Abstract
AST-120 (KREMEZIN®) consists of oral, spherical carbon particles that adsorb uremic toxins and their precursors within the gastrointestinal tract, allowing them to be excreted in the feces. Uremic toxins such as indoxyl sulfate and p-cresyl sulfate are abundant in the blood of chronic kidney disease (CKD) patients and are related to the progression of both CKD and cardiovascular disease. AST-120 was approved in Japan in 1991 followed by Korea (2004), Taiwan (2007) and the Philippines (2010) for treating uremic symptoms and prolonging the time to initiation of dialysis in patients with progressive CKD. In this review, we provide an overview of the past clinical data on AST-120 from 1982 to 2013. The effect of AST-120 for renal events was not supported in the primary analysis of randomized clinical trials. However, post-hoc analyses revealed significant differences between the AST-120 and control groups in the second Japanese phase III trial and in the multinational Evaluating Prevention of Progression in CKD (EPPIC) trials. Furthermore, inhibitory effects on the progression of CKD, as represented by amelioration in the estimated glomerular filtration rate (eGFR) decline and serum creatinine (sCr) elevation were suggested. These results suggest that AST-120 delays the decline in renal function. In addition, AST-120 may prolong the time to the initiation of dialysis, especially in patients with progressive CKD. For further verification of the clinical efficacy of AST-120, future study inclusion criteria should be determined carefully, defining progressive CKD using markers such as declines in eGFR and sCr elevation.
Keywords: AST-120, CKD, clinical trial, spherical carbon adsorbent, uremic toxin
Introduction
Chronic kidney disease (CKD) is a serious healthcare problem, which can lead to end-stage renal disease (ESRD) requiring dialysis or kidney transplantation. CKD is associated with high morbidity and mortality from cardiovascular disease [1]. The worldwide prevalence of ESRD is increasing yearly. Two major causes of CKD are diabetes and nephrosclerosis due to hypertension and current CKD treatments focus on managing blood sugar levels and blood pressure. AST-120 (KREMEZIN®), an alternative CKD treatment, is an oral spherical carbon adsorbent consisting of porous carbon particles that are 0.2–0.4 mm in diameter and insoluble in water. AST-120 treats uremic symptoms and prolongs the time to initiation of dialysis in patients with progressive CKD. In 1991, the Japanese regulatory agency approved AST-120, followed by Korea (2004), Taiwan (2007) and the Philippines (2010).
Uremic toxins are abundant in the blood of CKD patients [2,3]. The accumulation of microbiota-derived uremic toxins, such as indoxyl sulfate (IS) and p-cresyl sulfate (PCS), is related to the progression of CKD and cardiovascular disease [4–8]. One possible mechanism of the action of AST-120 is that it adsorbs uremic toxins and their precursors within the gastrointestinal tract, allowing them to be excreted in the feces before they can be absorbed into the bloodstream. For example, indole, a precursor of IS that is derived from the metabolism of tryptophan by bacteria within the gastrointestinal tract is adsorbed by AST-120, thereby attenuating IS accumulation in patients with CKD [9–11].
AST-120 has been prescribed to CKD patients for over two decades, and clinical data have been obtained from a number of prospective and retrospective studies [12,13]. The efficacy of AST-120 has been reviewed in some prior articles [14–17]. In the present review, we provide an overview of the past clinical data on AST-120 from 1982 to 2013, including post-hoc analyses, and have also included recent findings. Randomized clinical trials are included, which were conducted in the premarket period and became the basis for the drug approval in Japan. In addition, recent large trials, such as the multinational Evaluating Prevention of Progression in CKD (EPPIC) trials and the Kremezin Study against Renal Disease Progression in Korea (K-STAR), are included as shown in Table 1.
Table 1.
Summary of clinical data from randomized trials for AST-120.
| Trial name | Country | Reference | Approach | Patients | N | Treatment | Parameters | Results |
|---|---|---|---|---|---|---|---|---|
| PIII(I) | Japan | Koide et al. [18] | Double-blind, placebo-controlled | CKD (sCr 5–8 mg/dL) | 156 | Placebo + conventional treatment, AST-120 (3.6–7.2 g/day)+conventional treatment |
Investigator's assessment: global improvement, overall safety, global utility | After 24 weeks, there was no difference between AST-120 and placebo with regard to global improvement, overall safety, or global utility. |
| Hemodialysis score, improvement in Cr and hematocrit | There was no difference between AST-120 and placebo with regard to hemodialysis score, improvement in Cr, or hematocrit. | |||||||
| [post-hoc] Double-blind, placebo-controlled |
[subgroup analysis] Progressive CKD with significantly negative 1/Cr slope |
53 | 1/Cr | Compared with before trial, a highly significant attenuation of the 1/Cr slope was observed in the AST-120 group. No significant difference was observed in the placebo group. | ||||
| PIII(II) | Japan | Koide et al. [20] | Double-blind, placebo-controlled | CKD (sCr 5–8 mg/dL and ≥1.2 mg/dL increase during pretreatment observation period) | 244 | Placebo + conventional treatment, AST-120 (4.2–6.0 g/day)+conventional treatment |
Investigator's assessment: global improvement (based on 1/Cr slope and uremic symptoms), overall safety, global utility | After 24 weeks, global improvement and global utility of AST-120 were significantly superior to that of placebo. There was no difference between AST-120 and placebo with regard to overall safety. |
| 1/Cr | Compared with before the trial, a highly significant attenuation of the 1/Cr slope was observed in the AST-120 group. No significant difference was observed in the placebo group. | |||||||
