Abstract
To lock atmospheric CO2 at anthropogenic timescale, fast weathering silicates can be applied to soil to speed up natural CO2 sequestration via enhanced weathering. Agricultural lands offer large area for silicate application, but expected weathering rates as a function of soil and crop type, and potential impacts on the crops, are not well known. This study investigated the role of plants on enhanced weathering of wollastonite (CaSiO3) in soils. Using rooftop pot experiments with leguminous beans (Phaseolus vulgaris L.) and nonleguminous corn (Zea mays L.), CO2 sequestration was inferred from total inorganic carbon (TIC) accumulation in the soil and thermogravimetric analysis, and mineral weathering rate was inferred from alkalinity of soil porewater. Soil amendment with wollastonite promoted enhanced plant growth: beans showed a 177% greater dry biomass weight and corn showed a 59% greater plant height and a 90% greater dry biomass weight. Wollastonite-amended soil cultivated with beans showed a higher TIC accumulation of 0.606 ± 0.086%, as compared to that with corn (0.124 ± 0.053%). This demonstrates that using wollastonite as a soil amendment, along with legume cultivation, not only buffers the soil against acidification (due to microbial nitrogen fixation) but also sequesters carbon dioxide (12.04 kg of CO2/tonne soil/month, 9 times higher than the soil without wollastonite amendment).
1. Introduction
Atmospheric concentrations of carbon dioxide and other greenhouse gases (GHGs) have increased as a consequence of anthropogenic activities, resulting in a rise in overall global temperatures and frequency of extreme weather events.1 There is a global commitment to reduce GHG emissions, and carbon dioxide capture and storage (CCS) is seen as an essential strategy for reducing CO2 emissions. Among several approaches to CCS, enhanced weathering is a chemical storage route whereby CO2 is converted into CO32–/HCO3– by reaction with alkaline earth metal-oxide-rich minerals.2,3 The most suitable class of naturally occurring Ca- and Mg-containing minerals for CCS is silicates, owing to abundance, reactivity, and inertness of principal silicic byproduct ([SiOx(OH)4–2x]n). Even though silicates may not be as reactive as hydroxide minerals, they dominate the Earth’s crust, which makes them ideal candidates for weathering studies.4
In this study, the mineral wollastonite (nominally CaSiO3, but commonly found in association with other minerals, such as diopside (CaMgSi2O6)) was the main focus of investigation, because of its simple chemistry, high dissolution rate, and the ease of production of carbonated products due to the weaker bonding of Si to Ca ions.5,6 The world reserves of wollastonite are estimated to exceed 100 million tonnes with large reserves in China, Finland, India, Mexico, Spain, Canada, and the USA.7 For wollastonite, the enhanced weathering route is explained in eqs 1–3. In the pH range of ∼6.0–9.5, at an ambient temperature, according to the Bjerrum plot of the carbonate system, bicarbonate is the dominant species (eq 1). The metal ion (Ca2+) is liberated from the silicate by the proton (eq 2), and it ultimately reacts with the bicarbonate to precipitate as calcium carbonate (eq 3).8 Although this is the conventional description of the carbonation of silicate minerals, in regularly irrigated agricultural soil (the wollastonite application focus of this study), CaCO3 may not precipitate (solubility increases significantly as the pH drops below ∼6–8 at ambient conditions and is accentuated by the presence of salts) or may be redissolved into Ca2+ and 2HCO3– by excess carbonic acid (H2CO3). In this case, rather than remaining in the soil profile, the ions (Ca2+, HCO3–) gradually leach into the groundwater, and eventually into the oceans, where they are precipitated under alkaline conditions as calcium carbonates (eq 4)9
| 1 |
| 2 |
| 3 |
| 4 |
Kinetically, the mineral dissolution reaction (eq 2) is the weathering rate-limiting step10 governed by pH, whereas the carbonate precipitation/dissolution reactions are thermodynamically controlled. Increased CO2 capture requires higher calcium ions available for reaction with carbonic acid, and calcium release from wollastonite depends on the presence of protons. In agricultural soils, the type of plants and vegetation cover can contribute to enhancing the mineral weathering rate through several reactions that lead to soil acidity and consequently faster mineral dissolution. Organic acids (tartaric, oxalic, maleic, and citric acids) released as root exudates, microbial decay of plant litter, secretions from mycorrhizal symbionts (lichen acid, uronic acid, peptides, and amino acids), carbonic acid formed as a result of CO2 production during root respiration, and oxidation of soil organic matter microorganisms all lead to soil acidification.11,12 In this context, leguminous plants will have an added advantage as they provided more protons via nitrogen fixation process. During cultivation of leguminous plants, soil is acidified due to proton release from roots via nitrogen fixation (eq 5).13 The ammonia formed is assimilated to form amino acids and proteins, which dissociates to form organic anions (such as malate, citrate, and oxalate) and protons. These protons are transported to the rhizosphere to maintain a pH of 7 at the cytoplasm.14,15 These acids help to chelate and solubilize the otherwise normally insoluble elements such as iron and aluminum;16 hence, leguminous plants help to increase the soil fertility by fixing atmospheric nitrogen as well as by solubilizing essential micronutrients. Hence, protons from root exudates as well as nitrogen fixation can increase the dissolution of wollastonite to release Ca2+ ions and result in increased formation of calcium carbonate
| 5 |
This reaction is limited at a pH < 6.7; however, alkaline silicate mineral addition to agricultural soils can help in raising the pH,17 as silicate dissolution consumes protons (eq 2). Since an ideal soil pH for most of the plant is 5.0–6.5, excessive soil acidification by legumes or excessive alkalinization via application of mineral rock is undesirable.18 Hence, growing legumes in soil amended with alkaline minerals can result in a buffer system for optimal plant growth.
Although wollastonite weathering under laboratory conditions is well documented,19 no experimental data are available under crop conditions. The present study was carried out to determine the potential of wollastonite as a soil amendment for enhanced weathering, without posing a negative impact on plant growth. The main purpose of this study is to investigate the role of plants in enhanced weathering of wollastonite when applied to soils. Leguminous (beans) and nonleguminous (corn) plants were grown in soil amended with wollastonite. A hypothesis tested was whether the proton (H+) released by the beans plant (through eq 5) might stimulate Ca2+ release from wollastonite (eq 2). This study also investigated two effects of wollastonite application to soils: first, its effect on plant growth and second, the increase of inorganic carbon in soils amended with wollastonite. Carbon dioxide sequestration rate and extent were analyzed in terms of total inorganic carbon (TIC) content of soils, soil alkalinity, and thermogravimetric analysis (TGA) of soils.
2. Materials and Methods
2.1. Wollastonite Characterization
Wollastonite mineral sourced from Canadian Wollastonite’s Ontario mine was used for this study. Elemental composition of the wollastonite includes 26% silicon (55% SiO2), 18% calcium (26% CaO), 4.0% magnesium (9% MgO), 1.8% sulfur, 0.11% nitrogen, 0.10% P2O5, 0.10% K2O, 11 ppm copper, and 1.10 ppm zinc. Assuming all MgO is present as diopside (CaMgSi2O6), the estimated diopside content is 27.3 wt %; then, assuming that the remaining CaO is present as wollastonite (CaSiO3), the estimated wollastonite content is 39.2 wt %. This estimated mineralogical composition accounts for nearly two-thirds of the SiO2 content; thus, the remaining mineral mass is likely composed of free SiO2 and minor silicates and sulfates.
