Abstract
Recently, exosomes have been emerged as promising drug delivery carriers, while certain tissues are intrinsically resistant to exosomes. Therapeutically improving the drug delivery efficiency in these tissues/organs would certainly broaden the potential application of exosomes in future. Ultrasound-targeted microbubble destruction (UTMD) is a promising technique for non-invasive, targeted drug delivery. In this study, we explore the possibility that UTMD assists exosome delivery in these intrinsically resistant tissues. Mice were subjected to tail vein injection of DiR-labeled exosomes together with/without UTMD of SonoVueTM, followed by in vivo and ex vivo tracking of the exosomes. As expected, heart, adipose tissue, and skeletal muscle were found reluctant to exosomes from different origins. Targeted destruction of the ultrasound microbubbles (SonoVueTM) in the heart and adipose tissue region significantly increased the exosome infiltration and endocytosis there, as revealed by fluorescence imaging and confocal laser scanning microscope (CLSM). UTMD treatment 1 h prior to exosome injection failed to facilitate the exosome endocytosis in the targeted region, indicating that the transient promoting effects of UTMD. Moreover, increases of UTMD (numerous pulses) did not linearly enhance the exosome delivery. Together, our study here has established a novel strategy for targeted delivery of exosomes in the reluctant tissues, by combining the advantages of ultrasound microbubbles and exosomes in drug delivery.
Keywords: Targeted drug delivery, microbubbles, exosomes, efficiency, ultrasound-targeted microbubble destruction
Introduction
In the medicine field, gene therapy is the therapeutic delivery of nucleic acid into a patient's cells as a drug to treat disease. With the great advancement of RNAi, and gene editing with CRISPR/Cas9, gene therapy is now becoming a promising and possible strategy in the near future (Bak et al., 2018). One of the biggest hurdles of gene therapy is the challenge to non-invasively and locally deliver the gene drugs (Haussecker, 2014).
Exosomes, which are cell-derived vesicles of 30–150 nm in diameter, are emerging as a promising drug carrier (Contreras-Naranjo et al., 2017). In the physiological or pathological conditions, exosomes could transfer the bioactive molecules such as DNAs, RNAs, and proteins from the donor cells to the recipient cell, locally or distally (Kalluri, 2016). Moreover, the exosomes could be easily manipulated. The nucleic acids of interest could be either loaded by electroporation in the isolated exosomes or encapsulated during exosome biogenesis in the donor cells (Alvarez-Erviti et al., 2011; Barile & Vassalli, 2017). Exosomes have been reported to be resistant to the clearance by reticuloendothelial system and be able to cross multiple biological barrier (Alvarez-Erviti et al., 2011; EL Andaloussi et al., 2013). However, according to our preliminary data, exosomes remain resistant to certain tissues, with very low infiltration and endocytosed rate. Therapeutically increasing the exosomes delivery rate in these intrinsically refractory organs would certainly broaden the putative application of exosomes.
Recently, ultrasound-targeted microbubble destruction (UTMD) emerges as a novel means of tissue-specific gene delivery (Fujii et al., 2009, 2011). The approach delivers nucleic acids drug via the cavitation effect within the microvasculature of target tissues, which is especially of advantage for local delivery and the tissues with biological barriers (Hernot & Klibanov, 2008). However, the microbubbles should be loaded with the gene drugs prior to the UTMD. Currently, the ultrasound microbubbles are not efficient for gene loading and poor for long time preservation, restricting the potential application (Mullin et al., 2011; Tzu-Yin et al., 2013; Ma et al., 2017; Tamarov et al., 2017). In addition, the endocytosis efficiency is relatively low after the nucleic acids are released from the damaged microbubbles (Lee et al., 2012; Duan & Lam, 2013; Zhou et al., 2016).
In the current study, we explored the effects of targeted delivery of exosomes in the refractory tissues by using UTMD. With the assistance of the UTMD technique to regionally increase the vessel permeability, robust and localized delivery of the exosomes into the heart, adipose tissue, and muscle was achieved, shedding light on the gene therapy in these organs.
