ABSTRACT
A 20 year old male with past medical history of Type 1 Diabetes and Hypothyroidism presented to our hospital with severe hypocalcemia. His calcium was 5.8 mg/dl (normal range 8.6–10.3 mg/dl). He had been complaining of generalized weakness for weeks. Vital signs were within normal limits. Physical exam was significant for positive Chvostek sign. Other labs revealed low magnesium, low potassium, low vitamin D, low albumin, metabolic acidosis and low ferritin. He was started on supplements. Tissue transglutaminase antibody IgG was elevated. Upper gastrointestinal endoscopy showed scalloped and blunted duodenal mucosa. Duodenal biopsy showed villous blunting with intraepithelial lymphocytosis suggestive of celiac disease. He was started on gluten free diet. His symptoms improved and he was discharged home. Celiac disease can present in fulminant form with hemodynamic instability and is called celiac crisis. Celiac crisis is usually manifested by severe gastrointestinal manifestations, hypoproteinemia and metabolic and electrolyte disturbances requiring hospitalization. It is diagnosed by criteria proposed by Jamma et al. Celiac crisis is a rare presentation of celiac disease and is associated with high morbidity and mortality. Most of the cases respond to gluten withdrawal and nutritional suport and few require steroids.
Abbreviation: Type 1 DM -Type 1 Diabetes Mellitus
KEYWORDS: Celiac crisis, electrolytes, gluten
1. Introduction
Celiac disease is a multiple organ autoimmune disease precipitated by gluten proteins that affects small intestine in genetically predisposed children and adults [1]. The common manifestations of celiac disease are bulky, foul smelling diarrhea, abdominal distension and consequences of malabsorption such as failure to thrive, weight loss, anemia and osteopenia [2]. Celiac crisis is a life threatening manifestation of celiac disease especially in children and rarely in adults. Celiac crisis usually presents with severe diarrhea, low protein and severe metabolic and electrolyte derangements that require hospitalization and treatment [3,4]. Celiac crisis is associated with high morbidity and mortality [5]. It is important to have high index of suspicion of celiac crisis especially in people with undiagnosed celiac disease since it can be the initial presentation of celiac disease as in our case report.
2. Case report
A 20 year old male of mixed European descent with past medical history of Type 1 Diabetes and Hypothyroidism and vitamin D deficiency was called by his endocrinologist and told to go to emergency department after his labs showed severe hypocalcemia. His calcium was 5.8 mg/dl (normal range 8.6–10.3 mg/dl). His corrected calcium was 6.8 mg/dl and ionized calcium was 0.76 mmol/L (normal range 1.15–1.33 mmol/L). He had been complaining of generalized weakness for couple of weeks. Patient and his father reported history of bloating and swelling of feet. He denied any history of seizure like activity, nausea, vomiting, abdominal pain. He reported that his bowel movements were mostly 1–2 times a day light brown and formed. He had been on variable doses of magnesium for the last one year and would get loose bowel movements with high doses of magnesium. He was able to tolerate pasta without any symptoms. He denied history of weight loss, fever, chills. He had been taking calcium, magnesium and vitamin D supplement since his labs showed low calcium, magnesium and vitamin D levels one year ago. He was on insulin for Type 1 diabetes and levothyroxine for hypothyroidism. Family history was positive for hypothyroidism in both parents. Vital signs on admission revealed heart rate of 88/minute, respiratory rate of 18/minute, temperature of 98.4 F and blood pressure of 110/79 mm Hg. Physical exam was significant for 1+ bilateral lower extremity edema and positive Chvostek sign.
