommended information on discharge Target standard: an assessment (at least annually) of whether patients and healthcare professionals receive and understand the recommended discharge information | |||
| Consensus agreement | 96.3% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Low/very low | |||
7 Domain: Comfort, privacy, and dignity
7.1 Patient comfort assessment undertaken
| Performance measure | We recommend endoscopy services have procedures in place to assess the comfort of patients before, during, and after procedures | |||
| Domain | Comfort, privacy, and dignity | |||
| Category | Process | |||
| Rationale | Knowing what patients are experiencing is the first step to improving patient comfort Assessment of comfort should include both feedback from patients (or their carers) and also an assessment by staff, nurses, and endoscopists, with validated measures being used wherever possible | |||
| Standards | Minimum standard: agreed measures and processes to assess patient comfort before, during, and after all procedures Target standard: as for minimum standard | |||
| Consensus agreement | 100% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Moderate/low | |||
7.2 Action taken to improve patient comfort
| Performance measure | We recommend information on comfort is reviewed and fed back to endoscopists and staff and, where appropriate, action is taken to improve patient comfort levels | |||
| Domain | Comfort, privacy, and dignity | |||
| Category | Process | |||
| Rationale | As for Performance measure 7.1, plus action needs to be taken to protect patients from unnecessary pain | |||
| Standards | Minimum standard: information on patient comfort levels is collated, reviewed, and fed back to individual endoscopists and staff at least twice a year Target standard: when review of patient comfort identifies areas for improvement, action is taken to improve patient comfort within a reasonable time period (6–12 months) | |||
| Consensus agreement | 96.3% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Low/very low | |||
7.3 Environment suitable to preserve patient privacy
| Performance measure | We recommend that endoscopy services provide an environment and have processes in place that ensure the privacy and dignity of patients is respected and maintained | |||
| Domain | Comfort, privacy, and dignity | |||
| Category | Structure and process | |||
| Rationale | It is all too common for endoscopy teams to forget about the privacy and dignity of patients It is not possible to be prescriptive about what privacy and dignity means (it will be different in | |||
| different cultures and may be constrained by the physical nature of the unit); it is therefore advised that endoscopy services use patients and their carers who access the service to help define what is required, and subsequently test whether this is meeting patients’ needs by asking regularly about their experience | ||||
| Standards | Minimum standard: environment and processes conducive to ensuring patient privacy and dignity are in place based on national requirements, where they exist, and feedback from patients of what they expect Target standard: an annual review of patients’ perceptions of privacy and dignity | |||
| Consensus agreement | 100% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Not assessed | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Moderate to very low | |||
8 Domain: Staffing
8.1 Review of staffing in relation to activity
| Performance measure | We recommend that the endoscopy service undertakes regular reviews of staffing in relation to activity to identify gaps, and to improve the match between the skills of staff and the work undertaken | |||
| Domain | Staffing | |||
| Category | Process | |||
| Rationale | The qualitative and quantitative demands on an endoscopy service change with time, which means that the type and number of staff required to deliver the service is also likely to change, so regular review of the staffing of a service is essential if it is going to manage the demands placed upon it The European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA) has developed a European profile and a core curriculum for nurses working in endoscopy; however, it should be recognized that it is not only physicians and nurses who work in endoscopy units but also technicians and administrative staff, so it is essential that these roles are included in the review | |||
| Standards | Minimum standard: an annual review of staffing in relation to activity Target standard: staffing matches the recommendations of the review, with ad hoc reviews following any significant changes in service provision (such as new premises or new procedures) or in response to significant adverse events or shortfalls in quality that identify concerns about staffing levels | |||
| Consensus agreement | 96.3% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Not assessed | GRS/JAG accreditation Yes |
| Evidence grading | Low/very low | |||
8.2 New staff have an induction
| Performance measure | We recommend that all new staff (including new endoscopists) undertake an induction and orientation programme before working in the service | |||
| Domain | Staffing | |||
| Category | Process | |||
| Rationale | Each endoscopy unit is different, often with significant differences in culture, processes, and policies; therefore, to provide a safe, high-quality service, new recruits need to understand these | |||
| differences, even if they have worked in endoscopy previously Induction should be based on local, national, and professional guidance where it exists | ||||
