Abstract
Background
Opioid-induced bowel dysfunction is a complication of opioid therapy, in which constipation is the most common and problematic symptom. However, it is frequently under-recognised and thus effective management is often not instituted despite a number of treatment options.
Objective
The central objective of this study is to provide a summary of the pathophysiology and clinical evaluation of opioid-induced constipation and to provide a pragmatic management algorithm for day-to-day clinical practice.
Methods
This summary and the treatment algorithm is based on the opinion of a European expert panel evaluating current evidence in the literature.
Results
The pathophysiology of opioid-induced constipation is multi-faceted. The key aspect of managing opioid-induced constipation is early recognition. Specific management includes increasing fluid intake, exercise and standard laxatives as well as addressing exacerbating factors. The Bowel Function Index is a useful way of objectively evaluating severity of opioid-induced constipation and monitoring response. Second-line treatments can be considered in those with recalcitrant symptoms, which include gut-restricted or peripherally acting mu-opioid receptor antagonists. However, a combination of interventions may be needed.
Conclusion
Opioid-induced constipation is a common, yet under-recognised and undertreated, complication of opioid therapy. We provide a pragmatic step-wise approach to opioid-induced constipation, which should simplify management for clinicians.
Keywords: Opioid-induced constipation, gastroenterology, bowel dysfunction, management algorithm, gastro-intestinal motility
Introduction
Opioids are a class of potent analgesics, and their use has increased markedly in recent years.1 Although opioids are potent analgesics, they are not a panacea for all types of pain, and must be used appropriately in selected and supervised pain patients as part of a comprehensive, multi-modal, multi-disciplinary approach to treatment.2 More importantly, opioids are associated with a variety of bothersome side effects such as sedation, lethargy and pruritus, notwithstanding the considerable risk of addiction.3,4 Opioids also adversely impact the sensorimotor function of the gastrointestinal (GI) tract, via the action of exogenous opioid agonists, on the enteric nervous system (ENS).2,5 Such adverse effects limit dose escalation and can necessitate a switch in opioids or even cessation of therapy.2,6 The term opioid-induced bowel dysfunction (OIBD) encompasses a spectrum of symptoms including nausea, vomiting, bloating, gastro-oesophageal reflux-related symptoms and constipation.7,8
Opioid-induced constipation (OIC) is the most common subtype of OIBD that occurs in 51–87% of patients receiving opioids for cancer and between 41–57% patients receiving opioids for chronic non-cancer pain.9–11 OIC is associated with reduced work productivity, a decrease in quality of life and increased healthcare utilisation.12 OIC is often under-recognised and likely to be more troubling in younger rather than older patients.13,14 The Rome process has sought to systematise the definition of OIC, building upon previous proposals.15 The Rome IV criteria define OIC as new, or escalating, symptoms of constipation when initiating, changing or increasing opioid therapy with further clinical features, such as sensation of incomplete evacuation and fewer than three spontaneous bowel movements per week, see Table 1.16 The aims of this consensus article are to provide a focussed review of the pathophysiology, clinical evaluation and treatment of OIC, and pragmatic management recommendations that can be used in daily clinical practice.
Table 1.
The Rome IV diagnostic criteria for opioid-induced constipation.
| Diagnostic criteria |
|---|
| 1. New, or escalating, symptoms of constipation when initiating, changing or increasing opioid therapy that must include two or more of the following: (a) Straining during more than one quarter of defaecations. (b) Lumpy or hard stools (BSFS 1–2) more than one-quarter of the time. (c) Sensation of incomplete evacuation more than one-quarter of the time. (d) Sensation of anorectal blockage/obstruction in more than one-quarter of defaecations. (e) Manual manoeuvres to facilitate more than one-quarter of defaecations. (f) Fewer than three spontaneous bowel movements per week. 2. Loose stools rarely present without the use of laxatives. |
BSFS: Bristol Stool Form Scale.
Methods
A panel of seven European experts, chosen based upon their previous contributions to the area in terms of their clinical and academic experience, in the fields of neurogastroenterology, pain medicine and palliative medicine, met on two occasions to discuss, develop and agree on the contents of this statement. At the first meeting the broad aspects on the consensus statement were discussed and specific sections were assigned, where authors undertook a comprehensive literature review. The assigned sections were (a) definitions and diagnostic criteria (ADF), (b) pathophysiology of OIC (AMD, RDG), (c) clinical evaluation (GC and TOB), (d) patient reported outcome measures (ADF), (e) initial evaluation/standard laxatives (BM), (f) specific treatments (ADF, RDG, JT) as well as (g) pragmatic recommendations (all). Prior to the second meeting the various sections of the article were collated and circulated. At the second meeting, each section of the article was discussed in a workshop format and debated to achieve consensus. At this stage external input was given by a multi-disciplinary panel consisting of experts in neurogastroenterology, oncology and palliative medicine (see Acknowledgements). The pragmatic recommendations were based on expert opinion, considering existing recommendations and the clinical experience of the authors. Patient advocates were not involved in the development of this document. The final contents of this paper have been agreed upon by all of the members of the panel.
Pathophysiology of OIC
Opioid receptors in the GI tract
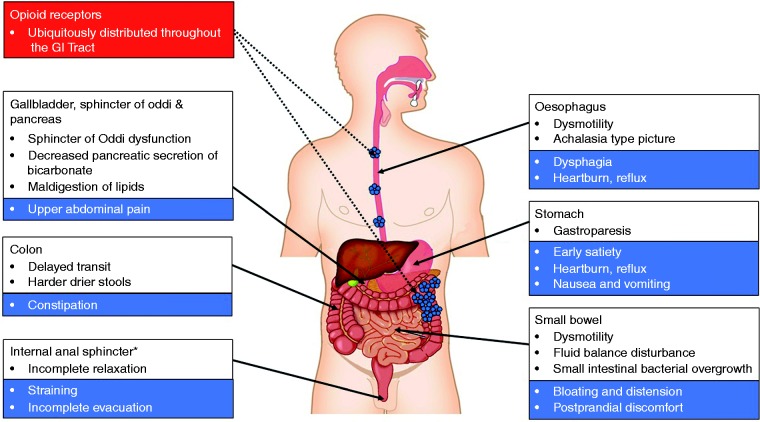
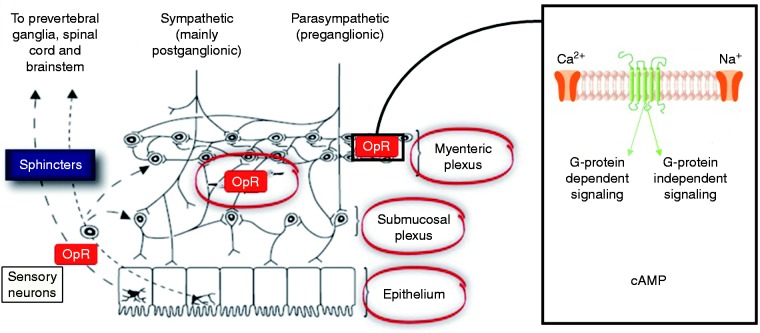
The opioid receptors, and their related ligands, exert a profound influence on GI physiology, see Figure 1. Opioid receptors, namely δ-, κ-, and μ-, are G-protein-coupled receptors widely distributed throughout the GI tract, with a relative distribution varying according to region and layer of the gut as well as with mammalian species considered.17–19 The majority of data is derived from animal studies which demonstrate that the highest densities of μ- and κ-receptors are located in the stomach and proximal colon.20 Whilst in humans the distribution of the different opioid receptors and subclasses has been less thoroughly investigated, μ-receptors are thought to be of central importance. Immunohistochemical studies have shown that μ-receptors are located on the cell membrane of submucosal and myenteric neurons and have been detected in mononuclear cells of the lamina propria, but not in epithelial cells.21 Various endogenous (e.g. enkephalins, endorphins and dynorphins) and exogenous (e.g. opioids) ligands can bind to opioid receptors, leading to their internalization, coupling to inhibitory Gi/Go proteins that activate or inhibit downstream messengers. Specifically, opioid agonists binding to the G-protein coupled μ- and δ-receptors close voltage-gated Ca2+ channels on presynaptic nerve terminals thereby reducing cyclic adenosine monophosphate (cAMP) and neurotransmitter release. In addition, they open K+ channels post-synaptically, resulting in neuronal hyperpolarization and inhibition of postsynaptic neurons.22 Taken together, opioid receptors affect GI function in a multifaceted manner through decreased neuronal excitability, see Figure 2.