Materials and methods
Exosome isolation
Harvested tissues were cut into small pieces before further culture in the FBS free medium for another 24–36 h, followed by exosome isolation. For the isolation of exosomes, supernatants or the serum were centrifuged at 500 g for 10 min to remove cells and then at 10,000 g for 20 min to eliminate the residual cellular debris. The resulting supernatant was regularly filtered through 0.4 μm filters. The sample was used to precipitate exosomes with Exoquick-TCTM kit. After that, exosomes were re-suspended in PBS or DMEM and stored at −80 °C. The morphology of isolated exosomes was analyzed by electron microscopy. Briefly, the exosomes were added onto the grid and stained with 2% uranyl acetate, followed by imaging with the transmission electron microscope (JEM-2000EX TEM, JEOL Ltd., Tokyo, Japan). Isolated exosomes were diluted to 500 ng/ml and subjected for size distribution analysis by Nanoplus.
Animal housing
Male C56BL/6 mice (8–10 weeks old, 22–25 g) were used for in vivo analyzing the effects of ultrasound targeted microbubble destruction on exosome redistribution. Mice were maintained in a room with 12-h light-dark cycle and the temperature kept between 22 and 24 °C. All animal experiments were carried out under protocols approved by the Animal Care and Use Committee of Fourth Military Medical University.
Ultrasound targeted microbubble destruction
SonoVueTM microbubble (Bracco Imaging) was injected via tail vein at the dose of 100 µL or otherwise indicated. Ultrasound was generated by a 0.66 MHz low-power US instrument (Gift from Chongqing Medical University) with the probe area of 4.5 cm2. The nominal spatial peak-temporal average (SPTA) intensity varied from 0.22 to 1.80 W/cm2. The probe was adjusted with a gel interface so that the focus was positioned at the aimed tissues. For ultrasound irradiation, mice were anesthetized with 2% isoflurane, and 100 µL SonoVueTM microbubble solution was infused into the tail vein slowly. Simultaneously, an ultrasound beam was delivered using the parameters as indicated.
Exosome tracking in vivo and ex vivo
For in vivo tracking exosomes, purified exosomes were first labeled with fluorescent dye DiR/DiI at the final concentration of 10 µM (Invitrogen, Carlsbad, CA) before tail vein injection. Labeled exosomes were then collected by centrifugation after washed with PBS. Mice with/without ultrasound targeted microbubble destruction at indicated sites were additionally injected with labeled exosomes (100 µg per mouse, at 100 µL in volume) via tail vein before, after or at the same time of UTMD.
The mice were sacrificed at the indicated time after ultrasound irradiation and exosome injection. Different tissues from the mice that injected with DiR-labeled exosomes were harvested for fluorescence imaging. IVIS® Lumina II in vivo imaging system was used for in vivo and ex vivo visualization of the exosomes as instructed. While different tissues from the mice that injected with DiI-labeled exosomes were harvested for sliced sections. For slice section staining, the specimens of different tissues were immediately harvested, embedded in optimal cutting temperature compound (OCT, Tissue-Tek, Torrance, CA). Tissue sections were fixed with 4% paraformaldehyde for 15 min and then stained with Hoechst (Invitrogen, Carlsbad, CA) for counterstaining of the cell nuclei. The whole process was conducted in dark. The fluorescence signal for the labeled exosomes and the blue nuclei were viewed by CLSM (ECLIPSE Ti, Nikon, Tokyo, Japan).
Statistical analysis
All the data are expressed as mean ± SEM. Student’s t test was used for two group comparison, and one-way ANOVA was used for multiple comparisons by Tukey’s post hoc test (Graphpad Prism 7.0, GraphPad Software, La Jolla, CA). p values of <.05 indicate statistical significance.
