Abstract
Background
Innovative approaches to improve diagnostic yield of endoscopic ultrasound-guided tissue acquisition (EUS-TA) have focused on needle design with development of fine-needle biopsy (FNB) needles with microcore-acquisition technology. Recently, a 20-gauge (20G) antegrade-cutting-side-bevelled biopsy needle (ProCore®) was developed for EUS-TA, but data about its diagnostic performance and histological capability are scant.
Objectives
We assessed the diagnostic performance and histologic retrieval rate of a new 20G antegrade-cutting-side-bevelled biopsy needle compared with a 22G reverse-side-bevelled needle for EUS sampling of solid pancreatic lesions.
Patients and methods
A retrospective analysis of 238 consecutively collected patients who underwent EUS-TA using a 20G or a 22G ProCore® needle, without rapid on-site evaluation (ROSE), was conducted at two centres.
Sensitivity, specificity, positive predictive value and negative predictive value were calculated. Histologic tissue retrieval was evaluated applying a scoring system for each case.
Results
Sensitivity and specificity were estimated as 98.4–100% in the 20G-, and 94.9–100% in the 22G-needle groups, respectively (p > 0.99). The 20G procured more histologic-grade tissues (92.6% vs 49.5%, p < 0.0001) achieved by a lower number of passes (2.64 vs 3.44, p < 0.0001) compared to the 22G.
Conclusions
Both side-bevelled FNB needles achieved a high diagnostic sensitivity. The 20G-side-bevelled needle obtained a significantly higher microcore retrieval rate.
Keywords: Endosonography, endoscopic ultrasound-guided tissue acquisition, fine-needle biopsy (FNB), pancreatic cancer, 20G ProCore®, solid pancreatic neoplasm
Key summary
Established knowledge on this subject includes the following:
Endoscopic ultrasound-guided tissue acquisition (EUS-TA) has gained a pivotal role in the management of pancreatic solid lesions.
Innovative approaches to improve the diagnostic yield of EUS-TA have focused on needle design with the development of fine-needle biopsy (FNB) needles with microcore-acquisition capability.
EUS-FNB with reverse-bevel technology showed only a slight advantage compared with standard fine-needle aspiration needles.
A new 20-gauge (20G) antegrade-cutting side-bevelled FNB needle has been designed to balance flexibility with a large calibre but scant data in the literature are available.
New findings of this study include the following:
The 20G antegrade-cutting side-bevelled needle acquires significantly more tissue microcores (more than 90% of cases) compared to the reverse-bevelled 22G needle.
Both 20G and 22G side-bevelled FNB needles have a high diagnostic rate in EUS-guided tissue acquisition of solid pancreatic lesions, even in the absence of rapid on-site evaluation.
Introduction
Twenty-five years after its introduction,1 endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has replaced percutaneous biopsy and achieved a pivotal role in the diagnostic management of pancreatic masses.2 EUS-FNA has a variable diagnostic sensitivity (54–96%), high specificity and accuracy ranging between 83% and 95%.3,4 Nonetheless, EUS-FNA has some limitations, like the inability or poor capability to provide histological samples for immunohistochemistry and ancillary techniques, and the need for rapid on-site evaluation (ROSE) to achieve an accuracy greater than 90%.5 Certainly, ROSE is useful in a learning curve setting for achieving an adequacy of more than 90%, and in low-volume centres.6
ROSE is not widely performed, however, largely because of the ever-increasing shortage of medical personnel7 and the requirement of specific expertise among pathologists.8
Both these factors have contributed to limit the widespread use of EUS in the community.
From the perspective of increasing the diagnostic accuracy by means of the retrieval of higher amounts of cellular material and the shift of approach from cytology to histology, the efforts for innovation have focused on needle design and technique variations. In particular, the evolution in needle design has led to the development of fine-needle biopsy (FNB) needles with microcore-acquisition technology for EUS-guided tissue acquisition (EUS-TA).
However, the first-generation FNB needles (Tru-Cut biopsy needle) failed because of poor manoeuvrability (QuickCore®, Cook Medical, Bloomington, IN, USA),9 whereas reverse-side-bevelled needles showed only minimal advantage in standard EUS-TA.10
More recently, three novel needles, designed to obtain cellular material that retains tissue architecture, have been developed for EUS-TA.
Two of them are forward-acquiring needles (SharkCore™, Covidien/Medtronic, Whiteley, UK; and Acquire™, Boston Scientific Corporation, Marlborough, MA, USA) with different designs of the cutting tip (fork-tip and Franseen-like tip, respectively), which have shown good histological yield,11–14 whereas the third one is a side-bevelled needle with a 20-gauge (20G) bore and an antegrade-cutting bevel (ProCore® 20G, Cook Medical, Limerick, Ireland). The 20G ProCore® has been designed to balance good flexibility with a large bore to improve tissue acquisition. Its histological capability has been recently assessed in an ex vivo animal study15 and in a prospective study involving a limited number of patients.16 However, data about its performance in histological material retrieval are scant. The aim of this work is to compare the diagnostic performance of 20G antegrade-cutting-side-bevelled needles and 22G reverse-side-bevelled needles (Figure 1) and their capability to achieve cellular material/microcore retrieval for the evaluation of solid pancreatic lesions.
