Abstract
Background
Despite significant progress in drug treatment, the prognosis of patients with advanced pulmonary arterial hypertension (PAH) remains extremely poor. Many preclinical studies have reported the efficacy of stem cell (SC) therapy for PAH; however, this approach remains controversial. The aim of this systematic review and meta-analysis is to assess the potential efficacy of SC therapy for PAH.
Methods
The Medline, EMBASE, Cochrane Library, and Web of Science databases were searched from inception to August 12, 2018. Preclinical studies that evaluated the use of SC therapy for PAH were included. The primary outcome was pulmonary haemodynamics, as assessed by measurement of the right ventricular systolic pressure (RVSP), mean pulmonary arterial pressure (mPAP), and/or mean right ventricle pressure (mRVP). The secondary outcomes included the weight ratio of the right ventricle to the left ventricle plus septum (RV/LV+S), the right ventricle to body weight ratio (RV/BW), the percentage of pulmonary arteriole area index (WA), and/or the percentage of medial wall thickness of the pulmonary arteriole (WT). The quality of outcomes was evaluated using the SYstematic Review Centre for Laboratory animal Experimentation (SYRCLE) bias risk tool. The inverse-variance method with random-effects modelling was used to calculate pooled weighted mean differences (WMDs) and 95% CIs. Statistical analysis was performed with STATA 14.0.
Results
Twenty-eight eligible articles (722 animals) were included. SC therapy reduced the pooled WMDs (95% CIs) of RVSP, mPAP, mRVP, RV/LV+S, RV/BW, WA, and WT for animals with PAH, with values of − 14.12 (− 14.63, − 13.61), − 11.86 (− 12.35, − 11.36), − 17.33 (− 18.10, − 16.56), − 0.10 (− 0.10, − 0.09), 0.23 (0.21, 0.24), − 13.66 (− 15.71, − 11.62), and − 7.96 (− 7.99, − 7.93), respectively.
Conclusions
SC therapy is effective for PAH in preclinical studies. These results may help to standardise preclinical animal studies and provide a theoretical basis for clinical trial design in the future.
Systematic review registration
PROSPERO (http://www.crd.york.ac.uk/PROSPERO).
Electronic supplementary material
The online version of this article (10.1186/s13287-019-1162-8) contains supplementary material, which is available to authorized users.
Keywords: Pulmonary hypertension, Stem cells, Animal model, Therapy, Meta-analysis
Background
Pulmonary arterial hypertension (PAH) is a progressive chronic disease with a high mortality rate [1], and the median survival of patients with idiopathic PAH was estimated to be 2.8 years [2]. This disease is characterised by progressively increasing PAH and vascular remodelling [3], which ultimately leads to right heart failure and death [4]. The pulmonary vasculature is also remodelled, with increased pulmonary vascular resistance and over-proliferation of pulmonary artery endothelial cells [5, 6]. In recent decades, the treatment of PAH has progressed significantly, as a deeper understanding of the underlying pathogenesis has been achieved [7–12]. However, the mortality of PAH remains high [12, 13]. Therefore, there is a considerable unmet medical need in the management of PAH.
Recently, cell-based gene therapies have attracted increasing interest due to their beneficial roles in ameliorating the progression of PAH [14–16]. Stem cells (SCs) are multipotent progenitor cells, and mesenchymal SCs are the preferred seed cells for cell-based therapy because of their strong expansion ability in culture, their reproducible potential, and their capacity to differentiate into bone, cartilage, muscle, or vascular smooth muscle cells, as well as other connective tissues [17–19]. The ultimate goal of SC therapy is to inhibit pulmonary vascular remodelling and excessive cell proliferation, slowing the development of pulmonary hypertension and the occurrence of right heart failure to achieve clinical improvement of cardiopulmonary function without severe adverse effects.
Many animal studies of PAH have been reported, with heterogeneous designs and conflicting outcomes. Nevertheless, preclinical studies are needed to assess the risk of new therapies and to predict safety, feasibility, and efficacy. Moreover, such studies can offer guidance concerning unresolved issues related to clinical cell therapy (i.e., the choice of cell type and dose, the route and timing of delivery, and follow-up after cell transplantation). Thus, we performed a systematic review of the pertinent literature, including a quantitative meta-analytical pooling of the data, to assess the effects of SC therapy on animals with PAH.
Methods
The author declares that all supporting data are available within this article and its online supplementary files. The study protocol is registered through PROSPERO (http://www.crd.york.ac.uk/PROSPERO), and the registration number is CRD42018103854, which can be found online at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=103854.
Data sources and search strategies
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria [20]. The PRISMA 2009 checklist is shown in Additional file 11: Table S1. A systematic literature search was conducted for animal studies on SC therapy and PAH that were published until 12 August 2018, using the Medline (www.ncbi.nlm.nih.gov/pubmed), EMBASE (www.embase.com), Cochrane Library (www.cochranelibrary.com), and Web of Science (http://apps.webofknowledge.com/WOS_GeneralSearch_input.do?) databases. The detailed search strategy is shown in Additional file 12: Table S2. The search was limited to English. We also hand-searched the references of the included articles. Literature searches were performed independently by Huo-Yan Liang and Bo Yuan.
Eligibility criteria
Studies were considered suitable for inclusion in this meta-analysis if (1) all PAH animal models were subjected to SC treatment (all species and sexes), (2) saline-treated PAH animal models were used as controls, and (3) the studies included data on one or more outcomes, such as right ventricular systolic pressure (RVSP), mean pulmonary arterial pressure (mPAP), mean right ventricle pressure (mRVP), the weight ratio of the right ventricle to the left ventricle plus septum (RV/LV+S), the right ventricle to body weight ratio (RV/BW), the pulmonary arteriole area index (WA), and the wall thickness of the pulmonary arteriole (WT). The exclusion criteria were as follows: (1) all inclusion criteria were not fulfilled, (2) the animals had comorbidities, (3) the animal models did not have PAH, (4) animal models with PAH did not receive SC treatment, (5) the study was a case study, a crossover study, or a study without a separate control group, (6) the study was a duplicate report, or (7) weighted mean differences (WMDs) with 95% CIs were not provided or could not be calculated.
Study selection and data extraction
The titles and/or abstracts of studies retrieved using the search strategy and studies from additional sources were screened independently by Huo-Yan Liang and Bo Yuan to identify studies that potentially met the inclusion criteria outlined above. Key data interpretation was conducted independently by Li-Feng Li and Tian Wang. Any differences were resolved through discussions with Quan-Cheng Kan and Le-Xin Wang. The extracted information was as follows: author (year of publication), animal characteristics (species, gender, sample size, and model), intervention characteristics (source, dosage, route, and timing of SC therapy), follow-up (observation time of outcomes after SC administration), and measures relevant to our primary and secondary outcomes.
Assessment of risk of bias
The risk of bias of eligible studies was assessed using the SYstematic Review Centre for Laboratory animal Experimentation (SYRCLE) bias risk tool [21]. The SYRCLE tool was adapted from the Cochrane Risk of Bias Tool to assess methodological quality using criteria specific to animal studies. The items in this tool include assessments for selection bias (sequence generation, baseline characteristics, and allocation concealment), performance bias (random housing and blinding), detection bias (random outcome assessment and blinded outcome assessment), attrition bias (completeness of outcome data), and reporting bias. For each included study, results of no, yes, and unclear risk of bias were scored as high, low, and unclear risk of bias, respectively.
Primary and secondary outcomes
The current gold standard for the diagnosis and evaluation of clinical PAH is direct pulmonary haemodynamic measures, as assessed by measurement of RVSP, mPAP, and mRVP through right heart catheterization. RV remodelling is a precursor of right heart failure and is characterised by decreased function and dilatation of the RV, and this change is strongly correlated with prognosis and survival in PAH patients [22]. We collected morphometric data on RV remodelling expressed as the RV/LV+S and RV/BW. In addition, we also collected data from other non-invasive measures obtained by echocardiography to evaluate cardiac structure and pulmonary haemodynamics, such as WA and WT. In this meta-analysis, the primary outcomes included RVSP, mPAP, and mRVP after SC administration. Secondary outcomes included RV/LV+S, RV/BW, WA, and WT.