Results
Exosomes preferentially distributed in reticuloendothelial system
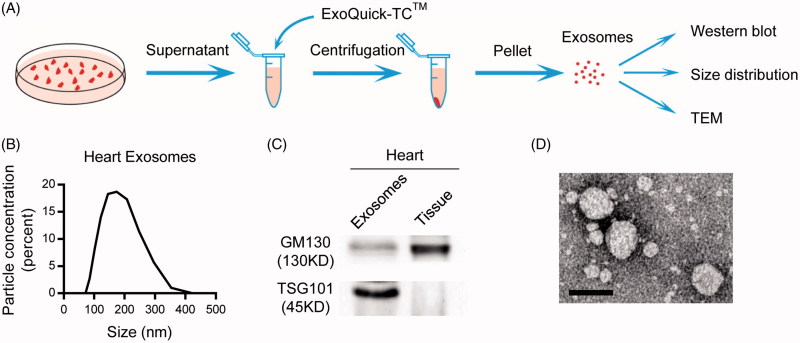
Exosomes have been recognized as a promising drug carrier for targeted delivery. We thus explored the in vivo distribution of exosomes from different origins. For example, the heart tissues were cut into small pieces, cultured in the serum free medium and the secreted exosomes were isolated (Figure 1(A)). Size distribution analysis revealed that the size ranged from 60 to 200 nm in diameter (Figure 1(B)). The exosomal inclusive marker TSG101 was founded expressed in the exosomes, while the exclusive marker GM130 was absent (Figure 1(C)), confirming the identity of the isolated exosomes. TEM analysis of the morphology further confirmed the exosomes identity (Figure 1(D)).
Figure 1.
Isolation and characterization of exosomes. (A) Schematic representation of the exosomes isolation procedure. (B) Size distribution of the isolated exosomes. (C) Western blot analysis of exosomal markers in the isolated exosomes and the parental tissues. (D) Representative TEM (transmission electron microscope) image of the isolated exosomes (scale bar =100 nm).
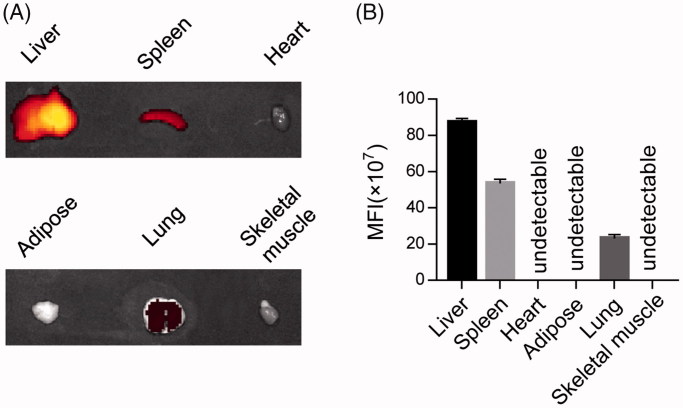
To track the in vivo distribution, isolated exosomes were labeled with DiR and injected via tail vein. Two hours later, the exosomes were found mainly localized in the liver, spleen, and lung, while rarely localized in the heart, adipose tissue, and skeletal muscles (Figure 2(A,B)), indicating that the exosomes mainly uptaken by the reticuloendothelial system in vivo. Similar results were observed in other tissue-derived exosomes.
Figure 2.
In vivo distribution of injected exosomes. (A) Fluorescence imaging distribution of the DiR labeled exosomes in different organs, including liver, spleen, heart, adipose, lung, skeletal muscle. Representative images of at least triplicate experiments. (B) Quantification of (A) (MFI: mean fluorescence intensity).
