Abstract
Background
Success and event rates of endoscopic ultrasound (EUS)-guided biliary drainage vary with techniques, and results from different studies remain inconsistent.
Objective
We conducted a proportion meta-analysis to evaluate the efficacy and safety of EUS-guided biliary drainage and compare the outcomes of current procedures.
Methods
We searched MEDLINE, Embase, Cochrane and Web of knowledge to identify studies reporting technical success, clinical success and complication rates of EUS-guided biliary drainage techniques to estimate their clinical and technical efficacy and safety.
Results
We identified 17 studies including a total of 686 patients. The overall clinical success and technical success rates were respectively 84% confidence interval (CI) 95% (80–88) and 96% CI 95% (93–98) for hepaticogastrostomy, and respectively 87% CI 95% (82–91) and 95% CI 95 (91–97) for choledochoduodenostomy. Reported adverse event rates were significantly higher (p = 0.01) for hepaticogastrostomy (29% CI 95% (24–34)) compared to choledochoduodenostomy (20% CI 95% (16–25)). Compared with hepaticogastrostomy, the pooled odds ratio for the complication rate of choledochoduodenostomy was 2.01 (1.25; 3.24) (p = 0.0042), suggesting that choledochoduodenostomy might be safer than hepaticogastrostomy.
Conclusion
The available literature suggests choledochoduodenostomy may be a safer approach compared to hepaticogastrostomy. Randomized controlled trials with sufficiently large cohorts are needed to compare techniques and confirm these findings.
Keywords: Biliary drainage, biliary obstruction, choledochoduodenostomy, endoscopy-guided, hepaticogastrostomy
Key summary
- Summarize the established knowledge on this subject.
- Endoscopic retrograde cholangiopancreatography (ERCP) with placement of transpapillary stent is the standard procedure for biliary decompression because of its high efficacy and low morbidity.
- Even when performed by an expert, ERCP-based deobstruction and stenting fails in 5% for one or several reasons.
- Endoscopic ultrasound (EUS)-guided biliary drainage is a recently developed alternative when ERCP fails.
- Success and event rates of EUS-guided biliary drainage vary with techniques, and results from different studies remain inconsistent
- What are the significant and/or new findings of this study?
- When bile duct access cannot be obtained as a result of failed cannulation or anatomical modification, EUS-guided biliary drainage can advantageously be used as an alternative to interventional radiology or surgery.
- Choledochoduodenostomy and hepaticogastrostomy are not statistically different regarding clinical and technical success.
- Choledochoduodenostomy appears to be a safer approach compared to hepaticogastrostomy.
Introduction
Endoscopic retrograde cholangiopancreatography (ERCP) with placement of a transpapillary stent is the standard procedure for biliary decompression because of its high efficacy and low morbidity.1 Even when performed by an expert, however, ERCP-based deobstruction and stenting fails in 5% for one or several reasons. In these cases, standard alternative approaches include surgical bypass and percutaneous transhepatic cholangiography and biliary drainage (PTBD). However, these procedures are associated with higher patient discomfort and prolonged hospital stay.2 PTBD can also require long-term exchanges of indwelling drains, a major source of discomfort for patients receiving palliative care.3 Surgical biliary decompression is associated with morbidity ranging from 9% to 67% and mortality up to 3% in the postoperative period.4–7 Endoscopic ultrasound-guided biliary drainage (EUBD) is a recently developed alternative to PTBD for patients in whom ERCP has failed. There are many reports regarding the safety, feasibility and clinical efficacy of EUBD. EUBD includes transenteric techniques, so-called EUS-guided choledochoduodenostomy (CDS) and EUS-guided hepaticogastrostomy (HGS), and anatomic or “natural” techniques, i.e. EUS-guided rendezvous (RDV) and EUS antegrade transpapillary drainage (AG). The transhepatic and extrahepatic approaches are different with respect to indications, techniques and complications, although many reports have analyzed the combined results of their case-mix including both intrahepatic and extrahepatic approaches. In the extrahepatic approach, the common bile duct is accessed mainly through the duodenum. Biliary drainage can therefore be achieved using either transluminal stent placement (choledochoduodenostomy) or transpapillary stent placement via the RDV technique, whereas in the intrahepatic approach, the left lobe of the liver is accessed from the upper gastric wall and more rarely from the distal esophagus or jejunum. Using this approach, biliary drainage can be achieved using either transluminal stent placement (hepaticogastrostomy) or transpapillary stent placement via the RDV technique or AG technique.8
Published studies have reported variable success rates and adverse events using EUS-guided biliary drainage techniques. In this study, we aimed to evaluate the clinical efficacy, technical efficacy and safety of EUBD transenteric approaches, i.e. choledochoduodenostomy and hepaticogastrostomy, whose technical principles are illustrated in Figure 1.
Figure 1.
