Abstract
As a key player of the protein quality control network of the cell, the molecular chaperone Hsp70 inhibits the aggregation of the amyloid protein tau. To date, the mechanism of this inhibition and the tau species targeted by Hsp70 remain unknown. This is partly due to the inherent difficulty of studying amyloid aggregates because of their heterogeneous and transient nature. Here, we used ensemble and single-molecule fluorescence measurements to dissect how Hsp70 counteracts the self-assembly process of the K18 ΔK280 tau variant. We found that Hsp70 blocks the early stages of tau aggregation by suppressing the formation of tau nuclei. Additionally, Hsp70 sequesters oligomers and mature tau fibrils with nanomolar affinity into a protective complex, efficiently neutralizing their ability to damage membranes and seed further tau aggregation. Our results provide novel insights into the molecular mechanisms by which the chaperone Hsp70 counteracts the formation, propagation, and toxicity of tau aggregates.
The aberrant aggregation of tau into intracellular deposits is thought to play a key role in the pathogenesis of various human tauopathies including Alzheimer’s disease (AD).1 During disease, tau forms large intracellular aggregates termed neurofibrillary tangles, and their abundance and localization in the brain correlates with cognitive decline.2,3 As part of the quality control machinery of the cell, molecular chaperones such as the highly abundant heat shock protein 70 (Hsp70) counteract the aggregation of amyloid proteins and target misfolded species for degradation.4
Over the past few decades, a robust body of literature has provided evidence for an important role of Hsp70 in the pathogenesis of AD and other tauopathies, including the formation of a stable Hsp70–tau complex under conditions of cell stress,5−7 the regulation of tau degradation,8,9 and the inhibition of tau aggregation by Hsp70.10−13 Accordingly, induction or overexpression of Hsp70 in various cell lines leads to a reduction of insoluble and hyperphosphorylated tau inside cells and facilitates the association of tau with microtubules and microtubule polymerization.9,14,15 Further, hippocampal sections from AD patients show elevated Hsp70 levels as compared to age-matched controls.13,14 These hippocampal sections have been found to be either immuno-positive for Hsp70 or for tau, suggesting that the presence of Hsp70 leads to a local reduction of insoluble tau.14 These findings illustrate the capacity of Hsp70 to prevent tau aggregation and target aberrant tau species for degradation. The inhibitory action on tau aggregation by Hsp70 was found to be independent of ATP/ADP and cochaperones.10−12
Currently it is not known which molecular steps of tau aggregation are inhibited by Hsp70 and which tau species are targeted by the chaperone. This is partly owed to the difficulty of studying protein aggregates as they are highly heterogeneous in nature and can populate rare and transient species such as small soluble oligomers. Highly sensitive single-molecule fluorescence methods have previously been employed to overcome these limitations and to study amyloidogenic proteins and their interactions at the single aggregate level.16−24 This has recently allowed an in-depth characterization of the oligomerization and fibrillization kinetics of K18 tau (a short tau construct containing the four aggregation prone repeat regions) and its pathological mutants P301L tau and ΔK280 tau.25 Particularly the deletion mutant ΔK280 tau was shown to have a pronounced oligomerization phase, during which early oligomeric species are highly populated before the onset of fibrillization. Because of the well-defined aggregation kinetics of this tau variant and the presence of two Hsp70 binding sites10 within K18 tau, we chose this construct to study how Hsp70 interacts with the different species formed during the aggregation of tau. We found that Hsp70 blocks the early stages of tau aggregation by suppressing the formation of small tau nuclei. Once tau fibrils are formed, they are sequestered with low nanomolar affinity (∼20 nM) into a protective complex by Hsp70, neutralizing the ability of tau to propagate by seeded aggregation. Finally, we also demonstrate that Hsp70 reduces the toxic properties of soluble tau oligomers towards lipid membranes. Taken together, our results show how the chaperone Hsp70 counteracts the formation, propagation, and toxicity of tau aggregates.
Results and Discussion
Hsp70 Is a Substoichiometric Inhibitor of Tau Aggregation
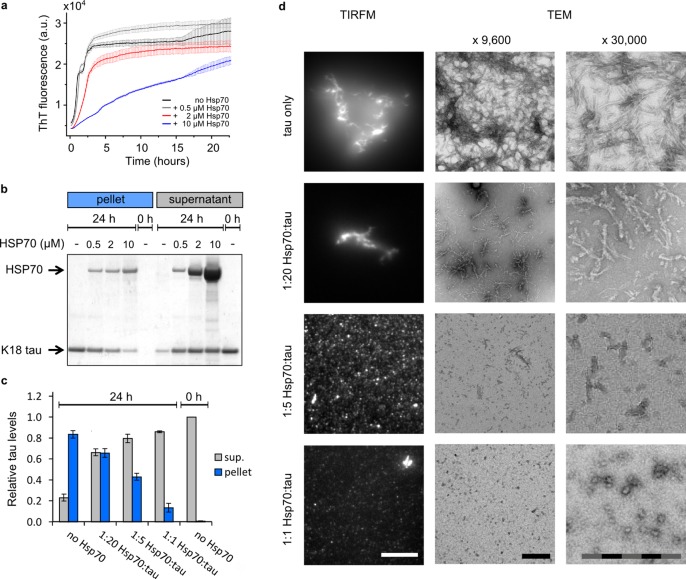
To confirm the inhibitory effect of Hsp70 on tau aggregation, the fibrillization of K18 ΔK280 tau was monitored in the absence and presence of Hsp70 using the reporter dye Thioflavin-T (ThT). ThT is a benzothiazole dye that exhibits enhanced fluorescence upon binding to beta-sheet rich amyloid fibrils. As expected, Hsp70 inhibited the aggregation of tau in a dose-dependent fashion when added to the aggregation mixture prior to the induction of aggregation (Figure 1a). To corroborate these findings, a sedimentation assay was used to measure the relative levels of insoluble K18 ΔK280 tau formed at increasing Hsp70 concentrations. In the absence of Hsp70, over 80% of the protein was found in the pellet after 24 h of aggregation. With increasing concentration of Hsp70, the relative amount of tau in the insoluble tau fractions decreased, and the majority of tau was detected in the supernatant (Figure 1b and c).
Figure 1.
Hsp70 inhibits tau aggregation in a substoichiometric manner. (a) The extent of heparin induced fibrillization of K18 ΔK280 tau (10 μM), monitored by Thioflavin-T (ThT) fluorescence over the course of the aggregation reaction.N = 3, error bars are s. e. m. (b) Sedimentation assay: soluble and insoluble K18 ΔK280 tau formed after 24 h at different concentrations of Hsp70. Representative SDS-PAGE analysis, coomassie staining. N = 3. (c) Relative protein levels in supernatant and pellet by densitometry of SDS-PAGE bands. N = 3, error bars are s. e. m. (d) The aggregates obtained at the end of the aggregation reactions visualized on a TIRF microscope (pFTAA staining) and by TEM. Representative images (N = 3). Scale bars: TIRFM 10 μm, TEM 500 nm.
We note here a discrepancy between the sedimentation assay and the ThT assay, with the latter showing similar fluorescence values after 1 day of aggregation for all four conditions. Therefore, we analyzed the composition of samples after 24 h by total internal reflection fluorescence (TIRF) microscopy and transmission electron microscopy (TEM). By using the dye pentameric formyl thiophene acetic acid (pFTAA), which binds to beta-sheet rich aggregates similar to the dye ThT, we can readily detect single fibrils and mature oligomers by TIRF microscopy.26,27
As evident from the TIRF images and electron micrographs, tau aggregates were smaller when aggregated with increasing Hsp70 concentrations, although the majority of aggregates still appeared to have high beta-sheet content, as evident by pFTAA staining (see Figure 1d). The majority of these small pFTAA-active aggregates remained in the supernatant during centrifugation at 16 000g, explaining the discrepancy between the recorded ThT-kinetics and the pelleting assay (see Figure S1). At the highest Hsp70 concentration (1:1 tau/Hsp70), small oligomeric species were observed by TEM with around 25 nm diameter. These species were still pFTAA-active but weaker, presumably due to their smaller size (see Figure 1d). The observation that tau aggregates appear smaller in the presence of Hsp70 indicates that the chaperone inhibits the elongation of tau aggregates. Therefore, we next set out to test this directly using kinetic measurements of elongation.