Figure 1.
A schematic summary of the effects of opioids on the gastro-intestinal (GI) tract. Opioid receptors are distributed throughout the GI tract. *The function of other GI sphincters can also be influenced opioids such as the lower oesophageal sphincter and pylorus.
Figure 2.
A highly schematic summary of the basic neural mechanisms leading to opioid-induced bowel dysfunction including constipation. Opioids bind to receptors expressed in the enteric nervous system. The overall result is a neuronal-mediated blockade of secretomotor gastro-intestinal (GI) function causing opioid-induced constipation (OIC). cAMP: cyclic adenosine monophosphate; OpR: opioid receptor.
Effects of opioids on GI motility
GI motility is dependent on a fine balance between excitatory and inhibitory neurotransmitters/neuromodulators mainly released by myenteric neurons that result in smooth muscle contraction and relaxation respectively. Excitatory motor neurons release acetylcholine and tachykinins (e.g. substance P), which evoke longitudinal smooth muscle contraction. This is in contrast to inhibitory motor neurons, which induce smooth muscle relaxation via nitric oxide and vasoactive intestinal polypeptide.8,23 Opioids inhibit the release of the neurotransmitters, which results in abnormal coordination of motility reflected by an increase in muscular tone and a decrease in the normal propulsive activity. In vivo human studies have shown that opioids exert a myriad of effect across the entire GI tract including dysmotility in the oesophagus and gallbladder, increased gastric tone, as well as retardation of gastric emptying, oro-caecal and colonic transit time.23–28
Effects of opioids on GI secreto-absorptive function
The GI tract secretes approximately 9–10 l of fluid per day (approximately 2 l saliva, 2.5 l gastric juice, 1–1.5 l bile, 2 l pancreatic juice and 1.5–2 l enteric secretion).29 Opioids exert a profound influence in the secretory and absorptive function of the GI tract through a number of mechanisms. For instance, opioids bind to receptors on secretomotor neurons in the submucosa of the GI tract and suppress acetylcholine and vasoactive intestinal peptide release, resulting in a decrease in chloride and water secretion into the lumen.23,30 In addition to secretory impairment, opioids may increase water absorption mainly via the prolonged stasis of intestinal content due to inhibition of gut motility. In the colon, a decreased faecal volume has a negative effect on motility – which results in propulsive contractions – as the intrinsic reflexes are dependent on mechanoreceptor activation.18 These effects can explain why patients in opioid therapy typically complain of harder, drier faeces and straining difficulties.
Effect of opioids on GI sphincters
In the human GI tract there are at least six anatomically or functionally characterised sphincters, i.e. the upper and lower oesophageal sphincters, pylorus, sphincter of Oddi, the ileo-caecal valve and the anal sphincters. Although the function of each these sphincters can be modulated by opioids, it is beyond the scope of this paper to examine all of these in detail, but we will highlight evidence around the anal sphincters. Opioid-induced dysfunction of anorectal function is characterised by increased contraction of the internal anal sphincter which, in turn, results in straining, haemorrhoids and/or a sense of incomplete evacuation. Taken together, this can lead to severe problems with defaecation and in the worst-case scenario colonic perforation may occur.31 For instance, loperamide has been shown to increase the tone of the internal anal sphincter and a third of patients treated with opioids report a sensation of anal blockage despite laxative treatment.10,32 In a recent study, Poulsen et al. reproduced these findings demonstrating that oxycodone inhibits anal sphincter relaxation, an effect that can be reversed by slow-release naloxone.33
Clinical evaluation
For most patients on opioids who present with ‘constipation’, it is likely that there are multiple potential factors contributing to the problem and it may not be easy, on initial assessment, to determine what contribution, if any, the opioid might be making to the overall symptom burden. As a basic principle, the assessing clinician must take a comprehensive history with particular focus on the baseline bowel habit and any changes that may have occurred subsequent to the introduction of an opioid. A detailed drug history is mandatory to identify medications that might be contributing to the problem. Where possible, the diagnosis of OIC should be made according the to the Rome criteria and in this regard, patients need to be questioned about bowel frequency, stool consistency and symptoms suggestive of disordered defecation such as straining at stool, sense of incomplete evacuation and faecal incontinence.16 An important consideration with respect to faecal incontinence is overflow diarrhoea due to opioid-induced faecal impaction.31 In addition to physical symptoms, addressing psychological aspects, such as a patient's underlying ideas and appreciation of their symptoms is also beneficial.34 Additional symptoms such as bloating, abdominal pain, nausea and vomiting suggestive of OIBD also need to be addressed. Causes of secondary constipation should be sought from the past medical history (e.g. prolonged physical inactivity, Parkinson's disease, advanced diabetes, etc.). A digital rectal examination is suggested in all patients consulting for OIC to exclude anorectal malignancy, faecal impaction and minor anal pathologies (e.g. anal fissure) which potentially may aggravate symptoms.35 Given the prevalence of OIC, we advocate that all patients initiating opioids, and those who are maintained on opioids, should have a regular systematic review of their bowel function. However, there remain a number of factors that act as barriers to the diagnosis of OIC being made, see Table 2.
Table 2.
Some of the barriers that exist in making the diagnosis of opioid-induced constipation (OIC).
| Putative barriers |
|---|
| 1. Lack of awareness among clinicians about OIC in patients on opioid therapy. 2. If clinicians are aware, they may not ask patients about constipation. 3. When considering constipation, most clinicians only ask questions about frequency of bowel movements, but symptoms such as bloating, straining, hard stool consistence, incomplete bowel movements and abdominal discomfort are more prevalent and bothersome, features reflecting the pan-enteric effects of OIBD. 4. Patients might feel ashamed to disclose their symptoms to clinicians. 5. Efforts to screen patients based on Rome IV criteria may not cover the whole spectrum of OIC. 6. Absence of a standard protocol for the treatment of OIC. |
OIBD: opioid-induced bowel dysfunction.