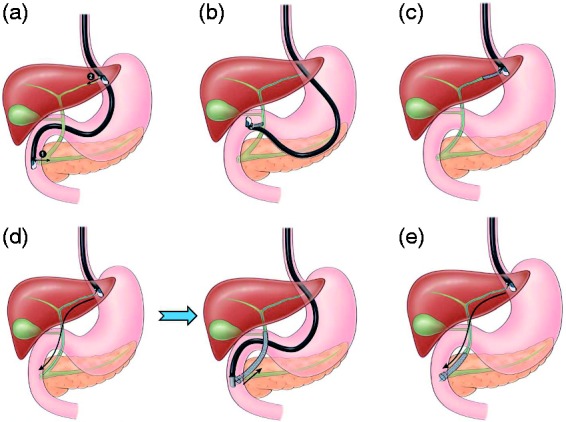
(a) 1. Transgastric. 2. Transduodenal. (b) Choledochoduodenostomy. (c) Hepaticogastrostomy. (d) Endoscopic ultrasound (EUS)-guided rendezvous technique. Step 1: transgastric bile duct puncture and antegrade transpapillary guidewire insertion (EUS scope) Step 2: retrograde biliary stenting over the transpapillary wire (duodenoscope). (e) Antegrade technique.
Methods
Data sources and search strategy
The systematic review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines9 and Meta-Analysis of Observational Studies in Epidemiology guidelines.10 PubMed, Web of Knowledge, Embase and Cochrane library were searched from January 2000 to January 2018. Animal and review studies were excluded. The following Medical Subject Headings, Emtree and keyword search terms were used in combination: biliary drainage, biliary stent, transpapillary biliary drainage, transluminal biliary drainage, choledochoduodenostomy, hepaticogastrostomy, endoscopic ultrasound guided, EUBD, EUS-guided biliary drainage, therapeutic EUS, endoscopic anterograde cholangiography, and interventional EUS. Published abstracts or unpublished data were not included.
Study selection and data extraction
All titles and abstracts of papers retrieved in the prespecified search were screened by two reviewers (A.H., A.S.). Titles and abstracts were screened independently based on the eligibility criteria. Disagreement between two reviewers was discussed with a third reviewer (F.P.). We included EUBD achieved with either CDS or HGS. Studies were included if they reported technical success, clinical success and adverse events. Studies were excluded if one of the latter items was missing. Studies reporting patients with duodenal stents or including fewer than 15 patients were excluded to prevent bias.11 Non-English language studies were excluded from this systematic review and meta-analysis as well as those with overlapping patients or performed in community hospitals. Extracted data included authors' names, year of publication, study design, patient demographics, causes of biliary obstruction, EUS puncture route, method of biliary drainage, technical success, clinical success and details of adverse events. Primary outcome was clinical success. We also excluded studies evaluating techniques other than CDS or HGS to avoid heterogeneity.
Statistical analysis
The primary outcome measure in this study was efficacy of EUBD as assessed by clinical success rate. Technical success rate and safety were secondary outcome measures. Weighted pooled rates were calculated for the primary outcomes of interest with corresponding 95% confidence intervals (CI). These were analyzed using the random-effects model (DerSimonian-Laird method).12 Heterogeneity across studies was assessed using the Cochran Q test and I2 statistics. A p value of < 0.1 for Cochran Q test was defined as indicating the presence of heterogeneity.13 I2, unlike Q, does not inherently depend on the number of studies considered, with values of 25%, 50% and 75% taken to indicate low, moderate and high levels of heterogeneity, respectively. Possible publication bias or other small study effects were disclosed using the Egger test.14 We did not conduct further statistical tests for funnel plot asymmetry because of the limited test power when fewer than 10 studies are included.11 In all cases, p < 0.05 was considered statistically significant. Descriptive data are presented as counts and percentages for categorical variables and mean (standard deviation (SD)) or median (interquartile range (IQR)) for continuous variables. Statistical analysis was performed using R (R version 4.0.2) on a Linux station (Ubuntu 15.04).
Results
Search results
A total of 995 abstracts were screened. Of these, 978 were excluded because they were not relevant or did not meet our inclusion criteria. Finally, 1715–31 studies met the inclusion criteria and were included in this meta-analysis. Eight studies were prospective uncontrolled studies,16–18,22,24,26,29,31 seven were retrospective19–21,23,25,28,30 and two were randomized controlled trials.15,27 Thirteen studies were single center and four were multicentric. Five studies described a cohort of both CDS and HGS with a sample size greater than 15.15,16,19,26,28 (Table 1). Therefore, these 17 studies described 13 HGS patient cohorts and 10 CDS cohorts. All cases of biliary obstruction were due to malignancy. The most frequent was pancreatic cancer with 87 (30.74%) and 85 (30.04%) patients undergoing CDS and HGS, respectively (Table 2). The agreement between reviewers for the collected data gave a Cohen kappa value of 1.0.
Table 1.
