ABSTRACT
Inflammatory myopathies are characterized by the skeletal muscle inflammation leading to symptoms of myopathy along with varying involvement of other organs such as lung, skin and joints. The strong association between inflammatory myopathies and malignancy has been well recognized. Recently, ‘cancer-associated myositis (CAM)’, has been proposed to be a paraneoplastic syndrome due to the anti-tumor immunity secondary to similar tumor and regenerating muscle antigens.
As the prognosis of myositis depends on the prognosis and treatment of the underlying malignancy, physicians must determine the degree of testing necessary to detect cancer both at myositis onset and thereafter. However, there are no clear guidelines regarding the best approach. Emerging medical evidence shows that identification of certain risk factors and serology patterns can be utilized to predict cancer risk in patients with myositis.
KEYWORDS: Inflammatory myopathies, dermatomyositis, cancer-associated myositis, p-155 antibody, malignancy screening
1. Introduction
Inflammatory myopathies are characterized by the skeletal muscle inflammation leading to symptoms of myopathy along with varying involvement of other organs such as lung, skin and joints. The strong association between inflammatory myopathies and malignancy has been well recognized. Recently, ‘cancer-associated myositis (CAM)’, has been proposed to be a paraneoplastic syndrome due to the anti-tumor immunity secondary to similar tumor and regenerating muscle antigens.
As the prognosis of myositis depends on the prognosis and treatment of the underlying malignancy, physicians must determine the degree of testing necessary to detect cancer both at myositis onset and thereafter. However, there are no clear guidelines regarding the best approach. Emerging medical evidence shows that identification of certain risk factors and serology patterns can be utilized to predict cancer risk in patients with myositis.
We report a case of dermatomyositis (DM) due to the dilemma we encountered when deciding how to screen for malignancy amongst the DM population. This case highlights the risk-factors which have been known to increase the likelihood of CAM as an attempt to emphasize the extra-vigilance that needs to be maintained by the physicians caring for such patients. Annual cancer surveillance may be required; as with other paraneoplastic disorders, the neoplasm may not reveal itself until after some time. This report also signifies the need to revise the current guidelines about cancer screening in myositis patients.
2. Case description
A 66-year old gentleman with history of hypertension, COPD, Barrett’s esophagus and hyperlipidemia presented with a skin rash, muscle weakness involving his upper arms and myalgias of 2-months duration. He reported that the rash started from his arms and then progressed to rest of his body. This was associated with weakness and tenderness in his arms and thighs. He was seen by his dermatologist who advised admission due to concerns of inflammatory myositis after performing a skin biopsy. Physical examination revealed a diffuse macular non-blanching non-pruritic rash (Figures 1&2).
Figure 1.
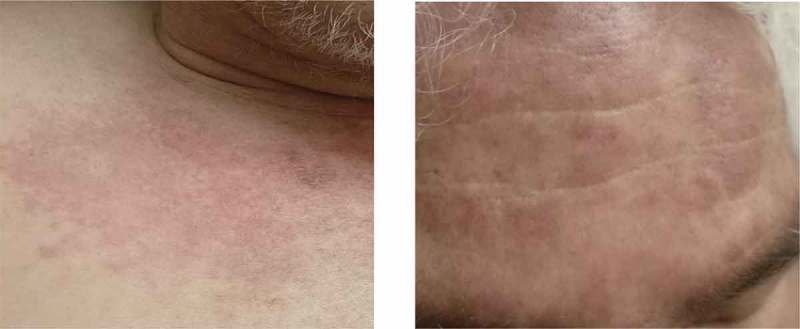
Rash seen on upper chest and forehead.
Figure 2.
