Abstract
Background
Endoscopic resection is now commonly used for removal of early gastrointestinal lesions. However, the risk of the procedure may be heightened by intraprocedural or delayed bleeding. A novel, self-assembling peptide (PuraStat®) was recently licensed for use as a haemostat.
Objective
The aim of this study was to assess the efficacy and safety of this haemostat when used to control intraprocedural bleeding or to prevent delayed bleeding in endoscopic resection.
Methods
PuraStat® was used on 100 patients undergoing endoscopic resection in a tertiary referral centre. The efficacy, safety, feasibility of use and delayed bleeding rates were measured.
Results
Forty-eight oesophageal, 31 colorectal, 11 gastric and 10 duodenal procedures were included. The mean lesion size was 3.7 cm and 30% of the patients were on antithrombotic therapy. Intraprocedural bleeding occurred in 64%. PuraStat® was an effective haemostat in 75% of these cases. Only a small amount was required for haemostasis (mean = 1.76 ml) and it took on average 69.5 seconds to stop a bleed. The delayed bleeding rate was 3%.
Conclusions
PuraStat® is an effective haemostat for use in controlling bleeds during endoscopic resection. It is safe, easy to use and did not interfere with the procedure.
Keywords: Delayed bleeding, endoscopic mucosal resection, endoscopic submucosal dissection, haemostasis, self-assembling peptide
Key summary
Summarise the established knowledge on this subject:
Intraprocedural and delayed bleeding are encountered during endoscopic resection.
Usual methods of managing these bleeds (e.g. diathermy) carry a risk of thermal injury.
A novel, synthetic, self-assembling peptide was recently licensed as a haemostat for use in mucosal bleeding from the gastrointestinal tract.
Limited data exist on its efficacy as a haemostatic agent.
What are the significant and/or new findings of this study?
This self-assembling peptide works as a haemostat in 75% of mild to moderate intraprocedural bleeds encountered.
The delayed bleeding rate is low (3%).
The time taken to achieve haemostasis using this peptide is just over a minute.
Introduction
Endoscopic resection (ER) techniques such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are increasingly used as first-line treatment for removal of early gastrointestinal (GI) neoplasia with good outcomes. Bleeding is a well-recognised complication of ER and may pose a challenge to the endoscopist both during the procedure and afterward if delayed bleeding (DB) is encountered. This risk can range between 1% and 15% depending on lesion location, comorbidities and anticoagulant/antiplatelet agent usage.1
Current methods of haemostatic control during ER such as electrocoagulation and clip placement may increase the risk of thermal injury or impede further resection. Whilst adrenaline injections are a useful adjunct to haemostasis, their benefits are often short lived and definitive treatment is still required. Repeated application of diathermy (e.g. via coagulation forceps) over the site may lead to a perforation. Synthetic topical haemostatic agents have been used to control intraprocedural bleeding (IPB) and prevent DB following ER with modest success.2,3 Most are supplied as spray powders that can be applied in a nontargeted fashion over the bleeding area or resection base. However, their opaque nature does not make them the ideal haemostatic agents for use during ER.
Recently, a novel, self-assembling peptide (PuraStat®; 3D Matrix Ltd, France) was developed for use as a haemostat for treatment of exudative haemorrhages from small vessels in the GI tract. It is built from a chain of three types of amino acids that bond together to form a peptide (Figure 1). This transparent gel peptide self-assembles into fibres that form an extracellular matrix. It is activated when it comes into contact with bodily fluid as a change in pH and salt concentration triggers nanofiber network formation. The matrix sticks to and seals the blood vessel thereby facilitating haemostasis as a mechanical barrier is formed. Recent studies have highlighted its potential role in ESD-induced gastric ulcer healing and in reducing DB through the formation of a protective mucosal barrier over the resection site.4 There are limited data, however, on its use as a primary haemostat.5
Figure 1.
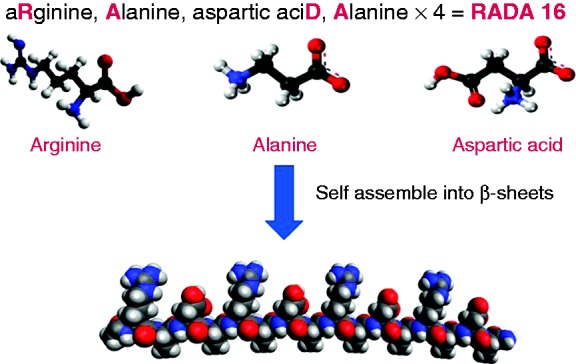
Building blocks of PuraStat®.