Characteristics of included studies.
| First author, yearref | Design | Setting | Country | Type of procedures | Age, mean (SD) | Male, n (%) | Sample size | Clinical success | Technical success | Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|
| Nakai, 201623 | Retrospective | Multicenter | Japan | HGS | 70 (63–77), (median, IQR) | 19 (58) | 33 | 33 | 33 | 3 |
| Kunda, 201620 | Retrospective | Multicenter | Europe | CDS | 73 (49–93), median (range) | 31 (54) | 57 | 54 | 56 | 4 |
| Park, 201527 | RCT | Multicenter | Korea | HGS | NR | NR | 20 | 18 | 20 | 5 |
| Poincloux, 201528 | Retrospective | Single center | France | HGS | NR | NR | 66 | 61 | 65 | 16 |
| CDS | 67 (10.3) | 18 (60) | 30 | 27 | 29 | 6 | ||||
| Umeda, 201531 | Prospective | Single center | Japan | HGS | 77 (NR) | 15 (65) | 23 | 23 | 23 | 4 |
| Artifon, 201515 | RCT | Single center | Brazil | HGS | 66.25 (14.28) | 11 (44) | 25 | 22 | 24 | 5 |
| CDS | 65.77 (15.74) | 11 (46) | 24 | 17 | 22 | 3 | ||||
| Song, 201429 | Prospective | Single center | Korea | CDS | 67 (48–82), median (range) | 8 (47) | 17 | 16 | 17 | 2 |
| Paik, 201424 | Prospective | Single center | Korea | HGS | NR | NR | 28 | 24 | 27 | 2 |
| Park, 201126 | Prospective | Single center | Korea | HGS | NR | NR | 31 | 27 | 31 | 6 |
| CDS | NR | NR | 26 | 22 | 24 | 5 | ||||
| Hara, 201117 | Prospective | Single center | Japan | CDS | 67.9 (10.9) | 7 | 18 | 17 | 17 | 4 |
| Cho, 201716 | Prospective | Single center | Korea | CDS | 64 (29–86), median (range) | 13 | 33 | 33 | 33 | 10 |
| HGS | 66.3 (44–82), median (range) | 16 | 21 | 18 | 21 | 14 | ||||
| Minaga, 201721 | Retrospective | Single center | Japan | HGS | 66 (NR) | 11 | 30 | 22 | 29 | 10 |
| Paik, 201725 | Retrospective | Multicenter | Korea and Japan | HGS | 67.6 (9.3) | 13 | 16 | 13 | 16 | 7 |
| Sportes, 201730 | Retrospective | Single center | France | HGS | 69.2 (NR) | 17 | 31 | 25 | 31 | 5 |
| Khashab, 201619 | Retrospective | Single center | United States | HGS | 63.6 (13.8) | 38 | 61 | 50 | 56 | 28 |
| CDS | 67.6 (13) | 32 | 60 | 51 | 56 | 16 | ||||
| Hara, 201318 | Prospective | Single center | Japan | CDS | 67.27 | 12 | 18 | 16 | 17 | 2 |
| Moryoussef, 201722 | Prospective | Single center | France | HGS | 68.8 (16.4) | 11 | 18 | 13 | 17 | 3 |
CDS: choledochoduodenostomy; HGS: hepaticogastrostomy; IQR: interquartile range; NR: not reported; RCT: randomized controlled trial; SD: standard deviation.
Table 2.
Diagnosis profile for choledochoduodenostomy and hepaticogastrostomy populations.
| Diagnosis | CDS (n = 117) | HGS (n = 204) |
|---|---|---|
| Ampulla of Vater cancer | 10 (8.55%) | 6 (2.94%) |
| Anastomotic biliary stricture | 0 (0%) | 3 (1.47%) |
| Breast cancer | 2 (1.71%) | 0 (0%) |
| Cholangiocarcinoma | 0 (0%) | 37 (18.14%) |
| Choledocholithiasis ± cholangitis | 0 (0%) | 5 (2.45%) |
| Colorectal cancer | 1 (0.85%) | 4 (1.96%) |
| Common bile duct cancer | 0 (0%) | 10 (4.9%) |
| Duodenal cancer | 6 (5.13%) | 5 (2.45%) |
| Gallbladder carcinoma | 2 (1.71%) | 12 (5.88%) |
| Gastric cancer | 3 (2.56%) | 6 (2.94%) |
| Hepatocellular carcinoma | 0 (0%) | 4 (1.96%) |
| Intraductal papillary mucinous neoplasm | 0 (0%) | 1 (0.49%) |
| Lymphoma | 0 (0%) | 1 (0.49%) |
| Metastases | 0 (0%) | 7 (3.43%) |
| Metastatic adenopathy | 3 (2.56%) | 15 (7.35%) |
| Neuroendocrine tumor | 1 (0.85%) | 1 (0.49%) |
| Others | 0 (0%) | 2 (0.98%) |
| Ovarian cancer/ uterus cancer | 2 (1.71%) | 0 (0%) |
| Pancreatic cancer | 87 (74.36%) | 85 (41.67%) |
CDS: choledochoduodenostomy; HGS: hepaticogastrostomy.