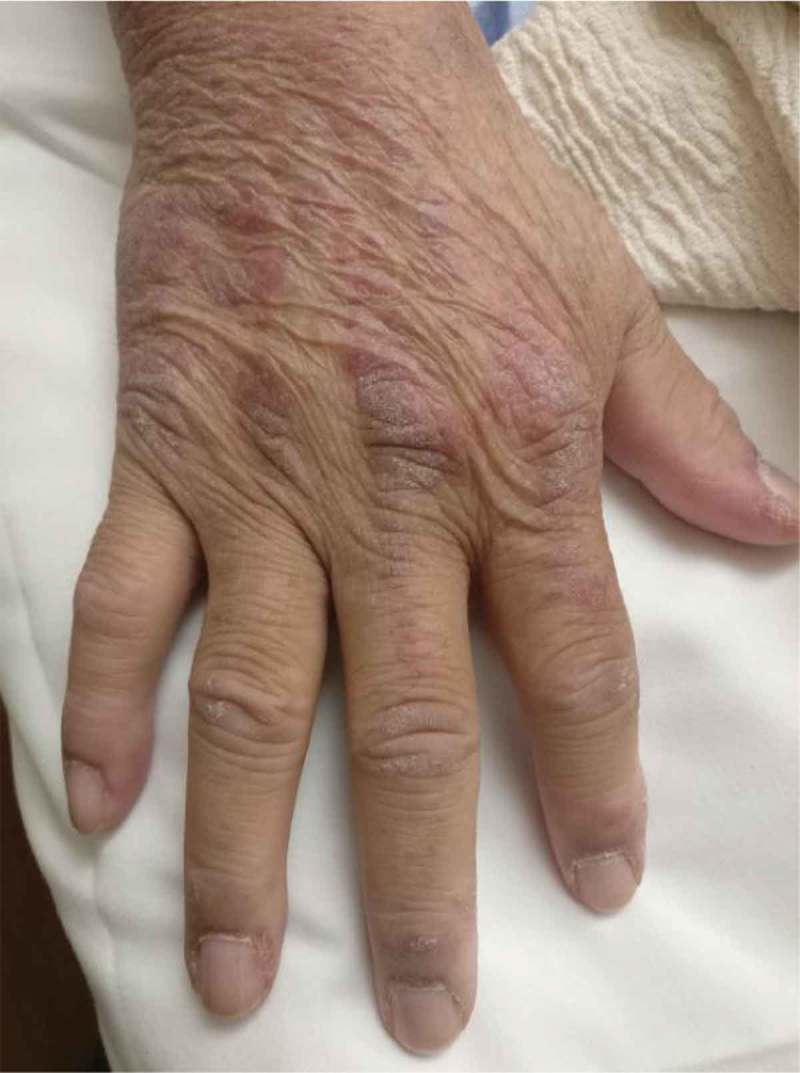
Gottron’s papules seen on the hands.
Gottron’s papules were also identified on his hands (Figure 2). He was also noted to have proximal muscle tenderness and weakness. Rest of the exam was unremarkable. Initial blood workup showed normal blood count, metabolic profile and urinalysis along with ESR of 14 mm/hour and CRP of 0.50 mg/dl. However, ALT was 110 IU/L and Creatine kinase levels were 2180 IU/L. Rest of the liver profile was normal. He was started on corticosteroids and underwent a complete myositis workup as shown in Table 1.
Table 1.
Result of the blood tests performed.
| Tests performed |
|---|
|
MRI right shoulder was performed which showed edema and enlargement of muscle groups. A muscle biopsy was subsequently performed but was unremarkable. Meanwhile, his skin biopsy showed severe cutaneous necrosis along with lymphocytic infiltration. Based on the clinical findings and skin biopsy results, the diagnosis of dermatomyositis was made although the autoimmune workup was unremarkable. The recommendation of age-/gender-appropriate cancer screening was followed. His colonoscopy done two years back was unremarkable for any malignancy while due to history of smoking, he had his CT chest done one year back which showed right lung nodules and an anterior mediastinal mass. CT chest was repeated which showed stable right lung nodules and a lobulated thymic mass (Figure 3.)
Figure 3.
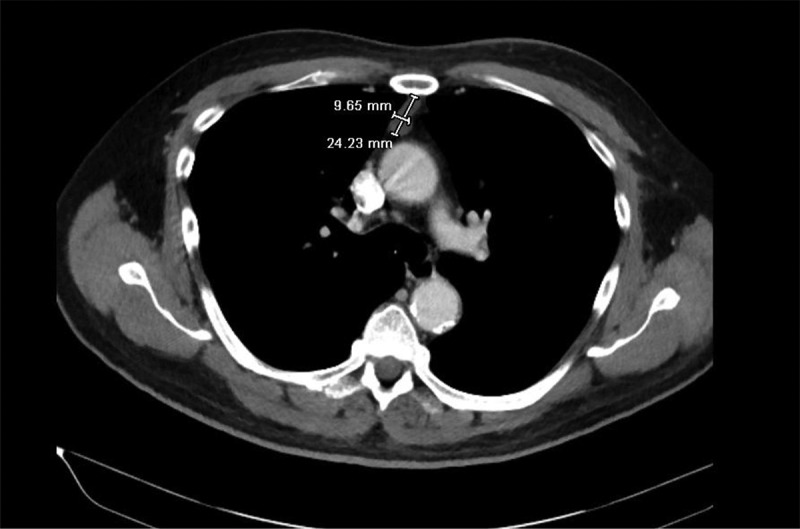
CT chest with contrast showing the anterior mediastinal mass.