| Initiation of dialysis | There was no difference between AST-120 and placebo with regard to initiation of dialysis. | |||||||
| Sanaka [21] | [post-hoc] Double-blind, placebo-controlled |
CKD (sCr 5–8 mg/dL and ≥1.2 mg/dL increase during pretreatment observation period) | 241 | Doubling of sCr or initiation of dialysis | A difference between AST-120 and placebo was observed in doubling of sCr or initiation of dialysis occurrence. | |||
| Koshikawa et al. [22] | [subgroup analysis] Progressive CKD with 1/Cr slope ≤ −250 × 10−5 dL·mg−1·week−1 |
121 | Initiation of dialysis | A difference between AST-120 and placebo was observed in the initiation of dialysis occurrence. | ||||
| CAP-KD | Japan | Akizawa et al. [24] | Randomized, controlled | Progressive CKD (sCr <5.0 mg/dL with negative 1/Cr slope) | 460 | Conventional treatment, AST-120 (6 g/day)+conventional treatment |
Primary endpoint: doubling of sCr, increase in sCr to ≥6.0 mg/dL, initiation of dialysis, transplantation, or death | After 56 weeks, there was no difference between AST-120 and conventional treatment with regard to primary endpoint. |
| Estimated CrCl | Estimated CrCl decreased significantly less with AST-120 versus conventional treatment. | |||||||
| eGFR | eGFR declined significantly less with AST-120 versus conventional treatment. | |||||||
| EPPIC-1, EPPIC-2 | North America, Latin America, and Europe | Schulman et al. [25] | Randomized, double-blind, placebo-controlled | Moderate to severe CKD (sCr 2.0–5.0 mg/dL for men and 1.5–5.0 mg/dL for women) | 2035 (EPPIC-1: 1020, EPPIC-2: 1015) |
Placebo + conventional treatment, AST-120 (9 g/day)+conventional treatment |
Primary endpoint: doubling of sCr, initiation of dialysis or transplantation | There was no difference between AST-120 and placebo with regard to primary endpoint in the pooled analysis of both trials. |
| eGFR | eGFR declined significantly less with AST-120 versus placebo in the pooled analysis of both trials. | |||||||
| Schulman et al. [26] | [post-hoc] Randomized, double-blind, placebo-controlled |
[subgroup analysis] Progressive CKD with UP/UCr ≥1.0, Hematuria(+) and ACEi/ARB use |
474 | Primary endpoint: doubling of sCr, initiation of dialysis or transplantation | A difference between AST-120 and placebo was observed in the primary endpoint occurrence in the pooled analysis of both trials. | |||
| Schulman et al. [27] | [subgroup analysis] Per-protocol subgroup from the USA |
464 | Primary endpoint: doubling of sCr, initiation of dialysis or transplantation | A difference between AST-120 and placebo was observed in the primary endpoint occurrence in the pooled analysis of both trials. | ||||
| K-STAR | Korea | Cha et al. [28] | Randomized, controlled | CKD stage 3 or 4 | 579 | Conventional treatment, AST-120 (6 g/day)+conventional treatment | Primary endpoint: doubling of sCr, 50% reduction in eGFR, or the initiation of renal replacement therapy | After 36 months, there was no difference between AST-120 and conventional treatment with regard to primary endpoint. |
| Cha et al. [29] | [post-hoc] Randomized, controlled |
[subgroup analysis] Per-protocol subgroup |
465 | Primary endpoint: doubling of sCr, 50% reduction in eGFR, or the initiation of renal replacement therapy | After 36 months, there was no difference between AST-120 and conventional treatment with regard to primary endpoint. |
ACEi/ARB: angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker; CKD: chronic kidney disease; sCr: serum creatinine; CrCl: creatinine clearance; eGFR: estimated glomerular filtration rate; UP/UCr: urinary protein-to-urinary creatinine ratio; USA: United States of America.
Premarket trials in Japan
To assess the efficacy and the safety of AST-120 in CKD patients, two phase III, double-blind, placebo-controlled, multicenter trials were conducted in Japan prior to approval of the drug in 1991.
First phase III trial
The first phase III trial of AST-120 evaluated its efficacy and safety in 156 patients with CKD at 25 hospitals from 1982 to 1983 [18]. The patients had serum creatinine (sCr) levels between 5 and 8 mg/dL. Each patient received AST-120 or a placebo for 24 weeks, in addition to conventional treatment. Each patient received 3.6 g/day for the first 4 weeks, and then the dosage was increased to 5.4 g/day, if possible for an additional 4 weeks. Thereafter, the dose could be increased up to 7.2 g/day at the investigator’s discretion. After 24 weeks, there were no differences between the AST-120 group and the placebo group with regard to final global improvement, overall safety, global utility, hemodialysis score, final sCr improvement and final hematocrit improvement ratings.
In a post-hoc analysis, the slope of the regression curve of the reciprocal of sCr (1/Cr slope) was analyzed. The slope of 1/Cr plotted against time indicates the rate of progression of CKD, such as a decrease in the number of functioning nephrons. A larger negative slope suggests a rapid rate of progression of CKD [19]. Among the patients in the study, 25 patients in the AST-120 group and 28 patients in the placebo group had significantly negative 1/Cr slopes at the beginning of the trial. Within this subset of patients, we found a significant attenuation in the 1/Cr slope over the course of the trial in the AST-120 group (−401 ± 343 to −178 ± 192, p < .01, W test: Wilcoxon matched pairs signed-rank test), whereas no significant change was seen in the placebo group (−315 ± 251 to −191 ± 204). These results suggested that it is possible to assess the efficacy of AST-120 within a short period of time, such as 24 weeks, in patients with fast-progressing CKD.
Second phase III trial
Informed by the above results, a second phase III trial was conducted from 1984 to 1986. This trial included a 24-week observation period prior to the 24-week double-blind treatment phase. Evidence of sCr levels within the range of 5–8 mg/dL, as well as an increase in sCr of ≥1.2 mg/dL during the 24-week observation period, were required for inclusion in the treatment phase of the study.