Patient-reported outcome measures in OIC
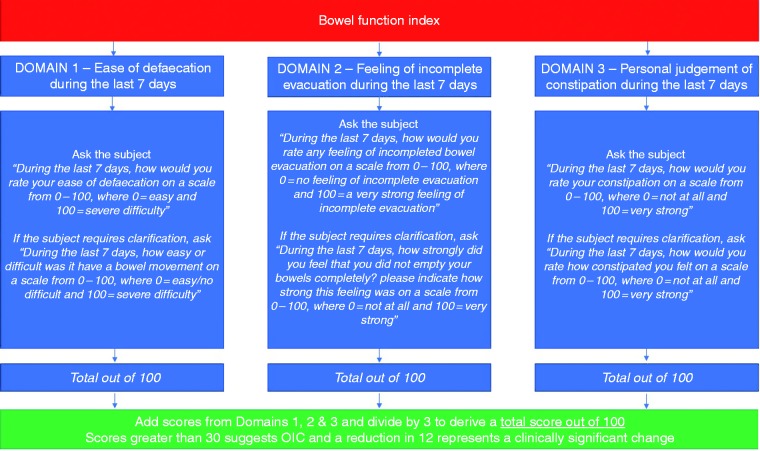
Although a plethora of patient reported outcome measures are available, such as the patient assessment of constipation symptoms (PAC-SYM),36 patient assessment of constipation quality of life (PAC-QoL)37 and the Knowles Eccersley Scott Symptom Score,38 many of these are too cumbersome for routine clinical practice.39 However, the Bristol Stool Form scale (BSFS) and the Bowel Function Index (BFI) are simple, brief and validated questionnaires that can be a useful adjunct to standard clinical evaluation as well as providing an objective assessment of treatment response. The BSFS evaluates stool consistency and is a widely used tool which pictorially describes stool ranging from type 7 to type 1, with the latter representing separate hard lumps of stool.40 BSFS type 1 and 2 would be consistent with, but not specific for, OIC. Other aspects of OIC can be assessed using the BFI, which contains three items evaluating the wider scope of OIC symptoms over the preceding week. These three items include ease of defaecation, feeling of incomplete bowel evacuation and the patient's own view of their constipation. Each are rated on a numerical scale from 0–100, giving a total combined score of 300, which is then divided by three to give an overall score out of 10041 (see Figure 3). Overall scores greater than 30 are consistent with OIC, and a change in score of ≥12 points represent a clinically meaningful change following an intervention.39,41,42
Figure 3.
The Bowel Function Index. OIC: opioid-induced constipation.
Investigations
In most cases, multiple investigations are not required in OIC. There is a paucity of data regarding the added utility of tests in patients with suspected OIC. Biochemical and haematological measures, such as electrolytes, calcium, haemoglobin concentration and thyroid function tests, can be of use in excluding electrolyte disturbance, anaemia, thyroid dysfunction, respectively, as an underlying cause of constipation.43 Colonoscopy should be reserved to those patients presenting with ‘red flag’ symptoms which include: (a) rectal bleeding, (b) iron-deficiency anaemia, (c) weight loss, (d) family history of colon cancer, (e) fever, and (f) age of onset after age 50 years.44 Radiological investigations, such as a plain abdominal radiograph or computed tomography, can be useful to identify marked faecal loading and impaction.45
Management of OIC
General measures
Prophylactic treatment of OIC with laxatives can be considered, although there is minimal evidence to support this view.46–48 However, more often than not laxatives are not co-prescribed; for instance, a Norwegian community study found that only 30% of cancer patients receiving opioids had a laxative co-prescription.49 Clearly, there is a role for the clinician commencing, changing or escalating the opioid to warn patients that constipation is a recognised side effect, although many patients never receive, or do not recall, this advice.50 Initial general measures include patient education, examining lifestyle factors (fluid intake and activity) and where possible identifying and modifying concurrent medications (such as iron supplements, calcium-channel blockers, anti-cholinergic agents, 5-hydroxytryptamine (5-HT)3 receptor antagonists or diuretics) which may exacerbate OIC. In some cases, switching the opioid or changing the route of administration can be useful. For example, tapentadol, a mixed-opioid agonist and noradrenaline-reuptake inhibitor, is associated with less constipation than oxycodone.51 In addition, the incidence of OIC may be numerically less with transcutaneous preparations of fentanyl in comparison to equipotent doses of oral morphine.52
Standard laxatives
Standard laxatives, such as osmotic agents (macrogol) and stimulants (bisacodyl, picosulphate and senna) are good first-line choices in the management of OIC. Additionally, a recent study reported that laxative side effects, such as gas, bloating/fullness and defaecatory urgency, are seen in up to 75% of patients and are more common in those under 40 years of age.53 Non-absorbable sugars, such as lactulose, can be fermented within the colon and exacerbate bloating and distension in OIC and should be avoided.54
Mu-opioid receptor antagonists
Opioid-receptor antagonists can alleviate the adverse effects of opioids on GI functions, but their central analgesic effects may also be antagonised if they cross the blood-brain barrier.55 The most readily well-known example is naloxone, commonly used as an intravenous reversal agent in the context of opioid over-dosing. Agents that block μ-opioid receptors in the GI tract, but do not enter the central nervous system (CNS), are expected to treat OIBD without diminishing central analgesic actions. Several opioid antagonists with local action within the gut or (outside the CNS) peripherally-acting μ-opioid receptor antagonists (PAMORAs) have become available and others are being developed. These have been shown to be safe and effective in treating OIC and are summarised in Table 3.56
Table 3.
Characteristics of the mu-opioid receptor antagonists.
| Drug | Mechanism of action | Route of administration | Licensed indication | Comments |
|---|---|---|---|---|
| Alvimopan | PAMORA | Oral | POI | Increased cardiovascular side effects – usage restricted to POI |
| Oxycodone hydrochloride and naloxone hydrochloride extended release | Combined opioid with a peripherally restricted opioid antagonist | Oral | OIC | In contrast to PAMORAs, which have potential effects in all tissues except for the CNS, the effect of this combination is mainly restricted to the ‘gut compartment’; there are reports of loss of selectivity with rapid dose up titration or rushing of tablets. |
| Methyl-naltrexone | PAMORA | Sub-cutaneous (oral equivalent under development) | OIC | To be used cautiously in patients with intra-abdominal malignancy or GI strictures due to reports of perforation. Single dose can be useful as a test to evaluate the contribution of opioids to constipation and other OIBD symptoms |
| Naloxegol | PAMORA | Oral | OIC | First orally administered PAMORA approved for OIC. |
| Naldemedine | PAMORA | Oral | OIC | Not yet available in the European Union |
CNS: central nervous system; GI: gastro-intestinal; OIC: opioid-induced constipation; PAMORA: peripherally-acting µ-opioid receptor antagonist; POI: post-operative ileus.