The particle size distribution of wollastonite was determined by laser diffraction (Malvern Mastersizer SM), and 90% of particles by volume were less than 25.9 μm in diameter (Figure S1). The loss on ignition, 0.50 wt % at 900 °C, was determined by thermogravimetric analysis (TGA); less than half of this loss occurs in the temperature range for CaCO3 decomposition, indicating negligible CO2 content.
The wollastonite powder was further characterized by N2 adsorption at 77 K in a physisorption analyzer (Autosorb iQ). Prior to adsorption measurement, the wollastonite sample was degassed in vacuum consecutively at 120 °C (30 min soaking time) and 350 °C (300 min soaking time). The isotherm (Figure S2) obtained showed a narrow H4 hysteresis loop, which is caused by capillary condensation and is indicative that wollastonite contains mesopores. The multipoint Brunauer–Emmett–Teller (BET) surface area is 20.28 m2/g, and the average pore diameter is 33.35 Å, which is characteristic of mesopore presence (though of relatively low cumulative pore volume given the BET value). The relatively low specific surface area makes it difficult for wollastonite to sequester CO2 directly from the gas phase (i.e., the atmosphere), as diffusion resistance into the mineral particle would be high, so carbonation kinetics would be very slow. Hence, the aqueous phase carbonation reaction occurring in the soil via eqs 1–4, which causes mineral dissolution and reprecipitation, is essential to achieve accelerated weathering.
2.2. Soil and Plant Selection
The agricultural soil was collected from a bean field at the intersection of Side Rd 12 and Concession Rd 4, close to the city of Guelph, Ontario (43°28′01.2″N 80°14′19.1″W). The soil was characterized for pH (4.94), total carbon (TC = 0.868%), total organic carbon (TOC = 0.808%), and total inorganic carbon (TIC = 0.060%), as described in Section 2.5.
Since beans and corn were grown in crop rotation on this particular farm, these plants were selected for this study. Beans (Phaseolus vulgaris L.) and corn (Zea mays L.) seeds were initially sowed on rockwool, inside a growth tent, maintained at 24/19 °C, light/dark cycle of 16/8 h, and humidity at 62%. After seed germination, the seedlings were transplanted into the soil.
2.3. Mineral Soil Amendment (MSA) and Experimental Setup
To determine a suitable wollastonite: soil amendment ratio for growing bean and corn, different amounts of wollastonite (10–2000 g) were added to a fixed mass of soil (8 kg, suitable for the size of the pots used). The variation of the pH of these wollastonite-amended soils was measured with respect to time to determine the amendment that results in a pH suitable for the growth of plants as well as for carbon dioxide sequestration (Figure S3). Geochemical modeling, using Visual Minteq v3.1, showed that at a pH of 7.23, i.e., obtained by mixing 1000 g of wollastonite with 8 kg of soil, the saturation index becomes positive, which is required for the precipitation of solid mineral phases, especially calcite in the case of wollastonite. Also, a high mixing ratio of wollastonite and soil is desirable to ensure that a sufficient amount of carbonate formation is taking place, so that its detection is possible via the TIC method (as discussed in Section 2.5).
Experimental pots were set up at a building rooftop in the University of Guelph, Ontario, Canada. The six pots set up included original soil (“soil”), wollastonite-amended soil (“MSA”), beans cultivated in original soil (“soil + bean”), beans cultivated in MSA (“MSA + bean”), corn cultivated in original soil (“soil + corn”), and corn cultivated in MSA (“MSA + corn”). Poly(vinyl chloride) pots of 9 L capacity, with a top diameter of 24 cm, were filled with soil or MSA. For the MSA, 1000 g of wollastonite powder was thoroughly mixed with 8 kg of soil prior to potting. No other chemical or mineral amendments (e.g., fertilizers) were used in these experiments. The seedlings, six per pot, were transplanted on Aug 20, 2017. At the start, all pots were supplied with adequate tap water, and later, watering spikes were used to maintain adequate soil moisture. The soil from each pot was sampled, over a period of 8 weeks, using a soil core sampler (1/2″ diameter) at five different points radially distributed and down to full depth and thoroughly mixed prior to chemical analyses. The experiment was terminated at the end of 8 weeks, during the first week of October, after which the temperature started to fall below 0 °C at night.
2.4. Plant Growth
The plant growth and development were analyzed based on the development stages. The vegetative (V1, V2, V3, V4) as well as reproductive stages (R1, R2, R3, R4) were recorded for beans, and the vegetative stages (V1, V2, V4, V6, V10, VT) were recorded for corn.20,21 V1 implies the development of the 1st leaf, V2–V10 = 2nd to the 10th leaf, and VT = appearance of the tassel (in corn). R1 and R2 imply the beginning of bloom and full bloom, respectively, whereas R3 and R4 imply the beginning of pod and full pod, respectively. At the end of the experimental run, after 55 days, the plants were harvested by cutting them just above the soil level. The main growth parameters recorded were plant height, stem width, leaf blade width, and fresh/dry biomass weight. Dry biomass weight was determined after drying the sample in a drying oven (Thermo Scientific) at 80 °C for 48 h.22
2.5. Chemical Analyses
The pH of MSA samples was determined using a suspension made up of various wollastonite–soil mixtures and 0.01 M CaCl2 solution, at a 1:5 mass ratio, which was placed on a shaker for 30 min to 72 h. At each measurement, the suspension was allowed to settle for 1 h before taking the pH measurement of the clear supernatant.23
Dried soil for other analyses was obtained by placing soil samples in a muffle furnace (Thermo Scientific F48055-60, Waltham, MA) maintained at 103 ± 2 °C for at least 15 h, which were then cooled in a desiccator containing silica gel. Dried samples were sieved through 200 μm mesh prior to analysis. To determine the total carbon (TC) in the samples, a Thermo Fisher FlashEA 1112 elemental analyzer was used. The solid sample is combusted with oxygen gas (99.995%) and helium (99.999%) in a dynamic flash combustion furnace; the resulting combustion gases are then passed through a gas chromatograph column, and a thermal conductivity detector quantifies the CO2 amount released from the solid sample.