The study sample included 244 patients with CKD from 41 hospitals. All patients received AST-120 or a placebo, in addition to conventional treatment, for 24 weeks [20]. The dose of AST-120 was 4.2 g/day for the first 2 weeks, which was then increased to 6.0 g/day. The drug was packed in sets of four cases: two cases of the drug and two cases of placebo. Each case contained 12 packs and each pack contained a 2-week supply of the drug or placebo. Patients were randomly assigned to receive a case of either the drug or the placebo. The controllers confirmed that the placebo packs were indistinguishable from the AST-120 packs, and a key code was retained by a double controller method. A third party contracted by the controller confirmed the contents and quality of the study drugs using a random sample.
The study analyzed 122 patients in the AST-120 group and 119 patients in the placebo group. The investigators assessed the improvement rating of the change in 1/Cr with time, improvement rating of uremic symptoms, global improvement rating, overall safety rating and global utility rating. Symptoms associated with CKD (anorexia, nausea, halitosis and itching) were assessed at study initiation and every 4 weeks during treatment. The 1/Cr slope was assessed by the evaluation committee.
After 24 weeks, the AST-120 group was significantly more likely to receive a rating of ‘improved’ or better in the change in 1/Cr compared to that of the placebo group (43% of the AST-120 group and 24% of the placebo group, p < .01, χ2 test). AST-120 therapy was associated with significantly higher scores for symptom improvement and significantly lower scores for symptom aggravation compared with that of the placebo. Within the AST-120 group, 22% received a rating of ‘improved’ in uremic symptoms compared to 8% in the placebo group (p < .01, χ2 test). A global improvement rating based on the improvement in the change of 1/Cr with time and uremic symptoms was 45% in the AST-120 group and 22% in the placebo group for ‘improved’ or better; thus, the AST-120 group showed significantly superior results compared to those from the placebo group (p < .001, χ2 test). No significant difference was observed between the two groups in overall safety rating. The frequencies of adverse events were comparable between the two groups (6.6% in the AST-120 group and 9.2% in the placebo group). The major treatment-related adverse events were gastrointestinal symptoms such as constipation and abdominal distension. There were no serious adverse events. A global utility rating of ‘moderately useful’ or better based on both the global improvement rating and overall safety rating was 48% in the AST-120 group and 23% in the placebo group; therefore, the AST-120 group showed significantly superior results versus the placebo group (p < .001, χ2 test).
For cases in which 1/Cr slope data were available in both the observation period and study period, the mean value was attenuated in the AST-120 group (119 cases, –329 ± 245 to –222 ± 378, p < .001, Wilcoxon matched pairs signed-rank test), whereas no significant change was seen in the placebo group (118 cases, –293 ± 184 to –274 ± 279).
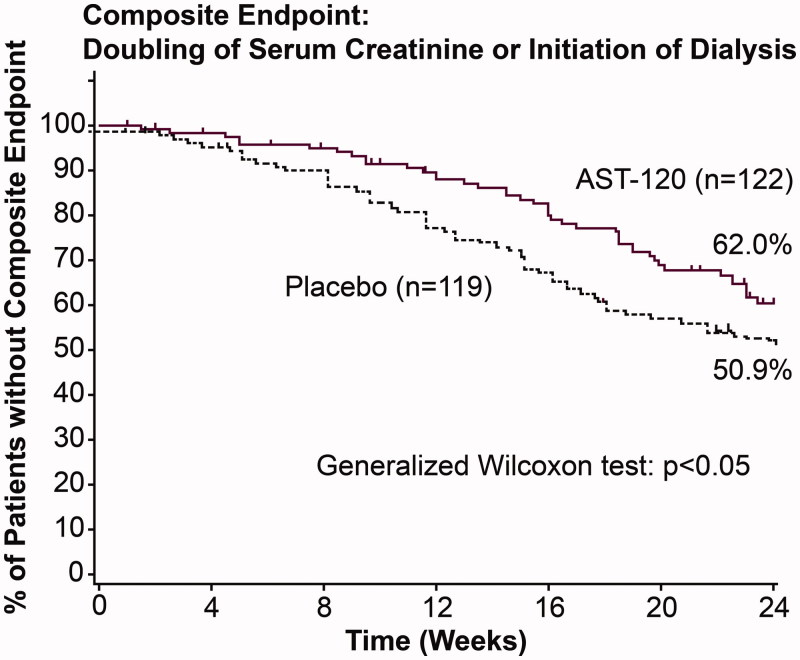
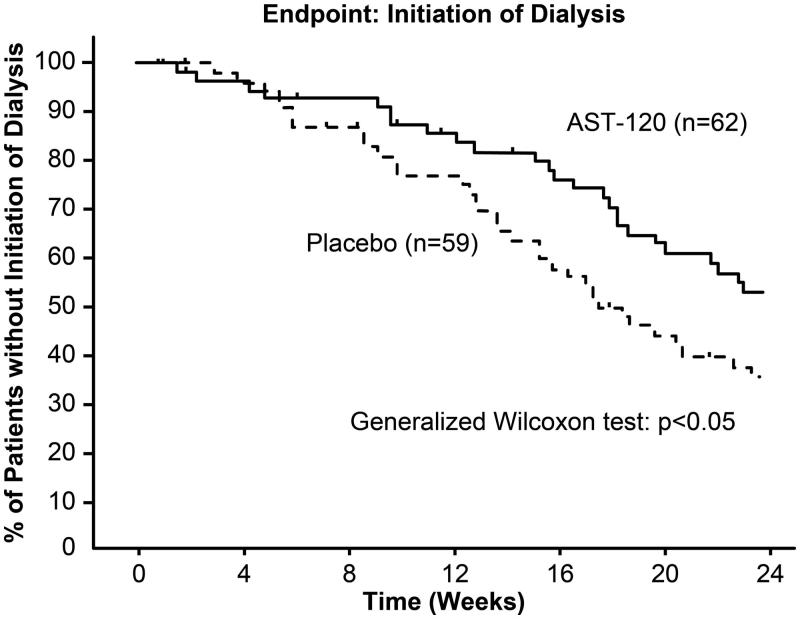
To assess the effect of AST-120 in delaying dialysis, the time to initiation of dialysis was compared between the AST-120 and placebo groups. The number of cases where dialysis was initiated was fewer in the AST-120 group than in the placebo group (39 patients in the AST-120 group versus 49 patients in the placebo group), but there was no significant difference in the time to initiation of dialysis between the two groups. However, a difference between the AST-120 and placebo groups was observed in the time to composite endpoint (i.e., doubling of sCr or initiation of dialysis, p < .05, Figure 1) [21]. The occurrence of doubling of sCr is used as a surrogate endpoint for the progression of CKD. In addition, subgroup analysis in progressive CKD with 1/Cr slope of ≤ −250 × 10−5 dL·mg−1·week−1 (62 patients in the AST-120 group and 59 patients in the placebo group) revealed a difference in the initiation of dialysis between the AST-120 and placebo groups (p < .05, Figure 2) [22].