UTMD redistributes exosomes into targeted tissues
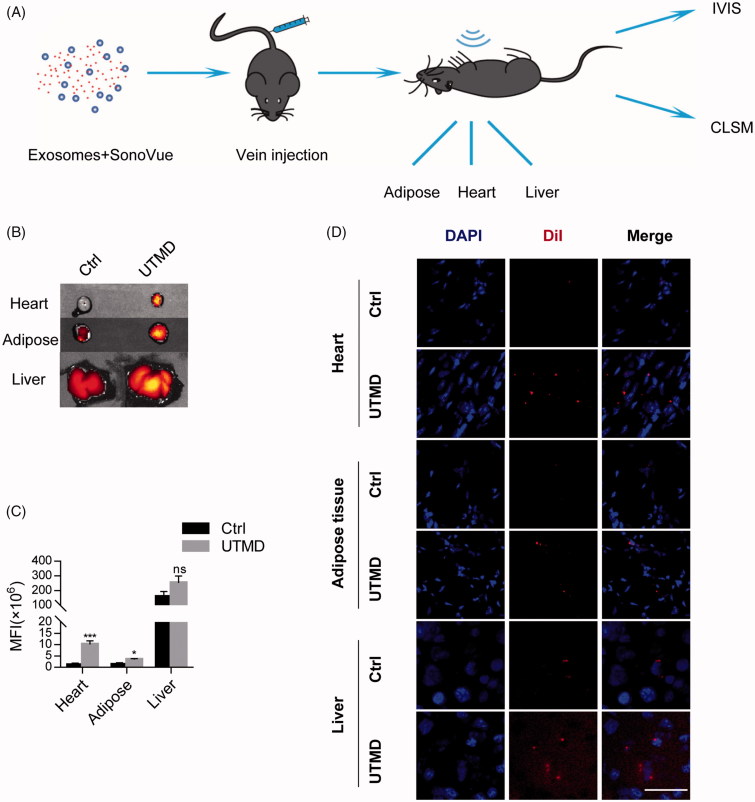
Enhancing localization of exosomes in the tissues like heart and adipose, is of fundamental importance for the treatment of associated diseases, especially when the prevalence of metabolic syndrome and cardiac disorders is considered. As UTMD could transiently destruct the microvasculature, we thus explored whether UTMD would facilitate exosomes infiltration in these naturally resistant organs. SonoVueTM microbubbles were thus injected together with the exosomes and UTMD was induced specifically in the heart, adipose tissue, and liver (Figure 3(A)). As expected, ex vivo luminescence imaging revealed that UTMD significantly increased the infiltration of exosomes in these organs (Figure 3(B,C)). Moreover, UTMD also slightly increased the localization of exosomes in the liver (Figure 3(B,C)), although the robust localization already observed in the basic condition. CLSM analysis of the tissue sections further confirmed the enhanced exosomes distribution in the UTMD targeted tissues (Figure 3(D)).
Figure 3.
UTMD promotes the uptake of injected exosomes in the targeted tissues. (A) Schematic representation of the experimental procedure. Labeled exosomes, together with the SonoVueTM microbubbles were injected via tail vein. UTMD was or was not induced in different tissues, including the heart, adipose tissue and liver. Distribution of the labeled exosomes was tracked by fluorescence imaging. (B) Fluorescence signal intensity in the tissues with or without UTMD. (C) Quantification of (B). (D) CSML image revealing the increased uptake of DiI-labeled exosomes in indicated tissues by UTMD. Nuclei were counterstained with Hoechst and scale bar =50 µm.