To determine total organic carbon (TOC), inorganic carbon must be first removed. For the removal of inorganic carbonates, 30 g of soil was added to 50 mL of deionized water, and 1 M HCl, enough to remove the small quantity of TIC present in the sample, was added to it until the pH dropped below 4. This suspension was filtered using Whatman paper (45 μm) after 10 min, followed by drying as described before. The elemental analysis of the acid-treated sample gives the total organic carbon (TOC) content in the sample. Total inorganic carbon (TIC) is calculated as the difference between TC and TOC.23
Semiquantitative determination of calcium carbonate in the sample can be estimated by using TGA. The dried samples were placed in a Thermo Scientific Nicolet 700 TGA-FTIR analyzer, where approximately 20 mg was heated from an ambient temperature to 1000 °C in nitrogen gas atmosphere at a heating rate of 10 °C/min. Nitrogen gas of high purity (99.99%) was supplied at a constant flow rate of 100 mL/min as an inert purge gas. The sample weight (W) as a function of temperature (T) is recorded and plotted, and calcium carbonate content is determined based on weight loss above 500 °C.19
Part of the CaCO3 formed by the reaction of CO2 with wollastonite may dissolve into carbonates (CO32–) and bicarbonates (HCO3–) in the soil porewater, according to a system of chemical equilibria. Hence, to account for this mode of CO2 sequestration as part of the CCS potential of mineral soil amendment, the alkalinity of the soil sample was determined by the titration method (Hach method 8221). Total carbonates (based on alkalinity measured in mEq/L and later converted into mg CaCO3/L) were estimated using eq 6(24)
| 6 |
where α1 and α2 are the speciation coefficients for carbonate/bicarbonate system, which depend on the pH (−log[H+]) of the soil (eqs 7–9)
| 7 |
| 8 |
| 9 |
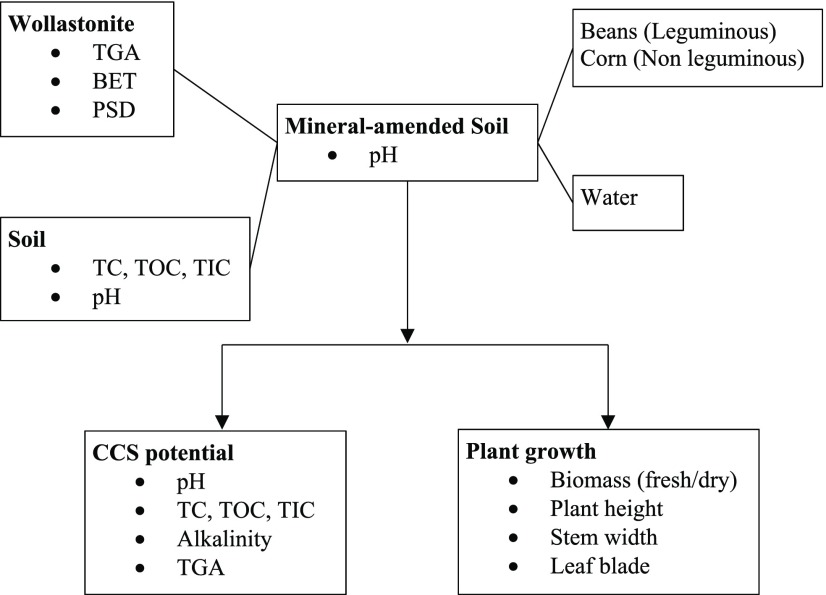
The values of Ka1 and Ka2 can be determined from the pKa value of bicarbonates (6.35) and carbonates (10.33). Total carbonate is also expressed in terms of mg Ca2+/L to give a measure of weathering, using the sum of calcium ions associated with carbonate ([Ca2+]/[CO32–] = 1:1) and bicarbonate ([Ca2+]/[HCO3–] = 1:2) ions. All chemicals used are of Fisher Scientific analytical grade. The overall schematic of the methodology is depicted in Figure 1.
Figure 1.
Overview of the methodology used in this study.
2.6. Data Analysis
All of the analysis readings were taken in triplicates, and mean results reported herein have been represented along with standard deviations. One-way analysis of variance with paired t-test comparison was primarily used to assess treatment differences between plant characteristics in various trials. P < 0.05 was used as the limit for statistical significance, unless otherwise stated.
3. Results
3.1. Plant Growth pH–Time Series
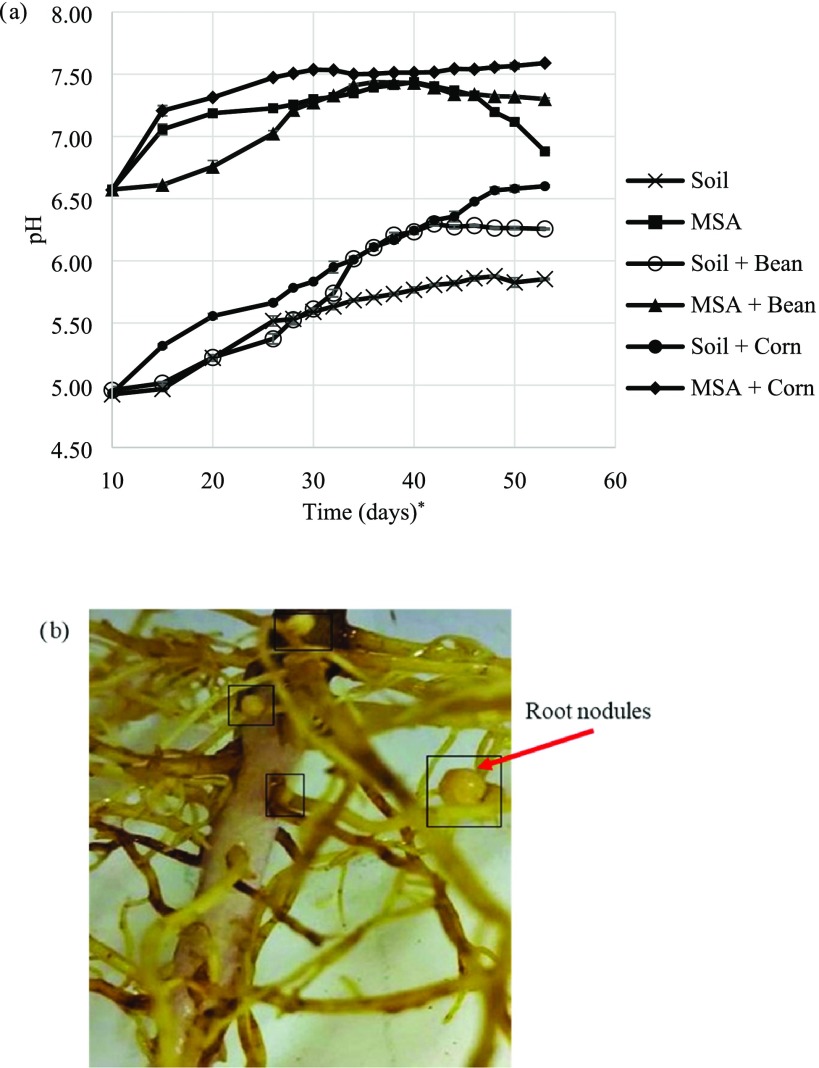
Change in pH in all pots was measured during the experimental run to analyze the effect of wollastonite addition (Figure 2a). Addition of wollastonite results in pH increase. The pH of MSA (7.06 ± 0.04, 15th day) is higher than that of soil (4.97 ± 0.02, 15th day). The pH of MSA + bean sample, in the range of 6.57–7.30, is higher than that of soil + bean (4.90–6.26), whose final value, in turn, is lower than the pH of soil + corn (6.60). The pH of MSA + bean lagged that of MSA + corn until the 38th day, after which the pH of MSA + bean again dropped. Early-stage pH buffering by the bean could be attributed to nitrogenase activity in the soil and root surface as nodulation commences, whereas the late-stage acidification could be attributed to the numerous fully formed root nodules (Figure 2b).25,26 The rhizobia that symbiotically work with leguminous crops, such as beans, aid in nitrogen fixation, which results in soil acidification due to proton release into the soil. Wollastonite addition balances this soil acidification, through partial dissolution, which releases alkaline earth elements, but the pH levels of MSA + bean remain lower throughout than those of MSA + corn. On the contrary, pH of MSA + corn is the highest at the end of the growth period (7.59 ± 0.01), indicating a dominant effect of the wollastonite alkalinity in regulating pH. Higher pH, however, does not necessarily indicate the greater extent of mineral weathering. The hypothesis being tested is that proton release during nitrogen fixation would facilitate wollastonite weathering and result in more calcium carbonate formation. This can be determined by measuring total inorganic carbon (TIC) in the soil samples (Section 3.3).
