Abstract
Malaria is a dangerous disease spread across several countries. Recent studies have focused on medicinal plants to discover alternative agents to the currently used drugs for malaria treatment. Here, we investigated the potential role of Indigofera oblongifolia leaf extract (IE) on hepatic inflammation in mice with Plasmodium chabaudi-infected erythrocytes. Female C57BL/6 mice were divided into three groups. The first group served as a control noninfected group, while the second and third groups were intraperitoneally injected with 106 erythrocytes parasitized by P. chabaudi. Mice from the third group were treated daily with a dose of 100 mg/kg of IE for 7 days. IE significantly reduced the number of leukocytes and apoptotic cells. The numbers of CD68-positive cells decreased in the livers of mice from the treatment group. Moreover, IE raised the hepatic antioxidant levels (glutathione and catalase) and reduced the levels of hepatic oxidative stress markers (malondialdehyde, nitric oxide, and reactive oxygen species). IE regulated some functions of the genes related to immune responses, including apoptotic genes (B-cell lymphoma-2, Bax, and caspase-3) and cytokine genes (interleukin-1β (IL-1β), IL-6, interferon-γ, and tumor necrosis factor-α). Therefore, IE exerts significant effects against malaria and protects the liver from injury caused by P. chabaudi via antioxidant and anti-inflammatory ways.
1. Introduction
Malaria is a dangerous disease spread across the world, especially in the developing and underdeveloped countries. Although major progress has been reported in the fight against malaria, about 3.2 billion people are at the risk of malaria infection worldwide [1]. According to the World Health Organization, more efforts have been directed to reduce malaria-induced death by 60% as well as to eliminate malaria from many countries since 2000 [1].
The liver is the first site of sporozoite development before infection of erythrocytes [2]. The sporozoites pass from the salivary glands of the mosquitoes to the host and are transmitted to the blood stream from the liver sinusoid. In the sinusoid, sporozoites cross the sinusoidal cell layer to infect hepatocytes and grow and develop into erythrocyte-invasive forms [3]. Several studies have investigated the effector role of the liver during blood-stage malaria [4–6]. Malaria infection is associated with both acquired immune [7, 8] and innate immune [9] responses, characterized with early and intense proinflammatory cytokine-mediated effector mechanisms that kill or remove parasite-infected cells [10].
Malaria induced by Plasmodium exhibits resistance to drugs [11]. The increase in the prevention and control measures adopted since 2010 has reduced the mortality rates associated with malaria by 29% [12]. In malaria-endemic areas, medicinal plants have been used for treatment [13]. Plant extracts have been shown to play an important role in the treatment of malaria, owing to the presence of active components against the malarial parasite [14].
Indigofera oblongifolia, a traditional plant used for medicinal purposes, is known for its analgesic and anti-inflammatory effects [15]. I. oblongifolia, called as hasr in Arabic, belongs to the family Fabaceae and is prominent in Asia and Africa [15]. I. oblongifolia leaf extract (IE) contains polyphenols, flavonoids, and organic acids [16]. IE showed antimalarial and antioxidant activities and provides protection to the spleen from the parasite P. chabaudi in mice [4–6]. In the present study, we demonstrate the role of IE in the modulation of cytokine expression and apoptosis in mouse liver infected with blood-stage malaria.
2. Materials and Methods
2.1. Preparation of the Extract
We collected fresh leaves of I. oblongifolia from Jazan, Saudi Arabia, in March 2018. The identity of this species was confirmed at the herbarium of King Saud University (code: 9028). Leaves were air dried at 40°C for 3 hours and ground into powder. The powder was incubated in 70% methanol at 4°C for 24 h. The extract was filtered and evaporated using an evaporator machine (Heidolph, Germany). In this experiment, distilled water was used to dissolve the powder [17].
2.2. Determination of Phenolics and Flavonoids in the Extract
Total phenolics and flavonoids were determined according to the method described by Kim et al. [18] and Dewanto et al. [19], respectively. Gallic acid was used as the standard for total phenolics, while quercetin was used as the standard for total flavonoids.
2.3. Experimental Animals
Adult 10- to 12-week-old female C57BL/6 mice were used as experimental animals. Mice were fed with a standard diet and water ad libitum. All experiments were approved by the state authorities and followed Saudi Arabian rules on animal protection.
2.4. Infection
Animals from the noninfected control group (8 mice) were orally inoculated with distilled water. The second and third groups of mice were intraperitoneally infected with 106 erythrocyte parasitized by Plasmodium chabaudi. The third group of mice was orally administered with 100 mg/kg of IE once daily for 7 days [4]. We chose this dose (100 mg/kg) based on our previous studies [4].