Hsp70 Inhibits Tau Elongation
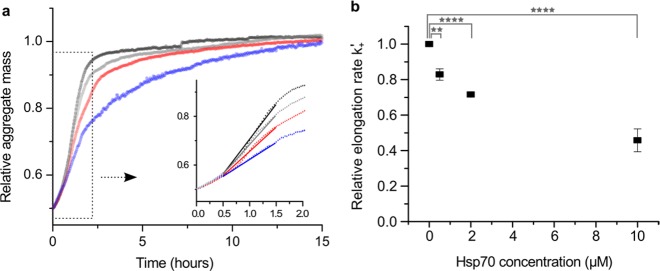
To test whether Hsp70 inhibits the elongation of K18 ΔK280 tau fibrils, a seeding assay was performed using a high concentration of tau seeds. Under these conditions, the initial aggregation kinetics are determined purely by the elongation of existing seeds, and thus the relative elongation rates k+′ for different samples can be extracted from fits of the initial aggregation rates28,29 (see Supporting Information). Here, tau seeds were prepared from a fibrillar tau sample, and these were used to seed monomeric tau in the presence of varying Hsp70 concentrations. The increase of fibril mass upon fibril elongation was monitored by ThT fluorescence. Indeed, the aggregation kinetics of tau were markedly slower in the presence of Hsp70 (Figure 2a). The relative elongation rate constants (k+′) of tau seeds were derived from linear fits of the initial aggregation kinetics (see Figure 2a inset). Plotting these constants as a function of Hsp70 concentration revealed a dose-dependent decrease of k+′ (Figure 2b), corroborating an inhibition of tau aggregate elongation by Hsp70.
Figure 2.
Hsp70 inhibits tau elongation. (a) Seeded aggregation of K18 ΔK280 tau in the presence of Hsp70. The increase of relative aggregate mass upon the addition of free monomers (10 μM) to existing seeds (10 μM) was measured over time by ThT fluorescence and normalized. Black line, tau only; gray line, + 0.5 μM Hsp70 (1:20 Hsp70/tau); red line, + 2 μM Hsp70 (1:5 Hsp70/tau); blue line, + 10 μM Hsp70 (1:1 Hsp70/tau). N = 3, error bars were omitted for the sake of clarity. Inset: Representative linear fits of the initial aggregation rate to derive the elongation rate of seeds as a function of Hsp70 concentration. (b) Relative elongation rates (k+′) of tau seeds as a function of Hsp70 concentration. Error bars: st. dev. of experimental repeats. Statistical test: One-way ANOVA; **p ≤ 0.01, ****p ≤ 0.0001.
smFRET Shows Inhibition of Tau Nucleation by Hsp70
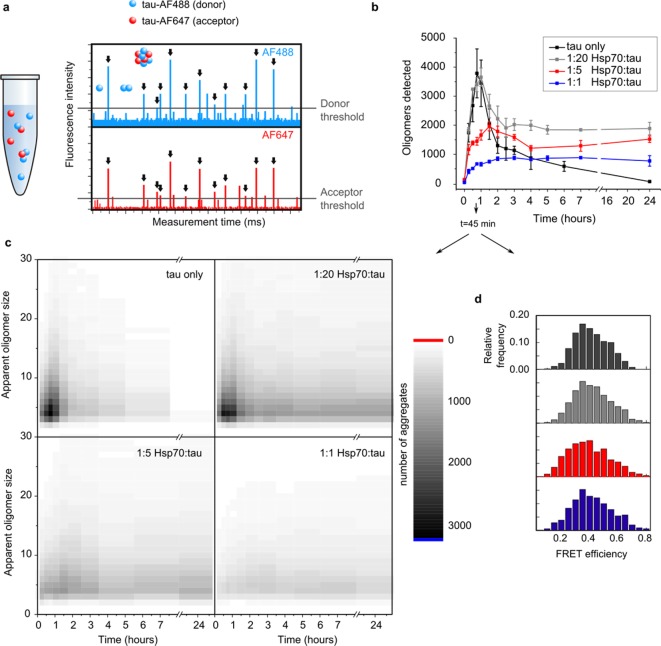
Next, we set out to test the effect of Hsp70 on the early stages of tau aggregation, specifically the formation of small oligomeric species. For this purpose, we employed a single-molecule Foerster Resonance Energy Transfer (smFRET) assay, which is able to detect even the smallest protein aggregates such as dimers or trimers in an excess of monomers.21 For this assay, a K18 ΔK280 tau sample was separated into two aliquots and labeled with two different fluorophores, a FRET donor (Alexa Fluor 488) and a FRET acceptor (Alexa Fluor 647). These monomeric versions tau-AF488 and tau-AF647 were then combined at equal amounts and coaggregated. In order to monitor the formation of oligomers, the mixture was diluted to picomolar concentration and analyzed on a dual-color confocal microscope. Single dual-labeled aggregates moving through the confocal volume one-by-one give rise to single FRET events. These can readily be counted and analyzed with regard to their approximate size and structure of the aggregate based on their intensity and FRET efficiency respectively (Figure 3a). This assay was conducted in the absence and presence of increasing Hsp70 concentrations to assess the effect of the chaperone on the nucleation and aggregation of tau.
Figure 3.
smFRET shows a reduced number of nuclei in the presence of Hsp70. (a) Typical smFRET spectrum obtained from dual-labeled protein aggregates on a confocal microscope. Single aggregates passing through the confocal volume gave rise to single FRET events which were quantified with regard to frequency, intensity, and FRET efficiency. (b) Evolution of K18 ΔK280 tau oligomers as a function of aggregation time. N = 3, error bars are s. e. m. (c) Apparent sizes of oligomers formed in the absence and presence of Hsp70 after 45 min of aggregation. Sizing reveals a strong decrease in the population of small nuclei formed in the first hour of aggregation. (d) FRET efficiencies of oligomers in the absence and presence of Hsp70 after 45 min of aggregation. Black, tau only; gray, 1:20 Hsp70/tau; red, 1:5 Hsp70/tau; blue, 1:1 Hsp70/tau.
When K18 ΔK280 tau (5 μM tau-AF488 and 5 μM tau-AF647) was aggregated in the absence of Hsp70, the protein rapidly nucleated into a population of oligomers after the addition of heparin (Figure 3b, black dots). The maximum number of oligomers was observed after less than 1 h, followed by a steady decline showing the transient nature of these oligomers. Strikingly, in the presence of Hsp70, the number of early oligomers was strongly reduced in a dose-dependent manner with a 1:5 substoichiometric concentration of Hsp70 leading to a reduction of early oligomeric species by approximately 50% (Figure 3b, colored dots). This was particularly evident for small oligomeric species (apparent size <10mer), which were depleted at the early time points (0–2 h) (see Figure 3c).
This marked reduction of early oligomeric species in the presence of the chaperone strongly suggests that primary nucleation of K18 ΔK280 tau is inhibited by Hsp70. Notably, besides the suppression of nucleation, our smFRET data also showed a stabilization of a fraction of early tau oligomers in the presence of Hsp70 (see Figure 3b, 24 h).
The FRET efficiencies observed for tau oligomers did not change in the presence of the chaperone, indicating that no structural change is induced by Hsp70 (Figure 3d).