Statistical analysis
The estimated effect sizes of primary and secondary outcomes were determined by WMDs and 95% CIs. WMDs, an ideal measure for continuous data, were calculated by the mean (M), standard deviation (SD), and sample number (N) in each study. The pooled WMD of each study was conducted using a fixed-effects model (inverse-variance) or a random-effects model (I-V heterogeneity) to generate forest plots. In addition, the Q test and I2 test were used to measure heterogeneity, which was not considered significant if P > 0.1 or I2 < 50%. Potential sources of heterogeneity, if significant, were further investigated by stratified analysis and meta-regression. Begg’s funnel plot [23] and Egger’s linear regression [24] were used to assess potential publication bias. Funnel plots were visually assessed for asymmetry. For Egger’s tests, P < 0.1 was considered significant to confirm the presence of a small study size. All analyses were performed using Stata 14.0 statistical software (Stata Corp LP, College Station, Texas 77,845, USA, serial number: 401406267051). Differences for which P < 0.05 (two-sided) were considered statistically significant.
Results
Study selection
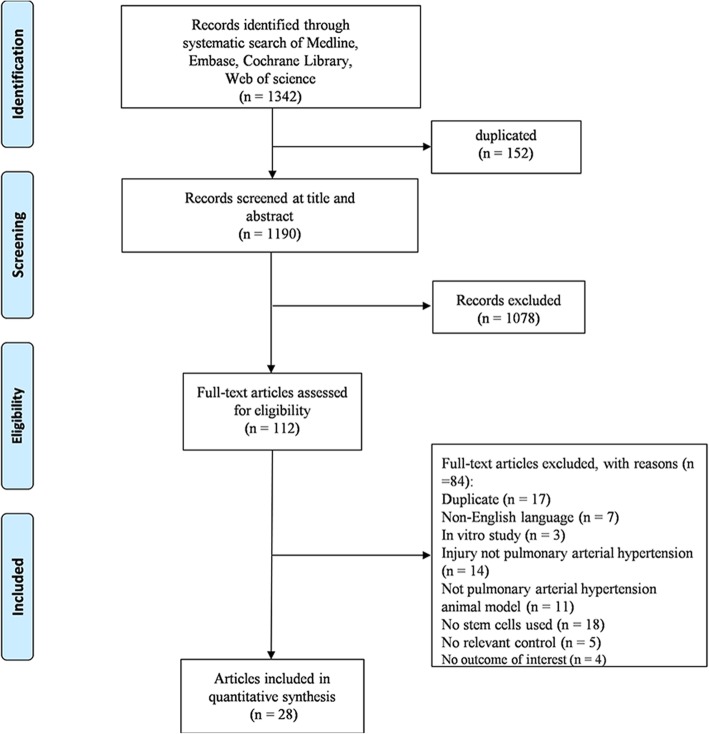
Using our search strategy, we initially identified 1342 records, and 1190 articles remained after duplicates were removed. After preliminary screening by title and abstract, 112 articles reporting the therapeutic potential of SCs for PAH were isolated for full-text review. However, 28 articles [14, 15, 25–50] enrolling 722 animals were ultimately included in this meta-analysis after study selection (Fig. 1).
Fig. 1.
Flow diagram of study selection
Study characteristics
The number of included articles reporting RVSP, mPAP, mRVP, RV/LV+S, RV/BW, WA, and WT was16 [14, 26, 28–33, 39, 40, 42, 45, 48–50], 12 [25, 30, 38, 41, 44, 47, 50], 14 [27, 34–37, 42, 50], 29 [25–27, 29–31, 33–36, 39–41, 43–45, 47, 48, 50], 16 [14, 29, 34–37, 42, 48, 50], 5 [27, 43, 44], and 23 [26, 27, 32–37, 40, 42–44, 49, 50], respectively. The animal model used in most studies was induced by surgical operation or by 40–60 mg/kg monocrotaline (MCT) administered intraperitoneally, subcutaneously, or via tail vein injection. The majority of the animals were male, 6-week-old, Sprague Dawley rats, and weighed 180–220 g. Regarding the intervention characteristics of the SCs, they mostly originated from human or rat bone marrow, adipose-derived mesenchymal tissue, or human umbilical cord blood-derived mesenchymal tissue. The doses of the interventions ranged from 105 to 107 SCs, which were injected intravenously, intratracheally, or intraperitoneally at least 3 days after induction of the PAH model. The observation time of the primary and secondary outcomes after SC administration was at least 1 week. In addition, several articles contained more than one study [34–38, 44]. The main characteristics of the 28 articles involved in this meta-analysis are shown in Tables 1 and 2.
Table 1.
General characteristics of preclinical studies investigating the efficacy of stem cells in models of PAH
| Author (year) | Species, strain, sex | No. of controls | No. of treated animals | PAH model | SC source | SC route | SC dose | Timing of SC therapy after PAH | Follow-up (weeks) |
|---|---|---|---|---|---|---|---|---|---|
| Lim et al.(2016) [39] | Rat, SPF, M | 5 | 5 | MCT (60 mg/kg) | hBM | Tail vein | 2.5 × 105 | 14 days | 2 weeks |
| Lee et al. (2017) [36] | Rat, SPD, M | 4 | 6 | MCT (60 mg/kg) | hUCB | Jugular vein | 3 × 105 | 7 days | 1 week |
| 3 weeks | |||||||||
| 4 | 6 | ||||||||
| 4 | 4 | 3 × 105 | 7 days | 1 week | |||||
| 3 weeks | |||||||||
| 4 | 4 | ||||||||
| Guo et al. (2013) [30] | Rat, SPD, M | 10 | 10 | MCT (60 mg/kg) | rBM | Jugular vein | 5 × 106 | 21 days | 3 weeks |
| Luan et al.(2012) [42] | Rat, SPD, NR | 20 | 20 | Surgical operation | rBM | Sublingual vein | 1–5 × 106 | 28 days | 2 weeks |
| Baber et al. (2006) [25] | Rat, SPD, M | 10 | 10 | MCT (60 mg/kg) | rBM | Intratracheal | 3 × 106 | 14 days | 3 weeks |
| Chen et al. (2014) [27] | Rat, SPD, NR | 7 | 7 | MCT (50 mg/kg) | rBM | Intravenous | 5 × 106 | 21 days | 2 weeks |
| Chen et al. (2016) [26] | Rat, Wistar, M | 10 | 10 | MCT (60 mg/kg) | rBM | Tail vein | 1 × 106 | 14 days | 3 weeks |
| Cheng et al. (2017) [29] | Rat, Lewis, M | 10 | 10 | MCT (60 mg/kg) | rBM | Tail vein | 3 × 106 | 21 days | 3 weeks |
| Huang et al.(2016) [31] | Rat, SPD, M | 6 | 6 | MCT (60 mg/kg) | iP | Tail vein | 2 × 106 | 14 days | 4 weeks |
| Chen et al.(2016) [28] | Rat, SPD, M | 8 | 8 | MCT (60 mg/kg) | hUCB | Caudal vein | 1 × 106 | 5 days | 3 weeks |
| Jiang et al.(2012) [32] | Rat, SPD, NR | 8 | 43 | MCT (60 mg/kg) | rBM | Tail vein | 4 × 106 | 3 days | 3 weeks |
| Kang et al. (2015) [33] | Rat, SPF, M | 10 | 10 | MCT (60 mg/kg) | hUCB | Tail vein | 2.5 × 105 | 14 days | 2 weeks |
| Kimet al (2012) [34] | Rat, SPD, M | 6 | 6 | MCT (60 mg/kg) | rBM | Tail vein | 2 × 107 | 7 days | 1 week |
| 6 | 6 | 3 weeks | |||||||
| Kim et al. (2016) [35] | Rat, SPD, M | 6 | 6 | MCT (60 mg/kg) | hUCB | External jugular vein | 3 × 106 | 7 days | 1 week |
| 3 weeks | |||||||||
| 6 | 6 | ||||||||
| 8 | 8 | ||||||||
| Liang et al. (2015) [38] | Rat, SPD, M | 10 | 10 | MCT (40 mg/kg) | rADM | Left external jugular vein | 1 × 106 | 7 days | 2 weeks |
| 8 | 10 | 3 weeks | |||||||
| Liu et al. (2015) [40] | Rat, SPD, F | 8 | 8 | MCT (60 mg/kg) | hUCB | Caudal vein | 1 × 106 | 5 days | 3 weeks |
| Luan et al. (2013) [43] | Rat, SPD, M | 10 | 10 | MCT (50 mg/kg) | rBM | Sublingual vein | 1 × 107 | 7 days | 23 weeks |
