Introduction
The recent development of immune checkpoint inhibitors such as PD-1/PD-L1 inhibitors and CTLA-4 inhibitors has revolutionized the approach to cancer therapy. However, with these advances comes a distinctive set of toxic effects, collectively named immune-related adverse events (IRAEs).
The most common cutaneous manifestations of IRAEs include morbilliform eruptions, lichenoid reactions, pruritus, eczema, and vitiligo.1 Nevertheless, these manifestations can affect any tissue in various combinations. Drug reaction with eosinophilia and systemic symptoms (DRESS) is one example of such presentation. Although initially reported with anticonvulsants, the list of potential causative agents for DRESS has considerably lengthened over the years. We present a patient with a novel case of nivolumab-associated DRESS, with a discussion on the current challenges in diagnosis, especially in the context of immune checkpoint inhibitors.
Case
A 66-year-old female under treatment with nivolumab for metastatic renal cell carcinoma presented with a rash and shortness of breath, pleuritic pain, cough, intermittent confusion, delirium, and general deterioration. She had been on nivolumab for 4 months, her latest dose being 1 week before presentation. Her other medications included metoprolol, tiotropium, denosumab, clotrimazole, insulin, dalteparin, pantoprazole, calcium carbonate, melatonin, and acetaminophen.
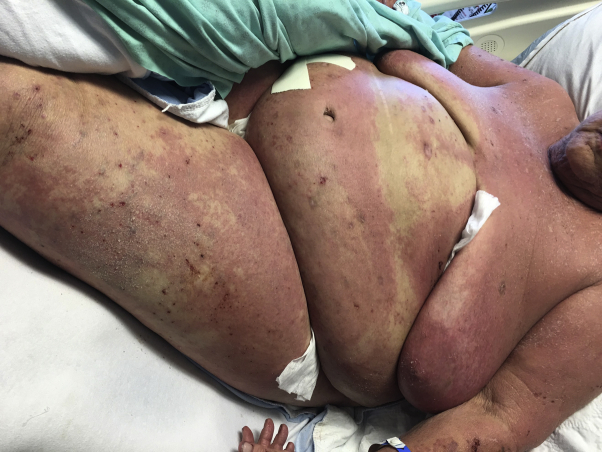
Physical examination found erythematous plaques covering more than 50% of body surface area with overlying erosions and excoriations on her arms, trunk, legs, and face (Fig 1). There were no pustules or bullae or mucosal involvement. There was no lymphadenopathy, although examination may have been inadequate given her severely obese body habitus. No fever was recorded, but she did have chills.
Fig 1.
Infiltrated erythematous plaques covering greater than 50% body surface area with erosions and excoriations.
She was lymphopenic (0.4 × 109/L) and had eosinophilia (0.73 × 109/L). During her admission, her eosinophil count continued to increase to 1.16 × 109/L. Troponins were elevated at 0.57 (normal, 0-0.04). Liver enzymes and thyroid-stimulating hormone level were normal.
A chest computed tomography scan showed a few nonspecific multifocal peripheral ground-glass changes resembling an interstitial lung disease such as cryptogenic organizing pneumonia or nonspecific interstitial pneumonia (Fig 2). On transthoracic echocardiography, the right ventricle appeared mildly enlarged and hypokinetic, but a cardiac biopsy was not performed. There was no change in her slightly impaired baseline renal function.
Fig 2.