(Figure reproduced with permission from 3D-Matrix Ltd.)
The aim of our study was to assess the safety, efficacy and technical feasibility of PuraStat® as a primary haemostat to control bleeding during ER. We also evaluated its impact on 30-day DB rates.
Methods
Patients
Between January 2016 and September 2017, PuraStat® was used on 100 patients undergoing complex ER at a referral centre. The complexity of the procedures related to the large lesion size (>3 cm), scarring or presence of neoplasia necessitating the use of ESD and higher risk of bleeding attributed to anticoagulant use and anatomical location. It was used as a haemostat for IPB and prophylactically over the resection base. Patients provided written informed consent for the procedure and institutional review board approval was obtained (South Central Hampshire Research Ethics Committee, 16/SC/0020, 23 January 2016). The research was carried out in accordance with the 1975 Declaration of Helsinki.
Data on patient demographics and anticoagulant or antiplatelet medication use were prospectively collected. Lesion size, location, nature of bleeding, volume of PuraStat® used and any additional haemostatic methods used were recorded. The ease of application and any interference of the gel in the ongoing resection were also recorded. Three grades of IPB were identified:
Grade 1: Ooze from a venous vessel (mild)
Grade 2: Nonspurting venous vessel bleed (moderate)
Grade 3: Spurting arterial vessel bleed (severe)
DB was defined as overt haemorrhage (melaena, haematochaezia) occurring up to 30 days postprocedure and requiring medical intervention (endoscopic/radiological/surgical management) with or without a blood transfusion.
Procedures (ESD, hybrid ESD, EMR)
All procedures were carried out with high-definition endoscopes (EG-760R, EC-760V/M; Fujifilm; GIF-HQ290, CF-HQ290L; Olympus). The ESD knives used were Flush (DK2618J Fujifilm), Dual or IT Knife (Olympus). A standard lifting solution (Gelofusin with 1 ml of 1:10,000 adrenaline and 2 ml 1% indigocarmine) was used in all cases.
Diathermy was delivered via the ERBE VIO 300D electrosurgical generator (Erbe; Tübingen, Germany). Mucosal incision was performed using Endocut I and submucosal dissection using swift coagulation. For EMR or snare resection during hybrid ESD, Endocut Q was used. Bleeding was treated with the knife or snare tip using forced/swift coagulation or Coagrasper (Olympus) in soft coagulation mode.
PuraStat® application for haemostasis and prophylaxis
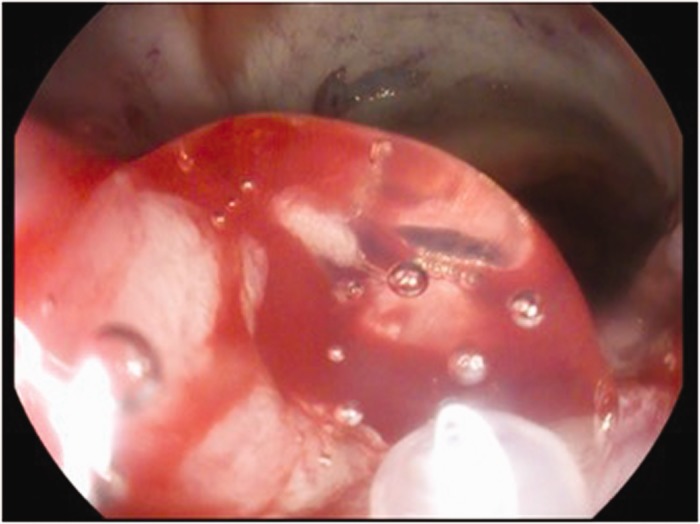
Three-ml prefilled syringes were connected to a 1600 mm (gastric) or 2200 mm (colonic) customised catheter inserted through the endoscope channel. PuraStat® was used as a primary haemostat when venous oozing was encountered. It was applied directly over the bleeding point (Figure 2) and the time taken for haemostasis was recorded. Diathermy was used as an additional haemostatic method if PuraStat® was not effective.