Interventional radiology was consulted for a thymic biopsy but due to concerns of difficult anatomical location, it could not be performed. Meanwhile, autoimmune profile for Myasthenia gravis and Lambert-Eaton syndrome was negative. He was discharged on oral corticosteroids after his muscle enzyme levels normalized.
He was seen in the rheumatology clinic few weeks later where due to persistent myopathy and initial negative autoantibody testing, further immunological workup was done. It showed p-155 antibody positivity which depicted a high likelihood for CAM. He was started on IVIG infusions and underwent a more detailed workup for malignancy including upper GI endoscopy, colonoscopy and PSA levels, all of which were unremarkable. His repeat chest CT with IV contrast showed resolution of the pulmonary nodules but persistence of the thymic mass. Therefore, he was referred to a thoracic surgeon for a thymic biopsy. In addition, he was advised that he would need a PET scan if the thymic mass is benign along with annual cancer surveillance. However, due to the high-risk anatomical location of the thymic mass, patient opted for serial imaging. The patient continues to follow closely with rheumatology while the thymic mass has remained stable.
The unusual clinical course of this patient led to many interesting learning points for our medical team. First, risk-stratification of patients with myositis can be done with the help of certain clinical features such as old age, prominent muscular/cutaneous symptoms, lower prevalence of comorbid interstitial pneumonia, poorer response to immunosuppressive therapies. Second, p-155 antibody is a recently discovered antibody which is not routinely tested in patients with dermatomyositis and can be missed easily. Its significance is shown by the fact that 67% of patients with anti-p155 positivity develop a malignancy in their life-time. Third, the general recommendation of age-/gender-appropriate cancer screening may not apply for DM patients who need more aggressive cancer testing at time of diagnosis. Even if the initial testing is negative, the patients need to undergo cancer surveillance on an annual basis for the next 3–4 years.
3. Discussion
Inflammatory myopathy/myositis (IM) refers to a group of heterogenous autoimmune disorders such as Polymyositis, Dermatomyositis (DM) and Inclusion-body myositis with characteristic immune-mediated muscle injury. Skeletal muscles are most commonly affected resulting in muscle weakness/tenderness and elevated enzymes (Creatine kinase, Aldolase). Additional involvement of skin, joints, vessels, heart and lung can also be seen [1].
Although considered to be an immune mediated process, a strong association between inflammatory myositis and cancer is well known- especially in patients with DM. The mechanism behind this myositis-cancer association is still unknown but is mainly speculated to be due to tumor-induced autoimmune and paraneoplastic processes. This has led to introduction of the term ‘Cancer-associated myositis’ (CAM) although the actual definition is still elusive. The evidence supporting this hypothesis is as follows: 1) Peak incidence of cancer is seen at initial presentation and during the first three years of myositis. 2) Myositis is resistant to standard treatment but usually resolves after the cancer is treated. 3) Relapse of cancer leads to relapse or development of myositis [2].
Generally, it is recommended that a pelvic/prostate examination, chest radiography and age-/gender-appropriate cancer screening (as per USPTF recommendations for general population) should be performed in all patients with recently diagnosed DM. However, data suggests that the standard ‘age appropriate’ guidelines may not be adequate in the DM population due to high pretest probability of cancer which necessitates a more aggressive approach for cancer detection [3]. Unfortunately, there is no clinical consensus regarding the modalities or frequency of testing in DM patients.
Recent studies have shown that presence of specific features in patients with IM may help in predicting the cancer risk (Table 2.) [4]
Table 2.
Risk factors associated with Cancer-associated myositis.
| High-risk features for cancer-associated myositis |
|---|
|
Furthermore, autoantibody testing particularly in dermatomyositis can assist physicians in risk-stratification. The antibodies detected can be either ‘Myositis-specific’ or ‘Myositis-associated’. Myositis-specific antibodies are detected primarily in dermatomyositis with varying clinical features. On the other hand, myositis-associated antibodies are found in other rheumatological diseases with overlapping features of DM (Table 3) .
Table 3.
Autoantibodies detected in Dermatomyositis.
| Types of autoantibodies | Clinical Features |
|---|---|
| Myositis-specific antibodies: | |
|
Interstitial lung disease, Raynaud’s phenomenon, arthritis, ‘Mechanic’s hands’, absence of muscle involvement Severe necrotizing myositis Acute-onset, good response to therapy Juvenile DM, calcinosis, ILD Cancer-associated DM |
| Myositis-associated antibodies: | |
|
Sjogren’s syndrome Sjogren’s syndrome/SLE Mixed connective tissue disease SLE Scleroderma |
One of the ‘Myositis-specific’ autoantibodies is the recently discovered anti-p155 antibody which is reactive against transcription intermediary factor-1-gamma (TIF1-gamma) protein involved in cell proliferation, immunity and carcinogenesis. So far, anti-p155 positivity has only been identified in patients with DM and is considered to be highly specific for CAM [5]. This is supported by a study done on cancer-associated DM which showed that patients positive for anti-p155 had 27-fold higher chances of developing cancer as compared to patients who were negative [6]. The carcinomas are detected in approximately 70% of patients who have DM and are anti-p155 positive [7].