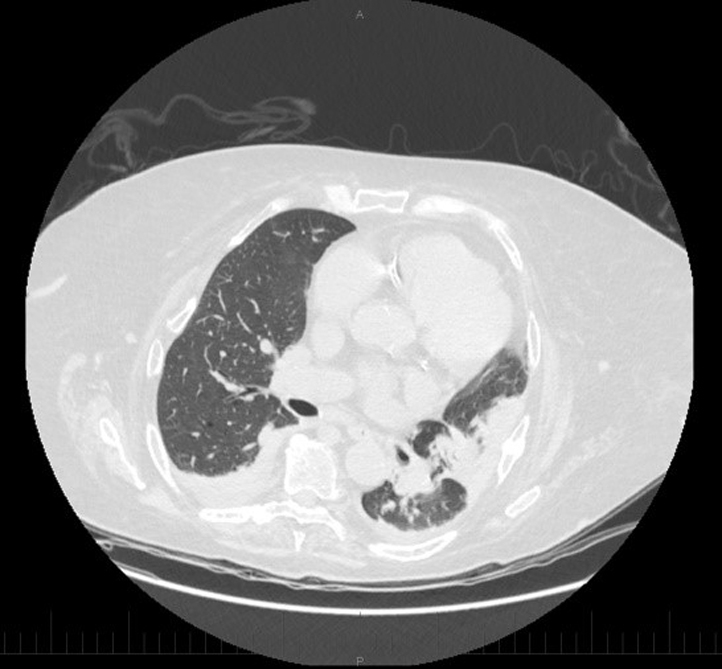
Chest computed tomography scan with peripheral ground-glass changes suggestive of cryptogenic organizing pneumonia.
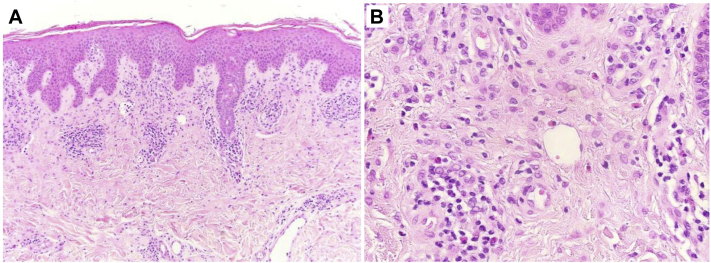
A skin biopsy found mild subacute spongiotic dermatitis, focal prominent parakeratosis, and a superficial perivascular lymphocytic infiltrate with numerous eosinophils (Fig 3). The changes were subtle, but the possibility of DRESS could not be excluded.
Fig 3.
A, Subacute spongiotic dermatitis with focal parakeratosis and superficial dermal perivascular lymphocytes. B, Higher magnification shows an eosinophil-rich superficial dermal inflammatory infiltrate. (Hematoxylin-eosin stain; original magnifications: A, ×10; B, ×40.)
The patient was initiated on 50 mg of intravenous methylprednisolone every 6 hours, but with no improvement after 3 days, the dose was increased to 1 g pulse for 3 days. She was then given 100 mg of oral prednisone daily with a subsequent taper. Her acute condition continued to improve, and nivolumab therapy was not restarted. A repeat thyroid-stimulating hormone level looking for delayed thyroid dysfunction could not be performed, as her cancer progressed and comfort care measures were instituted.
Discussion
Despite there being no reliable standard for diagnosis, criteria for DRESS have been developed taking into account clinical and laboratory abnormalities. The original criteria, proposed by Bocquet et al2 in 1996 has expanded to 2 more commonly used diagnostic criteria: the RegiSCAR and the J-SCAR. The RegiSCAR group has suggested inclusion criteria for hospitalized patients suspected to have DRESS, consisting of at least 3 of the following systemic features developing weeks to months after drug initiation: acute skin rash, fever greater than 38°C, lymphadenopathy, internal organ involvement, and hematologic abnormalities, including atypical lymphocytosis, eosinophilia, and thrombocytopenia.3 If a case is included based on those criteria, a further scoring system is applied to classify the case as excluded, possible, probable, or definite case of DRESS.4 In contrast, the J-SCAR criteria emphasizes the role of human herpes virus 6 reactivation and is more easily applied due to the reliance on simpler laboratory tests.
The proposed diagnostic criteria highlight different factors hypothesized to be involved with DRESS. Yet, some inconsistencies between them and the nonspecific appearance of DRESS on histopathology5 limit their utility in diagnosing the syndrome. Trends from retrospective data suggest that DRESS clinical characteristics vary depending on the causal drug, yet no clear unified outline can currently be defined for these multiorgan drug-induced reactions.3, 4, 5, 6
Based on eosinophilia, suggestive cutaneous rash, and internal organ involvement, our patient scored as a probable case of DRESS per the RegiSCAR criteria. Despite none of her active medications being listed in a literature review by Cacoub et al,7 nivolumab was the only new medication added in the preceding months, making it the most probable culprit drug.