Affinity of Hsp70 for Different Tau Aggregates
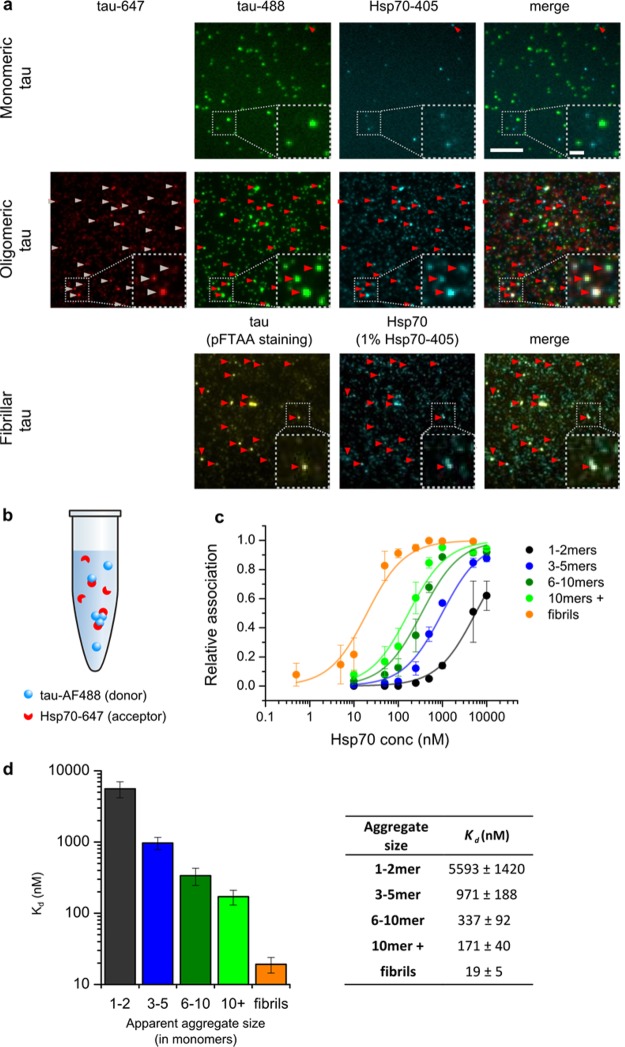
The data presented above suggest that Hsp70 binds and stabilizes different K18 ΔK280 tau aggregates such as oligomers and fibrils. In order to characterize this property in more detail, e.g., the binding stoichiometry and affinity, Hsp70 was labeled with Alexa Fluor 405 (AF405). Then, colocalization studies with different tau species were performed on a TIRF microscope. First, the binding of Hsp70-AF405 to labeled tau oligomers was assessed (1:5 Hsp70/tau stoichiometry). For TIRF imaging, the oligomeric sample was diluted and adsorbed onto a cover slide, allowing the visualization of individual dual-labeled oligomers. Hsp70-AF405 fluorescence also concentrated around these oligomers, despite incubating the sample at the exceedingly low protein concentrations needed for single-molecule imaging (picomolar). This demonstrates the high kinetic stability of the Hsp70-tau oligomer complex (Figure 4a, middle panel, arrows). By contrast, the level of colocalization of Hsp70 incubated with tau monomers was negligible under these conditions, suggesting rapid complex dissociation (see Figure 4a, top panel, arrows). Finally, binding of Hsp70 to mature tau fibrils was assessed. Again, the majority of fibrillar tau aggregates colocalized with Hsp70, suggesting that Hsp70 also forms a stable complex with tau fibrils (see Figure 4a bottom panel, arrows).
Figure 4.
Hsp70 affinity to K18 ΔK280 tau aggregates. (a) The binding of Hsp70-AF405 to different tau species was tested by TIRF microscopy. Representative images are shown (N = 3). Top panel: monomeric tau-AF488 incubated with Hsp70-AF405 under nonaggregating conditions. Middle panel: tau oligomers (tau-AF488/AF647 coaggregates), aggregated in the presence of Hsp70-AF405. Bottom panel: tau fibrils (pFTAA stain), incubated with 1.98 μM unlabeled Hsp70 and 0.02 μM Hsp70-AF405. Arrows: colocalization of tau species with Hsp70. Scale bar 10 μm, insets 2 μm. (b) Schematic of binding assays performed: tau-AF488 aggregates were kept at a constant concentration, and increasing amounts of Hsp70-AF647 were added until binding saturation was reached. The association between tau-AF488 and Hsp70-AF647 was measured by smFRET (AF488, donor; AF647, acceptor). (c) Saturation binding curves of Hsp70-AF647 to tau-AF488 aggregates. N = 3, error bars are st. dev. Lines: fits. (d) Dissociation constants extracted from fits shown in c. Error bars are st. dev.
In order to test the affinity of Hsp70 to different K18 ΔK280 tau aggregates in a more quantitative manner, saturation binding assays were performed under pseudo-equilibrium conditions using our smFRET assay (see Supporting Information for more details). This time, K18 ΔK280 tau-AF488 was used as a FRET donor and Hsp70 labeled with AF647 as a FRET acceptor (see Figure 4b). First, increasing amounts of Hsp70-AF647 were added to tau-AF488 oligomers and the association of the two proteins was measured for each concentration. Notably, the single-molecule resolution of our approach allowed us to assess the binding affinities of Hsp70 to tau oligomers of different sizes. The size of an aggregate was estimated based on the fluorescence intensity emitted by the complex.21 Importantly, this approach is an approximation which is limited by several factors such as fluorescence quenching and the nonhomogenous illumination intensity of the confocal volume. To take this into account, the oligomer sizes shown here are given as apparent oligomer sizes. The saturation binding curves and respective dissociation constants (Kd) obtained for oligomers of different sizes are shown in Figure 4c,d. These show that the affinity of Hsp70 increases as a function of oligomer size with an apparent Kd around 170 nM for large oligomers (10-mers and higher).
Finally, the same binding assay was performed with a fibrillar tau sample to assess whether the binding affinity to fibrils follows this trend or is less tight as previously reported.12 The binding curve obtained for tau fibrils was markedly shifted toward lower Hsp70 concentrations, and the resulting Kd value obtained for the binding of Hsp70 to fibrillar tau (19 ± 5 nM) was an order of magnitude lower than the one obtained for large oligomers (Figure 4c,d). These results demonstrate that the apparent binding affinity of Hsp70 to K18 ΔK280 tau aggregates increases as a function of aggregate size.
Stoichiometry of Hsp70-Binding to Tau Aggregates
Next, we studied the binding stoichiometry of Hsp70 to tau oligomers based on our single-molecule data. First, we analyzed the apparent number of Hsp70 molecules bound to each tau oligomer from our TIRF images. This revealed a linear correlation between the size of the tau oligomer and the number of Hsp70 molecules bound (see Figure S3a). At the concentrations used for our colocalization study, i.e., a 1:5 Hsp70/tau ratio, the average binding stoichiometry was one Hsp70 molecule per two tau monomers in an oligomer (see Figure S3b). To assess how this ratio changes as a function of Hsp70 concentration, we examined the FRET efficiencies obtained during the saturation binding smFRET experiment. If one assumes that the orientation of acceptor molecules to donor molecules (binding mode of Hsp70 molecules to tau aggregates) does not change as a function of Hsp70 concentration, the FRET efficiency measured for a tau-Hsp70 complex effectively reports on the number of acceptors (Hsp70 molecules) bound to the donor (tau; see schematic representation in Figure S3c). Indeed, the FRET efficiencies of Hsp70-tau complexes increased from around 0.15 at 10 nM Hsp70 to 0.65 at 1000 nM Hsp70, indicating that tau aggregates became increasingly decorated by Hsp70 molecules. At high acceptor concentrations, the FRET efficiency dropped, potentially due to nonspecific binding or fluorescence quenching. Notably, the FRET efficiencies of Hsp70-oligomer and Hsp70-fibril comlexes did not differ within error, indicating a similar mode of binding and surface density of Hsp70s to these different tau species (see Figure S3d).