Figure 2.
Application of PuraStat® through the delivery catheter.
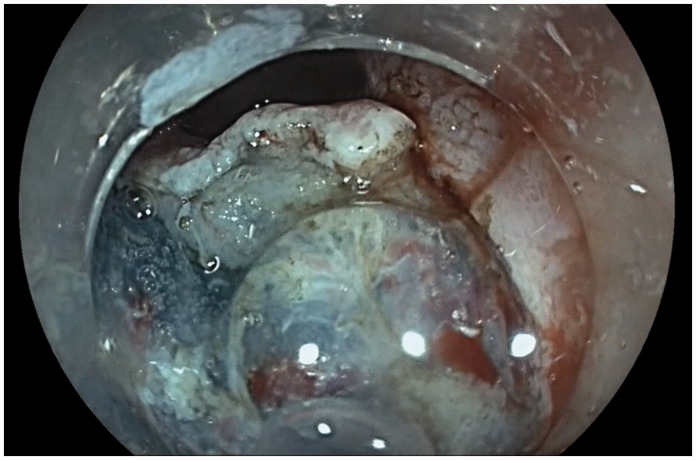
PuraStat® was applied over the resection base at the end of each procedure. To ensure sustained contact between the gel and the resection base, application was started from the antidependent side of the lateral edge of the base using gravity to allow the gel to slide into the centre. Gentle suction was used to bring the edges of the base closer to facilitate an even application and ensure complete coverage. It is important to note that the catheter is not retracted back into the scope for at least 15 seconds following deployment of PuraStat®. During this time, the endoscope position is maintained to observe the haemostatic effect. This prevents any gel dislodgement due to capillary action of catheter withdrawal or mechanical movement from the scope. Figure 3 highlights its transparent nature.
Figure 3.
Transparent nature of PuraStat®.
Follow-up
High-dose proton pump inhibitor therapy (40 mg omeprazole twice daily or equivalent) was administered to all patients undergoing upper GI ER for six weeks postprocedure. Anticoagulants or antiplatelets were reinstated 48 hours following resection according to the endoscopist's preference. Adverse events such as DB and perforation were recorded for 30 days postprocedure.
Statistical analysis
Descriptive statistics were used, continuous variables were expressed in terms of mean (with SD as a measure of variability) and categorical variables were expressed as proportions. Pearson's chi-squared test was used to compare proportions with p < 0.05 considered as significant.
Results
Patient, procedure and lesion characteristics
A total of 100 patients undergoing ER were included in the study (79 underwent ESD/hybrid ESD and 21 had EMR). The mean age of the patients was 69.3 years with a male majority (68% male, 32% female). Thirty per cent were on anticoagulant/antiplatelet therapy (nine warfarin, 19 aspirin or clopidogrel, and two novel oral anticoagulant).
Most resections were undertaken in the oesophagus (48%), followed by colorectum (31%), gastric (11%) and duodenum (10%). The mean lesion size was 3.67 cm (SD = 2.12) with a mean resection base area of 14.05 cm2 (SD = 16.47). The final resection histology confirmed high-risk neoplasia (carcinoma or high-grade dysplasia) in 59 lesions (58 in the upper GI tract and one in the lower GI tract) (Table 1).
Table 1.
Lesion characteristics according to location.