Figure 2.
(a) Soil pH–time series for all potting experiments, with and without plants, and (b) root nodules formed in MSA + bean sample (photograph taken on the 42nd day). (*Soil pH reading starts from the 10th day, which indicates the time when the seedlings were transplanted to the soil).
3.2. Wollastonite Impact on Plant Growth
Tracking of the development stages (Table S1) revealed that the plants performed identically in wollastonite-amended soil compared to those in the control (as-received soil) throughout the 8-week growth period. Differences are seen, however, when looking at the metrics for individual plant parts (Table 1). At the end of the growth period, corn grown in MSA had a 59% greater plant height and a 90% greater dry biomass weight. Similarly, MSA + bean plant sample showed a 177% greater dry biomass weight compared to the soil + bean sample. Both the plants showed better growth, in terms of plant height and biomass weight, in the wollastonite-amended soil (Figure 3). Although the bean plant grew to a similar height in both the amended and nonamended soils (262.5 ± 5.7 mm, P > 0.05), the leaf blade and stem widths were significantly greater in wollastonite amendment. Moisture content was slightly lower in the plants grown in amended soils, which correlates with denser plant tissue (i.e., plant size did not increase proportionally to dry weight). In addition, wollastonite-amended soils showed no signs of weed growth. These results suggest that wollastonite amendment does not present negative effects to plant growth (P > 0.05).
Table 1. Comparison of Plant Growth at the End of the Growth Period (55 Days); Average of up to Six Matured Seedlings.
| sample | stem width (mm) | plant height (cm) | leaf blade width (mm) | biomass fresh weight (g) | biomass dry weight (g) | moisture content (%) |
|---|---|---|---|---|---|---|
| soil+ bean | 2.4 ± 0.5 | 26.7 ± 2.8 | 34.7 ± 2.6 | 3.13 ± 0.5 | 0.33 ± 0.1 | 89.6 ± 0.5 |
| MSA + bean | 2.9 ± 0.3 | 25.9 ± 3.1 | 42.6 ± 2.8 | 5.73 ± 0.5 | 0.90 ± 0.1 | 84.1 ± 2.9 |
| soil + corn | 10.5 ± 1.3 | 17.0 ± 3.1 | 39.4 ± 5.5 | 13.70 ± 7.1 | 1.70 ± 0.6 | 82.4 ± 14.6 |
| MSA + corn | 10.2 ± 2.3 | 27.0 ± 13.5 | 38.5 ± 8.8 | 23.98 ± 19.3 | 3.23 ± 2.6 | 78.15 ± 24.3 |
Figure 3.
Comparison of above-ground growth of beans (left) and corn (right) in wollastonite-amended soil versus unamended soil, after 55 days.
3.3. Increased Soil Carbon Content
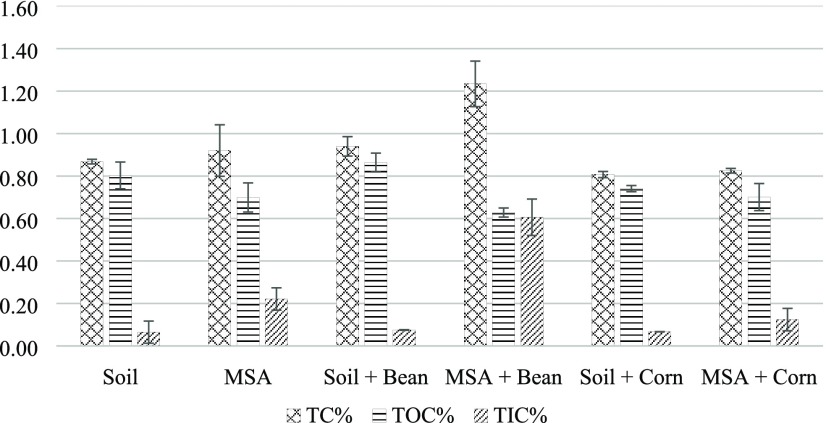
Figure 4 shows the soil carbon contents (TC, TOC, TIC) for all of the experimental pots recorded at the end of the growth period. MSA + bean soil samples showed the highest TIC of 0.606 ± 0.086%. In comparison, soil + bean soil showed a very low TIC accumulation of 0.075 ± 0.001%, which is similar to the soil (as-received) samples (0.065 ± 0.052%), but lower than the MSA samples (i.e., soil with wollastonite, but no plants) that showed a TIC accumulation of 0.221 ± 0.053%. MSA + corn sample had a TIC of 0.124 ± 0.053% TIC, which is not only lower than the MSA + bean samples, which might be due to the absence of protons to facilitate wollastonite dissolution, but also lower than the MSA samples. This implies that the type of plant, in addition to the amendment with wollastonite, has a significant impact on TIC accumulation. TOC contents in all experiments varied within a much narrower range (0.628–0.864%), with the main discernable trend being the three lowest values (0.628, 0.699, and 0.701%) seen in the three pots containing wollastonite.
Figure 4.
Soil carbon content (total, organic, and inorganic) at the end of the growth period (55 days).
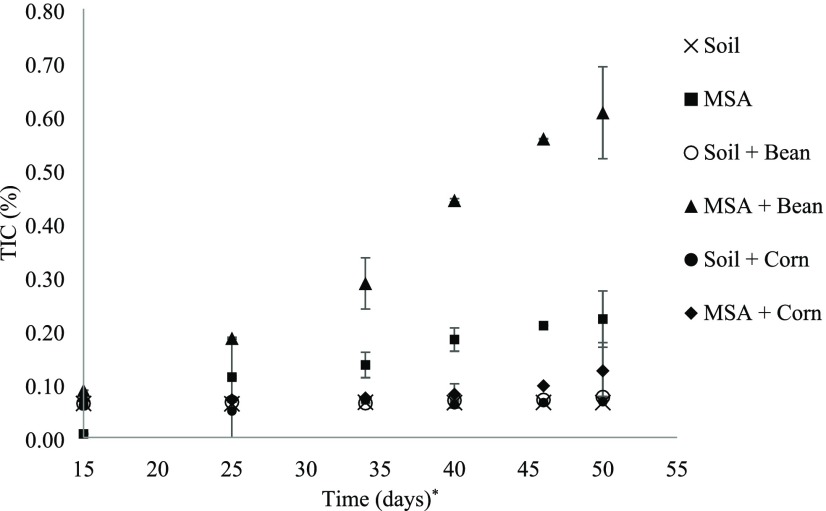
In the context of this study, the challenge seen with TOC is its long-term preservation in the soil as a carbon sink, as most organic compounds that make up TOC are highly unstable and can ultimately be released back to the atmosphere not only as CO2 but also as more potent GHGs (measured by the global warming potential (GWP)) such as methane (GWP100 years = 21), whereas nitrogen associated with organic matter and microbial activity can be emitted as even more potent nitrous oxide (GWP100 years = 310).27 On the other hand, TIC is the stable fraction of the soil carbon content, thus playing the dominant role in long-term carbon sequestration. Figure 5 shows that the TIC content for all potting samples increased throughout the growth period. The gradient of TIC increase (i.e., rate per unit time) for MSA + bean is 4 times greater than that for MSA.
Figure 5.
TIC accumulation over time for all plant growth experiments. (*TIC readings were recorded from the 15th day onwards).