Other labs on admission revealed magnesium of 1 mg/dl (normal 1.9–2.7 mg/dl), potassium of 3.1 meq/L (normal 3.5–5.1 meq/L), 25 Hydroxy vitamin D of 10.1 ng/ml (normal >20 ng/ml), parathyroid hormone of 140 pg/ml (normal 12–88 pg/ml), albumin of 2.7 g/dL (normal 3.5–5.7 g/dL), venous PH of 7.314 (normal 7.32–7.43), bicarbonate of 19.8 meq/L (normal 21–31 meq/L), ferritin of 7 ng/ml (normal 27–300 ng/ml), iron of 14 mcg/dL (normal 50–212 mcg/dL), iron sat of 7 (20–50%). His hemoglobin was 8.6 g/dL (14–17.5 g/dL), Mean Corpuscular Volume (MCV) 79 fl (normal 80–99 fl), Mean Cellular Hemoglobin (MCH) 24.8 pg (normal 27–34 pg) and total leucocyte count was 16,600/microL (normal 4800–10,800/microL) . Renal function was normal. Electrocardiogram revealed prolonged QT interval. Computerized Tomography (CT)scan of abdomen revealed diffuse enteritis with reactive mesenteric adenopathy.
He was started on calcium gluconate drip along with oral calcitriol and oral calcium carbonate. He was placed on Lantus 16 U nightly, Humalog 6 units with meals and sliding scale for management of Type 1 Diabetes Mellitus. He was placed on intravenous levothyroxine 75 mcg daily for management of hypothyroidism. When he presented his TSH was very high 383 uIU/ml (normal 0.5–5.3 uIU/ml) with low free T4 0.56 ng/dl (normal 0.58–1.64 ng/dl). He was given 100 mg hydrocortisone in the emergency room due to concern for myxedema and adrenal crisis. Since he was hemodynamically fine and his mental status was intact further doses of hydrocortisone was avoided. Magnesium, potassium and iron was repleted intravenously. Fractional excretion of potassium in urine was 7.34% (normal 4–16%). Fractional excretion for calcium and magnesium from urine were within normal limits. Tissue transglutaminase antibody IgG was elevated at >100 U/ml (normal 0–5 U/ml). He was positive for HLA DQ8 heterozygote. His electrolytes started improving after second day of hospitalization and he was switched to oral replacement. He underwent upper gastrointestinal endoscopy on third day of hospitalization. Findings showed scalloped and mildy blunted duodenal mucosa raising suspicion for celiac disease (see Figure 1). Biopsy showed chronic duodenitis with marked villous blunting with intraepithelial lymphocytosis suggestive of celiac disease (see Figure 2). Total lymphocyte count at duodenal biopsy was 40/100 epithelial cells. He was started on gluten free diet. His calcium, magnesium and potassium improved to normal range on fourth day of hospitalization. His symptoms improved. He was discharged home on oral calcitriol, calcium carbonate, high dose vitamin D, magnesium oxide and potassium chloride. He was advised to follow up with gastroenterology in 2–3 months with CT abdomen to see if enteritis improved with gluten free diet. His long term care plan was gluten free diet, oral calcium, vitamin D and magnesium supplements. He was advised to follow up regularly with Gastroenterology and Endocrinology in clinic.
Figure 1.
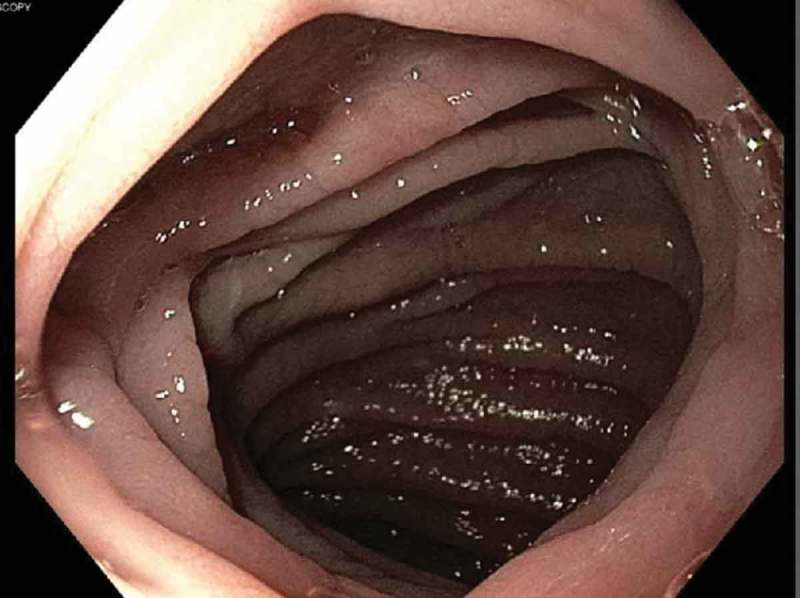
Upper GI endoscopy showing scalloped duodenal folds.