| Standards | Minimum standard: polices and systems that ensure all new staff have an induction appropriate to their role, including procedure-specific requirements Target standard: induction programmes include all temporary staff, such as locums, students, and trainees | |||
| Consensus agreement | 92.59% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Not assessed | Canada Not assessed | EU Not assessed | GRS/JAG accreditation Yes |
| Evidence grading | Not applicable | |||
8.3 Staff are adequately trained for their role(s)
| Performance measure | We recommend that the endoscopy service ensure that all staff (including the leadership team) have the necessary training and achieve the required competencies to undertake their roles | |||
| Domain | Staffing | |||
| Category | Process | |||
| Rationale | This is a basic requirement of any service within or outside of healthcare and there are two key aspects to this recommendation: ▪ the service needs to have instruments to assess competencies – or otherwise create them ▪ the service needs to have staff who are able to provide the training; in certain circumstances the service will not have the capability to carry out the training, in which case this should be ‘outsourced’ elsewhere, with the necessary resources to do this being identified | |||
| Standards | Minimum standard: access to service and procedure-specific competencies and methods to assess them Target standard: a training and assessment process that ensures the workforce is properly trained and competent, including procedure-specific education and training | |||
| Consensus agreement | 96.15% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Low/very low | |||
8.4 Methods in place to motivate staff
| Performance measure | We recommend the endoscopy service has methods in place to motivate staff to improve the service | |||
| Domain | Staffing | |||
| Category | Process | |||
| Rationale | Ultimately it is not possible to deliver a high-quality service if staff are not motivated to do this, so identifying good quality care and giving staff recognition for their contribution will help to motivate them Examples would be publicly recognizing when patients compliment individual members of staff or perhaps rewarding staff who make suggestions for service improvement when their idea is implemented Recognizing and rewarding motivates staff to excel | |||
| Standards | Minimum standard: processes for recognizing and rewarding the achievements of the team and its members Target standard: staff involvement in determining how to motivate staff to excel | |||
| Consensus agreement | 88.89% (1 disagree, 2 undecided) | |||
| PICO | Population: Any healthcare organization/unit/ department or any healthcare provider Intervention: Recognition/reward (of professionals) policies Control: No defined recognition/reward (of professionals) policies Outcome: Continued improvements in technique, quality, and safety of services/care provided | |||
| Concordance with other guidelines | ASGE Not assessed | Canada Not assessed | EU Not assessed | GRS/JAG accreditation Yes |
| Evidence grading | Not applicable | |||
It was not possible to evaluate the efficacy of the recognition/reward of professionals as none of the retrieved studies24,31,32 assessed the efficacy of this component alone: one study assessed the impact of financial incentives, in combination with other interventions, such as goal setting or WHO-5 (multimodal strategy consisting of five components: system change, training and education, observation and feedback, reminders in the hospital, and a hospital safety climate).
8.5 Confidential reporting is available to staff
| Performance measure | We recommend there is a process for confidential reporting, with action being taken for abuse of endoscopy staff by patients or other staff, including endoscopists, in line with institutional policies | |||
| Domain | Staffing | |||
| Category | Process | |||
| Rationale | Unfortunately, there are still reports of bullying, harassment, and verbal or other forms of abuse in all healthcare services It is advocated that there is zero tolerance of such behaviours and that offenders are dealt with promptly and effectively, even if this means withdrawing privileges to work in the service | |||
| Standards | Minimum standard: adherence to host organizational policies and processes on abuse from patients or staff, including endoscopists Target standard: action is taken in response to concerns about abuse | |||
| Consensus agreement | 96.3% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Not assessed | Canada Not assessed | EU Not assessed | GRS/JAG accreditation Yes |
| Evidence grading | Not applicable | |||
9 Domain: Patient involvement
9.1 Patient feedback is collected
| Performance measure | We recommend the endoscopy service gathers patient feedback at least annually | |||
| Domain | Patient involvement | |||
| Category | Process | |||
| Rationale | Patients are best placed to comment on what it is like to experience the service and, if the service is to become patient centred, it is essential patients are asked for their perspective The feedback should cover all aspects of the patient experience including booking, admission, comfort, privacy, dignity, and aftercare processes Surveys need to be frequent enough to truly reflect the service, and ideally their objectivity might be improved if they are gathered and reviewed by a body that has no stake in the service | |||
| Standards | Minimum standard: an annual patient feedback survey Target standard: a variety of formats to gather feedback from patients (such as verbal, written, and electronic feedback) | |||
| Consensus agreement | 92.59% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Very low | |||