Effects of the UTMD duration and timing on the delivery efficiency
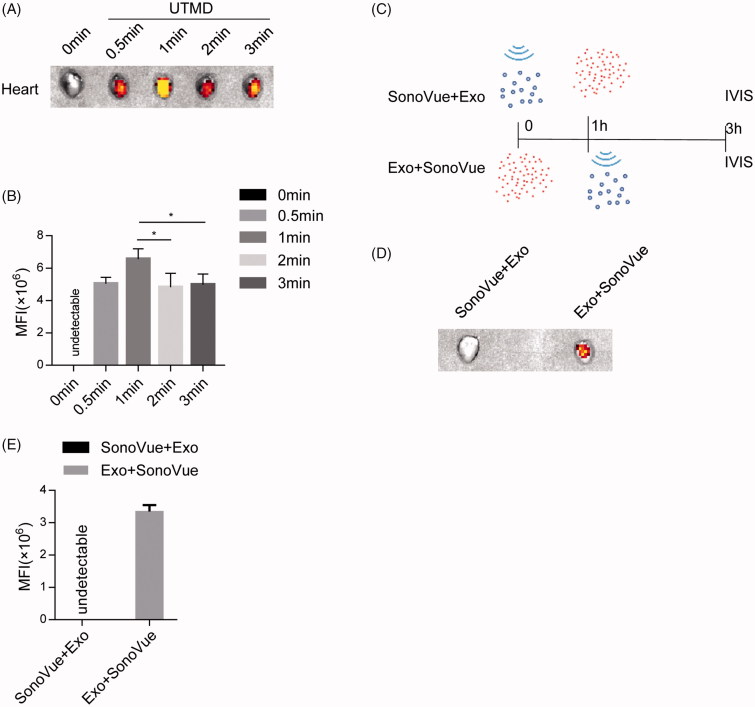
In view of above results, we next explored the effects of different UTMD duration on the exosome localization in the targeted heart tissue. Extension of the ultrasound radiation time from 0.5 min to 3 min did not increase the exosomes infiltration linearly as expected (Figure 4(A,B)), suggesting that the UTMD of 0.5 min or even less is enough for efficient delivery of exosomes to the targeted tissues.
Figure 4.
Effects of UTMD parameters on the uptake of injected exosomes in the targeted tissues. (A) Fluorescence imaging intensity reflecting the labeled exosomes distribution in the indicated tissues. Labeled exosomes with the SonoVueTM microbubbles were injected via tail vein, and UTMD was induced by pulsed ultrasound with different time durations. (B) Quantification of (A). (C) Schematic representation of the exosomes injection and imaging procedure. Exosomes were either injected before or after UTMD, followed by tracking with imaging. (D) Biodistribution of the labeled exosomes in the indicated tissues. Labeled exosomes was injected 1 h before or after the SonoVueTM microbubbles injection and destruction by UTMD. Simultaneous injection, followed by UTMD was also included. (E) Quantification of (D).
In the following experiments, we also explored the effect of UTMD initiation time point on delivery of exosomes. Similar as the simultaneous injection, UTMD stimulation 1 h after exosomes injection remained to facilitate the delivery of exosomes in the heart (Figure 4(C–E)), suggesting that there remain amounts of free circulating exosomes 1 h after injection. In contrast, when UTMD was conducted 1 h before exosomes injection, rare exosomes infiltration was seen in the targeted heart (Figure 4(C–E)), indicating that the UTMD effects on facilitated exosomes delivery is transient and could not be maintained over one hour. The transient effects further strengthen the safety of UTMD in promoting exosomes targeted delivery in the refractory organs.
Discussion
Our study here has revealed that targeted destruction of the microbubbles in the aimed region significantly facilitates the exosome endocytosis. To our knowledge, the study presented here for the first time solves the problem of delivering exosomes in the refractory tissues, such as heart and adipose tissues.