Figure 1.
Kaplan–Meier analysis of event-free rate in the second phase III trial for composite endpoint [21].
Figure 2.
Kaplan–Meier analysis of event-free rate in the second phase III trial based on subgroup analysis in progressive CKD with 1/Cr slope ≤ −250 × 10−5 dL·mg−1·week−1 [22].
The above results suggest that AST-120 prolongs the time to the initiation of dialysis. Based on these results, AST-120 was approved in Japan in 1991 for treating uremic symptoms and prolonging the time to the initiation of dialysis in patients with progressive CKD.
Post-marketing drug use investigation
After AST-120 was approved in Japan, an investigation of the drug’s use was conducted from 1992 to 1996 [23]. Data from 1865 CKD patients taking AST-120 (mean dosage of 4.9 g/day) was gathered from 331 hospitals and 1848 patients were analyzed. The global improvement rating, assessed by the same methodology as in the second phase III trial, was 52.3% for ‘improved’ or better. For 1777 cases in which the 1/Cr slope data were available both before and after administration of AST-120, there was a significant reduction in 1/Cr slope with AST-120 treatment (–445 ± 1,373 to –105 ± 381, p = .0001, Paired U-test). The major adverse drug reactions reported from pre and post-marketing studies in Japan were gastrointestinal symptoms, such as constipation, anorexia, nausea/vomiting and abdominal distension at 4.51%. Based on the results given above, the clinical efficacy of AST-120 was verified. AST-120 was approved upon reexamination in Japan in 1998.
Carbonaceous oral adsorbent’s effects on progression of CKD study
The Carbonaceous Oral Adsorbent’s Effects on Progression of CKD (CAP-KD) study was conducted in Japan from 2004 to 2007 to evaluate the efficacy and safety of AST-120 in 460 patients with progressive CKD (sCr <5.0 mg/dL with negative 1/Cr slope) [24]. All patients followed a low-protein diet and took antihypertensive medication [angiotensin-converting enzyme inhibitor (ACEi) and/or angiotensin II receptor blocker (ARB)], and were randomized to either a control group or a group treated with AST-120 (6 g/day). The composite primary endpoint was doubling of sCr, increase in sCr level to ≥6.0 mg/dL, need for dialysis or transplantation or death. Over the course of 56 weeks, the number of primary endpoint events (43 for control versus 42 for AST-120) and event-free survival did not differ between the two groups.
The estimated Cr clearance, one of the secondary endpoints, decreased more in the control group than in the AST-120 group (−15% per year versus −12% per year, relative to the baseline value, p = .001, linear mixed model). Furthermore, the estimated glomerular filtration rate (eGFR) decreased more in the control group than in the AST-120 group (p < .001, linear mixed model).
Similar to the results of previous studies, adverse events involving the gastrointestinal system were much more common in the group given AST-120. With those events excluded, numbers of adverse events in the 2 treatment groups were nearly equal.
Trials that took place outside of Japan
Dose-ranging study in the United States of America
From 2003 to 2004, a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study was conducted in the United States of America (USA) to examine the effects of 3 daily doses of AST-120 in patients with moderate to severe CKD, using serum IS as a parameter [9]. The 164 patients were randomly assigned to 1 of 3 doses of AST-120 (0.9, 2.1 or 3.0 g), or placebo, to be taken 3 times daily for 12 weeks. AST-120 decreased serum IS levels in a dose-dependent fashion. Based on these results, the dose of 3 g, 3 times daily was adopted as an optimal dose for the EPPIC trials.
Evaluating prevention of progression in CKD (EPPIC) trials
From 2007 to 2012, the multinational, randomized, double-blind, placebo-controlled EPPIC-1 and EPPIC-2 trials were conducted in North America, Latin America and Europe, to evaluate the effects of AST-120 on the progression of CKD when added to standard therapy [25]. The study included 2035 patients with moderate to severe CKD (sCr at screening, 2.0–5.0 mg/dL for men and 1.5–5.0 mg/dL for women), whose blood pressure was stable. If a patient was receiving antihypertensive therapy, the treatment must have included either an ACEi or an ARB unless contraindicated. Patients received either AST-120 (9 g/day) or a placebo. The primary endpoint was a composite of dialysis initiation, kidney transplantation and sCr doubling. The time to primary endpoint was similar between the AST-120 and placebo groups in each trial individually and in a pooled analysis of both trials. In the pooled analysis, a significant difference was observed in the change in eGFR from baseline, one of the secondary endpoints (p = .04, mixed-effect model).