9.2 Patient complaints and suggestions are reviewed
| Performance measure | We recommend there is a process for reviewing patient complaints and suggestions | |||
| Domain | Patient involvement | |||
| Category | Process | |||
| Rationale | Patient complaints and suggestions are a valuable source of patient feedback and should be used as a platform for improving the service | |||
| Standards | Minimum standard: a process to collect and collate patient complaints and suggestions Target standard: patient complaints and suggestions are reviewed | |||
| Consensus agreement | 96.15% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Yes | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Very low | |||
9.3 All patient feedback reviewed and acted upon
| Performance measure | We recommend that the service acts on both formal and informal feedback from patients to improve the service and to demonstrate it has addressed concerns when these are raised | |||
| Domain | Patient involvement | |||
| Category | Process | |||
| Rationale | Gathering feedback and reviewing complaints and suggestions is a waste of time if changes to improve the service are not made If patients and their carers see that their (and others’) feedback and concerns are recognized and acted upon, they are more likely to provide further feedback | |||
| Standards | Minimum standard: all formal and informal feedback from patients (see Performance measures 9.1 and 9.2) is reviewed and actions are agreed Target standard: formal and informal feedback indicates that action taken to improve patient experience has been effective | |||
| Consensus agreement | 96.3% | |||
| PICO | Not applicable | |||
| Concordance with other guidelines | ASGE Not assessed | Canada Yes | EU Yes | GRS/JAG accreditation Yes |
| Evidence grading | Very low | |||
Implementation – accelerating adoption of the guideline
A guideline will not benefit patients if it is not used. At first sight it might seem that it is the responsibility just of the leaders of an endoscopy service to implement this guideline; however, while the leaders of endoscopy services are in most control of their units, there are many other stakeholders who have an influence on how quickly the guidance can be adopted. We provide here an idea of who should take note of this guideline and what they might do to accelerate its adoption.
Patients
Patients (and their carers/representatives) have a role in advising endoscopy services and providing feedback on the quality of the service. Patients are encouraged to demand that endoscopy services should achieve the recommendations and ask for an explanation if they are not met.
Payers
Payers should require endoscopy services to achieve the recommendations and be able to demonstrate that they have been achieved.
Endoscopy staff
Endoscopy staff are well placed to know whether the recommendations have been followed and should challenge both the wider organization and the service itself if there are shortcomings.
Endoscopy team leaders
Endoscopy team leaders should use the recommendations to structure their approach to improving their service and acquiring the resources they need to meet the recommendations. The recommendations will empower them to manage poor performance and bad behaviours.
Other groups and departments
The effectiveness of an endoscopy service depends on pathways both into and out of the service, much of which can be outside the direct control of the endoscopy service itself. The department that perhaps impacts most on endoscopy is pathology; for example, processes for samples; timeliness and method of reporting; integration of pathology into performance reports (such as adenoma detection rate); and review of complex pathology. It is vital for the endoscopy service to both identify other relevant groups (such as its referrers) and departments, and collaborate with them both to optimize the care of patients and the use of resources.
Wider organizations
The organizations within which endoscopy services are delivered should have an interest in ensuring the recommendations are met and that their endoscopy service has the necessary resources both to deliver the recommendations and to demonstrate they have been achieved.
Patient advocacy groups
Patient advocacy groups can use the guideline to hold endoscopy services to account to ensure they are patient centred and delivering high-quality and safe endoscopy. These groups might create ‘key’ questions, based on the recommendations, for patients to ask of endoscopy services, thereby empowering them to ensure they receive a high-quality service.
National professional societies
These societies should decide whether they have a role in the governance of the endoscopy service in their country, or whether they see their role as supportive and educational. At the very least, they should be recommending services follow the guideline. At the other extreme, they might implement a process to assess whether services meet the recommendations, as currently occurs in the UK.
Ministries of health
Health ministries should require endoscopy services to meet the recommendations in this guideline and ideally set up a process to assess services to ensure the recommendations are met.
Regulators of health
It is only expected regulators of health will become interested in these guidelines if they become requirements within a quality assurance infrastructure, as is occurring in England. Currently, the health regulator in England (the Care Quality Commission) is working with professional accrediting bodies, such as the JAG, to use accreditation of services to inform, improve, and reduce the burden of regulation.