Alvimopan
Alvimopan, an orally administered PAMORA, has been demonstrated to be numerically superior to placebo in treating OIC, although its development has been discontinued.57 However, its long-term use has been associated with increased cardiovascular risk.58 In the USA, it is licensed for the management of post-operative ileus, where it has been shown to be effective and reduces the length of hospital stay, although its use is restricted.59
Oxycodone hydrochloride and naloxone hydrochloride extended-release combination
A fixed-ratio dose combination of oxycodone with extended-release naloxone is approved for the treatment of chronic pain, aiming at decreasing occurrence of OIC.60,61 The rationale for this approach is based on the slow release of naloxone allowing it to exert a local antagonist effect on opioid receptors in the GI tract, with a minimal impact on analgesia due to extensive first-pass metabolism in the liver.62 Several randomised placebo-controlled trials have shown the superiority of oxycodone/naloxone combination in comparison to oxycodone alone in maintaining bowel function, as quantified by the BFI, with equal analgesic efficacy and comparable safety.63–66 This combination tablet has been approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) to treat pain that is severe enough to require daily, around-the-clock, long-term opioid treatment, and for patients in whom alternative pain treatment options are inadequate.67,68 In comparison to oxycodone, the incremental cost-effectiveness ratio of oxycodone/naloxone has been reported to be £5800 per quality adjusted life year gained.69
Methylnaltrexone
Methylnaltrexone, a quaternary ammonium derivative of naltrexone, was the first PAMORA that became available. The N-methyl group restricts the ability to cross the blood-brain barrier due to its polarity and low lipid solubility and prevents central analgesia reduction. Sub-cutaneous methylnaltrexone was approved in 2008 as a subcutaneous injection for the treatment of OIC in patients with advanced (malignant) disease, who responded insufficiently to laxatives in a palliative-care setting and extended to include non-cancer patients in 2015.70 Recommended doses are 8 mg for patients up to 62 kg, and 12 mg for patients up to 114 kg.71 A meta-analysis of the available controlled trials with subcutaneous methylnaltrexone in patients with cancer pain and OIC confirmed benefit over placebo in improving bowel movements and reducing attendant GI symptoms such as abdominal cramps or flatulence.72 However, a number of GI perforations have been reported after administration of methylnaltrexone, mainly in patients with pre-existing GI disorders.73 In non-cancer pain patients, a four-week randomised placebo-controlled trial by Michna et al. using either daily or alternate day methylnaltrexone once daily showed a significant benefit over placebo in inducing rescue-free bowel movement with numbers needed to treat of five and 14 respectively.74 Moreover, this was associated with improvement in global symptom score, rectal symptoms (daily group only) and stool symptoms as assessed by the PAC-SYM questionnaire. However, cost and mode of administration are the main limitations for routine clinical use. The most commonly reported adverse events were abdominal pain, diarrhoea and nausea, as well as hyperhidrosis.74,75 Given the reported perforations, methylnaltrexone should be used cautiously in patients with a pre-existing risk for this such as those with intra-abdominal malignancy or established GI strictures.
Oral methylnaltrexone bromide is a formulation of methylnaltrexone bromide that was recently evaluated and that has recently received FDA approval for use in the treatment of OIC in adults with chronic non-cancer pain. A recently reported phase 3 study randomised patients to oral methylnaltrexone 150, 300 or 450 mg once daily against placebo. The most efficacious dose was 450 mg with 28.0% of administrations reaching the primary endpoint of a rescue-free bowel movement within four hours of dosing compared to 18.8% after placebo.76 In addition, the time to the first bowel movement after the first dose was significantly shorter with the 450 mg dose. Adverse events were mainly mild and largely GI-related, with analgesic efficacy being maintained.76
Naloxegol
Naloxegol is a pegylated derivative of naloxone. Pegylation induces P-glycoprotein transporter-substrate properties, thereby enhancing bio-availability and preventing passage across the blood-brain barrier. Two randomised, placebo-controlled, double-blind, parallel-group, multicentre, phase 3 trials in OIC patients with non-cancer pain demonstrated that naloxegol 25 mg was superior to placebo in achieving the primary response endpoint.77 The primary endpoint of the study was proportion of responders, defined as having ≥9 positive response weeks in the 12-week treatment period and ≥3 in the last four weeks of the 12-week treatment period. Naloxegol 25 mg also resulted in greater improvements in straining, stool consistency and frequency of days with complete spontaneous bowel movements compared to placebo. Significant benefit was also observed for several of the secondary endpoints with the 12.5 mg dose, but the primary endpoint was reached with the 12.5 mg dose in only one of the studies. In a 52-week, multicentre, open-label, randomised, parallel-group study, naloxegol 25 mg was found to be generally safe and well tolerated.78 The most common side effects were early-onset abdominal pain, diarrhoea and nausea, mostly mild to moderate in intensity, and transient after the first days.77–79 Naloxegol has been approved for OIC by the EMA and in non-cancer OIC by the FDA. In comparison to placebo, the incremental cost-effectiveness ratio of naloxone is £10,800 per quality adjusted life year gained.80
Naldemedine
Naldemedine is the newest orally available PAMORA, approved by the FDA for the treatment of OIC in adult patients. Two randomised controlled phase 3 trials in OIC subjects with chronic non-cancer pain showed that naldemedine 0.2 mg was superior to placebo in increasing the number of bowel movements over baseline.81 The primary responder endpoint of the study was reached in 47.6% compared to 34.6% (p = 0.002), and 52.5% compared to 33.6% (p < 0.0001) of the subjects with naldemedine versus placebo respectively in the COMPOSE I and II trial. A significant increase in spontaneous bowel movement frequency occurred during the first week of active treatment in both trials. GI-related adverse effects such as diarrhoea, nausea and abdominal pain were more prevalent in the naldemedine group but were mild to moderate in nature. In addition, there were no significant occurrences of opioid withdrawal symptoms or interference with the analgesic efficacy of opioids. A 52-week placebo-controlled study, COMPOSE III, was also conducted with 1246 patients randomised to either placebo or naldemedine 0.2 mg daily. Efficacy was evaluated with the PAC-SYM and PAC-QOL questionnaires at baseline, two, 12, 24, 36 and 52 weeks.82 Naldemedine resulted in significant increase in spontaneous bowel movements and significant improvement over placebo in all the subscales of the symptom and in quality of life questionnaires at all time-points. The adverse event profile and incidence were similar to that observed in the COMPOSE I and II trials. Naldemedine 0.2 mg was also studied in a two-week controlled trial in 193 cancer patients with OIC (COMPOSE IV).83 The proportion of spontaneous bowel movements responders (≥3 spontaneous bowel movements per week with an increase of ≥1 spontaneous bowel movements per week over baseline) was significantly greater with naldemedine (71.1% vs 34.4%, p < 0.0001). The study was followed by a 12-week open-label extension safety trial (COMPOSE V). Adverse-event profile and incidence were similar to previous studies in non-cancer patients.83
Other treatments
Lubiprostone
Lubiprostone activates chloride type 2 channels located on the apical membrane of epithelial cells in the GI tract resulting in an intraluminal efflux.84,85 Lubiprostone has been approved by the FDA for the treatment of OIC. In a 12-week randomised placebo-controlled trial of 418 patients with OIC, lubiprostone was associated with an increase in spontaneous bowel movements over placebo in comparison to baseline (3.3 vs 2.4, p = 0.005) at the pre-specified primary endpoint after eight weeks of treatment although this difference was not apparent at 12 weeks.86 In a further multicentre randomised controlled phase 3 trial, lubiprostone 24 mcg twice a day was compared to placebo for the treatment of constipation associated with non-methadone opioids in patients with chronic non-cancer-related pain in 431 subjects.87 Lubiprostone was associated with an increase in spontaneous bowel movements over placebo in comparison to baseline (3.2 vs 2.4, p = 0.001). The most common side-effects with lubiprostone are diarrhoea, nausea, vomiting and abdominal pain. A pooled analysis has shown that lubiprostone does not diminish the analgesic efficacy of opioids.88
Linaclotide
Linaclotide is a guanylate cyclase C receptor agonist which up regulates cyclic guanosine monophosphate (cGMP) within enterocytes resulting in intraluminal chloride secretion followed by water leading to a laxative effect.89 Moreover, animal studies have demonstrated a cGMP-mediated reduction in visceral afferent activity, which is mirrored by the analgesic effect seen in phase 3 clinical trials in patients with irritable bowel syndrome with constipation.90–92 A phase IIb study of linaclotide, in 254 patients receiving opioids for non-cancer pain, randomised participants to 145 or 290 mcg daily for eight weeks. Both doses of linaclotide resulted in a significant increase in spontaneous bowel movements, with the most common adverse event being diarrhoea.