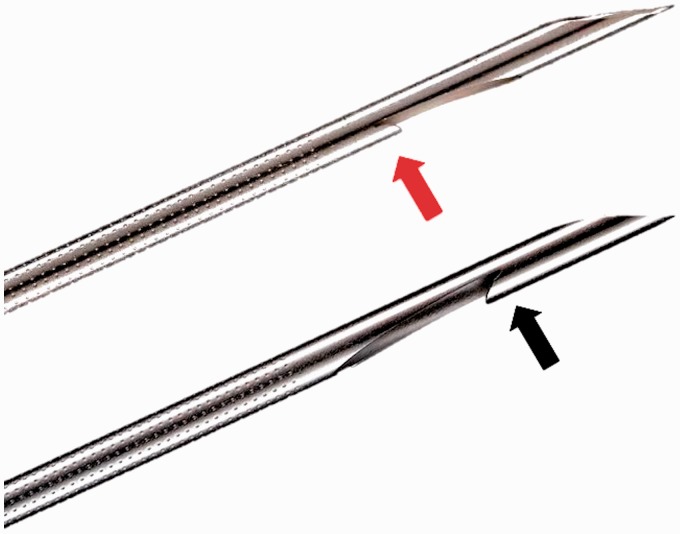
Figure 1.
The straight bevel of the 20-gauge ProCore® needle is located on the proximal side of the lateral window (red arrow). In the 22-gauge ProCore® needle, the reverse bevel is at the distal end of the side window (black arrow).
Patients and methods
A total of 238 consecutive patients, at least 18 years of age, who underwent EUS-FNB by 20G and 22G ProCore® for a pancreatic solid lesion between June 2017 and January 2018 were enrolled in two centres in a retrospective study. Of these patients, 112 were admitted to ‘Maggiore della Carità’ University Hospital (Novara, Italy) and 126 patients were admitted to The Pancreas Institute, G.B. Rossi University Hospital (Verona, Italy).
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (ethical review board of the province of Verona and Rovigo, registry number 63490, 28 December 2017) and with the Declaration of Helsinki of 1975, as revised in 2008. Informed consent was obtained from all patients for inclusion in the study.
EUS procedures
All patients gave their written informed consent prior to the EUS-FNB procedures. EUS-FNB was performed by expert endosonographers in patients under conscious or deep sedation, by using a conventional linear echoendoscope (EG-3870UTK, Pentax Europe GmbH, Hamburg, Germany or GF-UC180T, Olympus Medical System, Hamburg, Germany). Standard preliminary EUS evaluation was performed to determine the location, size, stage and vascular pattern of the lesion, after contrast injection (Sonovue®, Bracco, Milan, Italy). After excluding interposed vessels, EUS-FNB was performed by 20G or 22G EchoTip ProCore® HD Ultrasound Biopsy Needles (Cook Medical, Limerick, Ireland) with a slow-pull technique, and with the fanning technique, whenever possible.17
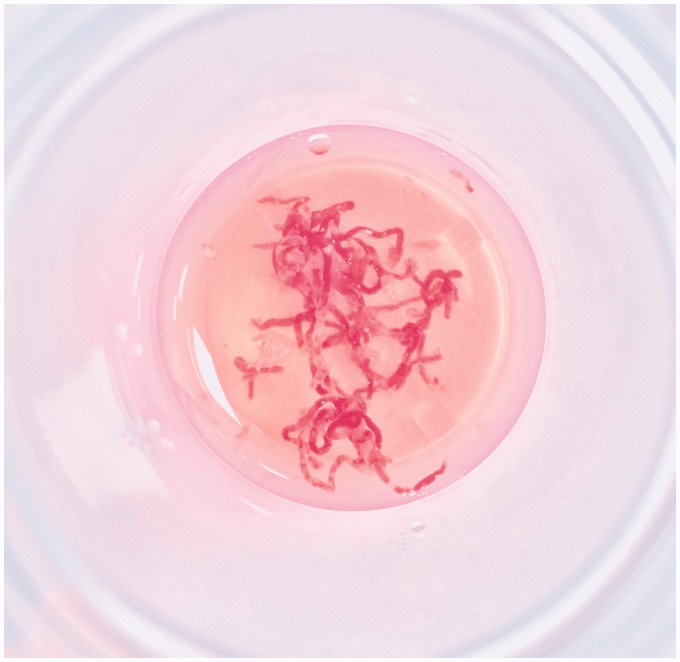
The number of passes was determined on the basis of the macroscopic on-site evaluation (MOSE) of acquired samples performed by the endosonographer.18 The procedure was stopped when sufficient material was obtained, presenting as worm-like yellowish specimens in the fixative vial (Figure 2), or after at least three passes, if MOSE was not satisfactory.