The percentage of parasitemia was determined in mice from groups 2 and 3 by Giemsa-stained blood smears collected from the tail vein [20].
2.5. Blood Collection
Blood was collected into heparinized tubes. Total leucocytes were counted using an automatic hematology analyzer HM5 (VetScan, USA).
2.6. Liver Samples
Seven days post infection, all the experimental mice were sacrificed. Liver tissues were excised and cut into small pieces.
For histological and immunohistochemical studies, liver tissues were fixed in 10% buffered formalin. For studying the antioxidant activity of IE, tissues were stored at −80°C. For gene expression experiment, liver tissues were stored in RNAlater (QIAGEN, Hilden, Germany).
2.7. Apoptosis Detection
The paraffin-embedded liver sections were assayed using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) Apoptosis Kit (GenScript, Piscataway, NJ, USA) according to the manufacturer's protocol [21].
2.8. Immunohistochemical Detection of CD68 Expression
Immunohistochemical detection of CD68-positive cells in liver sections was carried out. In brief, the deparaffinized sections were incubated in 10 mM citrate buffer solution for antigen retrieval, followed by the treatment of sections with 3% hydrogen peroxide (H2O2) in phosphate buffer solution for 5 min. Liver sections were blocked with 10% normal goat serum for 10 min at 37°C and overnight incubated at 4°C with equal amounts of normal goat IgG as a negative control. The avidin biotin affinity system was used to treat the liver sections, followed by washing and staining of the sections with the 3,3′-diaminobenzidine substrate.
2.9. Phagocytic Activity
Five mice from each group were intravenously injected with phosphate buffer containing 2.9 × 108 of green fluorescent beads (Duke Scientific, Palo Alto, CA, USA). Seven minutes later, all mice were sacrificed. Liver cryosections were prepared, and fluorescence intensity was determined using ImageJ software [22] after examination under an Olympus fluorescent microscope [23].
2.10. Antioxidant Activity in the Liver
Mouse liver tissues were homogenized in an ice-cold medium containing 300 mM sucrose and 50 mM Tris-HCl [24]. Centrifugation was carried out at 500 × g for 10 min at 4°C. The supernatant (10%) was used for different biochemical estimations.
Glutathione (GSH) level in the liver homogenate was determined according to the method described by Ellman [25]. The concentration of nitrite oxide (NO) in the liver homogenate was assayed according to the method described by Berkels et al. [26]. For evaluation of the lipid peroxidation level, the method described by Ohkawa et al. [27] was used. Catalase activity was determined according to the method described by Aebi [28].
2.11. Gene Expression
Total RNA from mouse liver was isolated using TRIzol (QIAGEN, Hilden, Germany). RNA was quantified using the ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) [22]. To process RNA for real-time quantitative polymerase chain reaction (RT-qPCR), we treated samples with DNase (Applied Biosystems, Darmstadt, Germany). Samples were then converted into cDNA using the reverse transcription kit (QIAGEN, Hilden, Germany). To carry out PCR, the ABI Prism 7500HT sequence detection system (Applied Biosystems, Darmstadt, Germany) with SYBR green PCR master mix from QIAGEN (Hilden, Germany) was used. The following genes were investigated: interleukin-1β (IL-1β) (Mm_Il1b_2_SG, cat. no. QT01048355), interleukin-6 (IL-6) (Mm_Il6_1_SG, cat. no. QT00138663), interferon gamma (INF-γ) (Mm_Ifng_1_SG, cat. no. QT01038821), tumor necrosis factor-alpha (TNF-α) (Mm_Tnf_1_SG, cat. no. QT00104006), Bcl2-associated X protein (Bax) (Mm_Bax_1_SG, cat. no. QT00102536), B-cell lymphoma 2 (Bcl2) (Mm_Bcl2_3_SG, cat. no. QT00156282), and caspase-3 (Casp3) (Mm_ Casp3_1_SG, cat. no. QT00260169). The used primers were purchased from QIAGEN. PCR reaction was performed as described by Dkhil et al. [4]. The 2−ΔΔCT method was used to determine the fold change in mRNA expression [29].
2.12. Statistical Analysis
Statistical comparison among the studied groups was carried out using one-way analysis of variance (ANOVA). Duncan's t-test and a statistical package program (SPSS version 17.0) were used. The statistical significance for all data was set at p ≤ 0.01.
3. Results
The concentration of phenolic compounds present in IE (gallic acid equivalent) was 77 ± 4 μg/mg, while the flavonoid content (quercetin equivalent) was 25 ± 3 μg/mg.