Prucalopride
Prucalopride is a selective 5-HT4 agonist which induces a prokinetic effect.93 In a four-week study of 196 patients with non-cancer pain-related OIC, prucalopride was numerically superior to placebo at increasing the number of spontaneous bowel movements per week.94 This effect was statistically significant after the first two weeks of treatment, but not after four weeks, with beneficial effects seen on symptoms and quality of life.
Pragmatic clinical recommendations
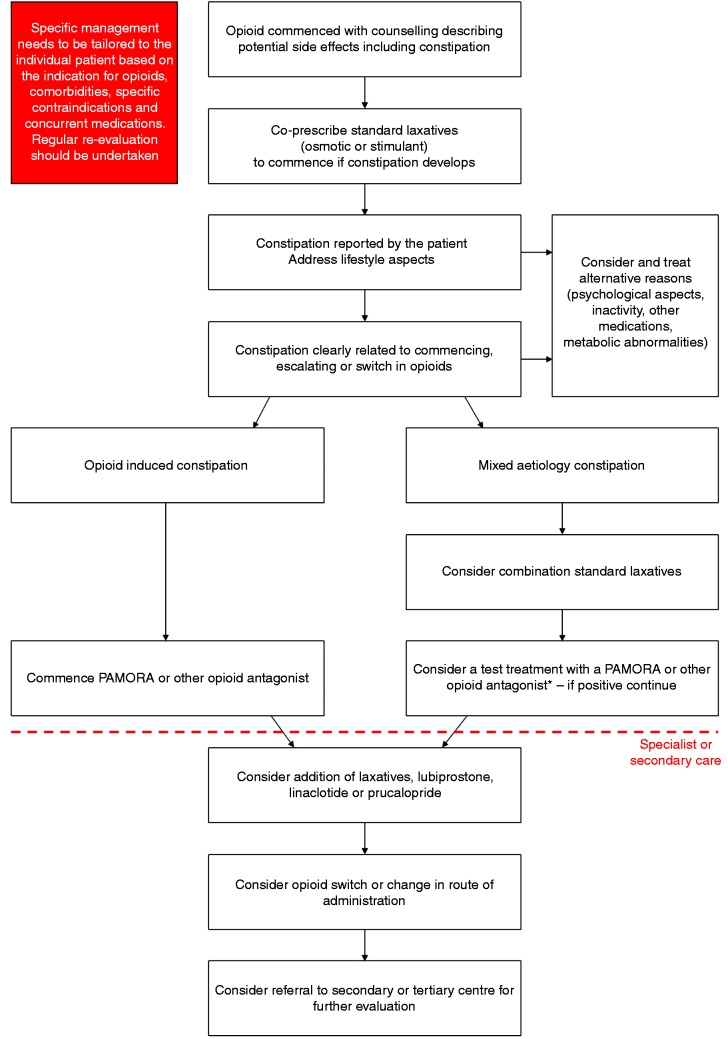
The first stage of managing OIC is appropriate counselling and education of patients as to the side-effects of opioids. We advocate co-prescription of a standard laxative, such as an osmotic or stimulant, when an opioid is commenced, escalated or switched which the patient can commence himself or herself should they develop constipation. Similarly, where possible, simple measures such as increasing fibre, exercise and fluid intake should be advised. Patients should be specifically asked about problematic opioid side effects, such as constipation, at each clinical review. Concurrently, alternative reasons for constipation symptoms should be considered such as inactivity, metabolic derangements and other medications. Although the clinical history is important, utilisation of the BFI is a useful tool in helping to identify OIC as well as monitoring response to any particular intervention.
It is useful to ascertain whether the constipation is related to the commencement, escalation or switch in opioid therapy. If the constipation is considered to be unrelated to the opioid then the switching to another class of simple laxatives may be appropriate, or introduction of a combination such as a stimulant and a stool softener. Should patients not respond to these measures then a test treatment with methylnaltrexone or a short trial of an opioid antagonist is useful. In contrast, if the constipation is considered to be secondary to the opioid therapy then treatment should be started with an opioid antagonist. The choice of the specific antagonist depends on the diagnosis, life expectancy, drug availability and patient preference.
Although there is no absolute consensus, we would suggest an early review (no more than one month) of the patient after the initiation of a treatment for OIC (independent of the frequency of pain management review), although this is clearly dependent on local resources. If at this point there is treatment failure and the patient is being managed in primary care, then referral to specialist/secondary care may be appropriate. Here escalation to more intensive laxative treatment or the addition of lubiprostone, linaclotide or prucalopride is advised. If these measures do not result in an improvement in constipation, the clinician should consider switching the opioid and/or changing the route of administration. Finally, if there is a lack of response, referral of such patients should be considered to tertiary centres where more detailed evaluation of GI physiology, such as anorectal manometry or other tests, can be undertaken. These management steps, summarised in Figure 4, build upon, and are aligned to, proposals made by Prichard et al., Drewes et al., Nelson et al., Argoff et al., Brenner et al. and Farmer et al.11,39,45,95–97 However, it should be stressed that the management of OIC always needs to be tailored to the individual patient based on their symptom profile and/or global clinical picture.
Figure 4.
A suggested pragmatic stepwise management suggestion for the management of opioid-induced constipation (OIC) in clinical practice. Treatment goals are to establish regular bowel function, improve quality of life and avoid complications, such as haemorrhoids, rectal prolapse and faecal impaction. Regular clinical re-evaluation should be undertaken, and the Bowel Function Index (BFI) can also be used as a useful adjunct.
*The length of the test treatment depends on the specific peripherally-acting µ-opioid receptor antagonist (PAMORA) or opioid antagonist. For instance, a two-week trial with naloxegol or a single test dose with subcutaneous methylnaltrexone may be appropriate. Following these pragmatic suggestions is dependent on cost, available expertise/technology and local practice circumstances.
Conclusions
The widespread use of opioids has been associated with a concomitant rise in dysfunction of the gut, which is often under-recognised and poorly managed. Successful management of OIC, and the side-effects following opioid therapy depends on its recognition and management should be based on a step-wise approach to treatment aimed at improving outcomes in this patient group.
Acknowledgements
The authors wish to acknowledge the input of Viola Andresen, Andrew Davies, Paul Farquhar-Smith, Antoine Lemaire, Juan Perez-Cajaraville and Carlos Jara Sanchez. All authors contributed to the article and have reviewed and revised the manuscript for important intellectual content.