Figure 2.
Appearance of a sufficient amount of tissue evaluated by the macroscopic on-site evaluation performed by the endosonographer during the procedure.
Acquired material underwent standard cell-block or histologic handling in accordance with the routine procedure of each institution. After processing, specimens were sectioned and stained with haematoxylin and eosin. Further immunostaining or molecular analysis was performed when necessary to achieve a definitive diagnosis. ROSE was not performed.
Diagnostic accuracy and definitions of cyto/histologic and final diagnosis
Samples were defined as nondiagnostic, benign, indeterminate (atypical, suspicious) or positive for malignancy. For statistical purposes, indeterminate samples were considered positive when suspicious and negative when atypical, according to European Society of Gastrointestinal Endoscopy guidelines.17
The final diagnosis of pancreatic ductal adenocarcinoma (PDAC), non-PDAC tumours or mass-forming pancreatitis was defined on the basis of histologic examination of the surgically resected specimens (if available) or was based on a combination of clinical and radiological follow-ups for at least six months.
Evaluation of the microcore retrieval
One experienced cytopathologist in each institution, blinded to the needle used during the procedure, reviewed the slides and applied the scoring system. Since the study was retrospective, all the slides were retrieved from pathology archives and reviewed for the purposes of the study.
A histologic specimen was defined by the presence of one or more than one ‘microcore’, defined as a fragment that allows for histologic and tissue architectural assessment19 at least 550 μm long at its greatest axis.20
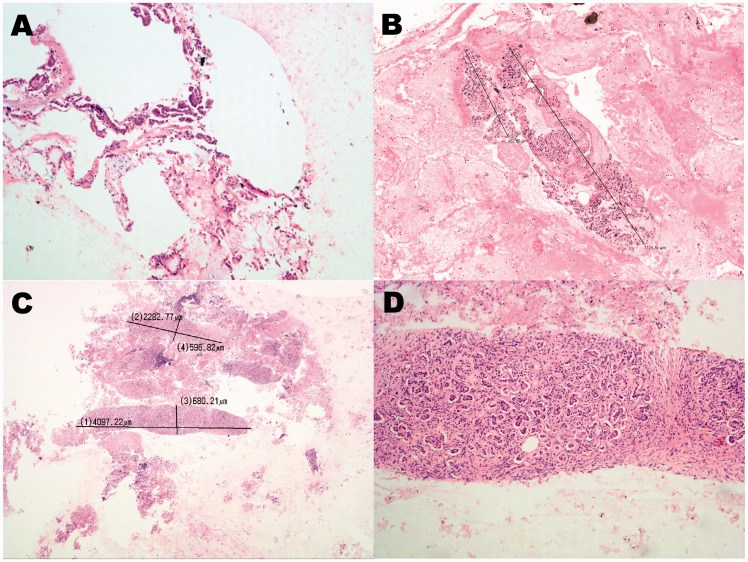
Samples not satisfying the definition of histologic specimens were classified as cytological specimens, characterised by the presence of cellular aggregates or microfragments. The length of intact histological fragments was measured by using dedicated software (D-Sight Software 2010, Menarini Diagnostics, Florence, Italy, and Nikon Digital Sight DS-L2, Nikon Corporation, Tokyo, Japan) (Figure 3).
Figure 3.
Histologic retrieval score. (a) Score 1: cellular aggregates of pancreatic adenocarcinoma, 100 × original magnification; (b) Score 2: single tissue fragment > 550 µm and one cellular aggregate <550 µm, 20 × original magnification. (c) Score 3: tissue fragments with length measurements of the two major cores (4.097 mm and 2.282 mm), 20 × original magnification; (d) Score 3: magnification of the longest microcore showed in (c), 100 × original magnification. Haematoxylin and eosin staining.
To evaluate tissue integrity and quantity, we modified the score system described by Gerke et al.,21 as reported in Table 1.
Table 1.