At the application rate of wollastonite to soil used, and according to wollastonite carbonation stoichiometry, the maximal % TIC value possible in these tests would be 1.293%. Even though the bottom of the pots had holes for excess water to escape, during the experimental run, there was no excess water draining out of the system; hence, it can be assumed that only evaporation contributed to water removal, thus allowing easier carbon accounting. To account for the partial dissociation of formed calcium carbonate and the dissolution of calcium from wollastonite without subsequent precipitation as carbonate, the alkalinity of the soil was determined (Figure S4). The TIC results are in consensus with the alkalinity analysis, which shows the highest alkalinity of 24.0 ± 0.06 mequiv/L for MSA + bean sample, which is equivalent to 535.2 ± 1.3 mg/L of total dissolved carbonate (CaCO3) in the soil solution. Thermogravimetric analysis (TGA) confirms that like alkalinity, there is the presence of greater quantities of solid-phase carbonates in the wollastonite-amended samples with plants. The decomposition temperature range for calcite is approximately 500–800 °C,19 and the TGA graph (Figure S5) shows greater mass losses in this high-temperature range for wollastonite-amended soil samples (0.23–0.39 wt %) versus samples not amended (0.06–0.14 wt %). A weight loss of 0.39 wt % for MSA + bean, which corresponds to the thermal liberation of CO2(g), indicates a CaCO3(s) content of 0.65 wt %, which compares well with the TIC value (Figure 5) determined for the same sample (0.606%). All of these results support that wollastonite carbonates better with beans (leguminous species), as the plant type used for cultivation, rather than corn (nonleguminous species).
4. Discussion
4.1. Importance of pH
The availability of plant nutrients and the soil’s capacity to absorb and temporarily retain them are often mediated by the soil pH. Generally, soil pH in a range of 5–8.5 is acceptable for cultivation. Highly weathered soil (as used in the present study) is characterized by low calcium (Ca) and high aluminum (Al) contents, and as a result the root growth will be impaired, and water and nutrient uptake by the plant will be affected, hence restricting crop growth.28 It is usually the extremes of acidity (3–4) or alkalinity (8–10) that cause soil infertility. In acidic soils (as used in the present study), the ionic forms K+, Ca2+, Mg2+, Na+, Mn2+, Fe2+, SO42–, and Cl– predominate, with complex forms of aluminum (Al) with organic ligands, F and OH.21 Hence, acidic soil pH is characterized with high levels of Al3+ ions. In agriculture, usually calcium or magnesium carbonates are needed to increase the pH of an acidic soil to the desired pH by liming. The indirect liming effect can be achieved by using wollastonite, which dissolves to form calcium carbonate during enhanced weathering. By the addition of wollastonite, the results of this study showed improvement in the pH of the acidic-weathered soil by supplying exchangeable Ca2+. In a crop application, this would improve the overall soil nutrient profile. This is evident from the present results, where beans and corn grown on wollastonite-treated soil (MSA) showed almost 3-fold better growth as compared to that grown on untreated soil.
As soil systems are not closed, they are in a state of dynamic chemical equilibria as the soil is subjected to liquid inputs/outputs by rainwater, atmospheric condensation and runoff, and gases entering and leaving the profile. The pH buffering capacity of the wollastonite-amended soil depends on its capacity to resist pH change in a dynamic system. Soil organic matter is concentrated in the top layer of the soil profile, where weakly dissociated organic acids buffer the soil pH. Below pH 5, pH buffering in many soils is due to decomposition of clays and other remaining aluminosilicates, if they are present. For soils with pH in the neutral to moderately acidic range (5–7), it is usually ion-exchange reactions associated with clays and organic matter that control pH. Above pH 6.5, the concentrations of bicarbonate and carbonate anions increase, which is due to the presence of partly dissolved alkaline and alkaline earth elements; in the case of wollastonite, calcium carbonate becomes the dominant pH buffering agent. The pH of MSA + bean sample is in the range of 6.57–7.30, and the pH of MSA + corn at the end of the growth period is 7.59. This implies that calcium carbonate is the main pH buffering agent at these pH ranges.
4.2. Role of Plants in Enhanced Weathering
One of the most important factors determining soil fertility is pH, which is also influenced by the type of plant cultivated and is controlled by the types and amounts of a variety of possible organic and inorganic root exudates.29 During cultivation of beans (legumes), the soil is acidified due to proton release from roots via nitrogen fixation, along with organic anions such as malate, citrate, and oxalate in the rhizosphere.14,15,30 Phenolics and aldonic acids exuded by the roots of N2-fixing legumes contribute to a net release of protons by signaling Rhizobiaceae bacteria to induce the formation of root nodules, where nitrogen is reduced to ammonia.29 As for the carbon cycle’s influence on pH, Nye concluded that the CO2 respired by roots and associated organisms causes a negligible pH gradient across the rhizosphere, since the partial pressure of CO2 next to the plant root is marginally higher than in the adjacent body of the soil.31 Hinsinger et al. further clarified why in acidic soils the contribution of root and microbial respiration to changes in rhizosphere pH can be neglected, by stating that since H2CO3 remains undissociated at acidic pH values due to its pK1 of 6.36, CO2 can only significantly affect the pH of neutral to alkaline soils.32 Since the concentration of H+ is in the order of 10–7 M at near-neutral pH, a change in pH at this range is accompanied by a very small change in ion concentration. Hence, the number of moles of organic acids produced in the process will be very small, in the order of micromoles to millimoles even after considering partial dissociation;33 so, the effect of the organic anions on TOC and TGA results can be assumed to be negligible.