The infection induced by P. chabaudi in female C57L/B6 mice causes an increase in leucocyte count; however, treatment with IE resulted in a significant difference in the leucocyte count (p < 0.05) (Figure 1).
Figure 1.
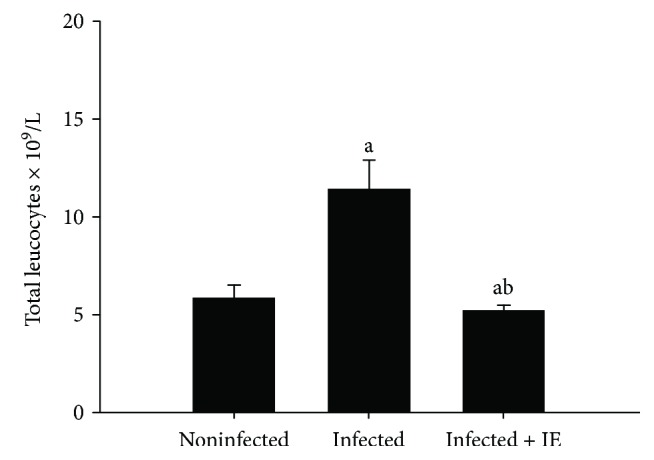
IE-induced changes in mouse leucocytes infected with P. chabaudi. Data are expressed as means ± SD; a: significant change at p ≤ 0.05 with respect to the control group; b: significant change at p ≤ 0.05 with respect to the infected (−IE) group.
Microscopic examination of liver sections following TUNEL staining (Figure 2) revealed the increase in the number of apoptotic cells in the samples from the infected group. On the contrary, the number of TUNEL-positive cells decreased by about 50% in the samples from the treatment group (Figure 3).
Figure 2.
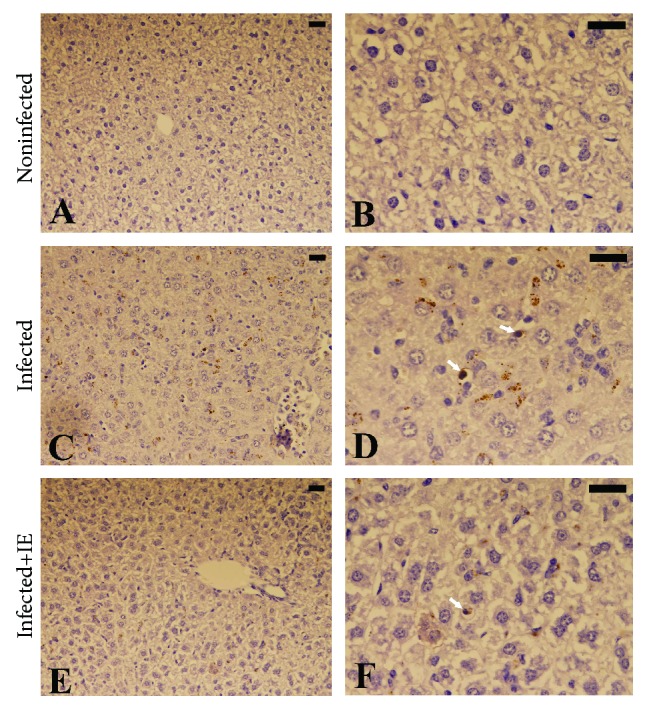
Apoptotic changes in mouse livers infected with P. chabaudi and treated with IE. (a, b) Control liver. (c, d) Infected liver with TUNEL apoptotic cells (white arrow) and hemozoin. (e, f) Liver from the treatment group with a few TUNEL-positive apoptotic cells. Scale bar = 25 μm.
Figure 3.
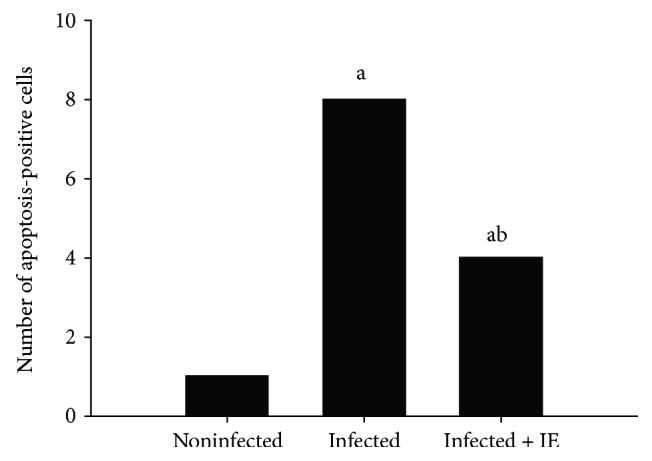
IE-induced changes in the number of apoptotic cells in the liver of mice infected with P. chabaudi on day 7. Data are expressed as means ± SD; a: significant change at p ≤ 0.05 with respect to the control group; b: significant change at p ≤ 0.05 with respect to the infected (−IE) group.