Scoring system used for the microcore retrieval evaluation.
| Tissue integrity/quantity score | |
|---|---|
| Score | Explanation |
| 0 | Insufficient material for interpretation |
| 1 | Only cytological interpretation possible |
| 2 | Sufficient material for good quality histological interpretation (1 core > 550 µm at greatest axis). |
| 3 | Sufficient material for high quality histological interpretation (>1 cores > 550 µm at greatest axis). |
Adverse events (AEs)
AEs were evaluated by using patients' electronic charts, and were classified as intraprocedural when detected during the EUS examination and postprocedural when observed within 72 hours after the procedure. Intraprocedural AE: (1) Self-limiting retroperitoneal or intraparietal bleeding after FNB procedure and (2) perforation. Postprocedural AE: (1) Bleeding (haemoglobin level lowering more than 2 g/dl as compared to that estimated in the preprocedural laboratory investigation); (2) acute pancreatitis (abdominal pain associated with an increase in serum pancreatic enzymes more than three times the upper normal limits and/or pancreatitis seen on imaging) and (3) perforation.
Statistical analysis
The estimated sample size (93 cases per group, minimum) was obtained assuming microcore retrieval in 50% of 22G-needles cases and microcore retrieval in 70% of 20G-needle cases, an alpha error of 0.05 (two sided) and a power of 0.80.22
Sensitivity, specificity, positive and negative predictive values, and accuracy were estimated and expressed as percentage with 95% confidence intervals (CIs). Inadequate samples were considered as false-negative cases. The chi-squared (χ2) without Yates' correction or the Fisher exact test was used for categorical data. The unpaired student t test was used to compare continuous variables. Statistical significance was determined by considering a p value < 0.05. Data were analysed by using STATA13 software (Stata Corp LP, College Station, TX, USA).
Results
Main clinicopathological data are summarised in Table 2. A total of 135 patients underwent EUS-FNB with a 20G ProCore® needle, and 103 patients with a 22G ProCore® needle. Sampling procedures were performed transduodenally in 151 patients (63.4%).
Table 2.
Study cohort populations' features and final diagnosis.
| All | 20G | 22G | p value | |
|---|---|---|---|---|
| N | 238 | 135 | 103 | >0.999b |
| Sex (M/F) | 132/106 | 74/61 | 58/45 | >0.510c |
| Age, year (±SD) | 66 ( ± 11.7) | 66 ( ± 11.3) | 65 ( ± 12.3) | >0.999b |
| Mean lesions size, mm (±SD) | 30.8 ( ± 11.8) | 32.4 ( ± 12.1) | 28.7 ( ± 11.1) | 0.0168 c |
| Lesions location, N (%) | ||||
| Head | 151 (63.4%) | 86 (63.7%) | 65 (63.1%) | 0.970b |
| Body | 47 (19.8%) | 27 (20.0%) | 20 (19.4%) | |
| Tail | 40 (16.8%) | 22 (16.3%) | 18 (17.5%) | |
| Access route | ||||
| Transduodenal | 151 (63.4%) | 86 (63.7%) | 65 (63.1%) | >0.999b |
| Transgastric | 87 (36.6%) | 49 (36.3%) | 38 (36.9%) | |
| Final diagnosis, N (%) | ||||
| PDAC | 166 (69.8%) | 102 (75.6%) | 68 (66%) | >0.999a |
| NET | 37 (15.5%) | 15 (11.1%) | 22 (21.4%) | |
| Mass-forming pancreatitis | 11 (4.6%) | 7 (5.2%) | 4 (3.9%) | |
| Other | 19 (8.0%) | 11 (8.1%) | 9 (8.7%) |
F: female; G: gauge; M: male; NET: neuroendocrine tumour; PDAC: pancreatic ductal adenocarcinoma; SD: standard deviation.
Fisher exact test.
Pearson χ2 test.
Unpaired student t test.
In the 20G-needle group, the mean number of passes was 2.64 ± 0.86, whereas in the 22G-needle group, the mean number of passes was 3.44 ± 1.07 (p = 0.0168, Table 3).
Table 3.
Microcore retrieval, diagnostic parameters and complications.
| 20G | 22G | p value | |
|---|---|---|---|
| Number of passes, mean (±SD) | 2.64 ± 0.86 | 3.44 ± 1.07 | <0.0001a |
| Samples adequacy (%) | 98.5% | 98.1% | >0.999a |
| Histological retrieval score | |||
| Score 0, N (%) | 2 (1.5) | 2 (1.9) | <0.0001a |
| Score 1, N (%) | 8 (5.9) | 74 (71.8) | |
| Score 2, N (%) | 31 (23.0) | 15 (14.6) | |
| Score 3, N (%) | 94 (69.6) | 12 (11.7) | |
| Core procurement yield, N (%) | 125 (92.6) | 51 (49.5) | <0.0001a |
| Diagnostic sensitivity, % | 98.4% | 94.9% | >0.999a |
| Diagnostic specificity, % | 100.0% | 100.0% | >0.999a |
| Intraprocedural complications, N (%) | |||
| Bleeding | 3 (2.2) | 4 (3.9) | – |
| Perforation | 0 | 0 | |
| Postprocedural complications, N (%) | |||
| Bleeding | 0 | 0 | – |
| Acute pancreatitis | 1 (0.7) | 2 (1.9) |
G: gauge.