In a post-hoc analysis, risk factors for the progression of CKD were explored in the pooled analysis of both trials. In a multivariate analysis using baseline parameters, sex, urinary protein-to-urinary creatinine ratio (UP/UCr), anemia, use of ACEi/ARB, hematuria, systolic blood pressure, region (i.e., North/Central/Latin America and Europe), and baseline sCr levels were associated with the primary endpoint, and UP/UCr, hematuria and region were associated with eGFR % change. It was shown that the common covariates for the primary endpoint and eGFR decline (i.e., UP/UCr ≥1.0 and hematuria), were independent risk factors for both ESRD occurrence and rapid disease progression in the EPPIC trials [26]. In addition, the cumulative event-free rates in the pooled placebo intent-to-treat (ITT) population were lowest in hematuria-positive patients with UP/UCr ≥1.0 at baseline [25]. In the subgroups with factors predicting rapid disease progression (i.e., UP/UCr ≥1.0 and hematuria) and use of ACEi/ARB, additional treatment with AST-120 reduced the risk of achieving the primary endpoint (hazard ratio (HR): 0.74, 95% confidence interval (CI): 0.56–0.96). The declines in eGFR from baseline in the AST-120 group were smaller than the declines in the placebo group (p = .035). These results suggest that treatment with AST-120 delays the time to the primary endpoint in patients with progressive CKD receiving standard therapy [26].
In another post-hoc analysis of the patients from the USA, there was a significant difference between the treatment groups in the time to achieve the primary endpoint (HR: 0.74, 95% CI: 0.56–0.97) in the per-protocol population with compliance rates of ≥67% [27]. For that reason, the time to achieve the primary endpoint in the American patients was similar to that projected before the study.
In safety population, there was virtually no difference in the rate of mild to severe treatment-emergent adverse events in 2 treatment groups. The most commonly reported treatment-related adverse events in the AST-120 groups occurred in the gastrointestinal system, which affected similar proportions of patients in the placebo groups [25].
Kremezin study against renal disease progression in Korea
From 2009 to 2013, the randomized, controlled Korean study, K-STAR was conducted with 579 patients with CKD stage 3 or 4 [28]. Patients were randomized to receive either conventional treatment in the control group or conventional treatment combined with AST-120 (6 g/day). The composite primary endpoint was doubling of sCr, 50% reduction in eGFR or the initiation of renal replacement therapy. Over 36 months, the two treatment groups were not different in the occurrence of the composite primary endpoint.
The result of a post-hoc analysis with the per-protocol group revealed that AST-120 patients with higher compliance had fewer composite primary outcomes. In addition, the eGFR was more stable in the AST-120 arm, especially in diabetic patients [29].
In safety population, the rates of severe adverse events were not different between two arms. Gastrointestinal symptoms were more frequent in the AST-120 arm [28].
Discussion
We reviewed existing clinical data on AST-120, including randomized clinical trials conducted in the premarket period.
The time to initiation of dialysis is utilized as a major outcome in many clinical studies of CKD. However, initiation of dialysis is a late event in CKD, requiring a long observation period and a large sample size to assess accurately. Alternative endpoints to reflect the time to initiation of dialysis are needed in clinical practice. The results of a meta-analysis using 35 cohorts supported using reduced eGFR declines (such as a 30% reduction over 2 years) as an alternative endpoint for CKD progression [30]. To assess the progression of CKD, sCr elevation is also frequently used as a marker. In the second phase III trial, there was a significant difference between the AST-120 and control groups in 1/Cr slope. Furthermore, significant differences between the AST-120 and control groups were observed for eGFR in the CAP-KD study and EPPIC trials. These results support an inhibitory effect of AST-120 on the progression of CKD.
In contrast, significant differences were not observed between the AST-120 and control groups in the time to renal events, such as the initiation of dialysis, in the clinical studies described above. One possible explanation for this is that the disease progression was more gradual than expected in those studies. In the EPPIC trials, the estimated median time to primary endpoints for the placebo groups was 124 weeks with power calculations, but the actual times were 189.0 and 170.3 weeks for EPPIC-1 and EPPIC-2, respectively [25]. In K-STAR, standard medical care was effective during the study period because the eGFR declines were significantly slowed after randomization in both the treatment and control groups. Subgroup analysis allowed us to observe a significant difference between the AST-120 and control groups for the time to initiation of dialysis in the second phase III trial, and for the time to composite endpoint in the EPPIC trials. These results suggest that AST-120 prolongs the time to the initiation of dialysis, especially in progressive CKD.
In future trials to verify the efficacy of AST-120 in prolonging the time to initiation of dialysis, the inclusion criteria should be set carefully; defining progressive CKD using markers such as sCr elevation and eGFR decline is reliable [30]. During the screening process, the progression of CKD should be confirmed using sCr and/or eGFR during a pretreatment observation period. In KDIGO guideline, a change in eGFR category confirmed by a minimal percentage of change in eGFR (25% or greater) over a 1-year period is suggested as a definition of CKD progression [31]. In a post-hoc analysis of EPPIC trials, eGFR of patients supposed to be rapid decliners (UP/UCr ≥1.0, hematuria and use of ACEi/ARB) in placebo group decreased about 25% during first 48-weeks of treatment period, similar to KDIGO suggestion [26]. In a post-hoc analysis of second phase III trial, the progression was defined as 1/Cr slope ≤ −250 × 10−5 dL·mg−1·week−1, using ≥7 measurements of sCr in 24-weeks. As for studies in which so few participants reached the primary endpoint and there was no difference between groups, negative 1/Cr slope using ≥4 measurements in 48-weeks was defined in inclusion criteria in CAP-KD study, and neither sCr elevation nor eGFR decline was evaluated for screening in EPPIC trials. Results of the above studies suggest that 1/Cr slope and/or eGFR could be used and more frequent measurements would bring more reliable evidence for the progression. On the other hand, it is reported that there are many CKD patients have a non-linear GFR trajectory or a prolonged period of nonprogression in contrast to the traditional paradigm of steady GFR progression over time [32]. Higher baseline eGFR, male sex, diabetes status, steeper eGFR slope, and non-renin-angiotensin aldosterone-system antihypertensives are supposed to be associated with a subsequent non-linear GFR trajectory [33]. Therefore, careful examination should be considered to evaluate the GFR decline in the interventional trials. In addition, the results of post-hoc analysis of the EPPIC trials suggested that urinary protein is associated with the progression of CKD. While urinary protein has been associated with CKD progression in several trials [34], more evidence from large-scale investigations should be obtained to use it as a surrogate endpoint.