| Luo et al. (2014) [44] | Rat, SPD, M | 8 | 8 | MCT (40 mg/kg) | rADM | Left jugular vein | 1 × 106 | 14 days | 1 week |
| 8 | 8 | 2 weeks | |||||||
| 8 | 8 | 3 weeks | |||||||
| Raoul et al. (2007) [45] | Mouse, C57BL/6, F | 5 | 5 | MCT (5 mg/kg) | rBM | Tail vein | 2.5 × 106 | 3 days | 3 weeks |
| Rathinasabapathy et al. (2016) [46] | Rat, SPD, M | 8 | 8 | MCT (50 mg/kg) | rADM | Jugular vein | 1 × 106 | 14 days | 2 weeks |
| Somanna et al. (2014) [47] | Rat, SPD, NR | 6 | 6 | MCT (60 mg/kg) | hADM | Intratracheal | 3 × 106 | 14 days | 2 weeks |
| Zhang et al. (2012) [49] | Mouse, ICR, NR | 10 | 10 | MCT (400 mg/kg) | rBM | Tail vein | 5 × 105 | 7 days | 2 weeks |
| Zhang et al. (2012) [50] | Rat, SPD, M | 9 | 9 | MCT (50 mg/kg) | hUCB | Sublingual vein | 3 × 106 | 7 days | 2 weeks |
| Umar et al. (2009) [48] | Rat, Wistar, F | 10 | 10 | MCT (60 mg/kg) | rBM | Jugular vein | 1 × 106 | 14 days | 2 weeks |
| 10 | 10 | 3 weeks | |||||||
| 10 | 10 | 4 weeks | |||||||
| Liu et al. (2011) [41] | Rat, Wistar, M | 17 | 16 | Surgical operation | rADM | Right jugular vein | 5 × 107 | 84 days | 2 weeks |
| Takemiya et al. (2009) [15] | Rat, Lewis, M | 10 | 10 | MCT (60 mg/kg) | rBM | Tail vein | 5 × 105 | 14 days | 2 weeks |
| Horimoto et al. (2005) [14] | Rat, SPD, M | 10 | 10 | MCT (60 mg/kg) | rBM | Right femoral vein | 1 × 106 | 7 days | 2 weeks |
| Lee et al. (2015) [37] | Rat, SPD, M | 8 | 8 | MCT (60 mg/kg) | hUCB | External jugular vein | 3 × 106 | 7 days | 1 week |
| 8 | 8 | 2 weeks | |||||||
| 8 | 8 | 3 weeks |
PAH pulmonary arterial hypertension, SPF specific-pathogen-free, SPD Sprague Dawley, M male, F female, NR not reported, SCs stem cells, hBM human bone marrow mesenchymal tissue, hUCB human umbilical cord blood-derived mesenchymal tissue, rBM rat bone marrow mesenchymal tissue, iP murine-induced pluripotent stem cells, BM bone marrow mesenchymal tissue, rADM rat adipose-derived stromal tissue, ADM adipose-derived stromal tissue
Follow-up (weeks) indicates the observation time of outcomes after stem cell administration
Table 2.
Detailed information on outcomes extracted from the included studies
| Author (year) | Total N, M, SD of Animals (treatment/control) | ||||||
|---|---|---|---|---|---|---|---|
| Primary outcomes | Secondary outcomes | ||||||
| RVSP | mPAP | mRVP | RV/LV+S | RV/BW | WA | WT | |
| N1, MD1, SD1; N2, MD2, SD2 | N1, MD1, SD1; N2, MD2, SD2 | N1, MD1, SD1; N2, MD2, SD2 | N1, MD1, SD1; N2, MD2, SD2 | N1, MD1, SD1; N2, MD2, SD2 | N1, MD1, SD1; N2, MD2, SD2 | N1, MD1, SD1; N2, MD2, SD2 | |
| Lim et al.(2016) [39] | 5,34.95,1.57;5,39.79,1.57 | 5,0.47,0.03;5,0.52,0.01 | |||||
| Lee et al. (2017) [36] | 6,16,1.63;4,29,3.63 | 6,0.31,0.02;4,0,36,0.0025 | 4,0.91,0.03;4,0.69,0.01 | 6,32.52,0.91;4,42.31,0.9 | |||
| 6,14,0.75;4,49.5,8 | 6,0.69,0.04;4,0.8,0.08 | 4,1.75,0.03;4,0.62,0.01 | 4,34.76,2.71;4,38.22,3.1 | ||||
| 4,14,0.75;4,27,2 | 4,0.32,0.0025;4,0.4,0.01 | 4,0.85,0.02;4,0.69,0.01 | 4,36.16,0.93;4,40.9,2.08 | ||||
| 4,13,1.75;4,44,1.75 | 4,0.62,0.02;4,0.81,0.06 | 4,1.52,0.04;4,0.62,0.01 | |||||
| Guo et al. (2013) [30] | 10,94.69,9.03;10,95.55,12.53 | 10,29.99,6.08;10,30.26,7.83 | 10,0.34,0.06;10,0.37,0.08 | ||||
| Luan et al.(2012) [42] | 20,43.83,2.13;20,56.84,1.54 | 20,26.82,2.13;20,41.37,2.24 | 20,28.34,1.98;20,41.7,6.17 | 20,0.547,0.04;20,0.613,0.07 | 20,20.83,5.49;20,45.21,4.37 | ||
| Baber et al. (2006) [25] | 10,33.48,6;10,44.3,6.35 | 10,0.47,0.03;10,0.6,0.02 | |||||
| Chen et al. (2014) [27] | 7,13.51,1.25;7,29.9,0.71 | 7,0.34,0.03;7,0.56,0.02 | 7,78.62,2.58;7,95.17,10.35 | 7,49.18,3.46;7,73.77,2.77 | |||
| Chen et al. (2016) [26] | 10,28,1.57;10,43,3.18 | 10,0.55,0.08;10,0.78,0.41 | 10,21.21,0.02;10,29.16,0.04 | ||||
| Cheng et al. (2017) [29] | 10,43.76,2.11;10,60.85,1.2 | 10,0.43,0.05;10,0.58,0.02 | 10, 0.42, 0.05;10, 0.51, 0.09 | ||||
| Huang et al.(2016) [31] | 6,47.99,5.53;6,66.7,1.74 | 6, 0.48, 0.04;6, 0.68, 0.09 | |||||
| Chen et al.(2016) [28] | 8, 29.01,1.67; 8,45.81,2.63 | 8,18.76,1.09; 8,30.44,2.23 | |||||
| Jiang et al.(2012) [32] | 43, 28.84, 1.35; 18, 40.2, 3.46 | 43, 0.38, 0.05; 18, 0.58, 0.03 | 43, 36.24, 1.51; 18, 53.47, 3.52 | ||||
| Kang et al. (2015) [33] | 10, 32.72, 1.19; 10, 42.24, 7.52 | 10, 0.5, 0.01; 10, 0.54, 0.07 | 10.32.4, 4; 10, 42, 7.4 | ||||
| Kimet al (2012) [34] | 6, 15.4, 3.8; 6, 38.4, 7.4 | 6, 0.33, 0.06; 6, 0.53, 0.04 | 6, 0.73, 0.02; 6, 0.82, 0.05 | 6, 29.3, 5.2; 6, 38.8, 1.9 | |||
| 6, 14.8, 3.6; 6, 42.3, 13 | 6, 0.64, 0.06; 6, 0.79, 0.04 | 6, 1.47, 0.02; 6, 1.7, 0.09 | 6, 35.7, 5.7; 6, 40.2, 5.4 | ||||
| Kim et al. (2016) [35] | 6, 16.4, 2.1; 6, 34.8, 7.3 | 6, 0.34, 0.27; 6, 0.38, 0.05 | 6, 0.75, 0.02; 6, 0.82, 0.05 | 6, 31.07, 4.71; 6, 38.84, 1.94 | |||
| 6, 13.4, 2.5; 6, 42.2, 13 | 6, 0.48, 0.1; 6, 0.79, 0.04 | 6, 1.3, 0.05; 6, 1.7, 0.07 | 6, 33.64, 3.57; 6, 40.19, 5.38 | ||||
| Liang et al. (2015) [38] | 10, 16.37, 0.94; 10, 26.88, 0.86 | ||||||
| 10, 18.26, 1.41; 8, 32.22, 0.71 | |||||||
| Liu et al. (2015) [40] | 8, 29.86, 2.87; 8, 47.13, 3.31 | 8, 17.53, 1.24; 8, 31.78, 2.16 | |||||
| Luan et al. (2013) [43] | 10, 31.57, 5.54; 10, 44.97, 4.26 | 10, 0.43, 0.05; 10, 0.59, 0.03 | 10, 45.69, 4.21; 10, 56.52, 4.5 | 10, 26.28, 3.95; 10, 36.56, 3.68 | |||
| Luo et al. (2014) [44] | 8, 18.63, 2.15; 8, 24.53, 2.9 | 8, 0.36, 0.04; 17, 0.43, 0.12 | 8, 58.23, 4.08; 8, 68.7, 3.43 | 8, 38.06, 4.15; 8, 46.09, 4.7 | |||
| 8, 23.07, 2.84; 8, 33.18, 3.28 | 8, 0.39, 0.04; 8, 0.49, 0.02 | 8, 62.97, 6.58; 8, 80.48, 6.19 | 8, 40.91, 5.24; 8, 57.26, 4.32 | ||||