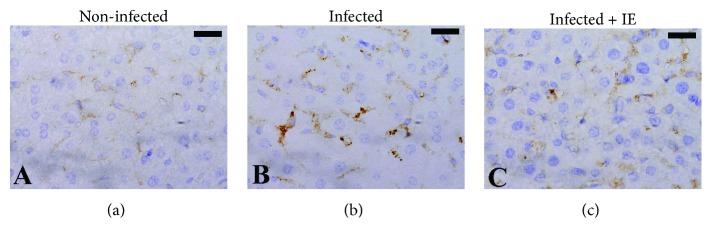
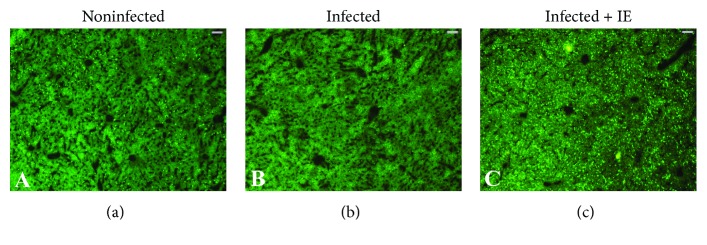
To evaluate the phagocytic activity during infection and after treatment of the infected mice with IE, we examined the number of CD68-positive cells in liver sections (Figure 4). IE treatment significantly decreased the number of CD68-positive cells in P. chabaudi-infected liver samples (Figure 4). The intensity of fluorescent particles as an indicator of phagocytic activity reduced in the liver sections from the infected group (Figure 5) but significantly increased in the liver sections from the IE-treated group (Figures 5 and 6).
Figure 4.
Liver section labeled with the macrophage detection anti-CD68 antibody from mouse infected with Plasmodium chabaudi. (a) Control mouse, (b) mouse infected with P. chabaudi, and (c) mouse infected with P. chabaudi and treated with IE. CD68-positive cells appeared brown. Scale bar = 25 μm.
Figure 5.
Phagocytic activity in the mouse liver. (a) Control liver with more fluorescent particles. (b) P. chabaudi-infected liver with reduced number of fluorescent particles. (c) IE-treated liver with a strong fluorescence signal. Bar = 25 μm.
Figure 6.
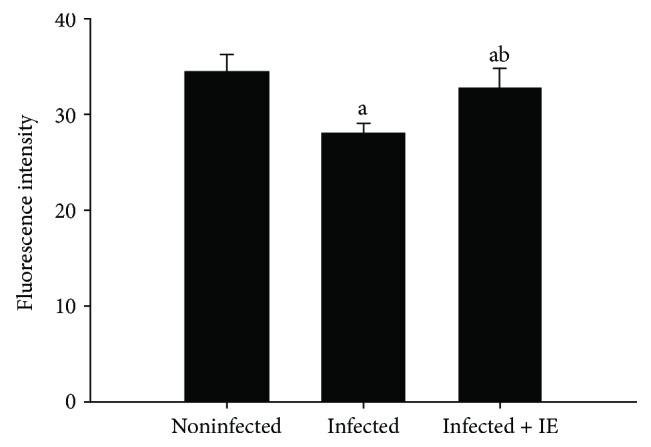
Semiquantitative evaluation of the fluorescence intensity of five cryosections per mouse. Data are expressed as means ± SD; a: significant change at p ≤ 0.05 with respect to the control group; b: significant change at p ≤ 0.05 with respect to the infected (−IE) group.
Biochemical analysis of mouse liver samples revealed a marked increase in the expression level of NO and MDA by approximately 89.91% and 53.33%, respectively, and a significant decrease (p ≤ 0.05) in the level of GSH and catalase activity (Table 1). IE treatment significantly reduced the infection-induced increase in NO and MDA levels and increased the level of GSH as well as the activity of catalase (Table 1).
Table 1.