| Location | Histology | Number | Mean lesion specimen size (cm) | Mean area of resection base (cm2) |
|---|---|---|---|---|
| Oesophagus | 48 | 3.19 | 10.89 | |
| Barrett's adenocarcinoma | 33 | |||
| Barrett's high-grade dysplasia | 10 | |||
| Intestinal metaplasia | 3 | |||
| Squamous dysplasia | 2 | |||
| Gastric | 11 | 4.73 | 19.57 | |
| Adenocarcinoma | 8 | |||
| High-grade dysplasia | 3 | |||
| Duodenum | 10 | 2.80 | 7.70 | |
| Adenoma | 9 | |||
| Neuroendocrine tumour | 1 | |||
| Colon | 16 | 3.02 | 7.88 | |
| Adenoma | 13 | |||
| Sessile serrated lesion | 2 | |||
| Hyperplastic | 1 | |||
| Rectum | 15 | 5.83 | 30.96 | |
| Adenoma | 15 |
Nature of bleeding and haemostatic efficacy of PuraStat®
Grade 1 and 2 bleeds were the most commonly encountered (noted in 62 resections (62%)) though bleeding did also arise from a spurting vessel in six (6%) cases. PuraStat® was used for IPB in 64/100 (64%) resections (33 oesophageal, five gastric, six duodenal, 20 colorectal) and applied prophylactically to cover the resection base in all 100 cases. It was effective in 48/64 (75%) of cases. This was stratified according to location where PuraStat® terminated bleeding in all duodenal, 81.8% of oesophageal, 80% of colonic, 60% of gastric and 40% of rectal IPB. When both modalities of ER were compared (ESD and EMR), we noted that there was no significant difference between the proportion of cases with IPB (64.5% vs 61.9%, p = 0.60). There was a greater proportion of Grade 1 bleeds in the EMR group compared to ESD (23.8% vs 6.3%, p < 0.02). Grade 3 (spurting) bleeds were the minority bleed type and seen in only 7.6% of ESD cases (Table 2).
Table 2.
Grade of bleed according to type of procedure.
| Procedure type | Bleed type |
||||
|---|---|---|---|---|---|
| Grade 1 alone | Grade 2 alone | Grade 3 alone | Grades 1 and 2 | No intraprocedural bleeding | |
| ESD and hybrid ESD (n = 79) | 5 (6.3%) | 2 (2.5%) | 6 (7.6%) | 38 (48.1%) | 28 (32.0%) |
| EMR (n = 21) | 5 (23.8%) | 1 (4.8%) | 0 | 7 (33.3%) | 8 (38.1%) |
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection.
PuraStat® was effective (with no additional haemostatic method) in 72.6% (45/62) of venous type (Grades 1 and 2) bleeds and 50% (3/6) of spurting (Grade 3) bleeds (p = 0.25). See Table 3.
Table 3.
Nature of bleeding and technical aspects of PuraStat® application.
| Oesophagus | Gastric | Duodenum | Colonic | Rectum | |
|---|---|---|---|---|---|
| N, % | 48 | 11 | 10 | 16 | 15 |
| Primary haemostat in mucosal oozing | 33 (68.8%) | 5 (45.5%) | 6 (60%) | 10 (62.5%) | 10 (66.7%) |
| Amount of PuraStat® used for haemostasis per lesion (mean, ml) | 1.53 | 3.7 | 1.78 | 1.31 | 1.98 |
| PuraStat® successfully achieved haemostasis | 27/33 (81.8%) | 3/5 (60%) | 6/6 (100%) | 8/10 (80%) | 4/10 (40%) |
| Average time taken to achieve haemostasis (seconds) | 69.4 | 26.7 | 50.7 | 102.5 | 69.7 |
| Amount of PuraStat® used for prophylactic coverage of resection base per lesion (mean, ml) | 2.57 | 3.31 | 2.33 | 1.86 | 2.82 |
| Amount used per cm2 (ml) | 0.24 | 0.17 | 0.30 | 0.24 | 0.10 |
DB
There were three DBs in this study – two following gastric ESD (both lesions were early gastric cancers located in the antrum) and one following oesophageal ESD for adenocarcinoma. PuraStat® had been used prophylactically in all cases and was required for intraprocedural haemostasis only in the oesophageal ESD, in which it terminated the bleeding successfully without diathermy. One gastric ESD bleed occurred five days postprocedure whilst the other occurred 10 days post. Both of these patients were on anticoagulants (warfarin/clopidogrel) that were restarted on day 2 postprocedure. They underwent urgent endoscopy upon presenting with melaena, and in one case endotherapy was required (bleeding from a visible vessel stemmed with adrenaline injection + clip placement). Neither required a blood transfusion and both were discharged thereafter.
The postoesophageal ESD bleed occurred on day 1 postprocedure, and endoscopy revealed a clot over the resection base but no active bleeding. No endotherapy or blood transfusion was required.
There was no haemodynamic instability in all three patients and none experienced a rebleed following the second endoscopy procedure (Table 4).
Table 4.