Overall clinical efficacy of EUBD technique
Thirteen studies described a hepaticogastrostomy biliary drainage technique with an overall number of 403 patients (Figure 2). The overall clinical success rate was 84% CI 95% (80–88). By using the I2 statistic, we did not find a significant heterogeneity (I2 = 19%, p = 0.26). The Egger test was found to be statistically significant (p = 0.018), which indicates publication bias. Nine studies described a CDS biliary drainage technique with more than 15 patients with an overall number of 283 patients. The overall clinical success rate was 87% CI 95% (82–91). By using the I2 statistic, we did not find a significant heterogeneity (I2 = 37%, p = 0.12). The Egger test was found to be statistically significant (p = 0.007), which indicates publication bias (Table 3). We could not find a significant difference between CDS and HGS (p = 0.37).
Figure 2.
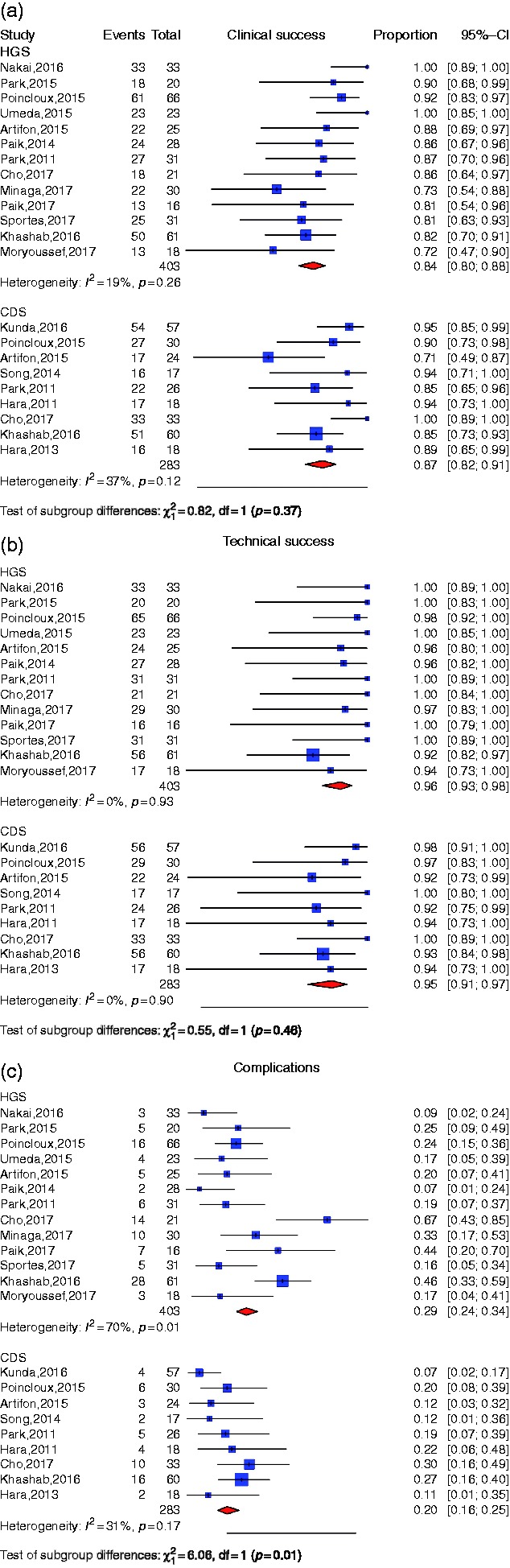
(a) Individual study proportion and pooled estimate for clinical success of choledochoduodenostomy (CDS) and hepaticogastrostomy (HGS). (b) Individual study proportion and pooled estimate for technical success of CDS and HGS. (c) Individual study proportion and pooled estimate for adverse event rate of CDS and HGS.
CI: confidence interval; df: degree of freedom.
Table 3.
Pooled estimate and odds ratio for clinical, technical success and adverse event rate for CDS and HGS.