Fisher exact test.
Intraprocedural (retroperitoneal or intraparietal) self-limiting bleeding was observed in seven (2.9%) patients. None of these patients experienced a significant reduction in haemoglobin levels or needed interventional treatment (transfusion or embolisation). Postprocedural mild acute pancreatitis occurred in three (1.3%) patients and was managed conservatively within two hospitalisation days (Table 3).
In the 20G-needle group, histologic specimens were adequate for diagnosis in 98.6% of cases and were evaluated as adequate for histologic interpretation in 125/135 (92.6%) patients. More than one microcore was procured in 94/135 (69.6%) patients (Table 3).
In the 22G-needle group, specimens were adequate for diagnosis in 98.1% of cases and were evaluated as adequate for histologic interpretation in 51 (49.5%) cases. More than one microcore was procured in 21/103 (20.4%) cases. A statistically significant difference between the two groups was observed both about core procurement yield (p < 0.0001) and histologic retrieval score (p < 0.0001).
Microcore retrieval rates for both calibre needles are summarised in Table 3.
Immunohistochemistry stainings were performed in 36 out of 20G-needle cases and in 39 out of 22G-needle cases (no statistically significant differences, p value = 0.318). It was generally performed to confirm the diagnosis in neuroendocrine tumours (NETs) and in suspected metastatic lesions. Molecular analysis was performed in three indeterminate cases: one case in the 20G-needle group and two cases in the 22G-needle group.
In the 20G-needle group, the sensitivity, specificity, negative predictive value and positive predictive value were estimated as 98.4% (95% CI, 94.5–99.8), 100% (95% CI, 59–100), 77.8% (95% CI, 40–97.2) and 100% (95% CI, 97.1–100), respectively.
In the 22G-needle group, the sensitivity, specificity, negative predictive value and positive predictive value were estimated as 94.9% (95% CI, 88.6–98.3), 100% (95% CI, 39.8–100), 44,4% (95% CI, 13.7–78.8) and 100% (95% CI, 96.2–100), respectively.
Discussion
In recent years, efforts have been focused on the possibilities of increasing the diagnostic accuracy of EUS-TA. The efficacy of the procedure depends on several preanalytical and postanalytical variables. These include an accurate pre-procedural clinical and radiological assessment, the expertise of the endoscopist and of the cytopathologist, the characteristics of the target lesion and the optimisation of the procedure including the sampling technique. The recent introduction of a 20G antegrade-cutting-side-bevelled needle has provided good flexibility for the use in EUS-TA, and the large-bore needle has enabled higher cellular material retrieval, including that of histologic fragments. Specimens containing tissue cores enable additional immunohistochemical staining and molecular analysis. This is beneficial when further characterisation of a tumour is needed, such as differential diagnosis between well-differentiated PDAC and chronic pancreatitis, grading of pancreatic neuroendocrine tumour (NET) or in case of rare conditions such as autoimmune pancreatitis, lymphoma or solid variant groove pancreatitis.23,24
Moreover, in the era of targeted therapy, the use of molecular analysis for predictive purposes is increasing and the availability of enough cellular material is crucial for all the required testing.
Ideally, molecular analysis can be performed both on histologic and cytological samples but it is still debated whether FNB-acquired samples are more reliable than FNA-acquired ones in terms of overall cellularity and tumour cell fraction.25
Concerning diagnostic sensitivity, the new 20G FNB needle has been poorly described in the available literature. In contrast, studies describing the diagnostic efficacy of the 22G reverse-side-bevelled needle have been well represented. A study by Fabbri et al., demonstrating the efficacy of the 22G side-bevelled needle, showed 80% diagnostic sensitivity for small pancreatic lesions (<2 cm).20 For larger lesions (mean diameter: 32.4 mm), Larghi et al. obtained an 87.5% diagnostic sensitivity with one pass.26 In a study by Alatawi et al., the diagnostic sensitivity ranged up to 97.8% with a mean of two passes (mean diameter: 32 mm).27
In our study, the 20G needle reached an excellent diagnostic sensitivity (98.4%) and high rate of histologic tissue retrieval (92.6%) with a mean of 2.64 passes.
The results obtained by the 20G ProCore® can be explained by two major differences in comparison with other side-bevelled needles: first, by its larger bore, and second, by the antegrade bevel design, which is different from other ProCore® needles, which have an opposite cutting edge (Figure 1).