Characteristics of delayed bleeds.
| Location | Oesophageal | Gastric antrum | Gastric antrum |
|---|---|---|---|
| Patient age (years) | 82 | 78 | 86 |
| Gender | Male | Male | Male |
| Specimen size (cm) | 2.5 | 6 | 6 |
| Histology | Intramucosal adenocarcinoma, pT1aM3 | High-grade dysplasia | Adenocarcinoma, pT1bSM1 |
| Anticoagulant use | Nil | Warfarin | Clopidogrel |
| Onset postprocedure | 1 day | 5 days | 10 days |
| Endoscopy findings | Clot over resection base | Bleeding from visible vessel | Clot over resection base |
| Endotherapy | No | Yes (6 ml 1:100,000 adrenaline injection + bipolar coagulation) | No |
| Blood transfusion | No | No | No |
| Rebleed postendoscopy | No | No | No |
The DB rate in colonic and duodenal ER was 0%.
Feasibility and technical outcomes
The average volume of PuraStat® used for IPB (per lesion) was 1.76 ml (SD = 1.40) whereas 2.56 ml (SD = 1.13) was the average required for complete coverage of the resection base. The average time taken to achieve haemostasis using PuraStat® was 69.5 seconds (SD = 68.5).
In all cases, the endoscopist rated PuraStat® as being ‘easy to apply’ with no instances of catheter blockage, catheter kinking or interference with visibility.
There were no instances of allergic reaction or hypersensitivity, pain or thromboembolic events related to PuraStat® use.
Discussion
ER is rapidly replacing major surgical resection thanks to development of new techniques like ESD. These procedures are associated with an increased risk of perforation and bleeding. Although these risks are mitigated by improved endoscopic skill, carbon dioxide insufflation and better endoscopes, the risk of IPB and DB remains high. PuraStat® is a novel agent with haemostatic properties. It has been used in cardiovascular surgery6,7 and in small ESD series5 with promising results.
Our study demonstrates that PuraStat® was effective as a single agent in controlling IPB in 75% of cases in which bleeding was encountered. We also identified the type of bleed for which it is more effective. Its haemostatic properties were best demonstrated in Grades 1 and 2 venous-type bleeds (where it stopped bleeding in 73%) compared to Grade 3 arterial-type spurting bleeds, for which it worked in only half the cases. Despite complete coverage of the bleeding point during a brisk bleed, the adhesive properties of the gel were hampered by the rate and volume of the bleed, thereby preventing adequate haemostasis. This observation has an important clinical implication as it can help the endoscopist choose the most appropriate haemostatic modality according to the grade of the bleed. It is also important to note that PuraStat® did not affect the use of any additional haemostatic modality (diathermy) or ongoing resection. This is due to its transparent nature and electrical conductivity of the gel in the presence of bodily fluids.
We also identified the average time taken to achieve haemostasis using PuraStat® (69.5 seconds). This is shorter than noted in a previous study of 16 patients undergoing ER of gastric tumours in which the time required for haemostasis was 105 ± 87 seconds.5 Our cohort was much larger with just a single endoscopist using the device – the growing cumulative experience may have led to a learning curve effect for its precise application enabling quicker haemostasis. It is also possible that the speed at which it works depends not only on the type of bleed but also on the location of the lesion as this differed in each anatomical location. The sample size outside the oesophagus and colon/rectum is not enough to draw any isolated clinical conclusion but suffice to say that PuraStat® works in just over a minute irrespective of the site. We believe that if haemostasis is not observed (despite adequate coverage of the bleeding point) after this time, then the endoscopist should apply another haemostatic modality.