| 8, 22.98, 3.24; 8, 36.38, 3.28 | 8, 0.41, 0.01; 8, 0.51, 0.01 | 8, 65.27, 5.45; 8, 84.01, 2.76 | 8, 41.91, 5.16; 8, 64.64, 3.86 | ||||
| Raoul et al. (2007) [45] | 5, 17.65, 2.16; 5, 27.77, 0.94 | 5, 0.3, 0.03; 5, 0.35, 0.02 | |||||
| Rathinasabapathy et al. (2016) [46] | |||||||
| Somanna et al. (2014) [47] | 6, 52.6, 2.79; 6, 56.24, 3.1 | 6, 053, 0.04; 6, 0.51, 0.03 | 6, 0.53, 0.04; 6, 0.51, 0.03 | ||||
| Zhang et al. (2012) [49] | 9, 32.85, 3.44; 9, 59.12, 4.32 | 9, 0.23, 0.01; 9, 0.31, 0.01 | 9, 29.97, 2.21; 9, 53.47, 3.52 | ||||
| Zhang et al. (2012) [50] | 10, 43.83, 2.13; 10, 56.84, 1.54 | 10, 26.82, 3.42; 10, 41.37, 2.24 | 10, 28.34, 1.98; 10, 41.7, 6, 17 | 10, 0.547, 0.041; 10, 0.613, 0.072 | |||
| Umar et al. (2009) [48] | 10, 31, 4; 10, 42, 17 | 10, 0.32, 0.07; 10, 0.47, 0.12 | 10, 0.64, 0.01; 10, 0.93, 0.21 | ||||
| Liu et al. (2011) [41] | 16, 19.83, 2.32; 17, 33.33, 5.36 | 16, 0.36, 0.04; 17, 0.43, 0.12 | |||||
| Takemiya et al. (2009) [15] | 10, 43.73, 3.37; 10, 65.56, 2.3 | ||||||
| Horimoto et al. (2005) [14] | 10, 42.3, 1.1; 10, 58.7, 1.86 | 10, 0.462, 0.029; 10, 0.59, 0.084 | |||||
| Lee et al. (2015) [37] | 8, 23.6, 7.6; 8, 36.5, 3.1 | 8, 1.43, 0.14; 8, 1.47, 0.18 | 8, 1.14, 0.21; 8, 1.01, 0.1 | 8, 34.2, 4; 8, 38.5; 4 | |||
| 8, 21.3, 4.9; 8, 37.2, 6.3 | 8, 2.18, 0.45; 8, 2.89, 0.43 | 8, 1.72, 0.33; 8, 1.62, 0.24 | 8, 33.2, 3.9; 8, 38.8, 6.3 | ||||
| 8, 16.5, 3.6; 8, 52, 19.7 | 8, 1.85, 0.28; 8, 2.88, 0.58 | 8, 1.81, 0.15; 8, 2.67, 0.04 | 8, 37.3, 1.5; 8, 32.3; 2.5 | ||||
N sample number, M mean, SD standard deviation, RVSP right ventricular systolic pressure, mPAP mean pulmonary arterial pressure, mRVP mean right ventricle pressure, RV/LV+S the weight ratio of the right ventricle to the left ventricle plus septum, RV/BW right ventricle to body weight ratio, WA pulmonary arteriole area index, WT wall thickness of pulmonary arteriole
Risk of bias
All 28 articles that met the inclusion criteria for this meta-analysis were included (Table 3). None of the experiments were judged as having a low risk of bias across all domains. All studies reported similar experimental and control groups at baseline, which reduced the risk of selection bias based on animal characteristics. Although the assignment of subjects to the experimental and control groups was random, none of the studies clearly described the method of random sequence generation. For this reason, the risk of bias in the sequence generation domain was judged as “unclear” in all studies. However, although none of the studies adequately described the method used to conceal allocation, the animals were randomly housed, the caregivers and investigators were blinded to the intervention each animal received, random outcome assessment was reported, and blinding of the outcome assessor was documented. Using the signalling questions provided, all studies were scored as having a low risk of attrition and reporting bias. Furthermore, we did not identify any additional sources of bias that were not already covered by the SYRCLE Risk of Bias tool.
Table 3.
SYRCLE risk of bias assessment for the included studies
| Author (year) | Random sequence generation? | Groups similar at baseline? | Allocation concealed? | Animals randomly housed? | Blinding of caregivers and/or examiners? | Random selection for outcome assessment? | Blinding of outcome assessor? | Incomplete outcome data addressed? | Free from selective outcome reporting? | Free from other bias? |
|---|---|---|---|---|---|---|---|---|---|---|
| Lim et al.(2016) [39] | U | L | U | U | U | U | U | L | L | L |
| Lee et al. (2017) [36] | U | L | U | U | U | U | H | L | L | L |
| Guo et al.(2013) [30] | U | L | U | U | U | U | U | L | L | L |
| Luan et al. (2012) [42] | U | L | U | U | U | U | U | L | L | L |
| Baber et al. (2006) [25] | U | L | U | U | U | U | U | L | L | L |
| Chen et al. (2014) [27] | U | L | U | U | U | U | U | L | L | L |
| Chen et al. (2016) [28] | U | L | U | U | U | U | U | L | L | L |
| Cheng et al. (2017) [29] | U | L | U | U | U | U | U | L | L | L |
| Huang et al. (2016) [31] | U | L | U | U | U | U | U | L | L | L |
| Chen et al. (2016) [26] | U | L | U | U | U | U | U | L | L | L |
| Jiang et al. (2012) [32] | U | L | U | U | U | U | U | L | L | L |
| Kang et al. (2015) [33] | U | L | U | U | U | U | U | L | L | L |
| Lee et al. (2015) [37] | U | L | U | U | U | U | U | L | L | L |
| Kim et al. (2012) [34] | U | L | U | U | U | U | U | L | L | L |
| Kim et al. (2016) [35] | U | L | U | U | U | U | U | L | L | L |
| Liang et al. (2015) [38] | U | L | U | U | U | U | U | L | L | L |
| Liu et al. (2015) [40] | U | L | U | U | U | U | U | L | L | L |
| Luan et al. (2013) [43] | U | L | U | U | U | U | U | L | L | L |
| Luo et al. (2014) [44] | U | L | U | U | U | U | U | L | L | L |
| Raoul et al. (2007) [45] | U | L | U | U | U | U | U | L | L | L |
| Rathinasabapathy et al. (2016) [46] | U | L | U | U | U | U | U | L | L | L |
| Somanna et al. (2014) [47] | U | L | U | U | U | U | U | L | L | L |
| Takemiya et al. (2009) [15] | U | L | U | U | U | U | U | L | L | L |
| Zhang et al. (2012) [49] | U | L | U | U | U | U | U | L | L | L |
| Zhang et al. (2012) [50] | U | L | U | U | U | U | U | L | L | L |
| Liu et al. (2011) [41] | U | L | U | U | U | U | U | L | L | L |
| Umar et al. (2009) [48] | U | L | U | U | U | U | U | L | L | L |
| Kanki et al.(2005) [14] | U | L | U | U | U | U | U | L | L | L |
H high risk of bias, L low risk of bias, U unclear risk of bias
Effect of SC therapy on PAH
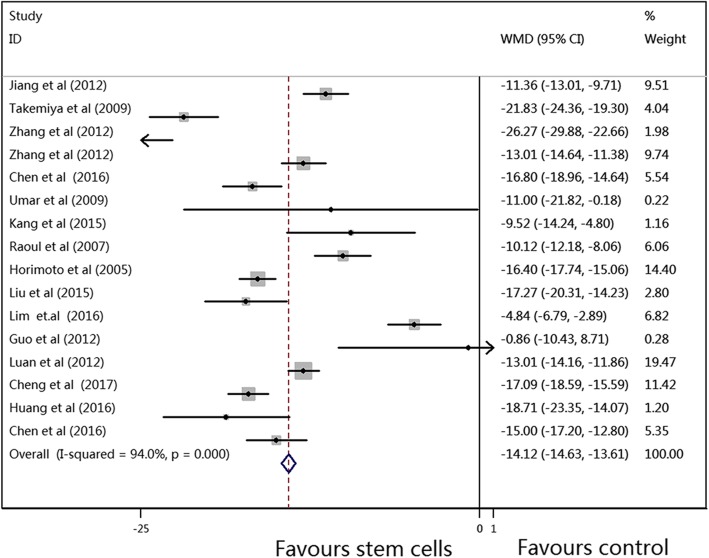
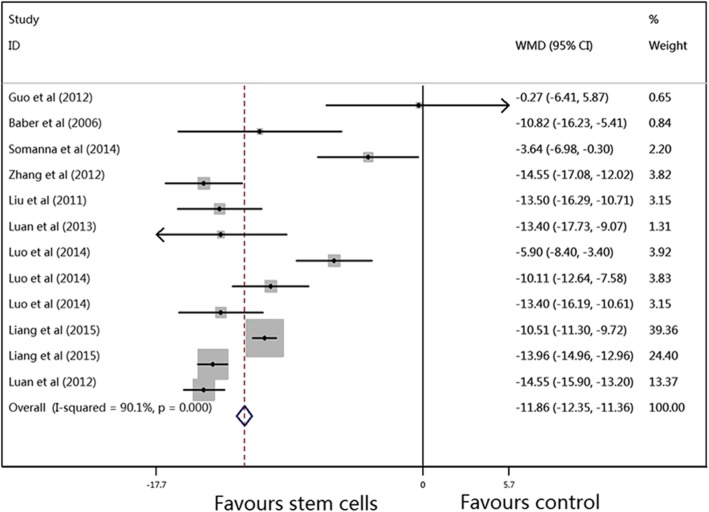