| Test for heterogeneity |
Test for publication bias |
||||||
|---|---|---|---|---|---|---|---|
| Outcome | Procedure | Proportion or OR (p value) | I 2 | Q | p valuea | Z statistic | p valueb |
| Clinical success | HGS | 84.16 (79.88; 87.67) | 18.6% | 14.74 | 0.256 | 2.78 | 0.018 |
| CDS | 86.91 (81.86; 90.72) | 36.82% | 12.66 | 0.124 | 3.4 | 0.007 | |
| CDS vs HGS | 1.01 (1.09;2.17) (0.97) | 23.7% | 5.24 | 0.263 | −0.59 | 0.594 | |
| Technical success | HGS | 95.98 (93.25; 97.63) | 0% | 5.82 | 0.925 | 6.34 | <0.001 |
| CDS | 94.68 (91.03; 96.89) | 0% | 3.54 | 0.896 | 2.81 | 0.026 | |
| CDS vs HGS | 1.48 (1.09; 2.17) (0.43) | 0% | 1.83 | 0.608 | 4.92 | 0.039 | |
| Complications | HGS | 25.4 (17.76; 34.92) | 70.49% | 40.67 | <0.001 | −2.18 | 0.052 |
| CDS | 20.08 (15.59; 25.48) | 30.76% | 11.55 | 0.172 | −2.76 | 0.028 | |
| CDS vs HGS | 2.01 (1.09; 2.17) (<0.001) | 0% | 3.84 | 0.428 | −0.45 | 0.681 | |
CDS: choledochoduodenostomy; HGS: hepaticogastrostomy; OR: odds ratio.
Cochran Q test.
Egger test.
Overall technical efficacy of EUBD technique
In the pooled patient population, the percentage of patients whose HGS procedure was a technical success was 96% CI 95% (93–98) (Figure 2). Heterogeneity was not significant (I2 = 0%, p = 0.93). The Egger test for assessing publication bias was found to be significant (p < 0.001). The percentage of patients with a technical success after CDS was 95% CI 95 (91–97). We did not find a significant heterogeneity (I2 = 0%, p = 0.9) but did find a signification publication bias (Egger test p = 0.026). The test for subgroup differences found no significant difference between CDS and HGS regarding technical success (p = 0.46) (Table 3).
Overall adverse event rate of EUBD technique
In the pooled patient population, the percentage of adverse events was 29% CI 95% (24–34) with HGS (Figure 2). Heterogeneity was significant (I2 = 70%, p < 0.01). However, the Egger test showed no evidence of a publication bias (p = 0.052) (Table 3). The most frequently reported side events were stent dysfunction (n = 34), bleeding or hemobilia (n = 12) and distal migration (n = 8) (Table 4).
Table 4.
Complication profile for choledochoduodenostomy vs hepaticogastrostomy.
| Adverse events | CDS (n = 253) | HGS (n = 317) |
|---|---|---|
| Abdominal pain (mild) | 1 (0.4%) | 4 (1.26%) |
| Abscess | 0 (0%) | 1 (0.32%) |
| Bilioma or bile leak | 2 (0.79%) | 6 (1.90%) |
| Bleeding or hemobilia | 5 (1.98%) | 12 (3.79%) |
| Cholangitis or sepsis | 5 (1.98%) | 6 (1.90%) |
| Cholecystitis | 0 (0%) | 1 (0.32%) |
| Distal migration | 4 (1.58%) | 8 (2.52%) |
| Hemobilia | 1 (0.4%) | 0 (0%) |
| Others | 1 (0.4%) | 0 (0%) |
| Pancreatitis | 2 (0.79%) | 0 (0%) |
| Perforation | 4 (1.58%) | 0 (0%) |
| Peritonitis | 7 (2.77%) | 6 (1.89%) |
| Pneumoperitoneum ( ± self-limited) | 6 (2.37%) | 6 (1.89%) |
| Sheared guide wire | 0 (0%) | 1 (0.32%) |
| Stent dysfunction (clogging/shrinkage/occlusion) | 10 (3.95%) | 34 (10.73%) |
| Stent misplacement | 0 (0%) | 2 (0.63%) |
The percentage of adverse events with CDS was 20% CI 95% (16–25). Heterogeneity was not significant (I2 = 31%, p = 0.17) but we did find a significant publication bias (Egger test = 0.028) and a significant subgroup difference (p = 0.01) (Table 3). The most frequently reported side events were stent dysfunction (n = 10), peritonitis (n = 7) and pneumoperitoneum (n = 6) (Table 4).
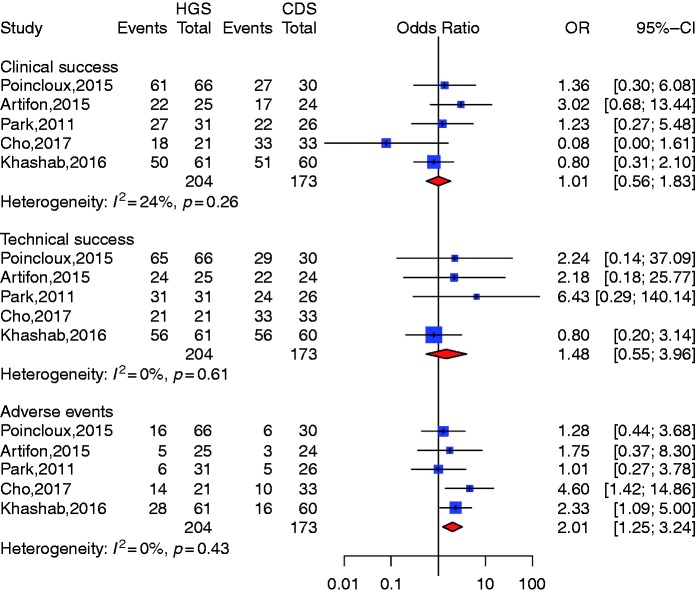
CDS vs HDS (Figure 3)
Figure 3.
