Introduction
Parry-Romberg Syndrome (PRS), or progressive hemifacial atrophy, is an uncommon disorder characterized by progressive unilateral loss of adipose tissue and underlying structures including muscle, cartilage, and bone, often with little or no sclerosis. PRS and morphea en coup de sabre (ECDS) have significant overlap, often coexist, and are likely different phenotypes of morphea.1 PRS usually presents in the first decade of life, but later presentations have been described.1 It is more common in females and the pathogenesis is not completely understood.1 Neurologic symptoms are the most common extracutaneous systemic manifestation. Bilateral disease occurs in rare instances.1 We describe a woman with profound bilateral facial atrophy whose presentation does not follow the typically reported disease course or histopathologic findings seen in PRS.
Report of a case
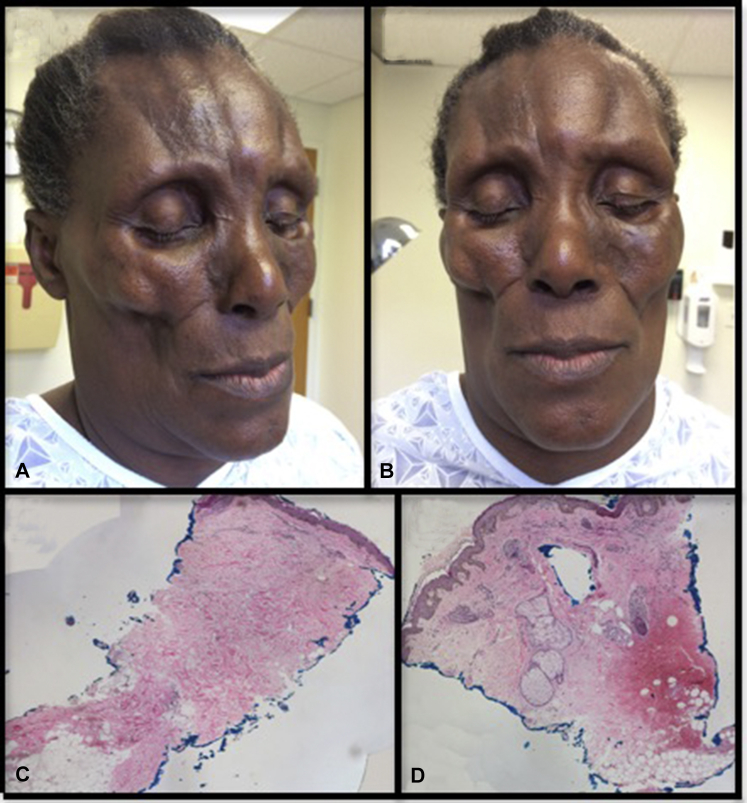
A 59-year-old Haitian woman who had moved to the United States 1 year before presentation was evaluated for bilateral facial atrophy. Approximately 20 years before presentation, she noted an itchy patch on the left cheek and subsequently had profound atrophy in this area. There was no history of trauma or injections to the affected area nor of preceding illness. Gradually over the last 2 decades, she experienced progressive bilateral facial atrophy (Fig 1). The affected areas were otherwise asymptomatic, and she continued to have full facial range of motion. In the 2 years before presentation, she had significant headaches and dizziness. On clinical examination, she had profound retroorbital and periorbital fat loss giving a sunken eye appearance and profound preauricular fat pad loss with cadaveric facies. Additionally, she had bilateral linear depressions with hyperpigmentation on the paramedian forehead and complete alopecia of bilateral eyebrows and frontal scalp. Scleroderma-like changes were not observed. The tongue, teeth, and gums were spared.
Fig 1.
Symmetrical subcutaneous fat loss of the cheeks, temples, forehead, and periorbital skin in a 59-year-old HIV-negative woman (A and B) with complete loss of peri-eccrine adipose tissue on punch biopsy of the left lesional cheek (C and D).
Medical history was notable only for hypertension, treated with chlorthalidone. Family history was negative for morphea or lipodystrophy. HIV testing was negative. Antinuclear antibodies, Scl-70 and SSA/SSB antibodies were negative. Urinalysis, C3 (171 mg/dL), and CH50 (>60 U/mL) were within normal limits. A punch biopsy of the left lesional cheek, down to visible fascia, found complete loss of peri-eccrine adipose tissue with mild chronic perifolliculitis and perivascular lymphocytic infiltrate. Findings consistent with morphea were not observed, and the remaining adipose tissue was unremarkable (Fig 1). Lupus band test and Alcian blue stain for stromal mucin were both negative. Magnetic resonance imaging (MRI) and computed tomography scan showed marked thinning of the skin and subcutaneous fat loss of the face with bilateral enophthalmos. Cerebral MRI found scattered T2 hyperintensities and chronic ischemic changes, both of which were stable over 1 year in consecutive MRIs.
Discussion
The clinical presentation of adult-onset bilateral facial atrophy suggests bilateral PRS. This syndrome classically presents in adolescence as a progressive unilateral loss of adipose tissue and underlying structures (muscle, cartilage, and bone) often with little or no sclerosis. Although most symptoms develop during the first decade of life, onset as late as 75 years of age has been reported.1 Females are 2 to 3 times more likely to be affected than males.1, 2 Bilateral disease is a relatively rare occurrence, observed in 2% to 7% of cases.1, 2 Seizures and headaches are the most common neurologic manifestations of PRS, reported in 10% to 40% and 20% of patients, respectively.1, 3 Neuroimaging in PRS frequently finds bilateral abnormalities that are highly variable, inconsistently associated with neurologic symptoms, and often do not correlate with cutaneous activity.4 Pathogenesis of these neurological findings in PRS is largely speculative and likely multifactorial.4 Proposed mechanisms include inflammatory and autoimmune etiologies.4 Because T2 hyperintensity on MRI is one of the imaging findings reported in PRS, neurologist review of our case proposed that her MRI findings could be due to PRS, although age-related changes cannot be excluded given that the patient is older than the typical PRS cohort.