Targeted delivery of drugs, especially gene drugs, is essential for the optimal therapy (Rupaimoole & Slack, 2017; Rosenblum et al., 2018). The ideal drug delivery system should be safe and efficient (Naldini, 2015). Canonical drug carriers such as viruses, liposomes, and ultrasound microbubbles have been extensively studied (Sawant & Torchilin, 2012; Karimi et al., 2016). Take ultrasound microbubbles as example, although with several advantages as drug delivery vehicles, the non-uniform particle size distribution and difficulty in encapsulation of functional drugs (Cavalli et al., 2013; Jin et al., 2013) has limited their applications. In addition, the free diffusion of the drugs after ultrasound mediated destruction also compromises the drug delivery efficiency. On the other hand, accumulating evidence suggests that exosomes can deliver multiple cargos from the donor cells to the recipient cells in a natural pathway for genetic material transfer (Jiang & Gao, 2017). Natural exosomes from many tissues or cells have been confirmed to be therapeutically effective for many different diseases (EL Andaloussi et al., 2013). In addition, exosomes could be easily manipulated by surface functionalization and cargo encapsulation. For these natural characteristics, exosomes are being explored as drug and gene delivery vehicles. However, exosomes are preferentially and mainly endocytosed by liver, spleen, bone marrow (Smyth et al., 2015), although it is founded to cross the blood–brain barrier and maternal–placental barrier (Rufino-Ramos et al., 2017; Nair & Salomon, 2018). We here solve the problem by combining the advantages of the two techniques. Our study here revealed that the clinically available diagnostic microbubble SonoVueTM significantly increased the endocytosis of exosomes in the refractory heart and adipose tissues. The study not only proposes a strategy for exosome delivery but also raises the possibility that diagnostic destruction of the microbubbles might also facilitate the endogenous exosomes endocytosis in the refractory tissues if UTMD occurs there.
As to the mechanism why UTMD facilitates the exosome delivery in the refractory tissues, we prefer the model that microbubbles destruction resultant cavitation effect is able to enhance cell membrane permeability, which in turn not only slows the local blood flow but also the uptake efficiency of recipient cells.
The primary limitation of this study is that we didn’t observe the effects of the system in treating myocardial disease models. Future work should evaluate the treatment effects in animal models by loading therapeutic drugs to native exosomes.
Together, our study here has established a novel strategy for targeted delivery of exosomes in the reluctant tissues, by combining the advantages of canonical ultrasound microbubbles and exosomes in drug delivery. Future studies refining the parameters for better efficacy and smaller side-effects would certainly forward the strategy in clinical settings.
Conclusion
Exosome drug delivery is a promising strategy for target therapy. But their resistance to certain tissues, such as heart and adipose tissue, limits the use in these areas. In this study, we for the first time revealed that SonoVueTM microbubble together with UTMD significantly increases the infiltration and endocytosis of exosomes these reluctant tissues. We also found that limit amount of UTMD is enough to promote exosome delivery and the promoting effects are transient, suggesting the safety of the proposed strategy. Overall, this study highlights the potent potential of UTMD in facilitating exosomes delivery in tissues like heart and adipose tissue, which is promising for cardiac diseases and metabolic syndrome management.
Funding Statement
This study was funded by National Natural Science Foundation of China NSFC81671690 to L. J. Yuan, NSFC 31771507 to G. D. Yang, and Major Clinical Renovation Project, Tangdu Hospital (No. 2013LCYJ003).
Acknowledgements
We are grateful to the technical help to Qianqian Chen and Jing Zhang from the Department of Ultrasound in Tangdu Hospital, Fourth Military Medical University.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alvarez-Erviti L, Seow Y, Yin H, et al. (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–5. [DOI] [PubMed] [Google Scholar]
- Bak RO, Gomez-Ospina N, Porteus MH (2018). Gene editing on center stage. Trends Genet 34:600–11. [DOI] [PubMed] [Google Scholar]
- Barile L, Vassalli G (2017). Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Therapeutics 174:63–78. [DOI] [PubMed] [Google Scholar]