The ‘absence’ of other myositis-specific/associated autoantibodies in DM (anti-Jo-1, anti-Scl, anti-RNP) has been shown to be highly sensitive for CAM. Chinoy et al. demonstrated in their study that the combination of the above-stated two autoantibody pattern had 100% sensitivity and negative predictive value for detecting cancer in patients with DM [8].
Our patient presented with signs of dermatomyositis which was confirmed on a skin biopsy showing severe necrosis. In addition, he was sixty-six years old, had no pulmonary features and showed little clinical improvement despite being started on corticosteroids and methotrexate. As all the commonly tested DM antibodies were found to be negative, anti-p155 antibody testing was performed which turned out to be positive. All these findings warranted that a more detailed workup for malignancy should be pursued as opposed to the usual age-/gender-appropriate screening.
Adenocarcinomas of the cervix, lung, ovaries, pancreas, bladder, and stomach are detected in up to seventy percent of cases [9]. However, CAM due to an underlying thymoma has been rarely reported so far [10]. EGD, colonoscopy, PSA levels and pan-CT scan were unremarkable for our patient apart from a thymic mass for which he was referred to surgery as outpatient for a biopsy. Thymic tumors are rare indolent tumors with reportedly variable outcomes. The 5-year survival rate ranges from 25–75% depending on the stage of the cancer [11].Till date, our patient is still following with the surgical team regarding the thymic mass.
As stated above, the risk of cancer is greatest within the first three years of myositis and the patients may require intensive annual cancer surveillance for 3–4 years. Therefore, it was decided that even if the thymic mass was found to be benign, the patient will need close outpatient observation along with a PET scan and annual cancer screening.
4. Conclusion
Our case highlights the fact that the current recommendations regarding cancer screening/detection in patients with DM need to be revised. Recent medical data suggests that in addition to initial age/sex appropriate cancer screening, all patients diagnosed with DM should undergo risk stratification for CAM. High-risk individuals should be aggressively worked up to detect an underlying malignancy at the time of diagnosis along with close observation during the first three to five years.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Ernste FC, Reed AM.. Idiopathic inflammatory myopathies: current trends in pathogenesis, clinical features, and up-to-date treatment recommendations. NEJM. 2013;88(1):83–105. [DOI] [PubMed] [Google Scholar]
- [2].Kang EH, Lee SJ, Ascherman DP, et al. Temporal relationship between cancer and myositis identifies two distinctive subgroups of cancers: impact on cancer risk and survival in patients with myositis. Rheumatology. 2016;55:1631–1641. [DOI] [PubMed] [Google Scholar]
- [3].Leatham HBS, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97(2):e9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lu X, Yang H, Shu X, et al. Factors predicting malignancy in patients with polymyositis and dermatomyostis: a systematic review and meta-analysis. PLoS One. 2014;9(4):e94128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ghirardello A, Borella E, Beggio M, et al. Myositis autoantibodies and clinical phenotypes. Auto Immun Highlights. 2014;5(3):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Trallero-Araguas E, Rodrigo-Pendas JA, Selva-O’Callaghan A, et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum. 2012;64(2):523–532. [DOI] [PubMed] [Google Scholar]
- [7].Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54(11):3682. [DOI] [PubMed] [Google Scholar]
- [8].Chinoy H, Fertig N, Oddis CV, et al. The diagnostic utility of myositis autoantibody testing for predicting the risk of cancer-associated myositis. Ann Rheum Dis. 2007;66(10):1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kaji K, Fujimoto M, Hasegawa M, et al. Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: an association with malignancy. Rheumatol. 2007;46:25–28. [DOI] [PubMed] [Google Scholar]
- [10].Dell’Amore A, Asadi N, Caroli G, et al. Paraneoplastic dermatomyositis as presentation of thymic carcinoma. Gen Thorac Cardiovasc Surg. 2013;61:422–425. [DOI] [PubMed] [Google Scholar]
- [11].Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol. 2010;5(10):S260–S2605. [DOI] [PMC free article] [PubMed] [Google Scholar]