In our study, no significant differences in diagnostic yield were observed between the two needle groups. This can be explained by the high diagnostic rate of cytological samples obtained by the 22G. However, our study was conducted at two referral centres with expert pathologists. The capability of the 20G needle to achieve a histologic-grade tissue sample instead of a cytological one could provide a higher sensitivity and a reduction in repeated procedures mainly in low-volume centres where a dedicated pathologist is often not available. Indeed, histologic specimens are usually easier to interpret than cytological samples by pathologists lacking expertise in pancreatic cytology. This hypothesis, however, must be confirmed by further studies.
The relation between needle calibre and histologic rate has been demonstrated by studies involving 19G standard needles.28,29 The 19G ProCore® needle itself demonstrated a high histologic capability and diagnostic accuracy in the first feasibility study for heterogeneous indications.28 However, it has not been largely utilised, and only one additional study has been published so far,29 suggesting a limitation of using a needle of such calibre.30 Difficulty in using a 19G needle transduodenally has been confirmed by a recent prospective multicentre study employing a 19G flexible needle. The authors reported a significant difference among participating centres regarding the feasibility of using 19G needles, concluding that the transduodenal route for the 19G needle cannot be routinely recommended.31 In our study, a transduodenal puncture was performed in 151 patients without any technical failure, confirming good flexibility and ease of usage.
The other novel characteristic of the 20G needle is the presence of a side bevel with an antegrade cutting edge. It can enable the procurement of a higher amount of tissue material as compared with that procured by using reverse-bevelled needles because it catches the tissue while pushing the needle forward, the most effective movement of the EUS-FNB procedure. A recent study has hypothesised that the side bevel, which goes in and out of the lesion during the sampling procedure, is able to cut the tumour surface more effectively than the needle tip within the tumour.32
Concerning microcore retrieval, the data currently reported in the literature about the new 20G needle are scant. A prospective study involving 53 patients has reported 96.2% of adequate tissue after one pass, performed by the slow-pull technique, without ROSE.16
However, available studies involving side-bevelled needles showed relevant differences in microcore retrieval, grossly ranging between 30% and 94%.18,33
These differences probably reflect different approaches to tissue acquisition (needle design and diameter, sampling technique) as well as differences in pathological definitions of microcore and measurement of tissue fragments.
In our study, we used a tissue microcore retrieval score, defining a core as an intact tissue fragment at least 550 µm in length at its greatest axis, and assessing the quantity of tissue in terms of cellular material and number of cores. This definition is in line with literature that shows a few similar definitions of microcore, which defined a fragment as approximately as large as a high-power microscopic field.20
Smear cell cytology scores, such as the Mair score, were developed previously; however, they poorly defined the slides derived by using FNB needles.34 Therefore, we modified the score described by Gerke et al.21 (Table 1).
This scoring system is simple to use in routine procedures, and it can easily distinguish between inadequate and adequate cytological samples and tissue-core samples.
At least one tissue core was obtained in 92.6% of cases and more than one in 69.6% of cases by using the 20G needle, demonstrating a high core retrieval rate. Our results are in accordance with those of the aforementioned study by Nishioka et al.,16 suggesting a higher histological procurement resulted by using the 20G needle as compared to that using small-bore needles.
Another possible advantage of using the 20G needle is the lower number of passes needed to obtain an adequate sample. In our study, the mean number of passes reported in the 20G needle group was 2.64, which was significantly different from that reported in the 22G-needle group (mean passes: 3.44), supporting the use of FNB needles as a valid alternative to ROSE-assisted EUS-TA in accordance with the recently issued European guidelines. In addition, AEs were similar among the two needle groups. Our study has some limitations: first, it is a retrospective evaluation of prospectively collected cases, hence it may be subjected to selection bias regarding the type or size of the needle. A comparison between the two groups did not show relevant differences in terms of sex, age, site of lesion or final diagnosis. A statistically significant difference was found only in the mean diameter of the lesion, which was 32.4 mm in the 20G-needle group and 28.7 mm in the 22G-needle group, reflecting the retrospective nature of the study. Second, one blinded pathologist was involved in each centre without centralisation of pathological evaluation. However, the acquired tissue was measured by dedicated software at both centres (Figure 3), thus the inclusion of cores was supported by an objective evaluation and the procedure was poorly influenced by the pathologist's subjective evaluation. Third, in this study we found an association between the use of the 20G needle and a lower number of passes necessary to achieve a diagnostic sample. However, only a prospective study in which every single pass is analysed will establish the number of passes necessary to be confident of an adequate diagnosis without ROSE.
Lastly, two different methods were used to process the retrieved specimens (cell-block or histology) in accordance with the routine procedure of each institution, thus creating a potential bias. The lack of criteria to confirm diagnosis of benign disease was overcome by confirmation on surgical specimen in two of 11 patients, and by laparoscopic and surgical biopsy in two of 11 patients. Seven out of 11 patients did not develop malignant evolution during follow-up.