Effect of IE on the level of hepatic glutathione (GSH), catalase (CAT), malondialdehyde (MDA), and nitric oxide (NO) in mice infected with P. chabaudi.
| Groups | GSH (mg/kg) | CAT (U/g) | MDA (nmol/g) | NO (μmol/g) |
|---|---|---|---|---|
| Noninfected | 11.8 ± 3.2 | 8.3 ± 0.9 | 4.1 ± 0.4 | 165.3 ± 17 |
| Infected (−IE) | 4.9 ± 0.1a | 5.4 ± 0.2a | 6.2 ± 0.2a | 313.9 ± 13.8a |
| Infected (+IE) | 11.6 ± 1.1ab | 18.1 ± 2.3ab | 3.9 ± 0.6b | 168.8 ± 1.1b |
Values are means ± SD. aSignificant against the noninfected group at p ≤ 0.05; bSignificant against the infected (−IE) group at p ≤ 0.05, (n = 8).
P. chabaudi-infected mice showed a significant upregulation in the mRNA expression of Bax, Bcl2, and Casp3 in liver tissues as compared to the noninfected mice (Figure 7). In contrast, a significant decrease in the mRNA levels of Bax, Bcl2, and Casp3 was observed in the mice following IE treatment (Figure 7).
Figure 7.
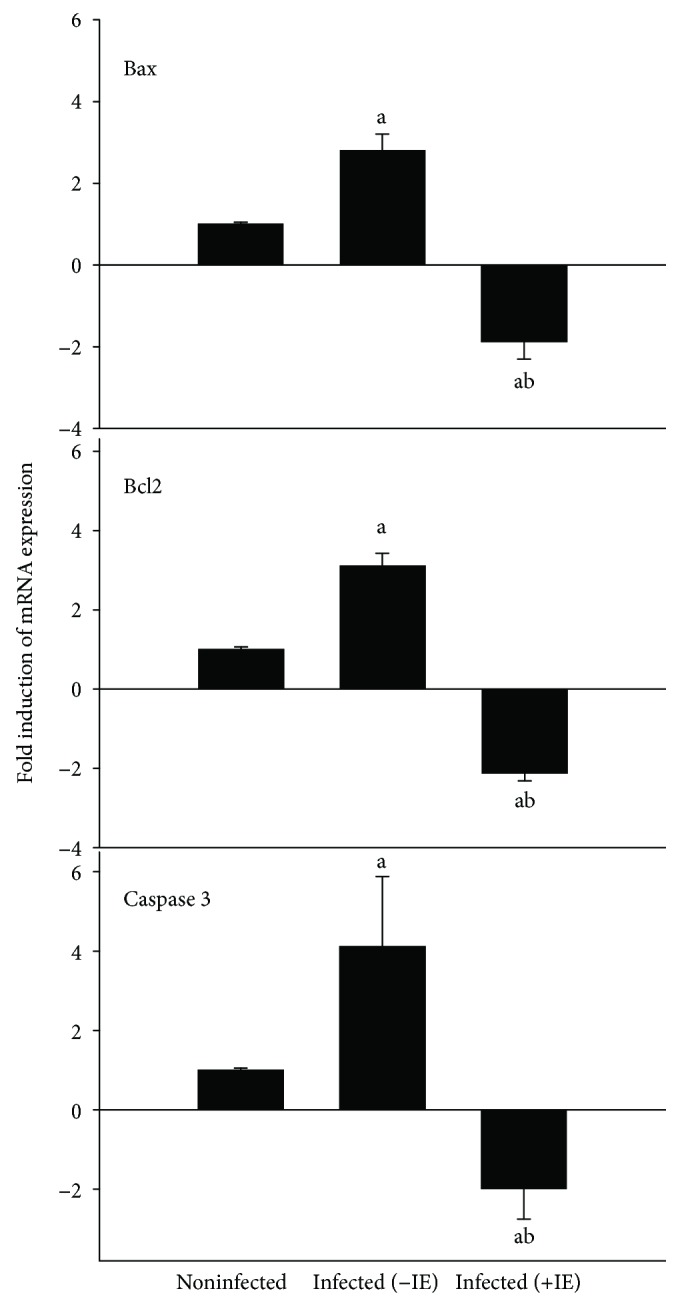
IE-induced changes in the expression level of apoptotic genes (Bcl2, Bax, and caspase-3) in the liver of mice infected with P. chabaudi. Data are expressed as means ± SD; a: significant change at p ≤ 0.05 with respect to the control group; b: significant change at p ≤ 0.05 with respect to the infected (−IE) group.