Comparison of choledochoduodenostomy (CDS) and hepaticogastrostomy (HGS).
CI: confidence interval; OR: odds ratio.
Five studies described both HGS and CDS.15,16,19,26,28 Compared with HGS the pooled odds ratio (OR) was 1.01 CI 95% (0.56–1.83) for CDS clinical success and 1.48 CI 95% (0.55; 3.96) for technical success, which indicated no significant difference between the two groups. Heterogeneity was not significant with I2 = 24% (p = 0.26) and I2 = 0% (p = 0.61) for clinical and technical success, respectively. Compared with HGS, however, the pooled OR for CDS was 2.01 (1.25; 3.24) (p = 0.0042) for complication rate, suggesting that CDS was a safer alternative compared to HGS. We could not identify a publication bias with symmetrical funnel plots and nonsignificant publication bias (Egger test for clinical and adverse event rate: p = 0.59 and p = 0.68, respectively). We identified a significant publication bias for technical success (p = 0.39) (Table 3).
Discussion
In 1996 Wiersema32 reported for the first time the use of EUS-guided cholangiography in seven patients who underwent successful EUS-guided cholangiography after failed ERCP with a 70% success rate. Five years later, Giovannini and colleagues33 reported the first experience of EUS-guided CDS with plastic stent placement in a patient with unresectable pancreatic cancer. Following this study, various EUS-guided biliary drainage results were reported.34,35 Mallery et al. in 200436 introduced the first EUS-guided RDV approach in two cases of obstructive jaundice due to malignancy after failed ERCP. Since then, many mostly small retrospective and prospective studies have been available and EUBD has been compared to PTBD. A recent meta-analysis37 comparing the efficacy and safety of EUBD to PTBD found that EUBD was associated with significantly better clinical success, a lower rate of postprocedure adverse events, and fewer reinterventions. Artifon et al. in 201215 published the first prospective randomized controlled trial comparing EUBD with PTBD. Twenty-five patients were randomized. All procedures were technically and clinically successful in both groups. There was no difference of complication rates between groups (p = 0.44), CDS (2/13; 15.3%) and PTBD (3/12; 25%). Those results suggested that EUBD can be an effective and safe alternative to PTBD with similar success, complication rates, cost and quality of life. Another study conducted by Khashab and colleagues19 compared the outcomes of 73 patients (22 EUBD and 51 PTBD). Technical success was higher for PTBD, but clinical success was similar, and adverse events were also higher for PTBD. Our results show that EUBD can be performed with high technical and clinical success rates but is associated with a 20% risk of adverse events. One advantage of the EUBD technique compared with PTBD is that EUBD can be performed in patients with ascitis and liver metastasis. The choice of the best approach to EUBD, i.e. essentially transgastric vs transduodenal, deserves a specific analysis.
Artifon et al.15 reported a randomized trial comparing the outcomes of CDS and HGS in 49 patients. The technical success rate was 91% for CDS and 96% for HGS (p = 0.61). Similarly, clinical success was comparable in both groups (77% vs 91%, respectively; p = 0.23). The overall adverse event rate was 16.3% (CDS, 12.5%; HGS, 20%). The authors concluded that CDS and HGS techniques provide similar efficacy and safety, and both are valid options for draining distal malignant biliary obstruction after failed ERCP. In our meta-analysis, however, we did not find a significant difference in technical or clinical success rates, but adverse event rate was significantly higher with HGS compared to CDS.
EUBD is technically difficult and currently complications rates remain relatively high compared to ERCP, so only endoscopists skilled both in EUS and ERCP should perform EUS-guided biliary and pancreatic drainage procedures when ERCP cannot be achieved. It is worthy of note that all studies originated from tertiary high-volume centers that employ highly qualified interventional endoscopists. Although Vila et al. in 201238 could not find an association of hospital type (community vs tertiary care) with a higher technical success or complication rate, we consider the feasibility of these techniques in community hospitals needs to be evaluated.
A previous meta-analysis was conducted very recently by Uemura et al.,39 which to our knowledge is the only previously published meta-analysis comparing HGS to CDS. This study has shown similar technical and clinical efficacy for both techniques, and contrary to the present study also found no difference in the adverse events rate (OR = 0.97 (0.60–1.56)). This different result could be explained by a fewer number of included studies leading to a smaller sample size and different eligibility criteria. Contrary to Uemura and colleagues,39 we chose to include articles that describe cohorts containing at least 15 patients, which may be more representative of high-volume centers describing a large number of patients treated by an experienced clinician familiar with the technique. Also, we chose to include only studies that described both clinical and technical success as well as adverse events. Although we found more adverse events with HGS than CDS, it is noteworthy that most of those are cases of stent dysfunction (34 occurrences with HGS vs 10 with CDS), which could have a stronger relation with the type of stent used than with the technique of biliary access. It was not possible to take into account this potential confounding factor since stent types were not always detailed in the studies.