ESGE
The next phase of work for the QIC Endoscopy Service Working Group of the ESGE will be to determine in what ways the ESGE can support its members and endoscopy services throughout Europe in the implementation of this guideline.
Addendum
There have been recent developments in the proposed methodology for assessing the strength of evidence supporting recommendations in clinical guidelines.33 This new thinking recognizes that there are situations/topics where it is obvious that the proposed recommendations would do substantially more good than harm, even if there are no studies available specifically designed to answer the clinical question. Guyatt et al., in their paper entitled ‘Guideline panels should not GRADE good practice statements’,33 state: ‘Good practice statements typically represent situations in which a large body of indirect evidence, made up of linked evidence including several indirect comparisons, strongly supports the net benefit of the recommended action’. In such cases it can be inappropriate, disproportionally time-consuming, and/or unrewarding to review the literature. Guyatt et al.33 propose that some recommendations be considered for re-classification as ‘good practice statements’ when:
the statement is clear and actionable
the message is really necessary
the net benefit is large and unequivocal
the evidence is difficult to collect and summarize
specific issues, such as equity, need to be considered
the rationale is explicit
the approach is better than formal GRADEing.
In the light of this new recommendation on the need for evidence supporting clinical guidelines, it is clear that many (if not most) of the quality measures included in this guideline could be classified as good practice statements. It was decided, because the project had been implemented using the classic GRADE methodology and the evaluation had already been done, to continue with the GRADE process. However, the latest perspective on when and when not to perform a literature review supports the working group’s approach and conclusions that it is appropriate to include quality measures that do not have a traditional evidence base.
The quality measures have not been formally assessed against the criteria proposed for ‘good practice statements’; however, it is evident that the majority would, with the exception of ‘considering equity’, meet the criteria. It is expected that future iterations of this guideline will adopt the latest recommendations and consider which quality measures should be GRADEd and which should be judged against criteria for good practice statements.
Supporting information
The detailed literature searches performed by an expert team of methodologists, as well as evolution and adaptation of the different PICOs and clinical statements during the Delphi voting process can be viewed in Supporting Information on the ESGE website.
Online content viewable at: https://www.esge.com/performance-measures-for-endoscopy-services.html
Acknowledgments
The authors gratefully acknowledge the contributions from: Dr. Stuart Gittens, ECD Solutions in the development and running of the web platform; Iwona Escreet and all at Hamilton Services for project administrative support; Debbie Johnston, Craic Consultancy Ltd, for support creating the minimum and target standards; the Scottish Intercollegiate Guidelines Network for hosting the critical appraisal module; and the Research Foundation-Flanders (FWO) for providing funding for Professor Raf Bisschops; also thank you to all members of the wider working group who attended meetings, participated in discussions, and voted on the measures including Bamakhrama K. (UAE), Fretwell I. (UK), Hamoudi W. (Jordan), Kariis T. (Estonia), Lauri A. (Italy), Madácsy L. (Hungary), Narra Figueiredo P. (Portugal), Nashat A. (Egypt), Ørmeci N. (Turkey), Paraskeva K. (Greece), Patchett S. (Ireland), Stefanovic M. (Slovenia), Wurm Johansson G.G. (Sweden), Seebach L. (Switzerland), Stirna D. (Latvia); Nurses: Bakic B. (Croatia), Beilenhoff U. (Germany), Christensen J.S. (Denmark), Jorgensen A. (Norway), Martisen K. (Norway), Petraki S. (Greece); Patient representative: Gore-Booth J. (France – EuropaColon). United European Gastroenterology (UEG) supplied co-funding and additional project governance to this endeavour.
Footnotes
Regular rounds by senior leaders providing an informal method for leaders to talk with front-line staff about safety issues in the organization and to show their support for staff-reported errors, ensuring two-way communication, with both the executives and the staff talking honestly and listening carefully. Walk rounds can be conducted in patient care departments (such as the emergency department, operating rooms, and radiology), the pharmacy, and laboratories. This approach, or a customized version of it, can easily be adopted in the endoscopy environment.