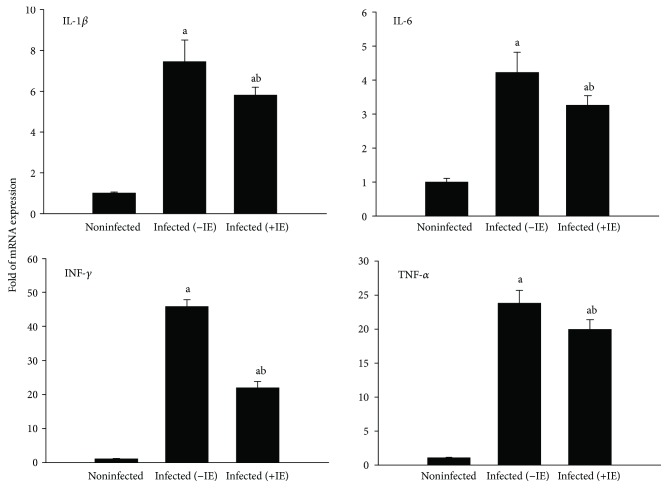
We observed that the infection induced upregulation in the mRNA expression of liver IL-1β, IL-6, IFN-γ, and TNF-α, while treatment of P. chabaudi-infected mice with IE resulted in a significant downregulation in the expression levels of these cytokines (Figure 8).
Figure 8.
IE-induced changes in the expression levels of hepatic IL-1β, IL-6, IFN-γ, and TNF-α genes in the mice infected with P. chabaudi on day 7. Data are expressed as means ± SD; a: significant change at p ≤ 0.05 with respect to the control group; b: significant change at p ≤ 0.05 with respect to the infected (−IE) group.
4. Discussion
Malaria remains as one of the dangerous diseases affecting people in several countries. IE reduces the induced parasitemia following P. chabaudi infection [4, 5, 30], owing mainly to the presence of an active antimalarial component, quinine [31].
Frevert and Nardin [2] reported that the liver is the first site of preerythrocytic development of Plasmodium. These authors documented that the liver serves as an effector against blood-stage malaria [2], wherein the liver endothelial system eliminates the parasitized erythrocytes possibly by phagocytosis [32]. Savil and Fadok [33] reported membrane changes in response to apoptosis that resulted in the formation of apoptotic bodies, which could be easily phagocytosed by macrophages. In the hepatic tissue, Kupffer cells comprise about 70-80% of the liver macrophages and may phagocytize the parasitized erythrocytes as well as hemozoin granules [34]. IE treatment increased the number of phagocytic cells and reduced P. chabaudi-induced parasitemia via phagocytosis.
Oxidative stress due to infection led to the activation of molecular pathways that drive inflammation and could directly induce tissue injury. In our previous work, we reported only that IE may effectively improve the liver histopathological changes associated with P. chabaudi infection [30]. Here, we reported for the first time that the induced pathological changes in the liver are due to parasite-induced oxidative stress that may cause cell damage and subsequently lead to cell death. In addition, IE ameliorated the apoptotic changes induced in response to the parasite infection.
In this study, we observed an increase in lipid peroxidation and depletion in the GSH level accompanied with the inhibition of antioxidant enzyme activities following malarial infection. Lipid oxidation results in cell membrane damage and loss of plasma membrane integrity, thereby causing cell death. Superoxide dismutase has a major role in scavenging O2 and may react with NO as an antioxidant. In addition, catalase degrades hydrogen peroxide (H2O2) into H2O and O2 and causes reduction in the level of ROS. Several studies have highlighted the antioxidant role of IE especially in the spleen and kidney [5, 6, 17]. In this study, the hepatic GSH level and catalase activity were significantly reduced upon infection, while treatment with IE resulted in the restoration of the GSH level and catalase activity. However, NO and MDA levels decreased after treatment with IE.
The liver is responsible for the systemic response to blood-stage malaria and produces cytokines, which are important in local immune response [35]. The number of leucocytes increased (Figure 1) in response to injuries caused by P. chabaudi [33]. IE is a natural product with anti-inflammatory, antioxidant, antimalarial, and hepatoprotective effects [5, 30]. Previous studies have documented that Indigofera oblongifolia contains active compounds such as flavonoids and alkaloids as well as the antimalarial compound, quinine [16]. The antioxidant and anti-inflammatory effects of IE were reported in rat liver with severe lead acetate-induced inflammation [16].
5. Conclusions
The present study highlights the antioxidant, anti-inflammatory, and antiapoptotic effects of IE in P. chabaudi-induced hepatic injury, as evident from the effects of IE on hepatic oxidative damage, regulation of inflammatory cytokines, and apoptotic gene expression.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support. This work was supported by the Deanship of Scientific Research at King Saud University through the research group project no. RG-198.