The differential diagnosis of PRS includes Barraquer-Simons syndrome (Table I). The diagnosis of atypical Barraquer-Simons syndrome was strongly considered because of the lack of morphea-like changes on pathology, bilateral symmetrical facial involvement, and no bone destruction. The two diseases may be associated, with some references classifying bilateral progressive facial atrophy as Barraquer-Simons syndrome.5, 6
Table I.
| Age of onset | Sex (F:M) | Body areas affected | Clinical features | Histopathologic features | Associated diseases | |
|---|---|---|---|---|---|---|
| Acquired partial lipodystrophy (Barraquer-Simons syndrome) | Childhood or adolescence, rarely adult | 3:1 | Decreased fat face, upper extremities, and trunk with cephalocaudal spread Increased fat hips, legs |
|
Two patterns:
|
|
| Parry-Romberg syndrome (progressive hemifacial atrophy) | Adolescence, average 13.6 years old | 3:1 | Forehead, nose, medial cheek, upper lip (total hemifacial involvement) |
|
|
|
| Morphea ECDS | Adolescence, average 10 years old | 2:1 – 3:1 | Frontoparietal and scalp without extension below eyebrow |
|
|
|
SLE, Systemic lupus erythematosus.
PRS is mostly a clinical diagnosis often supported by histopathology and imaging. The biopsies from our patient exhibited lipoatrophy without morphea changes. Although most cases of ECDS exhibit morphea findings (fibrosis and adnexal atrophy) on skin biopsy, these findings are found in a minority of PRS patients.7 It is possible that the patient has both PRS and ECDS, and if biopsies were taken from the forehead rather than the cheeks, changes of morphea would have been seen. ECDS and PRS share significant overlap. Although facial distribution of morphea-like changes may differentiate these entities, with ECDS characteristically above the eyebrows and PRS below, more than 50% of PRS patients were found to have concomitant ECDS.1 Additionally, neurological symptoms are reported in both conditions.1
The natural history of PRS is slow progression over several years followed by a plateau. The goal of therapy is to prevent disease progression. Activity of the disease in our patient was questionable, as there were no recent photographs. Because of the unexplained headaches and unclear rate of disease progression, the patient was started on methotrexate, 25 mg/week. She is treated by the neurology department for headaches and the ophthalmology department for enophthalmos and was referred to a plastic surgeon for a fat grafting procedure. This case is an unusual presentation of bilateral facial atrophy in an adult female most consistent with bilateral PRS.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Tollefson M.M., Witman P.M. En coup de sabre morphea and Parry-Romberg syndrome: a retrospective review of 54 patients. J Am Acad Dermatol. 2007;56(2):257–263. doi: 10.1016/j.jaad.2006.10.959. [DOI] [PubMed] [Google Scholar]
- 2.Stone J. Parry-Romberg syndrome: a global survey of 205 patients using the Internet. Neurology. 2003;61(5):674–676. doi: 10.1212/wnl.61.5.674. [DOI] [PubMed] [Google Scholar]
- 3.Amaral T.N., Peres F.A., Lapa A.T., Marques-Neto J.F., Appenzeller S. Neurologic involvement in scleroderma: a systematic review. Semin Arthritis Rheum. 2013;43(3):335–347. doi: 10.1016/j.semarthrit.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Doolittle D.A., Lehman V.T., Schwartz K.M., Wong-Kisiel L.C., Lehman J.S., Tollefson M.M. CNS imaging findings associated with Parry-Romberg syndrome and en coup de sabre: correlation to dermatologic and neurologic abnormalities. Neuroradiology. 2015;57(1):21–34. doi: 10.1007/s00234-014-1448-6. [DOI] [PubMed] [Google Scholar]
- 5.Payapvipapong K., Niumpradit N., Nakakes A., Buranawuti K. A rare case of acquired partial lipodystrophy (Barraquer-Simons syndrome) with localized scleroderma. Int J Dermatol. 2014;53(1):82–84. doi: 10.1111/j.1365-4632.2011.05435.x. [DOI] [PubMed] [Google Scholar]
- 6.Ruhin B., Bennaceur S., Verecke F., Louafi S., Seddiki B., Ferri J. [Progressive hemifacial atrophy in the young patient: physiopathologic hypotheses, diagnosis and therapy]Rev Stomatol Chir Maxillofac. 2000;101(6):287–297. [PubMed] [Google Scholar]
- 7.Orozco-Covarrubias L., Guzmán-Meza A., Ridaura-Sanz C., Carrasco Daza D., Sosa-de-Martinez C., Ruiz-Maldonado R. Scleroderma “en coup de sabre” and progressive facial hemiatrophy. Is it possible to differentiate them? J Eur Acad Dermatol Venereol JEADV. 2002;16(4):361–366. doi: 10.1046/j.1468-3083.2002.00442.x. [DOI] [PubMed] [Google Scholar]
- 8.Bolognia J., Jorizzo J.L., Schaffer J.V. Elsevier/Saunders; Edinburgh: 2012. Dermatology. [Google Scholar]
- 9.Tolkachjov S.N., Patel N.G., Tollefson M.M. Progressive hemifacial atrophy: a review. Orphanet J Rare Dis. 2015;10:39. doi: 10.1186/s13023-015-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]