Figure 2.
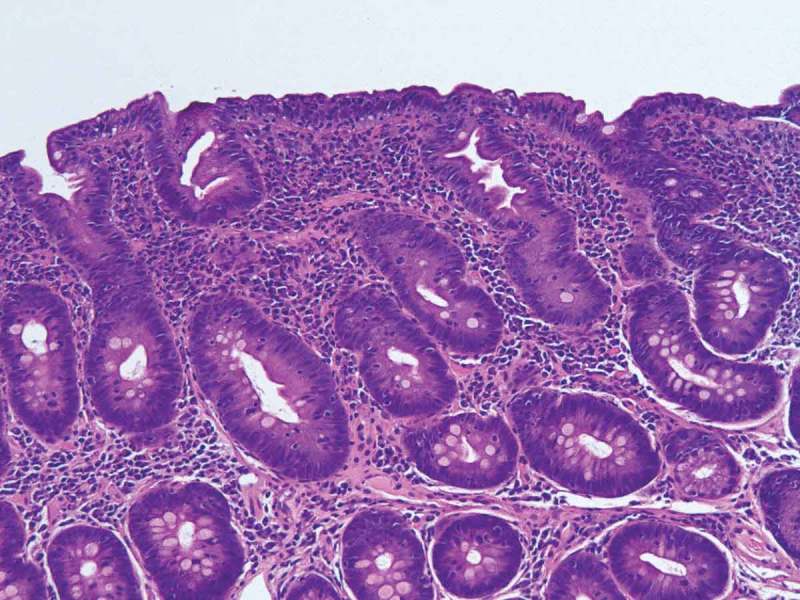
Duodenal biopsy image.
3. Discussion
Celiac disease (also called gluten sensitive enteropathy and nontropical sprue) is an immune mediated disease of small intestine characterized by malabsorption symptoms and villous atrophy precipitated by gluten protein [6]. Originally considered as a rare disease of childhood it now affects people of all age groups [6]. It was first reported by Samuel Gee in 1888 [7]. Celiac disease is closely associated with HLA-DQ2 and/or DQ8 gene loci hence it is believed to be caused by immune disorder in genetically susceptible population triggered by gliadin component of gluten [8]. Celiac disease is diagnosed by serologic evaluation (anti-tissue transglutaminase (TTG) antibody, anti endomysial antibody, anti Deaminated Gliadin Peptide (DGP) antibody), genetic testing for HLA DQ2/DQ8 and small intestinal biopsy [8]. Histologic findings include intraepithelial lymphocytes, loss of villi, flat mucosa and crypt hyperplasia [9,10].
Type 1 Diabetes and Hypothyroidism are the most common autoimmune diseases associated with celiac disease although there is no specific name for this condition [2]. Our patient did not have other features (adrenal insufficiency, hypoparathyroidism, candidiasis, hypogonadism) to qualify for autoimmune polyglandular syndrome type 1 or 2.
In most adult cases celiac disease presents as indolent course with gastrointestinal symptoms and nutritional abnormalities. Sometimes celiac disease can present as acute fulminant form called as celiac crisis. The term celiac crisis has been in use since the 1950s [11]. Celiac crisis is usually manifested by severe gastrointestinal manifestations, hypoproteinemia and metabolic and electrolyte disturbances requiring hospitalization [12]. Jamma et al. proposed a criteria for diagnosis of celiac disease [13]. The criteria requires acute onset of progression of gastrointestinal symptoms requiring hospitalization and/or parenteral nutrition with at least 2 of following (hemodynamic instability, neurologic dysfunction, renal dysfunction, metabolic acidosis, hypoproteinemia, electrolyte abnormalities and weight loss) [13]. In our case rapid onset of enteritis requiring hospitalization along with metabolic acidosis, hypoproteinemia and electrolyte abnormalities was sufficient for diagnosis of celiac crisis.