Abbreviations
- Bax:
Bcl2-associated X protein
- Bcl2:
B-cell lymphoma 2
- Casp3:
Caspase-3
- IE:
Indigofera oblongifolia leaf extract
- IL-1β:
Interleukin-1β
- IL-6:
Interleukin-6
- INF-γ:
Interferon gamma
- MDA:
Malondialdehyde
- NO:
Nitric oxide
- RT-qPCR:
Real-time quantitative polymerase chain reaction
- TNF-α:
Tumor necrosis factor-alpha
- TUNEL:
Terminal deoxynucleotidyl transferase dUTP nick end labeling.
Data Availability
The data set supporting our results is included within the article.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.World Health Organization. Global Malaria Programme. World Malaria Report 2017. 2017. June 2018, http://www.who.int/malaria/publications/world-malaria-report-2017/en/
- 2.Frevert U., Nardin U. E. Cellular effector mechanisms against Plasmodium liver stages. Cell Microbiology. 2008;10(10):1956–1967. doi: 10.1111/j.1462-5822.2008.01211.x. [DOI] [PubMed] [Google Scholar]
- 3.Yuda M., Ishino T. Liver invasion by malarial parasites--how do malarial parasites break through the host barrier? Cell Microbiology. 2004;6(12):1119–1125. doi: 10.1111/j.1462-5822.2004.00474.x. [DOI] [PubMed] [Google Scholar]
- 4.Dkhil M. A., Lubbad M. Y., Al-Shaebi E. M., Delic D., Al-Quraishy S. The antiplasmodial and spleen protective role of crude Indigofera oblongifolia leaf extract traditionally used in the treatment of malaria in Saudi Arabia. Drug Design, Development and Therapy. 2015;9:6235–6246. doi: 10.2147/dddt.s94673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lubbad M. Y., Al-Quraishy S., Dkhil M. A. Antimalarial and antioxidant activities of Indigofera oblongifolia on Plasmodium chabaudi-induced spleen tissue injury in mice. Parasitology Research. 2015;114(9):3431–3438. doi: 10.1007/s00436-015-4568-y. [DOI] [PubMed] [Google Scholar]
- 6.Al-Shaebi E. M., Taib N. T., Mubaraki M. A., et al. Indigofera oblongifolia leaf extract regulates spleen macrophage response during Plasmodium chabaudi infection. Saudi Journal of Biological Sciences. 2017;24(7):1663–1666. doi: 10.1016/j.sjbs.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trubowitz S., Mazek B. Plasmodium falciparum: phagocytosis by polymorphonuclear leukocytes. Science. 1968;162(3850):273–274. doi: 10.1126/science.162.3850.273. [DOI] [PubMed] [Google Scholar]
- 8.Celada A., Cruchaud A., Perrin L. H. Phagocytosis of Plasmodium falciparum-parasitized erythrocytes by human polymorphonuclear leukocytes. Journal Parasitology. 1983;69(1):49–53. doi: 10.2307/3281273. [DOI] [PubMed] [Google Scholar]
- 9.Serghides L., Kain K. C. Peroxisome proliferator-activated receptor γ-retinoid X receptor agonists increase CD36-dependent phagocytosis of Plasmodium falciparum-parasitized erythrocytes and decrease malaria-induced TNF-α secretion by monocytes/macrophages. The Journal of Immunology. 2001;166(11):6742–6748. doi: 10.4049/jimmunol.166.11.6742. [DOI] [PubMed] [Google Scholar]
- 10.Riley E. M., Wahl S., Perkins D. J., Schofield L. Regulating immunity to malaria. Parasite Immunology. 2006;28(1-2):35–49. doi: 10.1111/j.1365-3024.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- 11.Mehlhorn H., editor. 4th Ed. Vol. 1. Berlin: Springer; 2014. Encyclopedic reference of parasitology. [Google Scholar]
- 12.World Health Organization. Global Malaria Programme. World Malaria Report 2016. WHO; 2016. July 2017, http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/ [Google Scholar]
- 13.Gómez Castellanos J. R., Prieto J. M., Heinrich M. Red Lapacho (Tabebuia impetiginosa)--a global ethnopharmacological commodity? Journal of Ethnopharmacology. 2009;121(1):1–13. doi: 10.1016/j.jep.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Saxena N., Yadav N. K., Verma R. K. Traditional knowledge of medicinal plants used to cure gastro intestinal problems in Jalaun district of Uttar Pradesh, India. Journal of Medicinal Plants Studies. 2014;2:24–28. [Google Scholar]
- 15.Sharif A., Ahmed E., Malik A., et al. Lipoxygenase inhibitory constituents from Indigofera oblongifolia. Archives of Pharmacal Research. 2005;28(7):761–764. doi: 10.1007/BF02977339. [DOI] [PubMed] [Google Scholar]