Hsp70 Prevents Tau Oligomer Toxicity
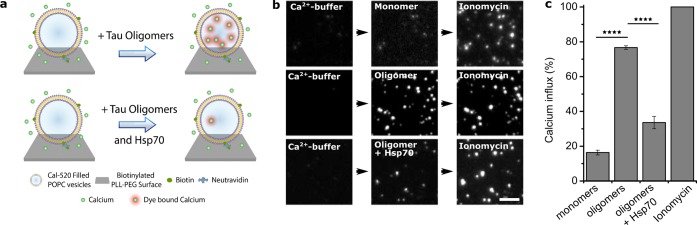
Tau oligomers are known to impair the integrity of artificial lipid bilayers.30 The high affinity binding of Hsp70 to K18 ΔK280 tau oligomers found here could serve as a mechanism to counteract such harmful interactions with membranes. In order to test this hypothesis, a single-vesicle permeabilization assay was conducted which allows us to study the ability of aggregates to permeabilize membranes.31 For this assay, lipid vesicles were loaded with Cal-520, a dye exhibiting increased fluorescence upon binding to Ca2+ ions. These vesicles were then immobilized onto a glass surface and incubated with the Ca2+-containing buffer L-15. Agents which are able to permeabilize the membrane of vesicles cause an influx of Ca2+ ions into the vesicles, leading to an increase in fluorescence intensity in those vesicles which can readily be detected on a TIRF microscope (see Figure 5a for a schematic). When vesicles were treated with 10 nM K18 ΔK280 tau oligomers, a strong influx of Ca2+ ions into the vesicles was observed (75% influx compared to the positive control ionomycin; Figure 5b,c). This finding confirms that tau oligomers are able to permeabilize lipid membranes as previously observed.30 At the same concentration, monomeric tau showed little effect on the vesicles (<20% influx). When tau was oligomerized in the presence of Hsp70, the previously observed leakage of Ca2+ into the vesicles by tau oligomers was strongly reduced to less than 35% Ca2+ influx, corresponding to about 50% of the initial oligomer response (see Figure 5c). This was also the case when Hsp70 was added to preformed tau oligomers just before the measurement (see Supporting Figure S4). These data show that Hsp70 neutralizes the ability of K18 ΔK280 tau oligomers to perturb lipid bilayers and suggest that tau toxicity could potentially be mitigated by acute upregulation or induction of Hsp70.
Figure 5.
Hsp70 counteracts the ability of K18 ΔK280 tau aggregates to permeabilize lipid vesicles. (a) Schematic of the membrane disruption assay. Single Cal520 filled vesicles are immobilized onto a glass cover slide. The addition of agents, which are able to disrupt the membrane of the vesicles, leads to an influx of Ca2+ ions from the buffer into these vesicles. The resulting increase of fluorescence intensity in each vesicle is detected on a TIRF microscope. (b,c) Hsp70 neutralizes the ability of K18 ΔK280 tau oligomers to impair lipid membranes. To test the effect of tau species—present at different times of the aggregation reaction—on membranes, either 10 nM monomeric tau, oligomeric tau, or Hsp70-tau oligomer complexes were added to the lipid vesicles. Membrane disruption was measured by an increase of fluorescence intensity in each vesicle. Ionomycin was used as a positive control to normalize the data (100% Ca2+ influx). N = 4, error are s.e.m. (b) Representative images (N = 4). Scale bar 5 μm. (c) Data from four independent experiments. Statistical test: One-way ANOVA; ****p ≤ 0.0001.
Summary: Mechanism of Aggregation Inhibition by Hsp70
In the past few decades, it has become clear that Hsp70 is a key regulator of tau aggregation and turnover.32 However, mechanistic details on how Hsp70 blocks tau aggregation, such as the microscopic steps inhibited and the preferred target species of Hsp70, remain unknown. Here, we used highly sensitive single-molecule methods to study the interaction of Hsp70 with the different species on the aggregation pathway of tau. Using the well-studied tetrapeptide repeat tau construct K18 ΔK280, which contains the core region of mature tau filaments33,34 and two Hsp70 binding sites,10 we showed that Hsp70 blocks both the nucleation and—more efficiently—the elongation of tau by sequestering aggregates efficiently into a tight complex.
Protective Sequestration of Tau Monomers
In the nucleation-conversion model of protein aggregation, which was recently shown to be applicable to K18 tau and the K18 mutant studied here,25 small oligomeric nuclei are formed during the early phase of aggregation, a fraction of which will convert to growth competent species and elongate by monomer addition. The remaining oligomers are in equilibrium with the monomers and will therefore dissociate over time. On the basis of the low affinity of Hsp70 to monomeric tau (micromolar Kd of Hsp70 to 0N4R tau monomers7,35), one would expect a weak inhibition of the nucleation process by Hsp70 present at micromolar concentrations in our aggregation reactions. This is consistent with our single-molecule data, showing an inhibition of nucleation only at the two highest Hsp70 concentrations tested. Notably, we found that Hsp70 stabilized a fraction of the oligomers present at the early stages of the aggregation process. We observed no change in FRET efficiencies of aggregates in the presence of Hsp70, suggesting that Hsp70 does not induce off-pathway tau species as observed for Aβ36 and polyglutamine proteins.37 The inhibitory action of Hsp70 on monomeric tau is likely a relevant factor in vivo, as the intracellular concentrations of both proteins are in the micromolar range, and thus the association of free cytosolic tau to Hsp70 is favored. Indeed, the constitutively expressed Hsp70 homologue Hsc70 was shown to rapidly engage tau monomers after destabilization of microtubules,15 showing the protective holdase nature of Hsp70 at the monomeric level (see Figure 6a). Notably, we could not observe direct binding of Hsp70 to tau monomers after diluting the complex to single-molecule concentrations (i.e., to picomolar protein concentrations), showing the transient nature of the Hsp70–tau monomer interaction. This finding is in agreement with a previous study which showed that the dissociation of tau monomers from the constitutively expressed variant Hsc70 occurs with an off-rate of 3.5 × 10–3 s–1 (half-life ∼ 3 min).10
Figure 6.
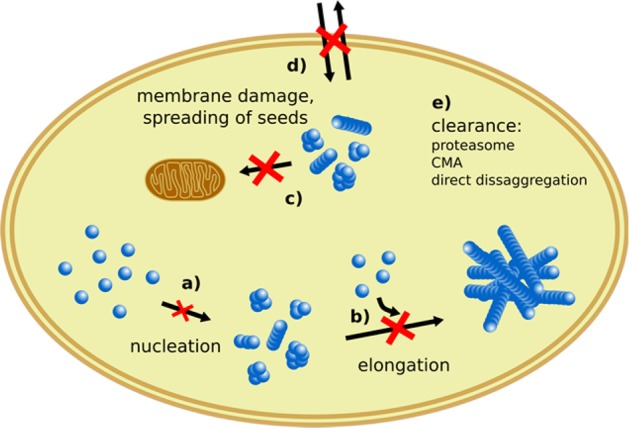
Hsp70 holdase function blocks tau aggregation, propagation, and toxicity. (a) Hsp70 inhibits the primary nucleation of tau by stabilizing monomeric and small oligomeric tau species. (b) Hsp70 sequesters growth competent seeds with high affinity and inhibits their elongation by monomer addition. (c) The binding of small oligomers by Hsp70 neutralizes their toxic properties such as membrane disruption. (d) Hsp70 may inhibit the spreading of seeds by sequestering fibrils and inhibiting their release and uptake. (e) The holdase function of Hsp70 may be the basis for the subsequent clearance of aberrant tau aggregates, e.g., via the proteasome, chaperone mediated autophagy (CMA), or direct disaggregation by Hsp70.