We also recognize the present meta-analysis study has several limitations. First, a significant publication bias has been found for a large part of the results. Second, most of the studies were retrospective, were not randomized or had only one arm. It is therefore difficult to compare the two drainage techniques that are performed in two different populations. Also, some interventional endoscopists might have a preferred route because of their personal experience or for anatomical reasons. HGS is the preferred route—or the only one available—in the presence of a proximal biliary stricture and/or after distal gastrectomy that prohibits access to the extrahepatic bile duct. CDS can be preferred in case of a native papilla, a dilated common bile duct and nondilated left intrahepatic ducts. Finally, studies were performed over different time periods in different countries with different equipment, populations and definitions of technical and clinical success. Because of this conceptual heterogeneity, the pooled estimate has to be interpreted cautiously.
We show in this meta-analysis and systematic review that when bile duct access cannot be obtained as a result of failed cannulation or anatomical modification, EUBD can advantageously be used as an alternative to interventional radiology or surgery. We did not find a significant difference between CDS and HGS regarding clinical and technical success, but the available literature suggests CDS is a safer approach compared to HGS. However, the above-mentioned limitations and biases in published studies warrant caution in the interpretation of this finding. Randomized controlled trials with sufficiently large cohorts are needed to compare techniques and refine these findings.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Not required
Informed consent
Not required
References
- 1.Kedia P, Gaidhane M, Kahaleh M. Technical advances in endoscopic ultrasound (EUS)-guided tissue acquisition for pancreatic cancers: How can we get the best results with EUS-guided fine needle aspiration? Clin Endosc 2013; 46: 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Born P, Rösch T, Triptrap A, et al. Long-term results of percutaneous transhepatic biliary drainage for benign and malignant bile duct strictures. Scand J Gastroenterol 1998; 33: 544–549. [DOI] [PubMed] [Google Scholar]
- 3.Winick AB, Waybill PN, Venbrux AC. Complications of percutaneous transhepatic biliary interventions. Tech Vasc Interv Radiol 2001; 4: 200–206. [DOI] [PubMed] [Google Scholar]
- 4.Khajanchee YS, Cassera MA, Hammill CW, et al. Outcomes following laparoscopic choledochoduodenostomy in the management of benign biliary obstruction. J Gastrointest Surg 2012; 16: 801–805. [DOI] [PubMed] [Google Scholar]
- 5.Luu C, Lee B, Stabile BE. Choledochoduodenostomy as the biliary-enteric bypass of choice for benign and malignant distal common bile duct strictures. Am Surg 2013; 79: 1054–1057. [PubMed] [Google Scholar]
- 6.Sohn TA, Lillemoe KD, Cameron JL, et al. Surgical palliation of unresectable periampullary adenocarcinoma in the 1990s. J Am Coll Surg 1999; 188: 658–666. discussion 666–669. [DOI] [PubMed] [Google Scholar]
- 7.Spanheimer PM, Cyr AR, Liao J, et al. Complications and survival associated with operative procedures in patients with unresectable pancreatic head adenocarcinoma. J Surg Oncol 2014; 109: 697–701. [DOI] [PubMed] [Google Scholar]
- 8.Artifon EL, Ferreira FC, Otoch JP, et al. EUS-guided biliary drainage: A review article. JOP 2012; 13: 7–17. [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 11.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000; 53: 1119–1129. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 13.Deeks JJ, Altman DG. Inadequate reporting of controlled trials as short reports. Lancet 1998; 352: 1908. [DOI] [PubMed] [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artifon EL, Marson FP, Gaidhane M, et al. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: Is there any difference? Gastrointest Endosc 2015; 81: 950–959. [DOI] [PubMed] [Google Scholar]
- 16.Cho DH, Lee SS, Oh D, et al. Long-term outcomes of a newly developed hybrid metal stent for EUS-guided biliary drainage (with videos). Gastrointest Endosc 2017; 85: 1067–1075. [DOI] [PubMed] [Google Scholar]
- 17.Hara K, Yamao K, Niwa Y, et al. Prospective clinical study of EUS-guided choledochoduodenostomy for malignant lower biliary tract obstruction. Am J Gastroenterol 2011; 106: 1239–1245. [DOI] [PubMed] [Google Scholar]
- 18.Hara K, Yamao K, Hijioka S, et al. Prospective clinical study of endoscopic ultrasound-guided choledochoduodenostomy with direct metallic stent placement using a forward-viewing echoendoscope. Endoscopy 2013; 45: 392–396. [DOI] [PubMed] [Google Scholar]