Declaration of conflicting interests
A Farmer reports personal fees from Kyowa Kirin and Allergan, during the conduct of the study. A Drewes reports personal fees from Kyowa Kirin, Astra-Zeneca, Mundipharma and Grünenthal. G Chiarioni reports personal fees from Kyowa Kirin and he is a member of the anorectal committee of the Rome Foundation. R De Giorgio reports personal fees from Shire, Sucampo, Coloplast and Takeda during the conduct of the study. T O'Brien reports personal fees from Mundipharma and Astra Zeneca during the conduct of the study. B Morlion reports personal fees from Kyowa Kirin, Mundipharma, Pfizer, Shionogi, Boehringer-Ingelheim, Bayer and Grünenthal. J Tack reports personal fees from Ironwood, Kyowa Kirin, Shionogi, Shire and Takeda during the conduct of the study. All authors received an honorarium and travel expenses for attending the workshops (except T O'Brien) funded by Kyowa Kirin.
Ethics approval
As this is a consensus statement, ethics approval was not required.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Informed consent
As this is a consensus statement, informed consent was not required.
References
- 1.Vadivelu N, Kai AM, Kodumudi V, et al. The opioid crisis: A comprehensive overview. Curr Pain Headache Rep 2018; 22: 16. [DOI] [PubMed] [Google Scholar]
- 2.Lee AA, Hasler WL. Opioids and GI motility–friend or foe?. Curr Treat Options Gastroenterol 2016; 14: 478–494. [DOI] [PubMed] [Google Scholar]
- 3.Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician 2008; 11: S105–S120. [PubMed] [Google Scholar]
- 4.O'Brien T, Christrup LL, Drewes AM, et al. European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur J Pain 2017; 21: 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: Systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005; 7: R1046–R1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porreca F, Ossipov MH. Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: Mechanisms, implications, and management options. Pain Med 2009; 10: 654–662. [DOI] [PubMed] [Google Scholar]
- 7.Ketwaroo GA, Cheng V, Lembo A. Opioid-induced bowel dysfunction. Curr Gastroenterol Rep 2013; 15: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brock C, Olesen SS, Olesen AE, et al. Opioid-induced bowel dysfunction: Pathophysiology and management. Drugs 2012; 72: 1847–1865. [DOI] [PubMed] [Google Scholar]
- 9.Glare P, Walsh D, Sheehan D. The adverse effects of morphine: A prospective survey of common symptoms during repeated dosing for chronic cancer pain. Am J Hosp Palliat Care 2006; 23: 229–235. [DOI] [PubMed] [Google Scholar]
- 10.Tuteja AK, Biskupiak J, Stoddard GJ, et al. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil 2010; 22: 424–e96. [DOI] [PubMed] [Google Scholar]
- 11.Drewes AM, Munkholm P, Simren M, et al. Definition, diagnosis and treatment strategies for opioid-induced bowel dysfunction–recommendations of the Nordic Working Group. Scand J Pain 2016; 11: 111–122. [DOI] [PubMed] [Google Scholar]
- 12.Bell T, Annunziata K, Leslie JB. Opioid-induced constipation negatively impacts pain management, productivity, and health-related quality of life: Findings from the National Health and Wellness Survey. J Opioid Manag 2009; 5: 137–144. [DOI] [PubMed] [Google Scholar]
- 13.Ducrotte P, Milce J, Soufflet C, et al. Prevalence and clinical features of opioid-induced constipation in the general population: A French study of 15,000 individuals. United European Gastroenterol J 2017; 5: 588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta A, Coyne KS, Datto C, et al. The burden of opioid-induced constipation in younger patients with chronic noncancer pain. Pain Med 2018. doi: 10.1093/pm/pny002. [DOI] [PubMed] [Google Scholar]
- 15.Gaertner J, Siemens W, Camilleri M, et al. Definitions and outcome measures of clinical trials regarding opioid-induced constipation: A systematic review. J Clin Gastroenterol 2015; 49: 9–16. [DOI] [PubMed] [Google Scholar]
- 16.Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016; doi: 10.1053/j.gastro.2016.02.031. . [DOI] [PubMed] [Google Scholar]
- 17.Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept 2009; 155: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurz A, Sessler DI. Opioid-induced bowel dysfunction: Pathophysiology and potential new therapies. Drugs 2003; 63: 649–671. [DOI] [PubMed] [Google Scholar]
- 19.Sternini C, Patierno S, Selmer IS, et al. The opioid system in the gastrointestinal tract. Neurogastroenterol Motil 2004; 16: 3–16. [DOI] [PubMed] [Google Scholar]
- 20.Fickel J, Bagnol D, Watson SJ, et al. Opioid receptor expression in the rat gastrointestinal tract: A quantitative study with comparison to the brain. Brain Res Mol Brain Res 1997; 46: 1–8. [DOI] [PubMed] [Google Scholar]
- 21.Galligan JJ, Sternini C. Insights into the role of opioid receptors in the GI tract: Experimental evidence and therapeutic relevance. Handb Exp Pharmacol 2017; 239: 363–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma SK, Nirenberg M, Klee WA. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A 1975; 72: 590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil 2004; 16: 17–28. [DOI] [PubMed] [Google Scholar]
- 24.Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther 2010; 31: 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penagini R, Allocca M, Cantu P, et al. Relationship between motor function of the proximal stomach and transient lower oesophageal sphincter relaxation after morphine. Gut 2004; 53: 1227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun WM, Read NW, Verlinden M. Effects of loperamide oxide on gastrointestinal transit time and anorectal function in patients with chronic diarrhoea and faecal incontinence. Scand J Gastroenterol 1997; 32: 34–38. [DOI] [PubMed] [Google Scholar]
- 27.Poulsen JL, Nilsson M, Brock C, et al. The impact of opioid treatment on regional gastrointestinal transit. J Neurogastroenterol Motil 2016; 22: 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson M, Poulsen JL, Brock C, et al. Opioid-induced bowel dysfunction in healthy volunteers assessed with questionnaires and MRI. Eur J Gastroenterol Hepatol 2016; 28: 514–524. [DOI] [PubMed] [Google Scholar]
- 29.Johnson LR, Barrett KE. Physiology of the gastrointestinal tract, 4th ed Burlington, MA: Elsevier Academic Press, 2006. [Google Scholar]
- 30.Galligan JJ, Akbarali HI. Molecular physiology of enteric opioid receptors. Am J Gastroenterol Suppl 2014; 2: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poitras R, Warren D, Oyogoa S. Opioid drugs and stercoral perforation of the colon: Case report and review of literature. Int J Surg Case Rep 2018; 42: 94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musial F, Enck P, Kalveram KT, et al. The effect of loperamide on anorectal function in normal healthy men. J Clin Gastroenterol 1992; 15: 321–324. [DOI] [PubMed] [Google Scholar]
- 33.Poulsen JL, Mark EB, Brock C, et al. Colorectal transit and volume during treatment with prolonged-release oxycodone/naloxone versus oxycodone plus macrogol 3350. J Neurogastroenterol Motil 2018; 24: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasricha PJ, Willis WD and Gebhart GF. Chronic abdominal and visceral pain: Theory and practice. 1st ed. Boca Raton, Florida, United States: CRC Press, 2006.