Inhibition of Aggregate Growth and Propagation by Hsp70
As demonstrated here, Hsp70 has a much higher affinity to aggregated tau species, with nanomolar Kd values for oligomers and fibrils. Therefore, the chaperone is likely to bind more tightly to growing aggregates, leading to an efficient inhibition of aggregate elongation. Consistent with this hypothesis, the elongation rate of tau seeds decreased significantly in the presence of Hsp70. Furthermore, increasing concentrations of Hsp70 in the reaction mixture led to a shortening of tau species observed after 24 h of aggregation, whereby at the highest Hsp70 concentration (1:1 tau/Hsp70), aggregates were found to be very small oligomeric species, presumably similar in size to initial converted oligomer. A sequestration of growth competent seeds and the inhibition of their elongation by Hsp70 has also been observed for α-synuclein38 and the yeast prion Ure2,39 suggesting that this process is one of the key mechanisms by which Hsp70 counteracts the propagation of aggregates and could be the basis for the strong antiaggregant effect of Hsp70 on tau and other proteins40 (see Figure 6b). In line with this hypothesis, hippocampal sections from AD patients are either immunoreactive for Hsp70 or fibrillar tau, suggesting that seeds can only grow and propagate in the absence of the chaperone.14 Despite this evidence for the protective effects of Hsp70, the role of Hsp70 in the propagation of pathological protein seeds remains unclear as the chaperone has been described to be able to amplify aggregates by fragmentation40,41 and aid the secretion42,43 or uptake of seeds.44,45
Binding mode of Hsp70 to Tau Aggregates
Interestingly, when we tested the binding of Hsp70 to different tau species by smFRET, we found no difference in FRET efficiencies for oligomers and fibrils, suggesting a similar binding mode to these two aggregate species. This indicates that the affinity of the chaperone increases only as a function of aggregate size and that structural differences such as beta-sheet content are less critical for this interaction. On the basis of our single-molecule data and a previous report showing that Hsp70s occupy amyloid fibrils all along the fibril axis,41 the apparent increase of affinity for larger aggregates is likely a result of an increased surface area on the aggregate which can be occupied by the chaperone. Since K18 tau lacks the long N- and C-terminal projection domains, it forms relatively compact fibrils.46,47 Given the lack of these relatively loose peptide stretches extending out from the fibril core and the relatively narrow substrate binding cleft of Hsp70, the tight binding to these dense filaments is noteworthy. This structural flexibility of Hsp70 for substrates of different levels of compaction (e.g., monomers vs fibrils) shown here is in agreement with a recent study on the bacterial Hsp70 homologue DnaK showing the ability of DnaK to bind to unfolded, partially folded, and near-native substrates.48 The high affinity of Hsp70 for tau aggregates may lay the basis for an efficient recruitment of factors initiating the degradation of tau such as the E3-ligase CHIP, which was shown to be involved in tau turnover.8,9
Hsp70 Counteracts Tau Toxicity by Direct Binding
While fibrillar tau species have emerged as the key molecular species for seeding the aggregation of free monomeric tau in recipient cells,49 soluble oligomers of tau are thought to confer damage to cells and play an important role in neurodegeneration.50−52 Here, we used lipid vesicles to show that the tight sequestration of small tau oligomers by Hsp70 mitigates their ability to confer damage to membranes. In a cell, this might serve to protect the cell membrane or membrane-rich organelles such as mitochondria or the endoplasmatic reticulum from toxic effects elicited by tau oligomers (Figure 6c). In a similar manner, the ability of tau aggregates to cross the cell membrane may be inhibited by Hsp70, hampering the trans-cellular propagation of tau (Figure 6d).
Conclusion
In summary, we showed here that Hsp70 efficiently blocks the aggregation and toxicity of tau by sequestering tau aggregates with high affinity. This interaction may lay the basis for a subsequent clearance of harmful and seeding competent tau species, e.g., by proteasomal degradation or chaperone mediated autophagy53 or direct disaggregation of the aggregates by a Hsp70-driven disaggregase system41,54 (Figure 6e).
Methods and Materials
Chemicals
Alexa Fluor 488 C5 maleimide, Alexa Fluor 647 C2 maleimide, Alexa Fluor 405 NHS ester, and Alexa Fluor 647 NHS ester were purchased from Molecular Probes. Heparin (low molecular weight heparin) was obtained from Fisher Scientific UK. Ammonium acetate, thioflavin T (ThT), and dithiothreitol (DTT) were purchased from Sigma. pFTAA was a kind gift from Therese Klingstedt.
Protein Expression, Purification, and Labeling
The K18 ΔK280 tau construct used in this work contains a deletion of lysine 280, and both natural cysteine residues were mutated to alanine. It contains a cysteine mutation at position 260 used for the covalent attachment of the dyes as previously described.25 The protein was kindly supplied by Professor St. George-Hyslop and labeled using Alexa Fluor 488 C5 or 647 C2 maleimide according to established protocols.
The expression and purification of Hsp70 was performed as previously described.55 To label Hsp70, the protein was reacted with Alexa Fluor 405 NHS ester or Alexa Fluor 647 NHS ester according to established protocols. The labeling efficiency was found to be 1.5 dyes/protein for Hsp70-AF405 by measuring the UV absorbance at 401 nm (εAF405 = 34 000 M–1 cm–1) and 1.3 dyes/protein for Hsp70-AF650 by measuring the UV absorbance at 650 nm (εAF650 = 250 000 M–1 cm–1) and the protein concentration determined by BCA assay.
Tau Aggregations and Preparation of Different Tau Species
All aggregation reactions were performed using 10 μM K18 ΔK280 tau (unlabeled aggregates) or 5 μM ΔK280 tau-AF488 + 5 μM ΔK280 tau-AF647 (dual-labeled aggregates) in 0.05 M ammonium acetate at pH 7 containing 1 mM DTT. To start the aggregation, 0.01 mg mL–1 of heparin was added to the samples (1:4 heparin/tau ratio). Samples were incubated at 37 °C without agitation for the indicated amounts of time. To obtain oligomeric tau, samples were incubated for 45 min and then transferred to an ice bath. The fraction of oligomers at this stage of the aggregation is around 10% as estimated by smFRET; i.e., the oligomer concentration is ∼1 μM (in monomer starting concentration). To obtain fibrillar tau, samples were incubated for 24 h. Under these conditions, the sample is predominantly fibrillar (≈90%) with negligible oligomer concentrations (see Figure 3b black dots, and ref (25)).
Monitoring Fibril Formation by Thioflavin-T Fluorescence
Hsp70 (0 μM, 0.5 μM, 2 μM or 10 μM) was added to K18 ΔK280 tau (10 μM) in a microwell plate (Corning 96-well half area clear bottom), and 10 μM ThT was added. Fibrillation was monitored by excitation at 440 nm and collecting fluorescence at 480 nm using a BMG FLUOstar OPTIMA plate reader.
Tau and Hsp70 Pelleting Assays
Hsp70 (0 μM, 0.5 μM, 2 μM or 10 μM) was added to K18 ΔK280 tau (10 μM) in a 200 μL PCR tube at 4 °C. The aggregation was started by heparin addition and continued for the indicated amounts of time at 37° C. To analyze the amount of soluble and insoluble protein after given periods of time, each mixture was centrifuged at 16 000g for 20 min. Supernatant and pellet fractions were then subjected to SDS-PAGE using 4–12% Bis-Tris gels (Bolt, Thermo Fisher Scientific) and protein bands stained with colloidal coomassie (BioRad). To quantify soluble and insoluble protein, densitometry was performed on the protein bands, and protein levels were normalized to a nonaggregated control containing 10 μM ΔK280 tau only.
Determining Elongation Rates of Tau Seeds in the Presence of Hsp70
Tau was fibrillated as described above. After 24 h, the fibrils were separated from soluble tau species by centrifugation at 16 000g for 15 min. The fibrils were then resuspended in a tenth of the original volume in 0.05 M ammonium acetate at pH 7 containing 1 mM DTT, vortexed for 10 s, and sonicated for 15 s in a water bath. Then, 10 μM seeds; 10 μM K18 ΔK280 tau; and 0 μM, 0.5 μM, 2 μM, or 10 μM Hsp70 were combined in an aggregation buffer containing 10 μM ThT and heparin in a microwell plate (Corning 96-well half area clear bottom). ThT kinetics were monitored in a plate reader as described above. To extract relative elongation rates from the recorded aggregation kinetics, the raw data were fitted as described in the Supporting Information.
smFRET Instrumentation and Data Acquisition
The confocal FRET instrument and the data acquisition have previously been described in detail.17,25
smFRET Analysis of Tau Oligomerization
In order to study the oligomerization of tau, equimolar amounts of monomeric AF488-labeled ΔK280 tau and AF647-labeled ΔK280 tau were combined at a concentration of 10 μM in a 200 μL PCR tube at 4 °C. To test the effect of Hsp70 on the aggregation of tau, indicated amounts of the chaperone were added prior to the initiation of aggregation. To start the aggregation, heparin was added to the aggregation mixture and the sample incubated at 37° C. The first time point (t0) was measured before the addition of heparin. For smFRET analysis, samples were diluted into 0.05 M ammonium acetate at pH 7 to a final protein concentration of 200 pM, and the sample was flowed through the channel of a microfluidic device mounted on a confocal setup as described previously.20 The data were analyzed as described previously,17,25 and a summary can be found in the Supporting Information.