Several large studies on ER outcomes have reported DB rates of between 4% and 9% for gastric ESD,8–10 6.2% for colonic EMR,11,12 2.7% for colonic ESD,13 5% to 20% for duodenal EMR,14–16 1.7% for Barrett's ESD17 and 1.1% for Barrett's EMR.18 Alternative techniques to prevent DB have been explored as advances in ER were made over the past decade. However, a recent meta-analysis of prophylactic clipping in colorectal ESD found no decrease in DB.19 A study of prophylactic coagulation after wide-field colonic EMR also found no significant difference in rates of DB compared to a control group (5.2% vs 8.0%).20 By contrast, prophylactic coagulation may be more effective in the upper GI tract in reducing DB following gastric ESD.21 Topical polysaccharide haemostats may have a role in preventing EMR-rated bleeding as evidenced by a study of 82 patients receiving EndoClot with a DB rate of 3.7%.3 One other study reports a DB rate of 6.2% following prophylactic application of PuraStat®, though this study included fewer lesions (56 patients with 65 lesions) with a majority resected via ESD (61.5%).22
The overall DB rate was 3% in this study. This is lower than anticipated in this high-risk group of patients, all of whose lesions were over 2 cm and 30% of whom were on antithrombotic therapy. Two of the three DBs occurred following ESD of early gastric antral cancers. Both lesions were 6 cm in size and the patients resumed anticoagulant therapy postprocedure, reflecting the skewed bleeding risk in this group. Previous studies have shown that a larger lesion size, antithrombotic therapy and location within the antrum are factors associated with higher rates of DB.23,24 Careful consideration should be given to the timing of restarting antithrombotic therapy in this group as no specific guidelines exist. Given the paucity of literature in this area, future prospective trials with a matched control group will be useful to determine whether the DB rate is reduced in all patients receiving PuraStat® prophylactically or if it is most useful in patients stratified as having a higher bleeding risk (e.g. those on interrupted antithrombotic therapy that will need to be resumed postendoscopy).
Our results show that PuraStat® is safe and easy to use – with no reported adverse events including allergic reactions. This is due to its synthetic nature. Unlike other synthetic haemostats, it is unique in being transparent and delivered through a fine catheter, thereby permitting targeted application of the gel over the bleeding vessel. There were no incidences of catheter blockage, and PuraStat® was delivered successfully to the desired site in all cases with no technical challenges. The endoscopist can accurately evaluate its efficacy as the bleeding can be seen to cease beneath the transparent layer of the gel. Further ER can continue once haemostasis has been achieved as the gel does not hinder the movement of the endoscopic knife during submucosal dissection and no detrimental impact on electrical conductivity through the gel was observed.
This study does, however, have several limitations. It was conducted in a single centre and lacked a matched control group. It does not stratify the type of patient or lesion that PuraStat® should be used as a first-line agent on. This was due to the lack of data on its haemostatic efficacy in ER which would have affected the design of a controlled trial. Only a single other pilot study evaluating its haemostatic efficacy was carried out though this was limited to a small group of patients (n = 12) undergoing gastric ESD.5 Our study provides new data on the efficacy of this haemostat in a range of ER procedures in various anatomical locations and establishes the type of bleed for which it is found to be more effective. The preliminary data gathered during this study can be used to design and conduct a prospective, randomised, controlled trial which will provide high-quality evidence to further guide the use of this haemostat.
To our knowledge, this is the largest study reporting haemostatic outcomes of this novel device for use during ER where bleeding can be encountered. We have shown that it is an effective haemostat in three-quarters of bleeds with an overall low rate of DB in this high-risk cohort. As its use increases in endoscopy, a randomised, controlled trial comparing PuraStat® with conventional haemostatic methods like diathermy is warranted to streamline its role as a haemostat during complex ER.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Institutional review board approval was obtained for this study (South Central Hampshire Research Ethics Committee, 16/SC/0020, 23 January 2016), and the research was carried out in accordance with the 1975 Declaration of Helsinki.
Informed consent
Patients provided written informed consent for the procedure.