CKD progression can be affected by treatment. ACEi/ARB are prescribed frequently for hypertensive therapy in CKD patients today, which slows the progression of CKD [35]. The efficacy of AST-120 was verified in CKD patients who were taking ACEi or ARB in the CAP-KD study and after subgroup analysis in the EPPIC trials. AST-120 and antihypertensive medications may work additively or synergistically on CKD, using different mechanisms. However, there are various antihypertensive medications, including ACEis, ARBs, calcium channel blockers and diuretics. The inclusion criteria in trials should standardize concomitant medications, antihypertensive medications in particular.
The background of CKD has changed during the two decades from the second phase III trial to the CAP-KD study and EPPIC trials. The ratio of patients with diabetic nephropathy in the control group increased from 5% in the second phase III trial [20] to 24% in the CAP-KD study [24] and 40.4% in the EPPIC trials [25], while the ratio of patients with nephritis decreased. In addition, the rate of renal function decline during the study period represented by sCr or eGFR was more rapid in the second phase III trial than in the CAP-KD study and EPPIC trials. In the second phase III trial and CAP-KD study, the urinary protein was neither in inclusion nor in exclusion criteria. Urinary protein/albumin concentration is known to be related to the incidence of renal insufficiency in diabetic nephropathy [36,37]. That is, the rate of CKD progression in patients with high urinary protein concentration is assumed to be more rapid than that with low urinary protein concentration. On the other hand, there are reports that among normoalbuminuric patients some patients do progressive [38]. In the above studies involving AST-120, diabetic nephropathy patients would have involved various urinary protein concentration and rate of CKD progression. Today, disease including various backgrounds in conventional diabetic nephropathy is named diabetic kidney disease. One possible explanation why the efficacy of AST-120 to reach the primary endpoint was not supported in recent studies is that the numerous CKD patients with diabetic kidney disease, whose rate of renal functional decline was various, extended the length of the study period. As mentioned above, defining CKD progression properly using sCr elevation and eGFR decline, medications background and the cause of CKD need to be considered.
One possible mechanism of the action of AST-120 is the removal of uremic toxins and their precursors in the intestines. The accumulation of uremic toxins, such as IS and PCS, has been shown to be related to the progression of CKD and cardiovascular disease. The oral administration of IS to uremic rats increased the sCr, blood urea nitrogen levels and the glomerular sclerosis index [4]. In addition to glomeruli, IS is toxic to various cells. IS increased oxidative stress, enhanced expression cytokine and inflammatory genes, and induced mediators for fibrosis in renal tubular cells [5]. Administration of IS increased oxidative stress in rat kidney and administration of AST-120 ameliorated oxidative stress in CKD rats [39]. IS may have a significant role in cardiovascular disease such as arteriosclerosis [6,7]. IS inhibited cell viability through oxidative stress in vascular endothelial cells, and promoted aortic calcification and aortic wall thickening in hypertensive rats [6]. From the cohort study with 139 CKD patients, it was suggested that the highest IS tertile is related to overall and cardiovascular mortality (p = .001 and .012, respectively) [8]. AST-120 adsorbs indole, a precursor of IS derived from the metabolism of tryptophan by bacteria within the gastrointestinal tract. In a dose-ranging study with 164 CKD patients in the USA, AST-120 decreased serum IS levels in a dose-dependent fashion [9]. Administration of AST-120 reduced accumulation of IS and oxidative stress followed by amelioration of endothelial dysfunction in CKD rats [40]. In nondiabetic CKD patients, administration of AST-120 reduced arterial stiffness and intima-media thickness [41]. Therefore, AST-120 attenuates IS accumulation in patients with CKD and may affect not only renal function, but also the cardiovascular system.
In addition to the above reports, clinical evidence for relationships among uremic toxins, CKD progression and CKD events, such as the initiation of dialysis is expected. In K-STAR, the AST-120-induced decrease in the serum IS concentration inversely correlated with the occurrence of composite primary outcomes [29]. These results support the association of IS with eGFR decline and CKD events.
In some reports, it was suggested that serum IS starts to decrease early after AST-120 administration [9,10]. The study period can be shortened when using IS as a surrogate endpoint. Therefore, it seems more reasonable to assess the efficacy of AST-120 by IS than by renal function and CKD events. Because AST-120 adsorbs various substances in addition to IS, novel markers that can be used to assess the progression of CKD or cardiovascular disease may be found in future research. Those substances may be associated with progressive factors such as hematuria and urinary protein, which were identified from post-hoc analyses of the EPPIC trials.
For CKD treatment, the approved dosage and administration of AST-120 is 6 g/day orally as capsules and fine granules. This high dosage makes it difficult for patients to take AST-120 for extended periods. The result of the post-hoc analysis with a per-protocol group in K-STAR revealed that there were fewer composite primary outcomes for AST-120 patients with higher compliance. This result suggests that it is necessary to improve medication adherence in order to maximize the efficacy of AST-120. In 2017, new dosage form (tablets) of AST-120 was developed and is expected to improve medication adherence.
In addition to adsorbents for uremic toxins derived from the diet, a low-protein diet is generally encouraged to prevent the progression of CKD [42,43]. AST-120 administration, concurrent with a low protein diet, was superior to a low protein diet alone in inhibiting the accumulation of IS [44]. In addition, the diagnosis of renal function at dialysis initiation is different among countries [27]. All of these differences may affect the results of clinical trials. For further verification of the efficacy of AST-120 in multinational trials, actual clinical parameters in each country should be comprehensively evaluated.