Competing interests
C. Bennett owns and works for Systematic Research Ltd., and received a consultancy fee from ESGE to provide scientific, technical, and methodological expertise for the present project (2014–2018). R. Bisschops has received: speaker’s fees from Covidien (2009–2014) and Fujifilm (2013); speaker’s fee and hands-on training sponsorship from Olympus Europe (2013–2014); speaker’s fee and research support from Pentax Europe; and an editorial fee from Georg Thieme Verlag as co-editor of Endoscopy. M. Bretthauer receives fees for being a member of the Norwegian government CRC screening advisory group (2012 to present) and receives funds from the American College of Physicians for editorial work for Annals of Internal Medicine. T. de Lange is a National Endoscopy Board’s member of the Norwegian Gastroenterology Association. M. Dinis-Ribeiro receives funds from Georg Thieme Verlag for editorial work for Endoscopy. M. Kaminski receives speakers and teaching fees and travel support from Olympus Erbe. C. Senore’s department received PillCAM2 Colon devices from Medtronics to conduct a comparative study (2014–2017); along with C. Belissario and S. Minozzi, he also received consultancy fees from ESGE to provide methodological expertise (PICOs evaluation, literature searches, and evidence summaries) for the present project (2014–2017). R. Valori is a director of AnderVal Ltd., a company providing endoscopy skills training (2015 to present). O. S. Balfaqih, G. Cortas, M. de Pater, D. Domagk, P. Eisendrath, P. Falt, C. Hassan, I. Koruk, A. Ono, C. J. Rees, N. Rustemović, M. Rutter, E. Schoon, C. Spada, and A. Veitch have no competing interests.
In brief
The European Society of Gastrointestinal Endoscopy (ESGE) and United European Gastroenterology (UEG) present a list of performance measures for endoscopy services, comprising 30 measures in nine domains. A systematic and scientifically based methodology was applied to substantiate the proposed measures with available evidence where possible. The measures include those related to the leadership, organization, and delivery of the service, as well as those associated with the patient journey. Each measure includes a recommendation for a minimum and target standard for endoscopy services to achieve. Adoption of these performance measures by all endoscopy services across Europe is recommended.
References
- 1.Corley DA, Jensen CD, Marks AR. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc 2011; 74: 656–665. [DOI] [PubMed] [Google Scholar]
- 2.Veitch AM, Uedo N, Yao K, et al. Optimizing early upper gastrointestinal cancer detection at endoscopy. Nat Rev Gastroenterol Hepatol 2015; 12: 660–667. [DOI] [PubMed] [Google Scholar]
- 3.Denis B, Gendre I, Sauleau EA, et al. Harms of colonoscopy in a colorectal cancer screening programme with faecal occult blood test: A population-based cohort study. Dig Liver Dis 2013; 45: 474–480. [DOI] [PubMed] [Google Scholar]
- 4.Rutter MD, Senore C, Bisschops R, et al. The European Society of Gastrointestinal Endoscopy Quality Improvement Initiative: Developing performance measures. Endoscopy 2016; 48: 81–89. [DOI] [PubMed] [Google Scholar]
- 5.Day LW, Cohen J, Greenwald D, et al. Quality indicators for gastrointestinal endoscopy units. VideoGIE 2017; 2: 119–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong D, Barkun A, Bridges R, et al. Canadian Association of Gastroenterology consensus guidelines on safety and quality indicators in endoscopy. Can J Gastroenterol 2012; 26: 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valori R, Rey JF, Atkin WS, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition – Quality assurance in endoscopy in colorectal cancer screening and diagnosis. Endoscopy 2012; 44(Suppl 3): SE88–SE105. [DOI] [PubMed] [Google Scholar]
- 8.Valori R. Quality improvements in endoscopy in England. Techn Gastrointest Endosc 2012; 14: 63–72. [Google Scholar]
- 9.JAG Joint Advisory Group on GI Endoscopy. Joint Advisory Group on Gastrointestinal Endoscopy (JAG) accreditation standards for endoscopy services. Available from: https://www.thejag.org.uk/Downloads/Accreditation/JAG%20accreditation%20standards%20for%20endoscopy%20services.pdf. Accessed 15 September 2018.
- 10.Sint Nicolaas J, de Jonge V, Korfage IJ, et al. Benchmarking patient experiences in colonoscopy using the Global Rating Scale. Endoscopy 2012; 44: 462–472. [DOI] [PubMed] [Google Scholar]
- 11.Sint Nicolaas J, de Jonge V, de Man RA, et al. The Global Rating Scale in clinical practice: A comprehensive quality assurance programme for endoscopy departments. Dig Liver Dis 2012; 44: 919–924. [DOI] [PubMed] [Google Scholar]
- 12.MacIntosh D, Dube C, Hollingworth R, et al. The endoscopy Global Rating Scale-Canada: Development and implementation of a quality improvement tool. Can J Gastroenterol 2013; 27: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segnan N, Patnick J, von Karsa L, eds. European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis. 1st edn. Luxembourg: Publications Office of the European Union: European Commission, 2010.