- 35.Talley NJ. How to do and interpret a rectal examination in gastroenterology. Am J Gastroenterol 2008; 103: 820–822. [DOI] [PubMed] [Google Scholar]
- 36.Slappendel R, Simpson K, Dubois D, et al. Validation of the PAC-SYM questionnaire for opioid-induced constipation in patients with chronic low back pain. Eur J Pain 2006; 10: 209–217. [DOI] [PubMed] [Google Scholar]
- 37.Marquis P, De La Loge C, Dubois D, et al. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol 2005; 40: 540–551. [DOI] [PubMed] [Google Scholar]
- 38.Knowles CH, Eccersley AJ, Scott SM, et al. Linear discriminant analysis of symptoms in patients with chronic constipation: Validation of a new scoring system (KESS). Dis Colon Rectum 2000; 43: 1419–1426. [DOI] [PubMed] [Google Scholar]
- 39.Argoff CE, Brennan MJ, Camilleri M, et al. Consensus recommendations on initiating prescription therapies for opioid-induced constipation. Pain Med 2015; 16: 2324–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997; 32: 920–924. [DOI] [PubMed] [Google Scholar]
- 41.Rentz AM, Yu R, Muller-Lissner S, et al. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid-induced constipation. J Med Econ 2009; 12: 371–383. [DOI] [PubMed] [Google Scholar]
- 42.Ueberall MA, Muller-Lissner S, Buschmann-Kramm C, et al. The Bowel Function Index for evaluating constipation in pain patients: Definition of a reference range for a non-constipated population of pain patients. J Int Med Res 2011; 39: 41–50. [DOI] [PubMed] [Google Scholar]
- 43.Bharucha AE, Pemberton JH, Locke GR., 3rd American Gastroenterological Association technical review on constipation. Gastroenterology 2013; 144: 218–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Black TP, Manolakis CS, Di Palma JA. ‘Red flag’ evaluation yield in irritable bowel syndrome. J Gastrointestin Liver Dis 2012; 21: 153–156. [PubMed] [Google Scholar]
- 45.Prichard D, Norton C, Bharucha AE. Management of opioid-induced constipation. Br J Nurs 2016; 25: S4–S5. S8–S11. [DOI] [PubMed] [Google Scholar]
- 46.Muller-Lissner S, Bassotti G, Coffin B, et al. Opioid-induced constipation and bowel dysfunction: A clinical guideline. Pain Med 2016; 18: 1837–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishihara M, Ikesue H, Matsunaga H, et al. A multi-institutional study analyzing effect of prophylactic medication for prevention of opioid-induced gastrointestinal dysfunction. Clin J Pain 2012; 28: 373–381. [DOI] [PubMed] [Google Scholar]
- 48.Plaisance L, Ellis JA. Opioid-induced constipation. Management is necessary but prevention is better. Am J Nurs 2002; 102: 72–73. [DOI] [PubMed] [Google Scholar]
- 49.Skollerud LM, Fredheim OM, Svendsen K, et al. Laxative prescriptions to cancer outpatients receiving opioids: A study from the Norwegian prescription database. Support Care Cancer 2013; 21: 67–73. [DOI] [PubMed] [Google Scholar]
- 50.Pottegård A, Knudsen T, van Heesch K, et al. Information on risk of constipation for Danish users of opioids, and their laxative use. Int J Clin Pharm 2014; 36: 291–294, . [DOI] [PubMed] [Google Scholar]
- 51.Baron R, Eberhart L, Kern KU, et al. Tapentadol prolonged release for chronic pain: A review of clinical trials and 5 years of routine clinical practice data. Pain Pract 2017; 17: 678–700. [DOI] [PubMed] [Google Scholar]
- 52.Tassinari D, Sartori S, Tamburini E, et al. Adverse effects of transdermal opiates treating moderate-severe cancer pain in comparison to long-acting morphine: A meta-analysis and systematic review of the literature. J Palliat Med 2008; 11: 492–501. [DOI] [PubMed] [Google Scholar]
- 53.Emmanuel A, Johnson M, McSkimming P, et al. Laxatives do not improve symptoms of opioid-induced constipation: Results of a patient survey. Pain Med 2017; 18: 1932–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basilisco G, Marino B, Passerini L, et al. Abdominal distension after colonic lactulose fermentation recorded by a new extensometer. Neurogastroenterol Motil 2003; 15: 427–433. [DOI] [PubMed] [Google Scholar]
- 55.Liu M, Wittbrodt E. Low-dose oral naloxone reverses opioid-induced constipation and analgesia. J Pain Symptom Manage 2002; 23: 48–53. [DOI] [PubMed] [Google Scholar]
- 56.Nee J, Zakari M, Sugarman MA, et al. Efficacy of treatments for opioid-induced constipation: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018; 16: 1569–1584.e2. . [DOI] [PubMed] [Google Scholar]
- 57.Irving G, Penzes J, Ramjattan B, et al. A randomized, placebo-controlled phase 3 trial (Study SB-767905/013) of alvimopan for opioid-induced bowel dysfunction in patients with non-cancer pain. J Pain 2011; 12: 175–184. [DOI] [PubMed] [Google Scholar]
- 58.Alvimopan (Entereg) for postoperative ileus. Med Lett Drugs Ther 2008; 50: 93–94. . [PubMed] [Google Scholar]
- 59.Xu LL, Zhou XQ, Yi PS, et al. Alvimopan combined with enhanced recovery strategy for managing postoperative ileus after open abdominal surgery: A systematic review and meta-analysis. J Surg Res 2016; 203: 211–221. [DOI] [PubMed] [Google Scholar]
- 60.Lowenstein O, Leyendecker P, Lux EA, et al. Efficacy and safety of combined prolonged-release oxycodone and naloxone in the management of moderate/severe chronic non-malignant pain: Results of a prospectively designed pooled analysis of two randomised, double-blind clinical trials. BMC Clin Pharmacol 2010; 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meissner W, Leyendecker P, Mueller-Lissner S, et al. A randomised controlled trial with prolonged-release oral oxycodone and naloxone to prevent and reverse opioid-induced constipation. Eur J Pain 2009; 13: 56–64. [DOI] [PubMed] [Google Scholar]
- 62.Burness CB, Keating GM. Oxycodone/naloxone prolonged-release: A review of its use in the management of chronic pain while counteracting opioid-induced constipation. Drugs 2014; 74: 353–375. [DOI] [PubMed] [Google Scholar]
- 63.Vondrackova D, Leyendecker P, Meissner W, et al. Analgesic efficacy and safety of oxycodone in combination with naloxone as prolonged release tablets in patients with moderate to severe chronic pain. J Pain 2008; 9: 1144–1154. [DOI] [PubMed] [Google Scholar]
- 64.Simpson K, Leyendecker P, Hopp M, et al. Fixed-ratio combination oxycodone/naloxone compared with oxycodone alone for the relief of opioid-induced constipation in moderate-to-severe noncancer pain. Curr Med Res Opin 2008; 24: 3503–3512. [DOI] [PubMed] [Google Scholar]
- 65.Lowenstein O, Leyendecker P, Hopp M, et al. Combined prolonged-release oxycodone and naloxone improves bowel function in patients receiving opioids for moderate-to-severe non-malignant chronic pain: A randomised controlled trial. Expert Opin Pharmacother 2009; 10: 531–543. [DOI] [PubMed] [Google Scholar]
- 66.Rauck RL, Hale ME, Bass A, et al. A randomized double-blind, placebo-controlled efficacy and safety study of ALO-02 (extended-release oxycodone surrounding sequestered naltrexone) for moderate-to-severe chronic low back pain treatment. Pain 2015; 156: 1660–1669. [DOI] [PubMed] [Google Scholar]
- 67.Food and Drug Administration (FDA). FDA approves new extended-release oxycodone with abuse-deterrent properties, https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/205777orig1s000ltr.pdf (2014, accessed 4 April 2017).