We reviewed past clinical data, consisting of randomized clinical trials of AST-120 that included the premarket period and post-hoc analyses. In several trials, the benefit of adding AST-120 was verified by primary analysis or post-hoc subgroup analysis. As for renal events, post-hoc analyses revealed significant differences between the AST-120 and control groups in the second phase III trial and in EPPIC trials, especially in patients with progressive CKD. Although current CKD treatments focus on managing blood sugar levels and blood pressure to treat two major causes of CKD, diabetes [e.g., dipeptidyl peptidase-4 inhibitors (DPP-4i)/sodium glucose transporter-2 inhibitor (SGLT-2i)] and nephrosclerosis (e.g., ACEi/ARB), there is no other alternative than AST-120 that can be used for renal failure caused by any primary disease only if the progression of disease is confirmed. However, among previous studies involving AST-120, the inclusion criteria (severity and progression of CKD), patient baseline characteristics (cause of CKD, concomitant medications and country), medication compliance, study duration and outcomes (event occurrence, 1/Cr slope and eGFR decline) were not identical. Among the above parameters, blood pressure controlled and hematocrit ≥30% were proposed as determinants of AST-120 efficacy in diabetic nephropathy [45]. UP/UCr ≥1.0, hematuria and use of ACEi/ARB were proposed in a post-hoc analysis of EPPIC trials [26]. Because there are few investigations until now, further investigations are expected in order to characterize the patients who could have benefit by AST-120 treatment. Standardization (e.g., with respect to the progression of CKD and antihypertensive medications) might be essential to evaluate the clinical efficacy of AST-120 properly. In addition, measurements of uremic toxins such as IS could be meaningful to detect the progression of CKD because the association of IS with eGFR decline and CKD events is suggested according to the mechanism of AST-120. Under the current circumstances in which above various parameters, especially the background of CKD and concomitant medications are changing over time, the benefit of adding AST-120 in patients with progressive CKD should be verified continuously in studies reflecting actual clinical parameters. Researches using real-world data such as the claims database, the large-scale cohort study and the disease registry are also expected because there could be enough data involving AST-120 use by many patients for a long time.
Conclusions
We reviewed existing randomized clinical trials on AST-120. Results from these trials suggest that AST-120 delays the decline in renal function. In addition, AST-120 may delay the initiation of dialysis in progressive CKD via renoprotection. To verify the clinical efficacy of AST-120 when used with standard therapy, further randomized clinical trials are necessary in which the inclusion criteria should be defined carefully, including defining progressive CKD and standardizing various parameters such as comedications and the cause of CKD.
Disclosure statement
All three authors are employees of Kureha Corporation.
No potential conflict of interest was reported by the authors.
References
- 1.Vanholder R, Massy Z, Argiles A, et al. . Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant. 2005;20:1048–1056. [DOI] [PubMed] [Google Scholar]
- 2.Vanholder R, De Smet R, Glorieux G, et al. . Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. [DOI] [PubMed] [Google Scholar]
- 3.Duranton F, Cohen G, De Smet R, et al. . Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012;23:1258–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med. 1994;124:96–104. [PubMed] [Google Scholar]
- 5.Vanholder R, Schepers E, Pletinck A, et al. . The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol. 2014;25:1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niwa T. Indoxyl sulfate is a nephro-vascular toxin. J Ren Nutr. 2010;20:S2–S6. [DOI] [PubMed] [Google Scholar]
- 7.Lekawanvijit S. Role of gut-derived protein-bound uremic toxins in cardiorenal syndrome and potential treatment modalities. Circ J. 2015;79:2088–2097. [DOI] [PubMed] [Google Scholar]
- 8.Barreto FC, Barreto DV, Liabeuf S, et al. . Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulman G, Agarwal R, Acharya M, et al. . A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of AST-120 (Kremezin) in patients with moderate to severe CKD. Am J Kidney Dis. 2006;47:565–577. [DOI] [PubMed] [Google Scholar]
- 10.Niwa T, Nomura T, Sugiyama S, et al. . The protein metabolite hypothesis, a model for the progression of renal failure: an oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int. 1997;52:S23–S28. [PubMed] [Google Scholar]
- 11.Toyoda S, Kikuchi M, Komatsu T, et al. . Impact of the oral adsorbent AST-120 on oxidative stress and uremic toxins in high-risk chronic kidney disease patients. Int J Cardiol. 2014;177:705–707. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T, Kawagoe Y, Ueda Y, et al. . Effects of oral adsorbent AST-120 in patients with chronic renal failure with or without diabetes. Ren Fail. 2004;26:99–101. [DOI] [PubMed] [Google Scholar]
- 13.Sato E, Tanaka A, Oyama J, et al. . Long-term effects of AST-120 on the progression and prognosis of pre-dialysis chronic kidney disease: a 5-year retrospective study. Heart Vessels. 2016;31:1625–1632. [DOI] [PubMed] [Google Scholar]
- 14.Schulman G, Vanholder R, Niwa T. AST-120 for the management of progression of chronic kidney disease. Int J Nephrol Renovasc Dis. 2014;7:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu HM, Sun HJ, Wang F, et al. . Oral adsorbents for preventing or delaying the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi J, Tanaka T, Inagi R. Effect of AST-120 in chronic kidney disease treatment: still a controversy?. Nephron. 2017;135:201–206. [DOI] [PubMed] [Google Scholar]