- 14.Brand CA, Barker AL, Morello RT, et al. A review of hospital characteristics associated with improved performance. Int J Qual Health Care 2012; 24: 483–494. [DOI] [PubMed] [Google Scholar]
- 15.Buljac-Samardzic M, Dekker-van Doorn CM, van Wijngaarden JD, et al. Interventions to improve team effectiveness: A systematic review. Health Policy 2010; 94: 183–195. [DOI] [PubMed] [Google Scholar]
- 16.Morello RT, Lowthian JA, Barker AL, et al. Strategies for improving patient safety culture in hospitals: A systematic review. BMJ Qual Saf 2013; 22: 11–18. [DOI] [PubMed] [Google Scholar]
- 17.Parand A, Dopson S, Renz A, et al. The role of hospital managers in quality and patient safety: A systematic review. BMJ Open 2014; 4: e005055–e005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suelflow E. Systematic literature review: An analysis of administrative strategies to engage providers in hospital quality initiatives. Health Policy Technol 2016; 5: 2–17. [Google Scholar]
- 19.Wong CA, Cummings GG, Ducharme L. The relationship between nursing leadership and patient outcomes: A systematic review update. J Nurs Manag 2013; 21: 709–724. [DOI] [PubMed] [Google Scholar]
- 20.Wong CA, Cummings GG. The relationship between nursing leadership and patient outcomes: A systematic review. J Nurs Manag 2007; 15: 508–521. [DOI] [PubMed] [Google Scholar]
- 21.Kaminski MF, Anderson J, Valori R, et al. Leadership training to improve adenoma detection rate in screening colonoscopy: A randomised trial. Gut 2016; 65: 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2013, pp. CD003543–CD003543. [DOI] [PubMed] [Google Scholar]
- 23.Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012, pp. CD000259–CD000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brouwers MC, De Vito C, Bahirathan L, et al. What implementation interventions increase cancer screening rates? A systematic review. Implement Sci 2011; 6: 111–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabatino SA, Habarta N, Baron RC, et al. Interventions to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers systematic reviews of provider assessment and feedback and provider incentives. Am J Prev Med 2008; 35: S67–S74. [DOI] [PubMed] [Google Scholar]
- 26.Rees CJ, Thomas-Gibson S, Bourke MJ, et al. Managing under-performance in endoscopy: A pragmatic approach. Gastrointest Endosc 2018; 88: 737–744. [DOI] [PubMed] [Google Scholar]
- 27.Algie CM, Mahar RK, Wasiak J, et al. Interventions for reducing wrong-site surgery and invasive clinical procedures. Cochrane Database Syst Rev 2015, pp. CD009404–CD009404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maaskant JM, Vermeulen H, Apampa B, et al. Interventions for reducing medication errors in children in hospital. Cochrane Database Syst Rev 2015, pp. CD006208–CD006208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parmelli E, Flodgren G, Fraser SG, et al. Interventions to increase clinical incident reporting in health care. Cochrane Database Syst Rev 2012, pp. CD005609–CD005609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald KM, Matesic B, Contopoulos-Ioannidis DG, et al. Patient safety strategies targeted at diagnostic errors: A systematic review. Ann Intern Med 2013; 158: 381–389. [DOI] [PubMed] [Google Scholar]
- 31.French SD, Green S, Buchbinder R, et al. Interventions for improving the appropriate use of imaging in people with musculoskeletal conditions. Cochrane Database Syst Rev 2010, pp. CD006094–CD006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luangasanatip N, Hongsuwan M, Limmathurotsakul D, et al. Comparative efficacy of interventions to promote hand hygiene in hospital: Systematic review and network meta-analysis. BMJ 2015; 351: h3728–h3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guyatt GH, Schünemann HJ, Djulbegovic B, et al. Guideline panels should not GRADE good practice statements. J Clin Epidemiol 2015; 68: 597–600. [DOI] [PubMed] [Google Scholar]