Testing the Binding of Hsp70 to Tau by TIRF Microscopy
To assess direct binding of Hsp70 to monomeric tau, 10 μM ΔK280 tau-AF488 was mixed with 2 μM Hsp70-AF405 in a 200 μL PCR tube, and the sample was incubated on ice for 10 min. To assess the direct binding of Hsp70 to oligomeric tau, dual-labeled tau oligomers were produced as described above in the presence of 2 μM Hsp70-AF405 in a 200 μL PCR tube. Then, samples were diluted into 0.05 M ammonium acetate at pH 7 to a tau concentration of 50–200 pM and adsorbed onto a glass cover slide for 15 min and imaged on a TIRF microscope using the appropriate illumination sources and emission filters.
To test the binding of Hsp70 to fibrillar tau, unlabeled ΔK280 tau was fibrillated as described above for 24 h. This fibrillar sample was then incubated with 1.98 μM unlabeled Hsp70 and 0.02 μM Hsp70-AF405 for 10 min on ice. Finally, the sample was diluted to a tau concentration of 25 nM into 0.05 M ammonium acetate at pH 7 containing 30 nM of the fibrillar stain pFTAA, adsorbed onto a glass cover slide for 15 min, and visualized by TIRF microscopy using the appropriate illumination sources and emission filters.
To control for bleed-through and cross-excitation of fluorophores, identical samples were prepared where only one of the interaction partners was fluorescently labeled. Both bleed-through and cross-excitation were negligible.
The TIRF microscope and the binding stoichiometry analysis are described in the Supporting Information.
Measuring Binding Affinities of Hsp70 to Tau by smFRET
Testing Association and Disassociation Kinetics
See the Supporting Information.
Saturating Binding Curves
Tau oligomers or fibrils were prepared as described above. Notably, to remove any nonfibrillar material, the fibrils were separated from soluble tau species by centrifugation at 16 000g for 15 min. The fibrils were then resuspended in 45 μL of 0.05 M ammonium acetate at pH 7 containing 1 mM DTT, vortexed for 10 s, and sonicated for 15 s in a water bath.
To perform binding saturation experiments, 100 nM oligomers or 1200 nM fibrils (initial monomer concentration) were mixed with increasing Hsp70 concentrations from 0.5 nM to 10 μM and incubated for 5 min on ice. Then, the sample was diluted to a 200 pM tau concentration, and the association was determined by smFRET (data analysis described in the Supporting Information).
Lipid Vesicles Permeabilization Assay
Tau samples were oligomerized as described above. The purification of lipid vesicles, their attachment onto glass coverslides, and the TIRF microscope used for imaging are described in detail in ref (31). To test the effect of tau on the lipid vesicles, 50 μL of either (i) 10 nM tau monomers, (ii) 10 nM tau oligomers, or (iii) 10 nM tau oligomers plus Hsp70 (2 nM) were added onto the coverslip and incubated for 10 min. Importantly, glass coverslips were not moved during the addition of samples. Then images were acquired. Next, 10 μL of a solution containing 1 mg mL–1 of ionomycin (Cambridge Bioscience Ltd.) was added and incubated for 5 min, and subsequently images of Ca2+-saturated single vesicles in the same fields of view were acquired. Vesicles were visualized by TIRF microscopy using the appropriate illumination sources and emission filters. The recorded images were analyzed using ImageJ56 to determine the fluorescence intensity of each spot under the three different conditions, namely, background (Fbackground), in the presence of a sample (Fsample), and after the addition of ionomycin (FIonomycin). The relative influx of Ca2+ ions due to the presence of the respective tau sample was then determined using the following equation:
| 1 |
Statistics
Statistical analysis was performed with OriginPro 2016. One-way ANOVA followed by a post hoc Tukey test was used. Differences were considered to be significantly different if p < 0.05.
Acknowledgments
D.K. acknowledges funding from the ERC (grant #669237). M.K. acknowledges fellowships from the Danish research council and the Lundbeck Foundation. F.K. acknowledges funding from the Augustus Newman foundation and the ERC. M.H.H. acknowledges funding from the Herchel Smith Fund and Christ’s College Cambridge. S.D. was funded by a Marie Skłodowska-Curie Individual Fellowship. P.F. acknowledges funding from the Boehringer Ingelheim Fonds and the Studienstiftung des deutschen Volkes. We acknowledge S. Qamar for providing the tau protein used for this study.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschembio.7b01039.
Figures S1–S4 and information on the association and disassociation kinetics of tau and Hsp70, the fitting process to obtain relative elongation rate constants, and the data analysis of the single-molecule assays performed for this study (PDF)
Author Present Address
‡ Aarhus Institute of Advanced Studies, Høegh-Guldbergs Gade 6B, building 1630, 310, 8000 Aarhus C, Denmark.
Author Present Address
§ Department of Biochemistry, University of Oxford, South Parks Road, Oxford OX1 3QU.
Author Contributions
† These authors contributed equally.
Author Contributions
F.K. designed, performed, and analyzed the single-molecule fluorescence experiments, bulk kinetic, sedimentation assays and SDS–PAGE. S.D. and P.F. developed and performed the single-vesicle permeabilization assayand analyzed the data. M.H.H., M.K. and S.L.S. assisted with the single-molecule fluorescence studies. D.K. supervised the project and edited the manuscript. All authors contributed to the writing and editing of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Goedert M. (2016) The ordered assembly of tau is the gain-of-toxic function that causes human tauopathies. Alzheimer's Dementia 12, 1040–1050. 10.1016/j.jalz.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Tomlinson B. E.; Blessed G.; Roth M. (1970) Observations on the brains of demented old people. J. Neurol. Sci. 11, 205–242. 10.1016/0022-510X(70)90063-8. [DOI] [PubMed] [Google Scholar]
- Arriagada P. V.; Growdon J. H.; Hedley-Whyte E. T.; Hyman B. T. (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42, 631–639. 10.1212/WNL.42.3.631. [DOI] [PubMed] [Google Scholar]
- Chiti F.; Dobson C. M. (2006) Protein Misfolding, Functional Amyloid, and Human Disease. Annu. Rev. Biochem. 75, 333–366. 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Johnson G.; Refolo L. M.; Wallace W. (1993) Heat-shocked neuronal PC12 cells reveal Alzheimer’s disease–associated alterations in amyloid precursor protein and tau. Ann. N. Y. Acad. Sci. 695, 194–197. 10.1111/j.1749-6632.1993.tb23051.x. [DOI] [PubMed] [Google Scholar]
- Wallace W.; Johnson G.; Sugar J.; Merril C. R.; Refolo L. M. (1993) Reversible phosphorylation of tau to form A68 in heat-shocked neuronal PC12 cells. Mol. Brain Res. 19, 149–155. 10.1016/0169-328X(93)90160-Q. [DOI] [PubMed] [Google Scholar]
- Young Z. T.; Rauch J. N.; Assimon V. A.; Jinwal U. K.; Ahn M.; Li X.; Dunyak B. M.; Ahmad A.; Carlson G. A.; Srinivasan S. R.; Zuiderweg E. R. P.; Dickey C. A.; Gestwicki J. E. (2016) Stabilizing the Hsp70-Tau Complex Promotes Turnover in Models of Tauopathy. Cell Chem. Biol. 23, 992–1001. 10.1016/j.chembiol.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura H.; Schwartz D.; Gygi S. P.; Kosik K. S. (2004) CHIP-Hsc70 Complex Ubiquitinates Phosphorylated Tau and Enhances Cell Survival. J. Biol. Chem. 279, 4869–4876. 10.1074/jbc.M305838200. [DOI] [PubMed] [Google Scholar]