- 16.Abdel Moneim A. E. Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS One. 2016;11(7, article e0158965) doi: 10.1371/journal.pone.0158965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dkhil M. A., Al-Khalifa M. S., Al-Quraishy S., Zrieq R., Abdel Moneim A. E. Indigofera oblongifolia mitigates lead-acetate-induced kidney damage and apoptosis in a rat model. Drug Design, Development and Therapy. 2016;10:1847–1856. doi: 10.2147/dddt.s105511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D. O., Chun O. K., Kim Y. J., Moon H. Y., Lee C. Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. Journal of Agricultural and Food Chemistry. 2003;51(22):6509–6515. doi: 10.1021/jf0343074. [DOI] [PubMed] [Google Scholar]
- 19.Dewanto V., Wu X. Z., Liu R. H. Processed sweet corn has higher antioxidant activity. Journal of Agricultural and Food Chemistry. 2002;50(17):4959–4964. doi: 10.1021/jf0255937. [DOI] [PubMed] [Google Scholar]
- 20.Mubaraki M. A., Dkhil M. A., Hafiz T. A., Khalil M. F., Al-Shaebi E. M., Delic D. Vitamin D receptor regulates intestinal inflammatory response in mice infected with blood stage malaria. Microbial Pathogenesis. 2018;117:299–303. doi: 10.1016/j.micpath.2018.02.048. [DOI] [PubMed] [Google Scholar]
- 21.Mubaraki M. A., Hafiz T. A., Al-Quraishy S., Dkhil M. A. Oxidative stress and genes regulation of cerebral malaria upon Zizyphus spina-christi treatment in a murine model. Microbial Pathogenesis. 2017;107:69–74. doi: 10.1016/j.micpath.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Krücken J., Mehnert L. I., Dkhil M. A., et al. Massive destruction of malaria-parasitized red blood cells despite spleen closure. Infection and Immunity. 2005;73(10):6390–6398. doi: 10.1128/IAI.73.10.6390-6398.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nolte C., Amores A., Nagy Kovács E., Postlethwait J., Featherstone M. The role of a retinoic acid response element in establishing the anterior neural expression border of Hoxd4 transgenes. Mechanisms of Development. 2003;120(3):325–335. doi: 10.1016/S0925-4773(02)00442-2. [DOI] [PubMed] [Google Scholar]
- 24.Tsakiris S., Schulpis K. H., Marinou K., Behrakis P. Protective effect of L-cysteine and glutathione on the modulated suckling rat brain Na+,K+-ATPase and Mg2+-ATPase activities induced by the in vitro galactosaemia. Pharmacological Research. 2004;49(5):475–479. doi: 10.1016/j.phrs.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Ellman G. L. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 26.Berkels R., Purol-Schnabel S., Roesen R. Measurement of nitric oxide by reconversion of nitrate/nitrite to NO. Methods in Molecular Biology. 2004;279:1–8. doi: 10.1385/1-59259-807-2:001. [DOI] [PubMed] [Google Scholar]
- 27.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 28.Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 29.Livak K., Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Al-Shaebi E. A., Dkhil M. A., Al-Quraishy S. Indigofera oblongifolia regulates the hepatic gene expression profile induced by blood stage malaria. Microbial Pathogenesis. 2018;119:170–182. doi: 10.1016/j.micpath.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Shahjahan M., Vani G., Shyamala Devi C. S. Protective effect of Indigofera oblongifolia in CCl4-induced hepatotoxicity. Journal of Medicinal Food. 2005;8(2):261–265. doi: 10.1089/jmf.2005.8.261. [DOI] [PubMed] [Google Scholar]
- 32.Krücken J., Delic D., Pauen H., et al. Augmented particle trapping and attenuated inflammation in the liver by protective vaccination against Plasmodium chabaudi malaria. Malaria Journal. 2009;8(1):p. 54. doi: 10.1186/1475-2875-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savil J., Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407(6805):784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 34.Kim T. S., Kang B. Y., Cho D., Kim S. H. Induction of interleukin-12 production in mouse macrophages by berberine, a benzodioxoloquinolizine alkaloid, deviates CD4 T cells from a Th2 to a Th1 response. Immunology. 2003;109(3):407–414. doi: 10.1046/j.1365-2567.2003.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wunderlich F., Al-Quraishy S., Dkhil M. Liver-inherent immune system: its role in blood-stage malaria. Frontiers in Microbiology. 2014;5:p. 559. doi: 10.3389/fmicb.2014.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set supporting our results is included within the article.