- 19.Khashab MA, Messallam AA, Penas I, et al. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc Int Open 2016; 4: E175–E181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunda R, Pérez-Miranda M, Will U, et al. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction using a lumen-apposing fully covered metal stent after failed ERCP. Surg Endosc 2016; 30: 5002–5008. [DOI] [PubMed] [Google Scholar]
- 21.Minaga K, Takenaka M, Kitano M, et al. Rescue EUS-guided intrahepatic biliary drainage for malignant hilar biliary stricture after failed transpapillary re-intervention. Surg Endosc 2017; 31: 4764–4772. [DOI] [PubMed] [Google Scholar]
- 22.Moryoussef F, Sportes A, Leblanc S, et al. Is EUS-guided drainage a suitable alternative technique in case of proximal biliary obstruction? Therap Adv Gastroenterol 2017; 10: 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakai Y, Isayama H, Yamamoto N, et al. Safety and effectiveness of a long, partially covered metal stent for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Endoscopy 2016; 48: 1125–1128. [DOI] [PubMed] [Google Scholar]
- 24.Paik WH, Park DH, Choi JH, et al. Simplified fistula dilation technique and modified stent deployment maneuver for EUS-guided hepaticogastrostomy. World J Gastroenterol 2014; 20: 5051–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paik WH, Lee NK, Nakai Y, et al. Conversion of external percutaneous transhepatic biliary drainage to endoscopic ultrasound-guided hepaticogastrostomy after failed standard internal stenting for malignant biliary obstruction. Endoscopy 2017; 49: 544–548. [DOI] [PubMed] [Google Scholar]
- 26.Park DH, Jang JW, Lee SS, et al. EUS-guided biliary drainage with transluminal stenting after failed ERCP: Predictors of adverse events and long-term results. Gastrointest Endosc 2011; 74: 1276–1284. [DOI] [PubMed] [Google Scholar]
- 27.Park DH, Lee TH, Paik WH, et al. Feasibility and safety of a novel dedicated device for one-step EUS-guided biliary drainage: A randomized trial. J Gastroenterol Hepatol 2015; 30: 1461–1466. [DOI] [PubMed] [Google Scholar]
- 28.Poincloux L, Rouquette O, Buc E, et al. Endoscopic ultrasound-guided biliary drainage after failed ERCP: Cumulative experience of 101 procedures at a single center. Endoscopy 2015; 47: 794–801. [DOI] [PubMed] [Google Scholar]
- 29.Song TJ, Lee SS, Park DH, et al. Preliminary report on a new hybrid metal stent for EUS-guided biliary drainage (with videos). Gastrointest Endosc 2014; 80: 707–711. [DOI] [PubMed] [Google Scholar]
- 30.Sportes A, Camus M, Greget M, et al. Endoscopic ultrasound-guided hepaticogastrostomy versus percutaneous transhepatic drainage for malignant biliary obstruction after failed endoscopic retrograde cholangiopancreatography: A retrospective expertise-based study from two centers. Therap Adv Gastroenterol 2017; 10: 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umeda J, Itoi T, Tsuchiya T, et al. A newly designed plastic stent for EUS-guided hepaticogastrostomy: A prospective preliminary feasibility study (with videos). Gastrointest Endosc 2015; 82: 390–396.e2. [DOI] [PubMed] [Google Scholar]
- 32.Wiersema MJ. Endosonography-guided cystoduodenostomy with a therapeutic ultrasound endoscope. Gastrointest Endosc 1996; 44: 614–617. [DOI] [PubMed] [Google Scholar]
- 33.Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy 2001; 33: 898–900. [DOI] [PubMed] [Google Scholar]
- 34.Siddiqui AA, Sreenarasimhaiah J, Lara LF, et al. Endoscopic ultrasound-guided transduodenal placement of a fully covered metal stent for palliative biliary drainage in patients with malignant biliary obstruction. Surg Endosc 2011; 25: 549–555. [DOI] [PubMed] [Google Scholar]
- 35.Yamao K, Bhatia V, Mizuno N, et al. EUS-guided choledochoduodenostomy for palliative biliary drainage in patients with malignant biliary obstruction: Results of long-term follow-up. Endoscopy 2008; 40: 340–342. [DOI] [PubMed] [Google Scholar]
- 36.Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest Endosc 2004; 59: 100–107. [DOI] [PubMed] [Google Scholar]
- 37.Sharaiha RZ, Khan MA, Kamal F, et al. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest Endosc 2017; 85: 904–914. [DOI] [PubMed] [Google Scholar]
- 38.Vila JJ, Pérez-Miranda M, Vazquez-Sequeiros E, et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointestinal Endoscopy 2012; 76: 1133–1141. [DOI] [PubMed] [Google Scholar]
- 39.Uemura RS, Khan MA, Otoch JP, et al. EUS-guided choledochoduodenostomy versus hepaticogastrostomy: A systematic review and meta-analysis. J Clin Gastroenterol 2018; 52: 123–130. [DOI] [PubMed] [Google Scholar]