- Petrucelli L.; Dickson D.; Kehoe K.; Taylor J.; Snyder H.; Grover A.; Lucia M. D.; McGowan E.; Lewis J.; Prihar G.; Kim J.; Dillmann W. H.; Browne S. E.; Hall A.; Voellmy R.; Tsuboi Y.; Dawson T. M.; Wolozin B.; Hardy J.; Hutton M. (2004) CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum. Mol. Genet. 13, 703–714. 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- Sarkar M.; Kuret J.; Lee G. (2008) Two motifs within the tau microtubule-binding domain mediate its association with the hsc70 molecular chaperone. J. Neurosci. Res. 86, 2763–2773. 10.1002/jnr.21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss K.; Combs B.; Patterson K.; Binder L. I.; Gamblin T. C. (2012) Hsp70 alters tau function and aggregation in an isoform specific manner. Biochemistry 51, 888–898. 10.1021/bi2018078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K. R.; Ward S. M.; Combs B.; Voss K.; Kanaan N. M.; Morfini G.; Brady S. T.; Gamblin T. C.; Binder L. I. (2011) Heat Shock Protein 70 Prevents both Tau Aggregation and the Inhibitory Effects of Preexisting Tau Aggregates on Fast Axonal Transport. Biochemistry 50, 10300–10310. 10.1021/bi2009147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara N.; Maeda S.; Yoshiike Y.; Mizoroki T.; Yamashita S.; Murayama M.; Park J.-M.; Saito Y.; Murayama S.; Takashima A. (2007) Molecular chaperone-mediated tau protein metabolism counteracts the formation of granular tau oligomers in human brain. J. Neurosci. Res. 85, 3098–3108. 10.1002/jnr.21417. [DOI] [PubMed] [Google Scholar]
- Dou F.; Netzer W. J.; Tanemura K.; Li F.; Hartl F. U.; Takashima A.; Gouras G. K.; Greengard P.; Xu H. (2003) Chaperones increase association of tau protein with microtubules. Proc. Natl. Acad. Sci. U. S. A. 100, 721–726. 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinwal U. K.; O’Leary J. C.; Borysov S. I.; Jones J. R.; Li Q.; Koren J.; Abisambra J. F.; Vestal G. D.; Lawson L. Y.; Johnson A. G.; Blair L. J.; Jin Y.; Miyata Y.; Gestwicki J. E.; Dickey C. A. (2010) Hsc70 Rapidly Engages Tau after Microtubule Destabilization. J. Biol. Chem. 285, 16798–16805. 10.1074/jbc.M110.113753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P.; Orte A.; Clarke R. W.; Bolognesi B.; Hook S.; Ganzinger K. A.; Meehan S.; Wilson M. R.; Dobson C. M.; Klenerman D. (2011) The extracellular chaperone clusterin sequesters oligomeric forms of the amyloid-β(1–40) peptide. Nat. Struct. Mol. Biol. 19, 79–83. 10.1038/nsmb.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orte A.; Clarke R.; Balasubramanian S.; Klenerman D. (2006) Determination of the fraction and stoichiometry of femtomolar levels of biomolecular complexes in an excess of monomer using single-molecule, two-color coincidence detection. Anal. Chem. 78, 7707–7715. 10.1021/ac061122y. [DOI] [PubMed] [Google Scholar]
- Cremades N.; Cohen S. I. A.; Deas E.; Abramov A. Y.; Chen A. Y.; Orte A.; Sandal M.; Clarke R. W.; Dunne P.; Aprile F. A.; Bertoncini C. W.; Wood N. W.; Knowles T. P. J.; Dobson C. M.; Klenerman D. (2012) Direct Observation of the Interconversion of Normal and Toxic Forms of α-Synuclein. Cell 149, 1048–1059. 10.1016/j.cell.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P.; Ganzinger K. A.; McColl J.; Weimann L.; Meehan S.; Qamar S.; Carver J. A.; Wilson M. R.; St. George-Hyslop P.; Dobson C. M.; Klenerman D. (2013) Single molecule characterization of the interactions between amyloid-β peptides and the membranes of hippocampal cells. J. Am. Chem. Soc. 135, 1491–1498. 10.1021/ja3103567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrocks M. H.; Li H.; Shim J.; Ranasinghe R. T.; Clarke R. W.; Huck W. T. S.; Abell C.; Klenerman D. (2012) Single Molecule Fluorescence under Conditions of Fast Flow. Anal. Chem. 84, 179–185. 10.1021/ac202313d. [DOI] [PubMed] [Google Scholar]
- Orte A.; Birkett N. R.; Clarke R. W.; Devlin G. L.; Dobson C. M.; Klenerman D. (2008) Direct characterization of amyloidogenic oligomers by single-molecule fluorescence. Proc. Natl. Acad. Sci. U. S. A. 105, 14424–14429. 10.1073/pnas.0803086105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Ying L.; Green J. J.; Balasubramanian S.; Klenerman D. (2003) Ultrasensitive coincidence fluorescence detection of single DNA molecules. Anal. Chem. 75, 1664–1670. 10.1021/ac026367z. [DOI] [PubMed] [Google Scholar]
- Weiss S. (1999) Fluorescence Spectroscopy of Single Biomolecules. Science 283, 1676–1683. 10.1126/science.283.5408.1676. [DOI] [PubMed] [Google Scholar]
- Moerner W. E. (2007) New directions in single-molecule imaging and analysis. Proc. Natl. Acad. Sci. U. S. A. 104, 12596–12602. 10.1073/pnas.0610081104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammas S. L.; Garcia G. A.; Kumar S.; Kjaergaard M.; Horrocks M. H.; Shivji N.; Mandelkow E.; Knowles T. P. J.; Mandelkow E.; Klenerman D. (2015) A mechanistic model of tau amyloid aggregation based on direct observation of oligomers. Nat. Commun. 6, 7025. 10.1038/ncomms8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelstaff J.; Ossola B.; Neher J. J.; Klingstedt T.; Nilsson K. P. R.; Goedert M.; Spillantini M. G.; Tolkovsky A. M. (2015) The fluorescent pentameric oligothiophene pFTAA identifies filamentous tau in live neurons cultured from adult P301S tau mice. Front. Neurosci. 9, 184. 10.3389/fnins.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrocks M. H.; Lee S. F.; Gandhi S.; Magdalinou N. K.; Chen S. W.; Devine M. J.; Tosatto L.; Kjaergaard M.; Beckwith J. S.; Zetterberg H.; Iljina M.; Cremades N.; Dobson C. M.; Wood N. W.; Klenerman D. (2016) Single-molecule imaging of individual amyloid protein aggregates in human biofluids. ACS Chem. Neurosci. 7, 399–406. 10.1021/acschemneuro.5b00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisl G.; Kirkegaard J. B.; Arosio P.; Michaels T. C. T.; Vendruscolo M.; Dobson C. M.; Linse S.; Knowles T. P. J. (2016) Molecular mechanisms of protein aggregation from global fitting of kinetic models. Nat. Protoc. 11, 252–272. 10.1038/nprot.2016.010. [DOI] [PubMed] [Google Scholar]