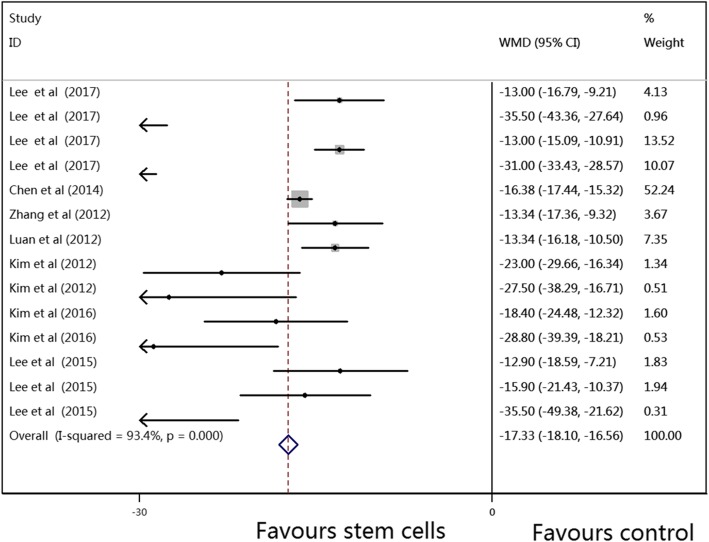
The results of the primary outcomes in this meta-analysis are shown in Figs. 2, 3 and 4. The pooled WMDs (95% CIs) for RVSP, mPAP, and mRVP were 14.12 (− 14.63, − 13.61), − 11.86 (− 12.35, − 11.36), and − 17.33 (− 18.10, − 16.56), and the P values were all < 0.001, which indicated that compared to vehicle treatment, SC therapy was significantly associated with reduced RVSP, mPAP, and mRVP values in the animal model of PAH. A random-effects model was used to perform this meta-analysis, as there was significant heterogeneity (I2 = 94.0%, 90.1%, and 93.4%, respectively) among the studies.
Fig. 2.
Meta-analysis of overall pooled WMDs with 95% CIs across studies for primary outcomes in PAH. Forest plot showing that SC therapy significantly reduced the RVSP in animals with PAH from a random-effects model. Abbreviations: WMD, weighted mean difference; RVSP, right ventricular systolic pressure; PAH, pulmonary arterial hypertension
Fig. 3.
Meta-analysis of overall pooled WMDs with 95% CIs across studies for primary outcomes in PAH. Forest plot showing that SC therapy significantly reduced the mPAP in animals with PAH from a random-effects model. Abbreviations: WMD, weighted mean difference; mPAP, mean pulmonary arterial pressure; PAH, pulmonary arterial hypertension
Fig. 4.
Meta-analysis of overall pooled WMDs with 95% CIs across studies for primary outcomes in PAH. Forest plot showing that SC therapy significantly reduced the mRVP in animals with PAH from a random-effects model. Abbreviations: WMD, weighted mean difference; mRVP, mean right ventricle pressure; PAH, pulmonary arterial hypertension
The results of secondary outcomes in this meta-analysis are shown in Additional file 1: Figure S1, Additional file 2: Figure S2, Additional file 3: Figure S3, and Additional file 4: Figure S4. The pooled WMDs (95% CIs) for RV/LV+S, RV/BW, WA, and WT were 0.10 (− 0.10, − 0.09), 0.23 (0.21, 0.24), − 13.66 (− 15.71, − 11.62), and − 7.96 (− 7.99, − 7.93), respectively, and the P values were all < 0.001, which indicated that compared to vehicle treatment, SC therapy was also significantly associated with reduced RV/LV+S, RV/BW, WA, and WT values in the animal model of PAH. A random-effects model was also used to perform this meta-analysis, as there was moderate or significant heterogeneity (I2 = 93.2%, 99.8%, 67.9%, and 97.5%, respectively) among the studies. In summary, based on the results of forest plots of the primary and secondary outcomes, we concluded that SC therapy is effective for PAH in animal studies.
Stratified analysis and meta-regression analysis
Stratified analysis was conducted on the primary outcomes according to source, route, dose, and timing of SC treatment after PAH and follow-up. However, we could not find the source of heterogeneity. To further investigate the unexplained heterogeneity across these studies, meta-regression was performed to simultaneously examine the impact of all variables on the study effect. However, for RVSP, no significant sources of heterogeneity were found. For mPAP, the method of establishing the PAH model and the dose of MCT were the sources of heterogeneity (P = 0.046). For mRVP, the follow-up time was the significant source of heterogeneity (P = 0.007).
Publication bias
Funnel plots and Egger’s linear regression tests were performed to evaluate publication bias in RVSP, mPAP, mRVP, RV/LV+S, RV/BW, and WT individually (Additional file 5: Figure S5, Additional file 6: Figure S6, Additional file 7: Figure S7, Additional file 8: Figure S8, Additional file 9: Figure S9, and Additional file 10: Figure S10). As the number of included studies that measured WA was small (< 10), we did not construct a funnel plot, as it may not have detected publication bias [51]. No significant publication bias was found for mPAP (Begg’s test, P = 0.244; Egger’s test, P = 0.423; Additional file 6: Figure S6), mRVP (Begg’s test, P = 0.003; Egger’s test, P = 0.335; Additional file 7: Figure S7), RV/LV+S (Begg’s test, P = 0.129; Egger’s test, P = 0.155; Additional file 8: Figure S8), RV/BW (Begg’s test, P = 0.752; Egger’s test, P = 0.186; Additional file 9: Figure S9), or WT (Begg’s test, P = 0.492; Egger’s test, P = 0.050; Additional file 10: Figure S10) in PAH. However, significant publication bias was found for RVSP (Begg’s test, P = 0.013; Egger’s test, P = 0.000; Additional file 5: Figure S5).
Discussion
Systematic reviews play a critical role in applying preclinical data to clinical practice. When combined with a meta-analysis of these experiments, the results can be assessed in a more methodical and objective manner. In preclinical studies of PAH animal models, SCs have been shown to improve pulmonary pressure, RV hypertrophy, and pulmonary artery endothelium over-proliferation [52]. Investigators using animal models of PAH have reported similarly promising results. Progress in regenerative medicine has led to the first clinical trial to evaluate the safety of autologous endothelial progenitor cells in PAH [53], but to date, SCs have not been used for clinical treatment in patients with PAH. To our knowledge, this is the first attempt to systematically collect and evaluate the current preclinical evidence supporting the use of SCs in animal models of PAH. Based on the results of this meta-analysis, we prove that SCs do indeed have potential therapeutic efficacy for reducing pulmonary artery pressure and RV remodelling in PAH animal models. Furthermore, these results are applicable across a range of experimental conditions.