- 17.Niwa T. The role of carbon adsorbent in the conservative management of chronic kidney disease. Panminerva Medica. 2017;59:139–148. [DOI] [PubMed] [Google Scholar]
- 18.Koide K, Koshikawa S, Yamane Y, et al. . Clinical evaluation of AST-120 in the treatment of chronic renal failure; multi-center, double-blind study in comparison with placebo. Clin Eval. 1987;15:487–525. [Google Scholar]
- 19.Mitch WE, Walser M, Buffington GA, et al. . A simple method of estimating progression of chronic renal failure. Lancet. 1976;2:1326–1328. [DOI] [PubMed] [Google Scholar]
- 20.Koide K, Koshikawa S, Yamane Y, et al. . Clinical evaluation of AST-120 on suppression of progression of chronic renal failure; multi-center, double-blind study in comparison with placebo. Clin Eval. 1987;15:527–564. [Google Scholar]
- 21.Sanaka T. The points to note in carbonaceous adsorptive agent. Kidney Dial. 2009;67:450–456. [Google Scholar]
- 22.Koshikawa S, Koide K, Yamane Y, et al. . The effect of AST-120 on delaying initiation of dialysis therapy in end-stage renal disease. Kidney Dial. 1992;32:783–794. [Google Scholar]
- 23.Akizawa T, Koide K, Koshikawa S. Effects of KREMEZIN® on patients with chronic renal failure; results of a nation-wide clinical study. Kidney Dial. 1998;45:373–388. [Google Scholar]
- 24.Akizawa T, Asano Y, Morita S, et al. . Effect of a carbonaceous oral adsorbent on the progression of CKD: a multicenter, randomized, controlled trial. Am J Kidney Dis. 2009;54:459–467. [DOI] [PubMed] [Google Scholar]
- 25.Schulman G, Berl T, Beck GJ, et al. . Randomized placebo-controlled EPPIC trials of AST-120 in CKD. J Am Soc Nephrol. 2015;26:1732–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulman G, Berl T, Beck GJ, et al. . Risk factors for progression of chronic kidney disease in the EPPIC trials and the effect of AST-120. Clin Exp Nephrol. 2018;22:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulman G, Berl T, Beck GJ, et al. . The effects of AST-120 on chronic kidney disease progression in the United States of America: a post hoc subgroup analysis of randomized controlled trials. BMC Nephrology. 2016;17:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cha RH, Kang SW, Park CW, et al. . A Randomized, controlled trial of oral intestinal sorbent AST-120 on renal function deterioration in patients with advanced renal dysfunction. Cjasn. 2016;11:559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cha RH, Kang SW, Park CW, et al. . Sustained uremic toxin control improves renal and cardiovascular outcomes in patients with advanced renal dysfunction: post-hoc analysis of the Kremezin study against renal disease progression in Korea. Kidney Res Clin Pract. 2017;36:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coresh J, Turin TC, Matsushita K, et al. . Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Astor BC, Lewis J, et al. . Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis. 2012;59:504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weldegiorgis M, de Zeeuw D, Li L, et al. . Longitudinal estimated GFR trajectories in patients with and without type 2 diabetes and nephropathy. Am J Kidney Dis. 2018;71:91–101. [DOI] [PubMed] [Google Scholar]
- 34.Heerspink HJ, Kröpelin TF, Hoekman J, et al. . Drug-induced reduction in albuminuria is associated with subsequent renoprotection: a meta-analysis. J Am Soc Nephrol. 2015;26:2055–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie X, Liu Y, Perkovic V, et al. . Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67:728–741. [DOI] [PubMed] [Google Scholar]
- 36.Agardh CD, Agardh E, Torffvit O. The association between retinopathy, nephropathy, cardiovascular disease and long-term metabolic control in type 1 diabetes mellitus: a 5 year follow-up study of 442 adult patients in routine care. Diabetes Res Clin Pract. 1997;35:113–121. [DOI] [PubMed] [Google Scholar]
- 37.Wada T, Haneda M, Furuichi K, et al. . Clinical impact of albuminuria and glomerular filtration rate on renal and cardiovascular events, and all-cause mortality in Japanese patients with type 2 diabetes. Clin Exp Nephrol. 2014;18:613–620. [DOI] [PubMed] [Google Scholar]
- 38.Moriya T, Suzuki Y, Inomata S, et al. . Renal histological heterogeneity and functional progress in normoalbuminuric and microalbuminuric Japanese patients with type 2 diabetes. BMJ Open Diab Res Care. 2014;2:e000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolati D, Shimizu H, Yisireyili M, et al. . Indoxyl sulfate, a uremic toxin, downregulates renal expression of Nrf2 through activation of NF-κB. BMC Nephrol. 2013;14:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Namikoshi T, Tomita N, Satoh M, et al. . Oral adsorbent AST-120 ameliorates endothelial dysfunction independent of renal function in rats with subtotal nephrectomy. Hypertens Res. 2009;32:194–200. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T, Kawagoe Y, Matsuda T, et al. . Oral ADSORBENT AST-120 decreases carotid intima-media thickness and arterial stiffness in patients with chronic renal failure. Kidney Blood Press Res. 2004;27:121–126. [DOI] [PubMed] [Google Scholar]
- 42.Klahr S, Levey AS, Beck GJ, et al. . The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of diet in renal disease study group. N Engl J Med. 1994;330:877–884. [DOI] [PubMed] [Google Scholar]
- 43.Effects of dietary protein restriction on the progression of moderate renal disease in the Modification of diet in renal disease study. J Am Soc Nephrol. 1996;7:2616–2626. [DOI] [PubMed] [Google Scholar]
- 44.Owada A, Nakao M, Koike J, et al. . Effects of oral adsorbent AST-120 on the progression of chronic renal failure: a randomized controlled study. Kidney Int Suppl. 1997;63:S188–S190. [PubMed] [Google Scholar]
- 45.Sanaka T, Akizawa T, Koide K, et al. . Protective effect of an oral adsorbent on renal function in chronic renal failure: determinants of its efficacy in diabetic nephropathy. Therapher Dial. 2004;8:232–240. [DOI] [PubMed] [Google Scholar]