In conclusion, both side-bevelled FNB needles achieved a satisfactory diagnostic sensitivity, although the 20G needle required a lower number of passes. Overall, the use of side-bevelled FNB needles might reduce the need for ancillary studies, marking a difference with previous reports involving FNA needles.35
The new 20G ProCore® needle enables adequate tissue procurement in 92.6% of cases with a significantly higher rate as compared to that obtained by small-bore needles, confirming its microcore capability. Its good flexibility also makes it amenable to histologic tissue procurement in difficult positions, such as the transduodenal route.
Acknowledgement
The funding agencies had no role in the collection, analysis and interpretation of data, or in writing the manuscript.
Declaration of conflicting interests
None declared.
Funding
This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC 5 per mille grant no: 12182), European Community FP7 Grant Cam-Pac (agreement no: 602783), the Italian Federation of Family Paediatricians (FIMP) and Ministero Salute (CUP_J33G13000210001).
Ethics approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (ethical review board of the province of Verona and Rovigo, registry number 63490, 28 December 2017) and with the Declaration of Helsinki of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all patients for inclusion in the study.
References
- 1.Vilmann P, Jacobsen GK, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc 1992; 38: 172–173. [DOI] [PubMed] [Google Scholar]
- 2.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017; 15: 1028–1061. [DOI] [PubMed] [Google Scholar]
- 3.Dumonceau JM, Deprez PH, Jenssen C, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline – Updated January 2017. Endoscopy 2017; 49: 695–714. [DOI] [PubMed] [Google Scholar]
- 4.Crino SF, Conti Bellocchi MC, Bernardoni L, et al. Diagnostic yield of EUS-FNA of small (≤15 mm) solid pancreatic lesions using a 25-gauge needle. Hepatobiliary Pancreat Dis Int 2018; 17: 70–74. [DOI] [PubMed] [Google Scholar]
- 5.Wani S, Mullady D, Early DS, et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: A prospective multicenter randomized controlled trial. Am J Gastroenterol 2015; 110: 1429–1439. [DOI] [PubMed] [Google Scholar]
- 6.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol 2011; 106: 1705–1710. [DOI] [PubMed] [Google Scholar]
- 7.van Riet PA, Cahen DL, Poley JW, et al. Mapping international practice patterns in EUS-guided tissue sampling: Outcome of a global survey. Endosc Int Open 2016; 4: E360–E370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jhala NC, Jhala DN, Chieng DC, et al. Endoscopic ultrasound-guided fine-needle aspiration. A cytopathologist's perspective. Am J Clin Pathol 2003; 120: 351–367. [DOI] [PubMed] [Google Scholar]
- 9.Thomas T, Kaye PV, Ragunath K, et al. Efficacy, safety, and predictive factors for a positive yield of EUS-guided Trucut biopsy: A large tertiary referral center experience. Am J Gastroenterol 2009; 104: 584–591. [DOI] [PubMed] [Google Scholar]
- 10.Wang F, Wang HL, Zhao Q. Endoscopic ultrasound-fine-needle biopsy is superior to endoscopic ultrasound-fine-needle aspiration in sampling pancreatic masses. Clin Gastroenterol Hepatol 2018; 16: 785–787. [DOI] [PubMed] [Google Scholar]
- 11.Adler DG, Witt B, Chadwick B, et al. Pathologic evaluation of a new endoscopic ultrasound needle designed to obtain core tissue samples: A pilot study. Endosc Ultrasound 2016; 5: 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kandel P, Tranesh G, Nassar A, et al. EUS-guided fine-needle biopsy using a novel fork-tip needle: A case-control study. Gastrointest Endosc 2016; 84: 1034–1039. [DOI] [PubMed] [Google Scholar]
- 13.Bang JY, Hebert-Magee S, Hasan MK, et al. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: Initial assessment. Dig Endosc 2017; 29: 338–346. [DOI] [PubMed] [Google Scholar]
- 14.Mitri RD, Rimbaş M, Attili F, et al. Performance of a new needle for endoscopic ultrasound-guided fine-needle biopsy in patients with pancreatic solid lesions: A retrospective multicenter study. Endosc Ultrasound. Epub ahead of print 24 August 2017. DOI: 10.4103/eus.eus_33_17. [DOI] [PMC free article] [PubMed]