References
- 1.Kataoka Y, Tsuji Y, Sakaguchi Y, et al. Bleeding after endoscopic submucosal dissection: Risk factors and preventive methods. World J Gastroenterol 2016; 22: 5927–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mourad FH, Leong RW. Role of hemostatic powders in the management of lower gastrointestinal bleeding: A review. J Gastroenterol Hepatol 2018; 33: 1445–1453. [DOI] [PubMed] [Google Scholar]
- 3.Huang R, Pan Y, Hui N, et al. Polysaccharide hemostatic system for hemostasis management in colorectal endoscopic mucosal resection. Dig Endosc 2014; 26: 63–68. [DOI] [PubMed] [Google Scholar]
- 4.Uraoka T, Ochiai Y, Fujimoto A, et al. A novel fully synthetic and self-assembled peptide solution for endoscopic submucosal dissection-induced ulcer in the stomach. Gastrointest Endosc 2016; 83: 1259–1264. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida M, Goto N, Kawaguchi M, et al. Initial clinical trial of a novel hemostat, TDM-621, in the endoscopic treatments of the gastric tumors. J Gastroenterol Hepatol 2014; 29(Suppl 4): 77–79. [DOI] [PubMed] [Google Scholar]
- 6.Masuhara H, Fujii T, Watanabe Y, et al. Novel infectious agent-free hemostatic material (TDM-621) in cardiovascular surgery. Ann Thorac Cardiovasc Surg 2012; 18: 444–451. [DOI] [PubMed] [Google Scholar]
- 7.Giritharan S, Salhiyyah K, Tsang G, et al. Feasibility of a novel, synthetic, self-assembling peptide for suture-line haemostasis in cardiac surgery. J Cardiothorac Surg 2018; 13: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Facciorusso A, Antonino M, Di Maso M, et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: A meta-analysis. World J Gastrointest Endosc 2014; 6: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lian J, Chen S, Zhang Y, et al. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc 2012; 76: 763–770. [DOI] [PubMed] [Google Scholar]
- 10.Park YM, Cho E, Kang HY, et al. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: A systematic review and metaanalysis. Surg Endosc 2011; 25: 2666–2677. [DOI] [PubMed] [Google Scholar]
- 11.Burgess NG, Metz AJ, Williams SJ, et al. Risk factors for intraprocedural and clinically significant delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol 2014; 12: 651–661. e1–e3. [DOI] [PubMed] [Google Scholar]
- 12.Burgess NG, Williams SJ, Hourigan LF, et al. A management algorithm based on delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol 2014; 12: 1525–1533. [DOI] [PubMed] [Google Scholar]
- 13.Fuccio L, Hassan C, Ponchon T, et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: A systematic review and meta-analysis. Gastrointest Endosc 2017; 86: 74–86.e17. [DOI] [PubMed] [Google Scholar]
- 14.Navaneethan U, Hasan MK, Lourdusamy V, et al. Efficacy and safety of endoscopic mucosal resection of non-ampullary duodenal polyps: A systematic review. Endosc Int Open 2016; 4: E699–E708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lépilliez V, Chemaly M, Ponchon T, et al. ER of sporadic duodenal adenomas: An efficient technique with a substantial risk of delayed bleeding. Endoscopy 2008; 40: 806–810. [DOI] [PubMed] [Google Scholar]
- 16.Tomizawa Y, Ginsberg GG. Clinical outcome of EMR of sporadic, nonampullary, duodenal adenomas: A 10-year retrospective. Gastrointest Endosc 2018; 87: 1270–1278. [DOI] [PubMed] [Google Scholar]
- 17.Yang D, Zou F, Xiong S, et al. Endoscopic submucosal dissection for early Barrett's neoplasia: A meta-analysis. Gastrointest Endosc 2018; 87: 1383–1393. [DOI] [PubMed] [Google Scholar]
- 18.Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett's esophagus-related neoplasia: A systematic review and pooled analysis. Gastrointest Endosc 2017; 85: 482–495.e4. [DOI] [PubMed] [Google Scholar]
- 19.Nishizawa T, Suzuki H, Goto O, et al. Effect of prophylactic clipping in colorectal ER: A meta-analysis of randomized controlled studies. United European Gastroenterology J 2017; 5: 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahin FF, Naidoo M, Williams SJ, et al. Prophylactic endoscopic coagulation to prevent bleeding after wide-field endoscopic mucosal resection of large sessile colon polyps. Clin Gastroenterol Hepatol 2015; 13: 724–730.e2. [DOI] [PubMed] [Google Scholar]
- 21.Park CH, Lee SK. Preventing and controlling bleeding in gastric endoscopic submucosal dissection. Clin Endosc 2013; 46: 456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pioche M, Camus M, Rivory J, et al. A self-assembling matrix-forming gel can be easily and safely applied to prevent delayed bleeding after ERs. Endosc Int Open 2016; 4: E415–E419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong J, Wei K, Deng J, et al. Effects of antithrombotic therapy on bleeding after endoscopic submucosal dissection. Gastrointest Endosc 2017; 86: 807–816. [DOI] [PubMed] [Google Scholar]
- 24.Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: Technical feasibility, operation time and complications from a large consecutive series. Dig Endosc 2005; 17: 54–58. [Google Scholar]