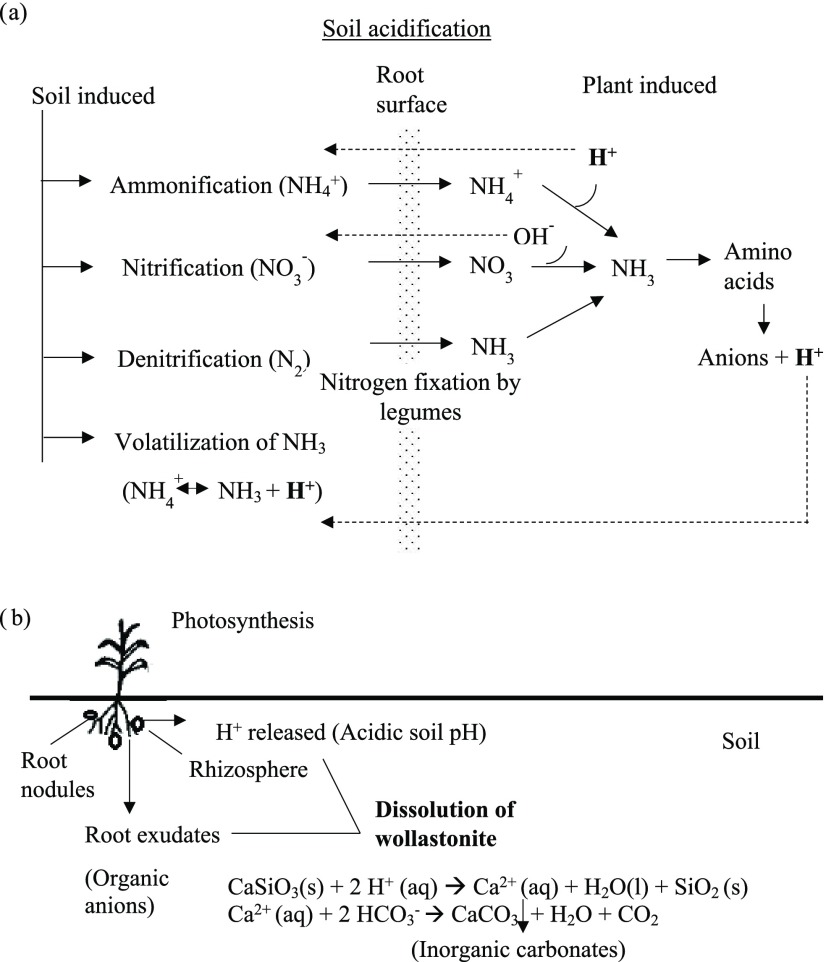
The process of proton generation during the soil nitrogen cycle is soil-induced as well as plant-induced. Soil-induced process transforms soil N via ammonification, nitrification, denitrification, and volatilization of ammonia (eqs 10–13). Ammonification is an enzymatically catalyzed microbial process, which produces ammonium (NH4+) by deamination of organic N (proteins, amino polysaccharides, and nucleic acids). Soil acidity is enhanced due to nitrification of NH4+ to nitrate ions, which results in the release of protons in the soil. However, denitrification, microbial reduction of nitrate to gaseous nitrogen (N2), is a proton consumption step, but soil acidity depends on the magnitude of nitrification rate and denitrification rate. Most soils have little ability to retain the nitrate and undergo nitrate leaching, hence reducing the magnitude of denitrification resulting in soil acidity. Lastly, ammonia volatilization from ammonium ions is accompanied by proton generation, thereby reducing the soil pH. The plant-induced process includes the uptake and assimilation of these ammonium, nitrate, and nitrogen ions by the roots to form ammonia via deprotonation of NH4+, reduction of NO3–, and nitrogen fixation of free nitrogen by legumes (Figure 6a). This ammonia is assimilated to form amino acids, and dissociation of the carboxylic group of the amino acid results in the generation of H+, which is transported to the soil to balance the cytoplasm pH (∼7).14 In the case of cultivation of nonlegumes, the nitrogen formed during denitrification cannot be fixed to ammonia in the absence of root nodules.
| 10 |
| 11 |
| 12 |
| 13 |
The plasmalemma H+-pump is the principal ion transport regulation mechanism in plant tissues,34 resulting in pH and electrical potential differences across the cell wall. Although protons are released into the soil, hydroxyl ions remain in the cytosol, which stimulates the carboxylation of phosphoenolpyruvate, leading to the production of organic anions that are released into the soil as root exudates.15 In most plant species, the most representative organic anion is malate (C2H4O(COO)22–). These protons and root exudates help in the dissolution of wollastonite, increasing Ca2+ ion release to form CaCO3(s) and Ca(HCO3)2(aq). Figure 6b illustrates the process of enhanced weathering by legumes. Using wollastonite as a soil amendment along with legume production not only avoids soil acidification (via pH buffering) but also sequesters carbon dioxide.
Figure 6.
(a) Soil acidification mechanism (after Bolan et al. (1991)14) and (b) role of legumes in enhanced weathering.
As seen in Figure 2a, the pH increases until fourth week, which is favorable for the precipitation of carbonates,35 but this precipitation formed on the surface of the wollastonite can passivate the surface, which might further prevent the reaction of the wollastonite’s core.36 The low pH, developed as a result of nitrogen fixation by rhizobia, initially on the root surface and later within root nodules, can leach out the calcium ions, which will then precipitate in the soil rather than the surface of the wollastonite. Soil pH is an important driving force in TIC accumulation, and geochemical modeling, using The Geochemist’s Workbench (version 12.0), helped to explain this mechanism.
Figure S6 shows the equilibrium of carbonate formation with respect to pH, and it was found that calcite precipitation is favored at a soil pH above 6.5. In this study, soil pH of the wollastonite-amended soil (MSA) is in the range of 6.57–7.30 for MSA + bean and 6.57–7.59 for MSA + corn, which implies that within this pH range, calcite precipitation will occur, and this explains the increase in TIC with time, from the beginning. Also, within this pH range, wollastonite is prone to dissolution, which is further increased at lower pH, as evidenced in the wollastonite saturation (Q/K, where Q is the reaction quotient and K is the equilibrium constant) plot given in Figure S7. This means that as calcium is consumed in the carbonation reaction, more calcium ions become available from wollastonite for further sequestering soil CO2, thus leading to continued increase in TIC, until the end of the experimental run.
Plant cover essentially holds the soil together, as well as its nutrients. The pH of MSA drops at the end of the experimental run, which can be probably due to the absence of plant cover in this experimental setup. In the absence of plants, the soil fertility decreases due to the washing away of the soil nutrients by rain.37 Additionally, the formed calcium carbonate might react with the excess carbonic acid (eq 4), and, rather than remaining in the soil profile, the ions (Ca2+, HCO3–) gradually leach out of the soil, thus lowering the pH. Hence, plants play an important role in enhanced weathering to capture atmospheric CO2 as well as to store it.
4.3. Wollastonite Application to Agricultural Land
Agricultural land offers a vast area for wollastonite application for carbon dioxide sequestration. The world’s arable land area is 13 963 743 km2 (FAO, 2015).38 Taking the wollastonite density of 2.84 g/cm3 and the theoretical CO2 sequestration capacity of 44 g CO2 per 116.2 g wollastonite (0.379 g/g),39 a total of 40 megatonnes of CO2 could be potentially sequestered by world’s wollastonite reserve of at least 100 million tonnes.7 This represents 11.6 kg CO2 per acre if all arable land is treated with the known reserves of wollastonite. The annual sequestration rate would be a function of the annual rate of wollastonite application and weathering rate, and evidently, if more wollastonite was applied per unit land area, more CO2 could be potentially sequestered.
In this study, wollastonite-treated soil used to grow beans showed a TIC accumulation of 0.606 ± 0.086% over 8 weeks (equivalent to 6.06 kg C/tonne soil or 12.04 kg CO2/tonne soil/month), which compares very favorably to Manning et al.’s data, who reported that a plot composed of compost and quarry fines showed a net rate of accumulation as inorganic carbonates for plots that used carbonate-free crushed rock to be of the order of 0.8 kg C/tonne soil annualy.40 Theoretically, 2.64 tonne of wollastonite sequesters 1 tonne of CO2, or 0.273 tonne of C So 0.125 tonne wollastonite per tonne soil (since 1000 g wollastonite/8 kg soil is used in this study) should sequester a maximum of 47.4 kg CO2/tonne soil or 12.9 kg C/tonne soil. Hence, it will take 0.33 years for complete carbonation of wollastonite in the soil amendment used in this study.
Owing to the experimental weather conditions observed in this study, limited by the low overnight temperature, the experimental run has to be cut short to 8 weeks. However, this time length may not be enough to track the growth cycle of corn (∼90 days); however, it is enough to track the growth cycle of beans (∼55 days), and for comparison purpose, the experimental period of 8 weeks for both the plants seems reasonable. The significance of the result reported in this study demonstrates that wollastonite application as a soil amendment has the potential to remove 47.4 kg of CO2/tonne soil/year, assuming that the crop conditions that resulted in the observed TIC accumulation over 8 weeks extend for several more weeks to allow full carbonation to complete within a year. Encouraged by the positive results of this work, the authors have started field trials on a soybean–corn field in Summer 2018 to ascertain the amount of CO2 sequestered annually via wollastonite amendment.