- 15.Mukai S, Itoi T, Katanuma A, et al. An animal experimental study to assess the core tissue aquisition ability of endoscopic ultrasound-guided histology needles. Endosc Ultrasound 2018; 7: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishioka N, Ogura T, Kurisu Y, et al. Prospective histological evaluation of a 20G core trap with a forward-cutting bevel needle for EUS-FNA of pancreatic lesions. Surg Endosc 2018; 32: 4125–4131. [DOI] [PubMed] [Google Scholar]
- 17.Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline – March 2017. Endoscopy 2017; 49: 989–1006. [DOI] [PubMed] [Google Scholar]
- 18.Iwashita T, Yasuda I, Mukai T, et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: A single-center prospective pilot study (MOSE study). Gastrointest Endosc 2015; 81: 177–185. [DOI] [PubMed] [Google Scholar]
- 19.Wani S, Muthusamy VR, McGrath CM, et al. AGA white paper: Optimizing endoscopic ultrasound-guided tissue acquisition and future directions. Clin Gastroenterol Hepatol 2018; 16: 318–327. [DOI] [PubMed] [Google Scholar]
- 20.Fabbri C, Luigiano C, Maimone A, et al. Endoscopic ultrasound-guided fine-needle biopsy of small solid pancreatic lesions using a 22-gauge needle with side fenestration. Surg Endosc 2015; 29: 1586–1590. [DOI] [PubMed] [Google Scholar]
- 21.Gerke H, Rizk MK, Vanderheyden AD, et al. Randomized study comparing endoscopic ultrasound-guided Trucut biopsy and fine needle aspiration with high suction. Cytopathology 2010; 21: 44–51. [DOI] [PubMed] [Google Scholar]
- 22.Machin D, Campbell MJ, Tan SB, et al. Sample size tables for clinical studies, 3rd ed Oxford: Wiley-Blackwell, 2009. [Google Scholar]
- 23.Al-Hawary MM, Kaza RK, Azar SF, et al. Mimics of pancreatic ductal adenocarcinoma. Cancer Imaging 2013; 13: 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crinò SF, Bernardoni L, Manfrin E, et al. Endoscopic ultrasound features of pancreatic schwannoma. Endosc Ultrasound 2016; 5: 396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy-Chowdhuri S, Chen H, Singh RR, et al. Concurrent fine needle aspirations and core needle biopsies: A comparative study of substrates for next-generation sequencing in solid organ malignancies. Mod Pathol 2017; 30: 499–508. [DOI] [PubMed] [Google Scholar]
- 26.Larghi A, Iglesias-Garcia J, Poley JW, et al. Feasibility and yield of a novel 22-gauge histology EUS needle in patients with pancreatic masses: A multicenter prospective cohort study. Surg Endosc 2013; 27: 3733–3738. [DOI] [PubMed] [Google Scholar]
- 27.Alatawi A, Beuvon F, Grabar S, et al. Comparison of 22G reverse-beveled versus standard needle for endoscopic ultrasound-guided sampling of solid pancreatic lesions. United European Gastroenterol J 2015; 3: 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: Results from a multicenter, pooled, cohort study. Gastrointest Endosc 2011; 73: 1189–1196. [DOI] [PubMed] [Google Scholar]
- 29.Iglesias-García J, Abdulkader I, Lariño-Noia J, et al. Evaluation of the adequacy and diagnostic accuracy of the histology samples obtained with a newly designed 19-gauge EUS histology needle. Rev Esp Enferm Dig 2014; 106: 6–14. [DOI] [PubMed] [Google Scholar]
- 30.Larghi A, Vázquez-Sequeiros E, Ricci R. Should EUS-guided tissue acquisition for histologic examination replace fine needle aspiration for cytologic examination? Another brick in the wall. Rev Esp Enferm Dig 2014; 106: 1–5. [DOI] [PubMed] [Google Scholar]
- 31.Attili F, Fabbri C, Yasuda I, et al. Low diagnostic yield of transduodenal endoscopic ultrasound-guided fine needle biopsy using the 19-gauge Flex needle: A large multicenter prospective study. Endosc Ultrasound 2017; 6: 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng B, Zhang Y, Chen Q, et al. Analysis of fine-needle biopsy versus fine-needle aspiration in diagnosis of pancreatic and abdominal masses: A prospective, multicenter, randomized controlled trial. Clin Gastroenterol Hepatol 2018; 16: 1314–1321. [DOI] [PubMed] [Google Scholar]
- 33.Ishiwatari H, Hayashi T, Kawakami H, et al. Randomized trial comparing a side-port needle and standard needle for EUS-guided histology of pancreatic lesions. Gastrointest Endosc 2016; 84: 670–678. [DOI] [PubMed] [Google Scholar]
- 34.Mair S, Dunbar F, Becker PJ, et al. Fine needle cytology: Is aspiration suction necessary? A study of 100 masses in various sites. Acta Cytol 1989; 33: 809–813. [PubMed] [Google Scholar]
- 35.Trisolini E, Armellini E, Paganotti A, et al. KRAS mutation testing on all non-malignant diagnosis of pancreatic endoscopic ultrasound-guided fine-needle aspiration biopsies improves diagnostic accuracy. Pathology 2017; 49: 379–386. [DOI] [PubMed] [Google Scholar]