- Cavalli R, Bisazza A, Lembo D (2013). Micro- and nanobubbles: a versatile non-viral platform for gene delivery. Int J Pharm 456:437–45. [DOI] [PubMed] [Google Scholar]
- Contreras-Naranjo JC, Wu HJ, Ugaz VM (2017). Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip 17:3558–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan F, Lam MG (2013). Delivery approaches of gene therapy in hepatocellular carcinoma. Anticancer Res 33:4711–8. [PubMed] [Google Scholar]
- EL Andaloussi S, Lakhal S, Mäger I, Wood MJA (2013). Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Delivery Rev 65:391–7. [DOI] [PubMed] [Google Scholar]
- S EL Andaloussi, Mager I, Breakefield XO, Wood MJ (2013). Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12:347–57. [DOI] [PubMed] [Google Scholar]
- Fujii H, Li S-H, Wu J, et al. (2011). Repeated and targeted transfer of angiogenic plasmids into the infarcted rat heart via ultrasound targeted microbubble destruction enhances cardiac repair. Eur Heart J 32:2075–84. [DOI] [PubMed] [Google Scholar]
- Fujii H, Sun Z, Li S-H, et al. (2009). Ultrasound-targeted gene delivery induces angiogenesis after a myocardial infarction in mice. JACC: Cardiovasc Imaging 2:869–79. [DOI] [PubMed] [Google Scholar]
- Haussecker D. (2014). Current issues of RNAi therapeutics delivery and development. J Controlled Release 195:49–54. [DOI] [PubMed] [Google Scholar]
- Hernot S, Klibanov AL (2008). Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev 60:1153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XC, Gao JQ (2017). Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm 521:167–75. [DOI] [PubMed] [Google Scholar]
- Jin Q, Wang Z, Yan F, et al. (2013). A novel cationic microbubble coated with stearic acid-modified polyethylenimine to enhance DNA loading and gene delivery by ultrasound. PloS One 8:e76544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R. (2016). The biology and function of exosomes in cancer. J Clin Invest 126:1208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Mirshekari H, Moosavi Basri SM, et al. (2016). Bacteriophages and phage-inspired nanocarriers for targeted delivery of therapeutic cargos. Adv Drug Delivery Rev 106:45–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Chung BH, Park TG, et al. (2012). Small-interfering RNA (siRNA)-based functional micro- and nanostructures for efficient and selective gene silencing. Acc Chem Res 45:1014–25. [DOI] [PubMed] [Google Scholar]
- Ma X, Bussonniere A, Liu Q (2017). A facile sonochemical synthesis of shell-stabilized reactive microbubbles using surface-thiolated bovine serum albumin with the Traut's reagent. Ultrasonics Sonochem 36:454–65. [DOI] [PubMed] [Google Scholar]
- Mullin L, Gessner R, Kwan J, et al. (2011). Effect of anesthesia carrier gas on in vivo circulation times of ultrasound microbubble contrast agents in rats. Contrast Media Mol Imaging 6:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Salomon C (2018). Extracellular vesicles and their immunomodulatory functions in pregnancy. Semin Immunopathol. doi: 10.1007/s00281-018-0680-2. [DOI] [PubMed] [Google Scholar]
- Naldini L. (2015). Gene therapy returns to centre stage. Nature 526:351–60. [DOI] [PubMed] [Google Scholar]
- Rosenblum D, Joshi N, Tao W, et al. (2018). Progress and challenges towards targeted delivery of cancer therapeutics. Nature Commun 9:1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufino-Ramos D, Albuquerque PR, Carmona V, et al. (2017). Extracellular vesicles: novel promising delivery systems for therapy of brain diseases. J Control Release 262:247–58. [DOI] [PubMed] [Google Scholar]
- Rupaimoole R, Slack FJ (2017). MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 16:203–22. [DOI] [PubMed] [Google Scholar]
- Sawant RR, Torchilin VP (2012). Challenges in development of targeted liposomal therapeutics. AAPS J 14:303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth T, Kullberg M, Malik N, et al. (2015). Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release 199:145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarov K, Sviridov A, Xu W, et al. (2017). Nano air seeds trapped in mesoporous janus nanoparticles facilitate cavitation and enhance ultrasound imaging. ACS Appl Mater Interfaces 9:35234–43. [DOI] [PubMed] [Google Scholar]
- Tzu-Yin W, Wilson KE, Machtaler S, Willmann JK (2013). Ultrasound and microbubble guided drug delivery: mechanistic understanding and clinical implications. CPB 14:743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhou G, Tian C, et al. (2016). Exosome-mediated small RNA delivery for gene therapy. Wires RNA 7:758–71. [DOI] [PubMed] [Google Scholar]