It is difficult to anticipate all impacts that the input of wollastonite may have on agricultural systems. Impacts are likely to vary widely depending on soil, crop, and climate characteristics. Based on Figure 2, it can be expected that the massive application of wollastonite will induce changes in soil pH and associated chemistry and this may affect the availability of plant nutrients. The rate at which natural silicate minerals dissolve in the soil is one of the fundamental uncertainties in the feasibility assessment of enhanced weathering.41,42 The release of potentially toxic elements during the dissolution of minerals may pose an environmental risk that would limit the application of certain minerals as a soil amendment for enhanced weathering. Some of these trace metals (particularly Ni, Mn, Cr, Si) present in the minerals are micronutrients required by the plants and therefore their presence is beneficial, as long as their concentration does not reach toxic levels.43
5. Conclusions
The study was conducted to determine the potential for enhanced weathering of wollastonite in agricultural soils. Through enhanced weathering, silicate mineral-derived pedogenic carbonate is generated to sequester CO2 from the environment. Though the process happens in the soil, the origin of the CO2 is the atmosphere; so, the direct removal of CO2 responsible for climate change is achieved. Enhanced weathering is promoted at a suitable pH range, a requirement that is shown in the present study to be especially satisfied when wollastonite is added to soil onto which plants are cultivated, resulting in an optimally tailored soil pH. Wollastonite amendment of soils not only showed increased TIC accumulation but also resulted in a better growth of beans and corns, as indicated by the biomass dry weight data. The co-benefits of wollastonite soil amendment (CO2 sequestration and improved crop yield) would encourage producers to effectively use this mineral to contribute toward global climate change mitigation without compromising their produce.
Acknowledgments
This research was financially supported by an NSERC Discovery Grant. The authors would like to thank Joanne Ryks, Ryan Smith, and Michael Speagle from the University of Guelph for their laboratory assistance.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02477.
Particle size distribution of Canadian wollastonite, determined by wet laser diffraction (Figure S1); nitrogen adsorption/desorption isotherm of Canadian wollastonite, determined for specific surface area and pore size analyses (Figure S2); variation of pH with time of various wollastonite-amended soils, including as-received soil and as-received wollastonite ( Figure S3); alkalinity and total carbonate of all potting experiments at the end of the growth period (55 days) (Figure S4); thermogravimetric analysis of all potting samples at the end of the growth period (55 days) (Figure S5); calcite saturation versus pH at different CO2 partial pressures (pCO2, 500–4000 Pa), generated using The Geochemist’s Workbench (Figure S6); Wollastonite and quartz saturation as a function of pH, generated using The Geochemist’s Workbench (Figure S7); development stages of beans and corn plants in wollastonite-amended and nonamended soils, in cumulative days from the start of growth period (Table S1) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Fischer E. M.; Knutti R. Anthropogenic contribution to global occurrence of heavy-precipitation and high-temperature extremes. Nat. Clim. Change 2015, 5, 560–564. 10.1038/nclimate2617. [DOI] [Google Scholar]
- Lackner K. S. A Guide to CO2 Sequestration. Science 2003, 300, 1677–1678. 10.1126/science.1079033. [DOI] [PubMed] [Google Scholar]
- Manning D. A. C.; Renforth P. Passive Sequestration of Atmospheric CO2 through Coupled Plant-Mineral Reactions in Urban soils. Environ. Sci. Technol. 2013, 47, 135–141. 10.1021/es301250j. [DOI] [PubMed] [Google Scholar]
- Railsback B. L.Some Fundamentals of Mineralogy and Geochemistry; Department of Geology, University of Georgia: Athens, Georgia, 2006. http://www.gly.uga.edu/railsback/FundamentalsIndex.html (accessed 2018/10/19).
- Palandri J. L., Kharaka Y. K.. A Compilation of Rate Parameters of Water-Mineral Interaction Kinetics for Application to Geochemical Modeling, U.S. Geological Survey Open File Report, 2004; p 1068. https://pubs.usgs.gov/of/2004/1068/pdf/OFR_2004_1068.pdf (accessed 2018-10-19).
- Schott J.; Pokrovsky O. S.; Spalla O.; Devreux F.; Gloter A.; Mielczarski J. A. Formation, growth and transformation of leached layers during silicate minerals dissolution: The example of wollastonite. Geochim. Cosmochim. Acta 2012, 98, 259–281. 10.1016/j.gca.2012.09.030. [DOI] [Google Scholar]
- Brioche A. S.Mineral Commodity Summaries – Wollastonite; U.S. Geological Survey, 2018. https://minerals.usgs.gov/minerals/pubs/commodity/wollastonite/mcs-2018-wolla.pdf (accessed 2018/10/19).
- Hangx S. J. T.; Spiers C. J. Coastal spreading of olivine to control atmospheric CO2 concentrations: A critical analysis of viability. Int. J. Greenhouse Gas Control 2009, 3, 757–767. 10.1016/j.ijggc.2009.07.001. [DOI] [Google Scholar]
- Köhler P.; Hartmann J.; Wolf-Gladrow D. A. Geoengineering potential of artificially enhanced silicate weathering of olivine. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 20228–20233. 10.1073/pnas.1000545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakopoulos E.; Santos R. M.; Chiang Y. W.; Vasilijie M. Influence of process parameters on carbonation rate and conversion of steelmaking slags – Introduction of the “carbonation weathering rate.”. Greenhouse Gases: Sci. Technol. 2016, 6, 470–491. 10.1002/ghg.1608. [DOI] [Google Scholar]
- Epihov D. Z.; Batterman S. A.; Hedin L. O.; Leake J. R.; Smith L. M.; Beerling D. J. N2-fixing tropical legume evolution: a contributor to enhanced weathering through the Cenozoic. Proc. R. Soc. B 2017, 284, 20170370 10.1098/rspb.2017.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrovsky O. S.; Shirokova L. S.; Bénézeth P.; Schott J.; Golubev S. V. Effect of organic ligands and heterotrophic bacteria on wollastonite dissolution kinetics. Am. J. Sci. 2009, 309, 731–772. 10.2475/08.2009.05. [DOI] [Google Scholar]
- Danyal K.; Shaw S.; Page T. R.; Duval S.; Horitani M.; Marts A. R.; Lukoyanov D.; Dean D. R.; Raugei S.; Hoffman B. M.; Seefeldt L. C.; Antony E. Negative cooperativity in the nitrogenase Fe protein electron delivery cycle. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, E5783–E5791. 10.1073/pnas.1613089113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolan N. S.; Hedley M.J.; White R. E. Processes of soil acidification during nitrogen cycling with emphasis on legume based pastures. Plant Soil 1991, 134, 53–63. 10.1007/BF00010717. [DOI] [Google Scholar]