There is 1 previous case of checkpoint inhibitor–associated DRESS with ipilimumab, a CTLA-4 inhibitor, in an elderly man being treated for melanoma,8 and 1 case in a 46-year-old man on combination ipilimumab and nivolumab for melanoma.9 Our patient differed from these 2 cases in sex, type of cancer being treated, and organs affected, with nivolumab being the sole suspected culprit drug.
The difficulty in using the proposed diagnostic criteria on novel drugs designed to target the immune system stems from the altered immune response that one would expect. Using leukocyte abnormalities in the criteria for diagnosis of DRESS may have been warranted with the initial culprit drugs. However, with the advent of immunomodulatory drugs, it is unclear whether the aforementioned criteria remain suitable for diagnosis.
DRESS is a type IV hypersensitivity reaction, whereby activated T cells play a central role.4 Checkpoint inhibitors enhancing the activation and activity of T cells could be interfering with cellular processing of drugs. This alteration in the immune system is comparable to that in individuals infected with HIV, in which the frequency of drug eruptions is higher when compared with the non-HIV population.10 Although large-scale studies on the prevalence and frequency of drug reactions with checkpoint inhibitors have yet to be conducted, it would be reasonable to assume that the adverse event profile of overactivation of the immune system has similarities with a suppressed immune system in a patient with HIV.
Conclusion
Of the IRAEs encountered with the emergence of checkpoint inhibitor therapies for otherwise prognostically dismal cancer patients, DRESS is a potentially ominous systemic reaction that requires early detection and immediate action. Based highly on clinical suspicion, it is probably underdiagnosed, thus making it important to remain cognizant of this syndrome, even when patients are not taking drugs previously known to cause these reactions.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
This article was a poster presentation at the Canadian Dermatology Association 2018 Annual Conference, Montreal, Quebec, Canada, June 21, 2018.
References
- 1.Collins L.K., Chapman M.S., Carter J.B., Samie F.H. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr Probl Cancer. 2017;41(2):125–128. doi: 10.1016/j.currproblcancer.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Bocquet H., Bagot M., Roujeau J.C. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS) Semin Cutan Med Surg. 1996;15(4):250–257. doi: 10.1016/s1085-5629(96)80038-1. [DOI] [PubMed] [Google Scholar]
- 3.Peyriere H., Dereure O., Breton H. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155(2):422–428. doi: 10.1111/j.1365-2133.2006.07284.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y.-C., Cho Y.-T., Chang C.-Y., Chu C.-Y. Drug reaction with eosinophilia and systemic symptoms: a drug-induced hypersensitivity syndrome with variable clinical features. Dermatologica Sinica. 2013;31(4):196–204. [Google Scholar]
- 5.Cho Y.-T., Yang C.-W., Chu C.-Y. Drug reaction with eosinophilia and systemic symptoms (DRESS): an interplay among drugs, viruses, and immune system. Int J Mol Sci. 2017;18(6):1243. doi: 10.3390/ijms18061243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chadly Z., Fredj N.B., Aouam K., Romdhane H.B., Boughattas N.A., Chaabane A. Drug rash with eosinophilia and systemic symptoms (DRESS) features according to the culprit drug. In: Allergy CaT, editor. 7th drug hypersensitivity meeting. BioMed Central; London: 2016. p. 31. [Google Scholar]
- 7.Cacoub P., Musette P., Descamps V. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588–597. doi: 10.1016/j.amjmed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Voskens C.J., Goldinger S.M., Loquai C. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the Ipilimumab Network. PLoS One. 2013;8(1):e53745. doi: 10.1371/journal.pone.0053745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirza S., Hill E., Ludlow S.P., Nanjappa S. Checkpoint inhibitor-associated drug reaction with eosinophilia and systemic symptom syndrome. Melanoma Res. 2017;27(3):271–273. doi: 10.1097/CMR.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 10.Yunihastuti E., Widhani A., Karjadi T.H. Drug hypersensitivity in human immunodeficiency virus-infected patient: challenging diagnosis and management. Asia Pac Allergy. 2014;4(1):54–67. doi: 10.5415/apallergy.2014.4.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]