- 68.European Medicines Agency. Oxynal-Targin and associated names, https://www.ema.europa.eu/en/medicines/human/referrals/oxynal-targin-associated-names(2014, accessed 4 April 2017).
- 69.Dunlop W, Uhl R, Khan I, et al. Quality of life benefits and cost impact of prolonged release oxycodone/naloxone versus prolonged release oxycodone in patients with moderate-to-severe non-malignant pain and opioid-induced constipation: A UK cost-utility analysis. J Med Econ 2012; 15: 564–575. [DOI] [PubMed] [Google Scholar]
- 70.Diego L, Atayee R, Helmons P, et al. Methylnaltrexone: A novel approach for the management of opioid-induced constipation in patients with advanced illness. Expert Rev Gastroenterol Hepatol 2009; 3: 473–485. [DOI] [PubMed] [Google Scholar]
- 71.Iyer SS, Randazzo BP, Tzanis EL, et al. Effect of subcutaneous methylnaltrexone on patient-reported constipation symptoms. Value Health 2011; 14: 177–183. [DOI] [PubMed] [Google Scholar]
- 72.Siemens W, Becker G. Methylnaltrexone for opioid-induced constipation: Review and meta-analyses for objective plus subjective efficacy and safety outcomes. Ther Clin Risk Manag 2016; 12: 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Candy B, Jones L, Goodman ML, et al. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Cochrane Database Syst Rev 2011, pp. CD003448, . [DOI] [PubMed] [Google Scholar]
- 74.Michna E, Blonsky ER, Schulman S, et al. Subcutaneous methylnaltrexone for treatment of opioid-induced constipation in patients with chronic, nonmalignant pain: A randomized controlled study. J Pain 2011; 12: 554–562. [DOI] [PubMed] [Google Scholar]
- 75.Mackey AC, Green L, Greene P, et al. Methylnaltrexone and gastrointestinal perforation. J Pain Symptom Manage 2010; 40: e1–e3. [DOI] [PubMed] [Google Scholar]
- 76.Rauck R, Slatkin NE, Stambler N, et al. Randomized, double-blind trial of oral methylnaltrexone for the treatment of opioid-induced constipation in patients with chronic noncancer pain. Pain Pract 2017; 17: 820–828. [DOI] [PubMed] [Google Scholar]
- 77.Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med 2014; 370: 2387–2396. [DOI] [PubMed] [Google Scholar]
- 78.Webster L, Dhar S, Eldon M, et al. A phase 2, double-blind, randomized, placebo-controlled, dose-escalation study to evaluate the efficacy, safety, and tolerability of naloxegol in patients with opioid-induced constipation. Pain 2013; 154: 1542–1550. [DOI] [PubMed] [Google Scholar]
- 79.Webster L, Chey WD, Tack J, et al. Randomised clinical trial: The long-term safety and tolerability of naloxegol in patients with pain and opioid-induced constipation. Aliment Pharmacol Ther 2014; 40: 771–779. [DOI] [PubMed] [Google Scholar]
- 80.National Institute for Health and Care Excellence. Naloxegol for treating opioid-induced constipation – final appraisal determination, https://www.nice.org.uk/guidance/ta345/documents/constipation-opioidinduced-naloxegol-final-appraisal-determination-document2 (2015, accessed 4 April 2017).
- 81.Hale M, Wild J, Reddy J, et al. Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): Two multicentre, phase 3, double-blind, randomised, parallel-group trials. Lancet Gastroenterol Hepatol 2017; 2: 555–564. [DOI] [PubMed] [Google Scholar]
- 82.Tack J, Camilleri M, Cai B, et al. OP284 Patient-reported outcomes with naldemedine long-term treatment of opioid-induced constipation in subjects with chronic non-cancer pain. United European Gastroenterol J 2017; 5: A121. [Google Scholar]
- 83.Katakami N, Harada T, Murata T, et al. Randomized phase III and extension studies of naldemedine in patients with opioid-induced constipation and cancer. J Clin Oncol 2017; 35: 3859–3866. [DOI] [PubMed] [Google Scholar]
- 84.Cuppoletti J, Malinowska DH, Tewari KP, et al. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol 2004; 287: C1173–1183. [DOI] [PubMed] [Google Scholar]
- 85.Raschi E, De Ponti F. Lubiprostone: Pharmacokinetic, pharmacodynamic, safety and regulatory aspects in the treatment of constipation-predominant irritable bowel syndrome. Expert Opin Drug Metab Toxicol 2014; 10: 293–305. [DOI] [PubMed] [Google Scholar]
- 86.Cryer B, Katz S, Vallejo R, et al. A randomized study of lubiprostone for opioid-induced constipation in patients with chronic noncancer pain. Pain Med 2014; 15: 1825–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jamal MM, Adams AB, Jansen JP, et al. A randomized, placebo-controlled trial of lubiprostone for opioid-induced constipation in chronic noncancer pain. Am J Gastroenterol 2015; 110: 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spierings EL, Brewer RP, Rauck RL, et al. Lubiprostone for opioid-induced constipation does not interfere with opioid analgesia in patients with chronic noncancer pain. Pain Pract 2017; 17: 312–319. [DOI] [PubMed] [Google Scholar]
- 89.Lee N, Wald A. The pharmacokinetics, pharmacodynamics, clinical efficacy, safety and tolerability of linaclotide. Expert Opin Drug Metab Toxicol 2011; 7: 651–659. [DOI] [PubMed] [Google Scholar]
- 90.Castro J, Harrington AM, Hughes PA, et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3',5'-monophosphate. Gastroenterology 2013; 145: 1334–1346.e11. [DOI] [PubMed] [Google Scholar]
- 91.Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: A 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol 2012; 107: 1702–1712. [DOI] [PubMed] [Google Scholar]
- 92.Rao S, Lembo AJ, Shiff SJ, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol 2012; 107: 1714–1724. quiz: 1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garnock-Jones KP. Prucalopride: A review in chronic idiopathic constipation. Drugs 2016; 76: 99–110. [DOI] [PubMed] [Google Scholar]
- 94.Sloots CE, Rykx A, Cools M, et al. Efficacy and safety of prucalopride in patients with chronic noncancer pain suffering from opioid-induced constipation. Dig Dis Sci 2010; 55: 2912–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nelson AD, Camilleri M. Opioid-induced constipation: Advances and clinical guidance. Ther Adv Chronic Dis 2016; 7: 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brenner DM, Stern E, Cash BD. Opioid-related constipation in patients with non-cancer pain syndromes: A review of evidence-based therapies and justification for a change in nomenclature. Curr Gastroenterol Rep 2017; 19: 12. [DOI] [PubMed] [Google Scholar]
- 97.Farmer AD, Bruckner Holt CE, Downes TJ, et al. Pathophysiology, diagnosis, and management of opioid-induced constipation. Lancet Gastroenterol Hepatol 2018; 3: 9. [DOI] [PubMed] [Google Scholar]