- Flagmeier P.; Meisl G.; Vendruscolo M.; Knowles T. P. J.; Dobson C. M.; Buell A. K.; Galvagnion C. (2016) Mutations associated with familial Parkinson’s disease alter the initiation and amplification steps of α-synuclein aggregation. Proc. Natl. Acad. Sci. U. S. A. 113, 10328–10333. 10.1073/pnas.1604645113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach K.; Hilbrich I.; Schiffmann A.; Gärtner U.; Krüger M.; Leonhardt M.; Waschipky H.; Wick L.; Arendt T.; Holzer M. (2012) Tau Oligomers Impair Artificial Membrane Integrity and Cellular Viability. J. Biol. Chem. 287, 43223–43233. 10.1074/jbc.M112.396176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagmeier P.; De S.; Wirthensohn D. C.; Lee S. F.; Vincke C.; Muyldermans S.; Knowles T. P. J.; Gandhi S.; Dobson C. M.; Klenerman D. (2017) Ultrasensitive Measurement of Ca(2+) Influx into Lipid Vesicles Induced by Protein Aggregates. Angew. Chem., Int. Ed. 56, 7750–7754. 10.1002/anie.201700966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y.; Koren J.; Kiray J.; Dickey C. A.; Gestwicki J. E. (2011) Molecular chaperones and regulation of tau quality control: strategies for drug discovery in tauopathies. Future Med. Chem. 3, 1523–1537. 10.4155/fmc.11.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bergen M.; Friedhoff P.; Biernat J.; Heberle J.; Mandelkow E.-M.; Mandelkow E. (2000) Assembly of τ protein into Alzheimer paired helical filaments depends on a local sequence motif (306VQIVYK311) forming β structure. Proc. Natl. Acad. Sci. U. S. A. 97, 5129–5134. 10.1073/pnas.97.10.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik C. M.; Novak M.; Thøgersen H. C.; Edwards P. C.; Runswick M. J.; Jakes R.; Walker J. E.; Milstein C.; Roth M.; Klug A. (1988) Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 85, 4506–4510. 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. D.; Scaglione K. M.; Prensner J.; Gillies A. T.; Chinnaiyan A.; Paulson H. L.; Jinwal U. K.; Dickey C. A.; Gestwicki J. E. (2012) Analysis of the Tau-Associated Proteome Reveals That Exchange of Hsp70 for Hsp90 Is Involved in Tau Degradation. ACS Chem. Biol. 7, 1677–1686. 10.1021/cb3002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C. G.; Wisén S.; Gestwicki J. E. (2006) Heat Shock Proteins 70 and 90 Inhibit Early Stages of Amyloid β-(1–42) Aggregation in Vitro. J. Biol. Chem. 281, 33182–33191. 10.1074/jbc.M606192200. [DOI] [PubMed] [Google Scholar]
- Muchowski P. J.; Schaffar G.; Sittler A.; Wanker E. E.; Hayer-Hartl M. K.; Hartl F. U. (2000) Hsp70 and Hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc. Natl. Acad. Sci. U. S. A. 97, 7841–7846. 10.1073/pnas.140202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprile F. A.; Arosio P.; Fusco G.; Chen S. W.; Kumita J. R.; Dhulesia A.; Tortora P.; Knowles T. P. J.; Vendruscolo M.; Dobson C. M.; Cremades N. (2017) Inhibition of α-Synuclein Fibril Elongation by Hsp70 Is Governed by a Kinetic Binding Competition between α-Synuclein Species. Biochemistry 56, 1177–1180. 10.1021/acs.biochem.6b01178. [DOI] [PubMed] [Google Scholar]
- Xu L.-Q.; Wu S.; Buell A. K.; Cohen S. I. A.; Chen L.-J.; Hu W.-H.; Cusack S. A.; Itzhaki L. S.; Zhang H.; Knowles T. P. J.; Dobson C. M.; Welland M. E.; Jones G. W.; Perrett S. (2013) Influence of specific HSP70 domains on fibril formation of the yeast prion protein Ure2. Philos. Trans. R. Soc., B 368, 20110410. 10.1098/rstb.2011.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarev V. F., Mikhaylova E. R., Guzhova I. V., and Margulis B. A. (2017) Possible Function of Molecular Chaperones in Diseases Caused by Propagating Amyloid Aggregates. Front. Neurosci. 11, DOI: 10.3389/fnins.2017.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X.; Carroni M.; Nussbaum-Krammer C.; Mogk A.; Nillegoda N. B.; Szlachcic A.; Guilbride D. L.; Saibil H. R.; Mayer M. P.; Bukau B. (2015) Human Hsp70 Disaggregase Reverses Parkinson’s-Linked α-Synuclein Amyloid Fibrils. Mol. Cell 59, 781–793. 10.1016/j.molcel.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine S. N.; Zheng D.; Sabbagh J. J.; Martin M. D.; Chaput D.; Darling A.; Trotter J. H.; Stothert A. R.; Nordhues B. A.; Lussier A.; Baker J.; Shelton L.; Kahn M.; Blair L. J.; Stevens S. M.; Dickey C. A. (2016) DnaJ/Hsc70 chaperone complexes control the extracellular release of neurodegenerative-associated proteins. EMBO J. 35, 1537–1549. 10.15252/embj.201593489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T.; Suzuki M.; Fujikake N.; Popiel H. A.; Kikuchi H.; Futaki S.; Wada K.; Nagai Y. (2015) Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc. Natl. Acad. Sci. U. S. A. 112, E2497–E2506. 10.1073/pnas.1412651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova E. Y.; Meshalkina D. A.; Aksenov N. D.; Pchelin I. M.; Martynova E.; Margulis B. A.; Guzhova I. V. (2015) The discovery of Hsp70 domain with cell-penetrating activity. Cell Stress Chaperones 20, 343–354. 10.1007/s12192-014-0554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couceiro J. R.; Gallardo R.; De Smet F.; De Baets G.; Baatsen P.; Annaert W.; Roose K.; Saelens X.; Schymkowitz J.; Rousseau F. (2015) Sequence-dependent internalization of aggregating peptides. J. Biol. Chem. 290, 242–258. 10.1074/jbc.M114.586636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barghorn S.; Zheng-Fischhöfer Q.; Ackmann M.; Biernat J.; von Bergen M.; Mandelkow E. M.; Mandelkow E. (2000) Structure, microtubule interactions, and paired helical filament aggregation by tau mutants of frontotemporal dementias. Biochemistry 39, 11714–11721. 10.1021/bi000850r. [DOI] [PubMed] [Google Scholar]
- Wegmann S.; Medalsy I. D.; Mandelkow E.; Müller D. J. (2013) The fuzzy coat of pathological human Tau fibrils is a two-layered polyelectrolyte brush. Proc. Natl. Acad. Sci. U. S. A. 110, E313–E321. 10.1073/pnas.1212100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashaghi A.; Bezrukavnikov S.; Minde D. P.; Wentink A. S.; Kityk R.; Zachmann-Brand B.; Mayer M. P.; Kramer G.; Bukau B.; Tans S. J. (2016) Alternative modes of client binding enable functional plasticity of Hsp70. Nature 539, 448–451. 10.1038/nature20137. [DOI] [PubMed] [Google Scholar]
- Jackson S. J.; Kerridge C.; Cooper J.; Cavallini A.; Falcon B.; Cella C. V.; Landi A.; Szekeres P. G.; Murray T. K.; Ahmed Z.; Goedert M.; Hutton M.; O’Neill M. J.; Bose S. (2016) Short Fibrils Constitute the Major Species of Seed-Competent Tau in the Brains of Mice Transgenic for Human P301S Tau. J. Neurosci. 36, 762–772. 10.1523/JNEUROSCI.3542-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves C. A.; Castillo-Carranza D. L.; Sengupta U.; Clos A. L.; Jackson G. R.; Kayed R. (2011) Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol. Neurodegener. 6, 39. 10.1186/1750-1326-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S. M.; Himmelstein D. S.; Lancia J. K.; Binder L. I. (2012) Tau oligomers and tau toxicity in neurodegenerative disease. Biochem. Soc. Trans. 40, 667–671. 10.1042/BST20120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniyappan S.; Chandupatla R. R.; Mandelkow E.-M.; Mandelkow E. (2017) Extracellular low-n oligomers of tau cause selective synaptotoxicity without affecting cell viability. Alzheimer's Dementia 13, 1270–1291. 10.1016/j.jalz.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Morawe T.; Hiebel C.; Kern A.; Behl C. (2012) Protein Homeostasis, Aging and Alzheimer’s Disease. Mol. Neurobiol. 46, 41–54. 10.1007/s12035-012-8246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillegoda N. B.; Kirstein J.; Szlachcic A.; Berynskyy M.; Stank A.; Stengel F.; Arnsburg K.; Gao X.; Scior A.; Aebersold R.; Guilbride D. L.; Wade R. C.; Morimoto R. I.; Mayer M. P.; Bukau B. (2015) Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation. Nature 524, 247–251. 10.1038/nature14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P.; Ambasta R. K.; Veereshwarayya V.; Rosen K. M.; Kosik K. S.; Band H.; Mestril R.; Patterson C.; Querfurth H. W. (2007) CHIP and HSPs interact with beta-APP in a proteasome-dependent manner and influence Abeta metabolism. Hum. Mol. Genet. 16, 848–864. 10.1093/hmg/ddm030. [DOI] [PubMed] [Google Scholar]
- Schneider C. A.; Rasband W. S.; Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.