Only a handful of cases of celiac crisis in adults have been reported in literature [13]. Jamma et al. reported 12 cases of celiac crisis [13]. 11 of those patients had positive antibodies and all patients had positive histology findings. 50% of the 12 patients responded to gluten withdrawal and nutritional support and rest of the patients needed steroids [13]. In our case, the patient improved with gluten withdrawl and nutritional support. Celiac crisis is often precipitated by stress stimulus like surgery, infection or pregnancy [14,15]. In our case no such precipitant could be found.
Celiac crisis is associated with high morbidity and mortality [5]. In most of the cases celiac crisis presents as the initial manifestation of celiac disease [12] as in our case. Clinicians should have a high index of suspicion of celiac crisis in someone presenting with gastrointestinal disturbances, hypotension, electrolyte and metabolic derangements after common causes have been ruled out.
Disclosure statement
No potential conflict of interest was reported by the authors. The authors do not have affiliation with or financial involvement with an organization or entity with a financial interest in or financial conflict with subject matter or materials discussed in the manuscript.
References
- [1].Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McAllister BP, Williams E, Clarke K.. A comprehensive review of celiac disease/gluten-sensitive enteropathies. Clin Rev Allergy Immunol. June2018. DOI: 10.1007/s12016-018-8691-2. [DOI] [PubMed] [Google Scholar]
- [3].Mones RL, Atienza KV, Youssef NN, et al. Celiac crisis in the modern era. J Pediatr Gastroenterol Nutr. 2007;45(4):480–483. [DOI] [PubMed] [Google Scholar]
- [4].Fasano A, Catassi C.. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001;120(3):636–651. [DOI] [PubMed] [Google Scholar]
- [5].Ozaslan E, Köseoğlu T, Kayhan B. Coeliac crisis in adults: report of two cases. Eur J Emerg Med Off J Eur Soc Emerg Med. 2004;11(6):363–365. [DOI] [PubMed] [Google Scholar]
- [6].Green PHR, Cellier C. Celiac disease. N Engl J Med. 2007;357(17):1731–1743. [DOI] [PubMed] [Google Scholar]
- [7].Smits BJ. History of coeliac disease. BMJ. 1989;298(6670):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119(1):234–242. [DOI] [PubMed] [Google Scholar]
- [9].Rubin CE, Brandborg LL, Phelps PC, et al. Studies of celiac disease. I. The apparent identical and specific nature of the duodenal and proximal jejunal lesion in celiac disease and idiopathic sprue. Gastroenterology. 1960;38:28–49. [PubMed] [Google Scholar]
- [10].Fry L, Seah PP, McMinn RM, et al. Lymphocytic infiltration of epithelium in diagnosis of gluten-sensitive enteropathy. Br Med J. 1972;3(5823):371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Andersen DH, Di Sant’agnese PA. Idiopathic celiac disease. I. Mode of onset and diagnosis. Pediatrics. 1953;11(3):207–223. [PubMed] [Google Scholar]
- [12].de Almeida Menezes M, Cabral V, Silva Lorena SL. Celiac crisis in adults: a case report and review of the literature focusing in the prevention of refeeding syndrome. Rev Espanola Enfermedades Dig Organo Of Soc Espanola Patol Dig. 2017;109(1):67–68. [DOI] [PubMed] [Google Scholar]
- [13].Jamma S, Rubio-Tapia A, Kelly CP, et al. Celiac crisis is a rare but serious complication of celiac disease in adults. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2010;8(7):587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bai J, Moran C, Martinez C, et al. Celiac sprue after surgery of the upper gastrointestinal tract. Report of 10 patients with special attention to diagnosis, clinical behavior, and follow-up. J Clin Gastroenterol. 1991;13(5):521–524. [DOI] [PubMed] [Google Scholar]
- [15].Malnick SD, Atali M, Lurie Y, et al. Celiac sprue presenting during the puerperium: a report of three cases and a review of the literature. J Clin Gastroenterol. 1998;26(3):164–166. [DOI] [PubMed] [Google Scholar]