- Mengel K.; Schubert S. Active Extrusion of Protons into Deionized Water by Roots of Intact Maize. Plant Physiol. 1985, 79, 344–348. 10.1104/pp.79.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X.; Turner N. C.; Song L.; Gu Y.; Wang T.; Li F. Soil carbon sequestration by three perennial legume pastures is greater in deeper soil layers than in the surface soil. Biogeosciences 2016, 13, 527–534. 10.5194/bg-13-527-2016. [DOI] [Google Scholar]
- Taylor L. L.; Beerling D. J.; Quegan S.; Banwart S. A. Simulating carbon capture by enhanced weathering with croplands: an overview of key processes highlighting areas of future model development. Biol. Lett. 2017, 13. 10.1098/rsbl.2016.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiyeh R. M.; Subler S.; Edwards C. A.; Bachman G.; Metzger J. D.; Shuster W. Effects of vermicomposts and composts on plant growth in horticultural container media and soil. Pedobiologia 2000, 44, 579–590. 10.1078/S0031-4056(04)70073-6. [DOI] [Google Scholar]
- Huijgen W. J. J.; Witkamp G.; Comans R. N. J. Mechanisms of aqueous wollastonite carbonation as a possible CO2 sequestration process. Chem. Eng. Sci. 2006, 61, 4242–4251. 10.1016/j.ces.2006.01.048. [DOI] [Google Scholar]
- Hanway J. J. Growth Stages of Corn. Agron. J. 1963, 55, 487–492. 10.2134/agronj1963.00021962005500050024x. [DOI] [Google Scholar]
- Lancashire P. D.; Bleiholder H.; Van Den Boom T.; Langelüddeke P.; Stauss R.; Weber E.; Witzenberger A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. 10.1111/j.1744-7348.1991.tb04895.x. [DOI] [Google Scholar]
- Jones D. L.; Chesworth S.; Khalid M.; Iqbal Z. Assessing the addition of mineral processing waste to green waste-derived compost: An agronomic, environmental and economic appraisal. Bioresour. Technol. 2009, 100, 770–777. 10.1016/j.biortech.2008.06.073. [DOI] [PubMed] [Google Scholar]
- Pansu M.; Gautheyrou J.. Handbook of Soil Analysis; Springer: Berlin, 2006. [Google Scholar]
- Schnoor J.Environmental Modeling: Fate and Transport of Pollutants in Water, Air, and Soil; John Wiley & Sons: New York, 1996. [Google Scholar]
- Sato T.; Kaneta Y.; Furuta N.; Kobayashi H.; Shindo H.; Ota T.; Sato A. Effect of soil physical properties on soybean nodulation and N2 fixation at the early growth stage in heavy soil field in Hachirougata Polder, Japan. Soil Sci. Plant Nutr. 2003, 49, 695–702. 10.1080/00380768.2003.10410327. [DOI] [Google Scholar]
- Tajima R.; Lee O. N.; Abe J.; Lux A.; Morita S. Nitrogen-Fixing Activity of Root Nodules in Relation to Their Size in Peanut (Arachis hypogaea L.). Plant Prod. Sci. 2007, 10, 423–429. 10.1626/pps.10.423. [DOI] [Google Scholar]
- Wattenbach M.; Martino D.; Smith P.; Smith J.; McCarl B.; Ogle S.; McAllister T.; Towprayoon S.; Gwary D.; Schneider U.; Scholes B.; Rice C.; Cai Z.; Kumar P.; Janzen H.; Sirotenko O.; Romanenkov V.; Pan G.; O’Mara F.; Howden M. Greenhouse gas mitigation in agriculture. Philos. Trans. R. Soc., B 2008, 363, 789–813. 10.1098/rstb.2007.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman G. P.; Burkett D. C.; Coventry R. J. Amending highly weathered soils with finely ground basalt rock. Appl. Geochem. 2002, 17, 987–1001. 10.1016/S0883-2927(02)00078-1. [DOI] [Google Scholar]
- Dakora F. D.; Phillips D. A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. 10.1023/A:1020809400075. [DOI] [Google Scholar]
- Yan F.; Schubert S.; Mengel K. Soil pH changes during legume growth and application of plant material. Biol. Fertil. Soils 1996, 23, 236–242. 10.1007/BF00335950. [DOI] [Google Scholar]
- Nye P. H. Changes of pH across the rhizosphere induced by roots. Plant Soil 1981, 61, 7–26. 10.1007/BF02277359. [DOI] [Google Scholar]
- Hinsinger P.; Plassard C.; Tang C.; Jaillard B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 2003, 248, 43–59. 10.1023/A:1022371130939. [DOI] [Google Scholar]
- Lipton D. S.; Blanchar R. W.; Blevins D. G. Citrate, Malate, and Succinate Concentration in Exudates from P-Sufficient and P-Stressed Medicago sativa L. Seedlings. Plant Physiol. 1987, 85, 315–317. 10.1104/pp.85.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelet B.; Boutry M. The Plasma Membrane H+-ATPase (A Highly Regulated Enzyme with Multiple Physiological Functions). Plant Physiol. 1995, 108, 1–6. 10.1104/pp.108.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo F.; Ruiz-Agudo C.; Ibañez-Velasco A.; Rodrigo Gil-San Millán R.; Navarro J. A. R.; Ruiz-Agudo E.; Rodriguez-Navarro C. The Carbonation of Wollastonite: A Model Reaction to Test Natural and Biomimetic Catalysts for Enhanced CO2 Sequestration. Minerals 2018, 8, 209 10.3390/min8050209. [DOI] [Google Scholar]
- Santos R. M.; Van Bouwel J.; Vandevelde E.; Mertens G.; Elsen J.; Van Gerven T. Accelerated mineral carbonation of stainless steel slags for CO2 storage and waste valorization: Effect of process parameters on geochemical properties. Int. J. Greenhouse Gas Control 2013, 17, 32–45. 10.1016/j.ijggc.2013.04.004. [DOI] [Google Scholar]
- Cerri C. C.; Volkoff B.; Andreaux F. Nature and behaviour of organic matter in soils under natural forest, and after deforestation, burning and cultivation, near Manaus. For. Ecol. Manage. 1991, 38, 247–257. 10.1016/0378-1127(91)90146-M. [DOI] [Google Scholar]
- FAO. FAOSTAT Land Use; Food and Agriculture Organization of the United Nations, 2015. http://www.fao.org/faostat/en/#data/EL (accessed on 2018/02/03).
- Tai C. Y.; Chen W.-R.; Shih S.-M. Factors affecting wollastonite carbonation under CO2 supercritical conditions. AIChE J. 2006, 52, 292–299. 10.1002/aic.10572. [DOI] [Google Scholar]
- Manning D. A. C.; Renforth P.; Lopez-Capel E.; Robertson S.; Ghazireh N. Carbonate precipitation in artificial soils produced from basaltic quarry fines and composts: An opportunity for passive carbon sequestration. Int. J. Greenhouse Gas Control 2013, 17, 309–317. 10.1016/j.ijggc.2013.05.012. [DOI] [Google Scholar]
- Renforth P.; Manning D. A. C. Laboratory carbonation of artificial silicate gels enhanced by citrate: Implications for engineered pedogenic carbonate formation. Int. J. Greenhouse Gas Control 2011, 5, 1578–1586. 10.1016/j.ijggc.2011.09.001. [DOI] [Google Scholar]
- Renforth P.; Manning D. A. C.; Lopez-Capel E. Carbonate precipitation in artificial soils as a sink for atmospheric carbon dioxide. Appl. Geochem. 2009, 24, 1757–1764. 10.1016/j.apgeochem.2009.05.005. [DOI] [Google Scholar]
- Barral Silva M. T.; Silva B. M.; García-Rodeja E.; Vázquez Freire N. Reutilization of granite powder as an amendment and fertilizer for acid soils. Chemosphere 2005, 61, 993–1002. 10.1016/j.chemosphere.2005.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.