SCs significantly improved PAH and RV pressure. Our results showed that SCs could ameliorate pulmonary artery resistance and inhibit the over-proliferation of pulmonary epithelial cells, which is consistent with studies that used SCs to treat adult animal models of lung diseases. For instance, a meta-analysis performed by Zhao et al. [54] found a positive effect on pulmonary artery resistance in lung disease. In our study, the mPAP results yielded a WMD of − 11.86, which is comparable to the values obtained in the majority of studies but is different from the values obtained by Guo et al. [30]. Taken together, these findings support the potential use of SC therapy in preclinical studies of PAH.
This meta-analysis suggests that further studies should be performed to elucidate the ideal MSC dose, as the outcomes for mRVP suggested doses between 0.5 × 106 and 1 × 106 SCs, while the outcomes for mPAP and RVSP showed optimal doses between1 × 106 and 3 × 106. Similarly, there was a discrepancy regarding the most effective route of delivery, with the mPAP and RVSP results favouring the sublingual vein route, while the mRVP studies favoured external jugular vein injection. These variables are exceedingly relevant to future patient applications from a clinical perspective. As such, our findings should be used to guide future preclinical or clinical trials when determining the optimal SC characteristics for successful outcomes.
In all comparisons, significant heterogeneity in treatment effects was found among studies. This heterogeneity can be expected in studies such as ours that are based on a limited number of included studies and have potential bias in study selection. Funnel plots and Egger’s test reported the outcomes of RVSP and confirmed the presence of publication bias. Study quality may also be affected if our primary outcome measure was not the focus of the preclinical study. Such variations in study design could account for the heterogeneity found in this meta-analysis.
We performed a meta-regression analysis to assess the impact of these variables and consider sources of heterogeneity. The results of this analysis suggest that the mPAP, mRVP, and RVSP outcomes were indeed associated with moderator variables in the included study. However, it is also important to consider the limitations of meta-regression. In this meta-analysis, there are relatively few studies but many possible study characteristics that could explain the heterogeneity. Without significant power, it is possible to arrive at false-positive conclusions. Meta-regression is intended to generate hypotheses regarding heterogeneity rather than fully explain heterogeneity. For this reason, it is difficult to truly ascertain the variables with the most promising effects given the current collection of studies.
The SYRCLE Risk of Bias tool highlighted notable deficiencies in reporting across all studies. None of the 28 articles included in this meta-analysis were considered to have a low risk of bias based on the reporting domains included in this tool. As discussed, domains were scored as having a low risk of bias only if the authors specifically stated these details in their published manuscripts. Therefore, it is possible that the studies utilised such methods in their studies but simply failed to report them. This meta-analysis emphasises this widespread shortcoming and suggests a need for higher reporting standards when publishing, specifically for preclinical translational studies. We suggest using a checklist such as the SYRCLE Risk of Bias tool when designing future preclinical studies to minimise internal reporting bias.
The strengths of this meta-analysis are obvious. First, this is the first meta-analysis of the effect of SCs on PAH in animal models. Second, we conducted a systematic literature search and followed a published protocol to ensure a diligent and rigorous review process. Third, data from studies including large samples were pooled in this meta-analysis, increasing its robustness. Furthermore, our primary outcomes of pulmonary artery and RV pressure are widely applicable to future preclinical and clinical trials.
In addition, this meta-analysis has several limitations. For example, the included studies are limited only to studies that have already been published. Unpublished data may exist that would alter our results. While we have made every effort to thoroughly search the current literature, it is possible that we may have missed relevant studies. Additionally, this meta-analysis is limited by relatively small data sets due to strict inclusion criteria, with external publication bias across these studies. Our study did not include studies that used microvesiclesor medium-derived SCs. Finally, we are unable to comment on the clinical safety of SC therapy, as none of the included studies thoroughly investigated SC dose-effects on PAH in animal models. While immunogenicity is less of a concern with SC therapy, other significant risks exist. For instance, SCs, especially induced pluripotent stem cells (iPSCs), have been associated with malignant transformation, tumour growth, and a higher overall degree of metastasis [55–57]. Therefore, iPSCs should be considered carefully for the future treatment of PAH, although none of the studies indicated that iPSCs promoted tumourigenicity in PAH. Although complications have been observed in humans receiving SCs, meta-analysis has not shown a direct correlation between SCs and acute toxicity, systemic failure, malignancy, or death [58–60]. Although there have been clinical trials on the safety and efficacy of SCs for acute respiratory distress syndrome and other respiratory diseases, as well as progenitor cells for pulmonary hypertension, SCs have not yet been used to treat pulmonary hypertension. Therefore, further research is needed to define the dose of SCs for standardised preclinical studies or clinical trials. Despite these limitations, our results reflect the widespread tendencies in this field of research.
Conclusions
These findings highlight the effects of SCs on pulmonary artery and RV stress and pulmonary artery and RV remodelling in animal models. Furthermore, these results may help to standardise preclinical animal studies and provide a theoretical basis for future SC clinical trial designs.
Additional files
Figure S1. Meta-analysis of overall pooled WMDs with 95% CIs across studies for secondary outcomes in PAH. Forest plot showing that SC therapy significantly reduced the RV/LV+S in animals with PAH from a random-effects model. Abbreviations: PAH, pulmonary arterial hypertension; RV/LV+S, the weight ratio of the right ventricle to the left ventricle plus septum; WMD, weighted mean difference. (TIF 1900 kb)
Figure S2. Meta-analysis of overall pooled WMDs with 95% CIs across studies for secondary outcomes in PAH. Forest plot showing that SC therapy significantly reduced the RV/BW in animals with PAH from a random-effects model. Abbreviations: PAH, pulmonary arterial hypertension; RV/BW, right ventricle to body weight ratio; WMD, weighted mean difference. (TIF 987 kb)
Figure S3. Meta-analysis of overall pooled WMDs with 95% CIs across studies for secondary outcomes in PAH. Forest plot showing that SC therapy significantly reduced the WA in animals with PAH from a random-effects model. Abbreviations: PAH, pulmonary arterial hypertension; WA, pulmonary arteriole area index; WMD, weighted mean difference. (TIF 358 kb)
Figure S4. Meta-analysis of overall pooled WMDs with 95% CIs across studies for secondary outcomes in PAH. Forest plot showing that SC therapy significantly reduced the WT in animals with PAH from a random-effects model. Abbreviations: PAH, pulmonary arterial hypertension; WT, wall thickness; WMD, weighted mean difference. (TIF 1560 kb)
Figure S5. Funnel plot indicates significant publication bias regarding mPAP in animal studies of PAH. (TIF 347 kb)
Figure S6. Funnel plot indicates no significant publication bias regarding mPAP in animal studies of PAH. (TIF 356 kb)
Figure S7. Funnel plots demonstrating no significant publication bias among the included studies for mRVP in PAH. (TIF 347 kb)
Figure S8. Funnel plots demonstrating no significant publication bias among the included studies for RV/LV+S in PAH. (TIF 357 kb)
Figure S9. Funnel plots demonstrating no significant publication bias among the included studies for RV/BW in PAH. (TIF 340 kb)
Figure S10. Funnel plots demonstrating no significant publication bias among the included studies for WT in PAH. (TIF 360 kb)
Table S1. The PRISMA 2009 checklist. (DOCX 26.7 kb)
Table S2. The detailed search strategy. (DOCX 17.6 kb)
Acknowledgements
We thank the Chinese Evidence Based Medicine Center at West China Hospital of Sichuan University for providing the Stata 14.0 statistical software.
Funding
This study was supported by the Natural Science Foundation of Henan Province (Grant No. 182300410369), Science and Technology Innovation Talents in Universities of Henan Province (Grant No. 16IRTSTHN021), Scientific and Technological Innovation leaders in Central Plains (Grant No. 194200510017), Provincial Ministry Co-construction Project from Medical Scientific and Technological Research Program of Henan Province (Grant No. SBGJ2018020), the "51282" Project Leaders of Scientific and Technological Innovative Talents from Health and Family Planning Commission in Henan Province (2016-32).
Availability of data and materials
All supporting data are included in the article and its additional files.
Abbreviations
- mPAP
Mean pulmonary arterial pressure
- mRVP
Mean right ventricle pressure
- PAH
pulmonary arterial hypertension
- RV/BW
Right ventricle to body weight ratio
- RV/LV+S
The weight ratio of the right ventricle to the left ventricle plus septum
- RVSP
Right ventricular systolic pressure
- SCs
Stem cells
- SYRCLE
SYstematic Review Centre for Laboratory animal Experimentation
- WA
Pulmonary arteriole area index
- WMD
Weighted mean difference
- WT
Wall thickness of pulmonary arteriole
Authors’ contributions
All the authors contributed extensively to the work presented in this article. TWS, XFD, and HYL conceived the study. HYL and BY performed the literature search and analysed the data. LFL and TW contributed to key data interpretation. XFD and HYL contributed to the study protocol and wrote the article. QCK and LXW resolved any differences through discussions. TWS revised the article. The corresponding author had full access to all of the data and has final responsibility for the article submitted for publication. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to publication of the present manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xian-Fei Ding, Email: dingxianfei2009@163.com.
Huo-Yan Liang, Email: push2017@126.com.
Bo Yuan, Email: 1944649712@qq.com.
Li-Feng Li, Email: lilifeng0317@163.com.
Tian Wang, Email: wangtianinno@163.com.
Quan-Cheng Kan, Email: kanquancheng@126.com.
Le-Xin Wang, Email: lwang@csu.edu.au.
Tong-Wen Sun, Email: suntongwen@163.com.
References
- 1.Swaminathan AC, Dusek AC, McMahon TJ. Treatment-related biomarkers in pulmonary hypertension. Am J Respir Cell Mol Biol. 2015;52(6):663–673. doi: 10.1165/rcmb.2014-0438TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 3.Pereira SL, Kummerle AE, Fraga CA, Barreiro EJ, Rocha Nde N, Ferraz EB, et al. A novel Ca2+ channel antagonist reverses cardiac hypertrophy and pulmonary arteriolar remodeling in experimental pulmonary hypertension. Eur J Pharmacol. 2013;702(1–3):316–322. doi: 10.1016/j.ejphar.2013.01.050. [DOI] [PubMed] [Google Scholar]
- 4.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30(20):2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 5.Mori H, Park IS, Yamagishi H, Nakamura M, Ishikawa S, Takigiku K, et al. Sildenafil reduces pulmonary vascular resistance in single ventricular physiology. Int J Cardiol. 2016;221:122–127. doi: 10.1016/j.ijcard.2016.06.322. [DOI] [PubMed] [Google Scholar]
- 6.Fukumoto Y, Shimokawa H. Recent progress in the management of pulmonary hypertension. Circulation J. 2011;75(8):1801–1810. doi: 10.1253/circj.CJ-11-0567. [DOI] [PubMed] [Google Scholar]
- 7.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet (London, England) 2001;358(9288):1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 8.Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347(5):322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 9.Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165(6):800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 10.Galie N, Badesch D, Oudiz R, Simonneau G, McGoon MD, Keogh AM, et al. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46(3):529–535. doi: 10.1016/j.jacc.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 11.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 12.Pulido T, Adzerikho I, Channick RN, Delcroix M, Galie N, Ghofrani HA, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369(9):809–818. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
- 13.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL registry. Chest. 2012;142(2):448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 14.Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E, et al. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006;114(1 Suppl):I181–I185. doi: 10.1161/CIRCULATIONAHA.105.001487. [DOI] [PubMed] [Google Scholar]
- 15.Takemiya K, Kai H, Yasukawa H, Tahara N, Kato S, Imaizumi T. Mesenchymal stem cell-based prostacyclin synthase gene therapy for pulmonary hypertension rats. Basic Res Cardiol. 2010;105(3):409–417. doi: 10.1007/s00395-009-0065-8. [DOI] [PubMed] [Google Scholar]
- 16.Chai S, Wang W, Liu J, Guo H, Zhang Z, Wang C, et al. Leptin knockout attenuates hypoxia-induced pulmonary arterial hypertension by inhibiting proliferation of pulmonary arterial smooth muscle cells. Transl Res. 2015;166(6):772–782. doi: 10.1016/j.trsl.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Wang CH, Cherng WJ, Yang NI, Kuo LT, Hsu CM, Yeh HI, et al. Late-outgrowth endothelial cells attenuate intimal hyperplasia contributed by mesenchymal stem cells after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28(1):54–60. doi: 10.1161/ATVBAHA.107.147256. [DOI] [PubMed] [Google Scholar]
- 18.Westerweel PE, Verhaar MC. Directing myogenic mesenchymal stem cell differentiation. Circ Res. 2008;103(6):560–561. doi: 10.1161/CIRCRESAHA.108.184374. [DOI] [PubMed] [Google Scholar]
- 19.Minguell JJ, Allers C, Lasala GP. Mesenchymal stem cells and the treatment of conditions and diseases: the less glittering side of a conspicuous stem cell for basic research. Stem Cells Dev. 2013;22(2):193–203. doi: 10.1089/scd.2012.0417. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62(25 Suppl):D22–D33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 24.Stuck AE, Rubenstein LZ, Wieland D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ. 1998;316(7129):469. doi: 10.1136/bmj.316.7129.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, et al. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292(2):H1120–H1128. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Yang H, Yue H, Strappe P, Xia P, Pan L, et al. Mesenchymal stem cells expressing eNOS and a Cav1 mutant inhibit vascular smooth muscle cell proliferation in a rat model of pulmonary hypertension. Heart Lung Circ. 2017;26(5):509–518. doi: 10.1016/j.hlc.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Chen JY, An R, Liu ZJ, Wang JJ, Chen SZ, Hong MM, et al. Therapeutic effects of mesenchymal stem cell-derived microvesicles on pulmonary arterial hypertension in rats. Acta Pharmacol Sin. 2014;35(9):1121–1128. doi: 10.1038/aps.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, Huang JH, Liu XR, Liu JF. Mesenchymal stem cells transplantation improves functions of circulating endothelial progenitor cells in a rat model of pulmonary hypertension. Int J Clin Exp Med. 2016;9(2):2057–2066. [Google Scholar]
- 29.Cheng G, Wang X, Li Y, He L. Let-7a-transfected mesenchymal stem cells ameliorate monocrotaline-induced pulmonary hypertension by suppressing pulmonary artery smooth muscle cell growth through STAT3-BMPR2 signaling. Stem Cell Res Ther. 2017;8(1):34. doi: 10.1186/s13287-017-0480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Y, Su L, Li Y, Guo N, Xie L, Zhang D, et al. The synergistic therapeutic effect of hepatocyte growth factor and granulocyte colony-stimulating factor on pulmonary hypertension in rats. Heart Vessel. 2014;29(4):520–531. doi: 10.1007/s00380-013-0395-1. [DOI] [PubMed] [Google Scholar]
- 31.Huang WC, Ke MW, Cheng CC, Chiou SH, Wann SR, Shu CW, et al. Therapeutic benefits of induced pluripotent stem cells in monocrotaline-induced pulmonary arterial hypertension. PLoS One. 2016;11(2):e0142476. doi: 10.1371/journal.pone.0142476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang L, Song XH, Liu P, Zeng CL, Huang ZS, Zhu LJ, et al. Platelet-mediated mesenchymal stem cells homing to the lung reduces monocrotaline-induced rat pulmonary hypertension. Cell Transplant. 2012;21(7):1463–1475. doi: 10.3727/096368912X640529. [DOI] [PubMed] [Google Scholar]
- 33.Kang H, Kim KH, Lim J, Kim YS, Heo J, Choi J, et al. The therapeutic effects of human mesenchymal stem cells primed with sphingosine-1 phosphate on pulmonary artery hypertension. Stem Cells Dev. 2015;24(14):1658–1671. doi: 10.1089/scd.2014.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim KC, Lee HR, Kim SJ, Cho MS, Hong YM. Changes of gene expression after bone marrow cell transfusion in rats with monocrotaline-induced pulmonary hypertension. J Korean Med Sci. 2012;27(6):605–613. doi: 10.3346/jkms.2012.27.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KC, Lee JC, Lee H, Cho MS, Choi SJ, Hong YM. Changes in Caspase-3, B cell leukemia/lymphoma-2, interleukin-6, tumor necrosis factor-α and vascular endothelial growth factor gene expression after human umbilical cord blood derived mesenchymal stem cells transfusion in pulmonary hypertension rat models. Korean Circulation J. 2016;46(1):79–92. doi: 10.4070/kcj.2016.46.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee H, Kim KC, Choi SJ, Hong YM. Optimal dose and timing of umbilical stem cells treatment in pulmonary arterial hypertensive rats. Yonsei Med J. 2017;58(3):570–580. doi: 10.3349/ymj.2017.58.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee H, Lee JC. The effect of umbilical cord blood derived mesenchymal stem cells in monocrotaline-induced pulmonary artery hypertension rats. J Korean Med Sci. 2015;30(5):576–585. doi: 10.3346/jkms.2015.30.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang M, Li H, Zheng S, Ning J, Xu C, Wang H, et al. Comparison of early and delayed transplantation of adipose tissue-derived mesenchymal stem cells on pulmonary arterial function in monocrotaline-induced pulmonary arterial hypertensive rats. Eur Heart J Suppl. 2015;17:F4–F12. doi: 10.1093/eurheartj/suv049. [DOI] [Google Scholar]
- 39.Lim J, Kim Y, Heo J, Kim KH, Lee S, Lee SW, et al. Priming with ceramide-1 phosphate promotes the therapeutic effect of mesenchymal stem/stromal cells on pulmonary artery hypertension. Biochem Biophys Res Commun. 2016;473(1):35–41. doi: 10.1016/j.bbrc.2016.03.046. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Han Z, Han Z, He Z. Mesenchymal stem cells suppress CaN/NFAT expression in the pulmonary arteries of rats with pulmonary hypertension. Exp Ther Med. 2015;10(5):1657–1664. doi: 10.3892/etm.2015.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu K, Liu R, Cao G, Sun H, Wang X, Wu S. Adipose-derived stromal cell autologous transplantation ameliorates pulmonary arterial hypertension induced by shunt flow in rat models. Stem Cells Dev. 2011;20(6):1001–1010. doi: 10.1089/scd.2010.0222. [DOI] [PubMed] [Google Scholar]
- 42.Luan Y, Zhang X, Kong F, Cheng GH, Qi TG, Zhang ZH. Mesenchymal stem cell prevention of vascular remodeling in high flow-induced pulmonary hypertension through a paracrine mechanism. Int Immunopharmacol. 2012;14(4):432–437. doi: 10.1016/j.intimp.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Luan Y, Zhang X, Qi TG, Cheng GH, Sun C, Kong F. Long-term research of stem cells in monocrotaline-induced pulmonary arterial hypertension. Clin Exp Med. 2014;14(4):439–446. doi: 10.1007/s10238-013-0256-3. [DOI] [PubMed] [Google Scholar]
- 44.Luo L, Lin T, Zheng S, Xie Z, Chen M, Lian G, et al. Adipose-derived stem cells attenuate pulmonary arterial hypertension and ameliorate pulmonary arterial remodeling in monocrotaline-induced pulmonary hypertensive rats. Clin Exp Hypertens. 2015;37(3):241–248. doi: 10.3109/10641963.2014.954710. [DOI] [PubMed] [Google Scholar]
- 45.Raoul W, Wagner-Ballon O, Saber G, Hulin A, Marcos E, Giraudier S, et al. Effects of bone marrow-derived cells on monocrotaline- and hypoxia-induced pulmonary hypertension in mice. Respir Res. 2007;8:8. doi: 10.1186/1465-9921-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rathinasabapathy A, Bruce E, Espejo A, Horowitz A, Sudhan DR, Nair A, et al. Therapeutic potential of adipose stem cell-derived conditioned medium against pulmonary hypertension and lung fibrosis. Br J Pharmacol. 2016;173(19):2859–2879. doi: 10.1111/bph.13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Somanna NK, Wörner PM, Murthy SN, Pankey EA, Schächtele DJ, Hilaire RCS, et al. Intratracheal administration of cyclooxygenase-1-transduced adipose tissue-derived stem cells ameliorates monocrotaline-induced pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol. 2014;307(8):H1187–H1H95. doi: 10.1152/ajpheart.00589.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umar S, de Visser YP, Steendijk P, Schutte CI, Laghmani el H, Wagenaar GT, et al. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009;297(5):H1606–H1616. doi: 10.1152/ajpheart.00590.2009. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Liao S, Yang M, Liang X, Poon MW, Wong CY, et al. Improved cell survival and paracrine capacity of human embryonic stem cell-derived mesenchymal stem cells promote therapeutic potential for pulmonary arterial hypertension. Cell Transplant. 2012;21(10):2225–2239. doi: 10.3727/096368912X653020. [DOI] [PubMed] [Google Scholar]
- 50.Zhang ZH, Lu Y, Luan Y, Zhao JJ. Effect of bone marrow mesenchymal stem cells on experimental pulmonary arterial hypertension. Exp Ther Med. 2012;4(5):839–843. doi: 10.3892/etm.2012.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ (Clinical research ed) 2006;333(7568):597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7:7. doi: 10.1186/s13287-015-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu JH, Wang XX, Zhang FR, Shang YP, Tao QM, Zhu JH, et al. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Pediatr Transplant. 2008;12(6):650–655. doi: 10.1111/j.1399-3046.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhao R, Su Z, Wu J, Ji HL. Serious adverse events of cell therapy for respiratory diseases: a systematic review and meta-analysis. Oncotarget. 2017;8(18):30511–30523. doi: 10.18632/oncotarget.15426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yagi H, Kitagawa Y. The role of mesenchymal stem cells in cancer development. Front Genet. 2013;4:261. doi: 10.3389/fgene.2013.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: key players in cancer progression. Mol Cancer. 2017;16(1):31. doi: 10.1186/s12943-017-0597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng J, Zhang Y. Cell transplantation for spinal cord injury: tumorigenicity of induced pluripotent stem cell-derived neural stem/progenitor cells. Stem Cells Int. 2018;2018:5653787. [DOI] [PMC free article] [PubMed]
- 58.Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125. doi: 10.1186/s13287-016-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu Y, Li M-Y, Feng T, Feng R, Mao R, Chen B-L, et al. Systematic review with meta-analysis: the efficacy and safety of stem cell therapy for Crohn’s disease. Stem Cell Res Ther. 2017;8(1):136. doi: 10.1186/s13287-017-0570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yubo M, Yanyan L, Li L, Tao S, Bo L, Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: a meta-analysis. PLoS One. 2017;12(4):e0175449. doi: 10.1371/journal.pone.0175449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Meta-analysis of overall pooled WMDs with 95% CIs across studies for secondary outcomes in PAH. Forest plot showing that SC therapy significantly reduced the RV/LV+S in animals with PAH from a random-effects model. Abbreviations: PAH, pulmonary arterial hypertension; RV/LV+S, the weight ratio of the right ventricle to the left ventricle plus septum; WMD, weighted mean difference. (TIF 1900 kb)
Figure S2. Meta-analysis of overall pooled WMDs with 95% CIs across studies for secondary outcomes in PAH. Forest plot showing that SC therapy significantly reduced the RV/BW in animals with PAH from a random-effects model. Abbreviations: PAH, pulmonary arterial hypertension; RV/BW, right ventricle to body weight ratio; WMD, weighted mean difference. (TIF 987 kb)
Figure S3. Meta-analysis of overall pooled WMDs with 95% CIs across studies for secondary outcomes in PAH. Forest plot showing that SC therapy significantly reduced the WA in animals with PAH from a random-effects model. Abbreviations: PAH, pulmonary arterial hypertension; WA, pulmonary arteriole area index; WMD, weighted mean difference. (TIF 358 kb)
Figure S4. Meta-analysis of overall pooled WMDs with 95% CIs across studies for secondary outcomes in PAH. Forest plot showing that SC therapy significantly reduced the WT in animals with PAH from a random-effects model. Abbreviations: PAH, pulmonary arterial hypertension; WT, wall thickness; WMD, weighted mean difference. (TIF 1560 kb)
Figure S5. Funnel plot indicates significant publication bias regarding mPAP in animal studies of PAH. (TIF 347 kb)
Figure S6. Funnel plot indicates no significant publication bias regarding mPAP in animal studies of PAH. (TIF 356 kb)
Figure S7. Funnel plots demonstrating no significant publication bias among the included studies for mRVP in PAH. (TIF 347 kb)
Figure S8. Funnel plots demonstrating no significant publication bias among the included studies for RV/LV+S in PAH. (TIF 357 kb)
Figure S9. Funnel plots demonstrating no significant publication bias among the included studies for RV/BW in PAH. (TIF 340 kb)
Figure S10. Funnel plots demonstrating no significant publication bias among the included studies for WT in PAH. (TIF 360 kb)
Table S1. The PRISMA 2009 checklist. (DOCX 26.7 kb)
Table S2. The detailed search strategy. (DOCX 17.6 kb)
Data Availability Statement
All supporting data are included in the article and its additional files.