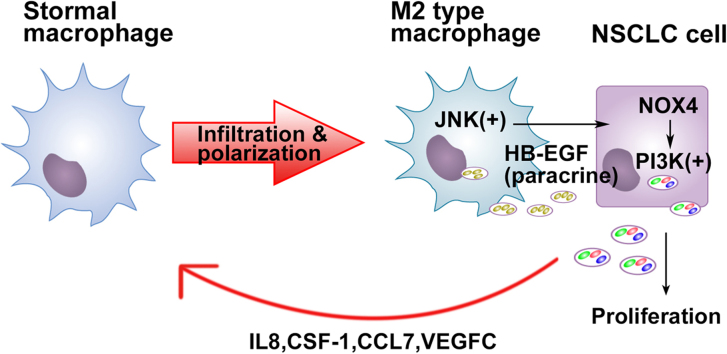
Abstract
M2-type tumor-associated macrophages (TAMs) infiltration contributes to cancer malignant progression. However, the mechanisms for controlling recruitment and M2 polarization of macrophages by cancer cells are largely unclear. NADPH oxidase 4 (NOX4) is abundantly expressed in non-small cell lung cancer (NSCLC) and mediates cancer progression. NOXs are in close relation with cancer-related inflammation, nevertheless, whether tumoral NOXs influence microenvironmental macrophages remains undentified. This study found that there was a close association between NOX4 expression and macrophage chemotaxis in patients with NSCLC analyzed using TCGA RNA-sequencing data. NOX4 in NSCLC cells (A549 and Calu-1 cell lines) efficiently enhanced murine peritoneal macrophage migration and induces M2 polarization. Immunohistochemical analysis of clinical specimens confirmed the positive correlation of NOX4 and CD68 or CD206. The mechanical study revealed that tumoral NOX4-induced reactive oxygen species (ROS) stimulated various cytokine production, including CCL7, IL8, CSF-1 and VEGF-C, via PI3K/Akt signaling-dependent manner. Blockade of the function of these cytokines reversed NOX4 effect on macrophages. Specifically, the results showed that tumoral NOX4-educated M2 macrophages exhibited elevated JNK activity, expressed and released HB-EGF, thus facilitating NSCLC proliferation in vitro. Pretreatment of macrophages with JNK inhibitor blocked tumoral NOX4-induced HB-EGF production in M2 macrophages. Finally, in a xenograft mouse model, overexpression of NOX4 in A549 cells enhanced the tumor growth. Elimination of ROS by NAC or inhibition of NOX4 activity by GKT137831 suppressed tumor growth accompanied by reduction in macrophage infiltration and the percentage of M2 macrophages. In conclusion, our study indicates that tumoral NOX4 recruits M2 TAMs via ROS/PI3K signaling-dependent various cytokine production, thus contributing NSCLC cell growth.
Keywords: NOX4, Macrophages, NSCLC, M2 polarization, Chemotaxis
Graphical abstract
Highlights
-
•
NOX4 has a novel function that affects cancer progression via action on TAM.
-
•
There exists a NOX4-dependent crosstalk between NSCLC cells and M2 macrophages.
-
•
GKT137831 has anti-cancer potential for targeting cancer microenvironmental TAM.
1. Introduction
Lung cancer is the leading cause of cancer mortality worldwide, and non-small cell lung cancer (NSCLC) accounts for up to 80% of all lung cancer cases with poor prognosis. It is urgent to explore new and more effective targets against NSCLC progression.
Cancer cells have increased levels of reactive oxygen species (ROS) in comparison to their normal counterparts. A major source of ROS in cancer cells is produced by NADPH oxidases (nicotinamide adenine dinucleotide phosphate oxidase, NOXs) [1]. NOXs are a family of membrane bound enzyme complexes that have the primary function to generate superoxide (O2•-) or hydrogen peroxide (H2O2). NOXs consist of seven members: NADPH oxidase 1 (NOX1), NOX2 (gp91phox), NOX3, NOX4, NOX5, Duox1, and Duox2 [2]. NOXs have been confirmed to be closely correlated with cancer development and progression [3]. Our previous studies show that NOX4 is abundantly expressed in NSCLC cells and contributes to NSCLC progression through ROS/PI3K pathway activation [4] and promotion of glycolysis [5]. Nevertheless, the comprehensive mechanisms for NOX4-mediated NSCLC malignant progression remain largely unknown.
Tumor-associated macrophages (TAMs) are a major component of the leukocyte infiltration of many solid tumors. There are two well-established polarized macrophage phenotypes, including classically activated macrophages (M1) and alternatively activated macrophages (M2) [6]. The infiltration of M2 TAMs has been positively associated with tumor progression and has also been described as an inhibitor of inflammatory responses in most types of human cancer including NSCLC [7], [8]. Also in vitro, M2 macrophages encourage growth of various tumor cells [9] and promote tumor cell survival [10]. Tumor cells induce macrophage infiltration in tumor tissues and skew macrophages to a M2 phenotype to facilitate their malignant progression, however, the underlying mechanisms for controlling recruitment and M2 polarization of macrophages by cancer cells are largely unclear.
Interestingly, a recent report by Mongue-Din et al. showed that cardiomyocyte NOX4 skews macrophage toward an M2-polarized phenotype, thus protecting against myocardial infarction-induced cardiac remodeling [11]. Besides, a previous study revealed that NOX-induced ROS in macrophages play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages [12]. Nevertheless, whether tumoral NOXs influence microenvironmental macrophages remains undentified. Therefore, this study was aimed to explore the potential role of tumoral NOX4 in microenvironmental macrophages and its significance in NSCLC cell growth.
2. Materials and methods
2.1. Bioinformatic tools
All expression profiling data of mRNA analyzed in this study were downloaded from The Cancer Genome Atlas (TCGA, http://cancergenome.nih.gov/). The differentially expressed genes between the NOX4 low and NOX4 high (one-fourth cutoff) were subjected to GO analysis performed with DAVID Bioinformatics Resources 6.7 (http://david.abcc.ncifcrf.gov/) and Gene set enrichment analysis (GSEA) analysis with GSEA v2.0.13 software.
2.2. Specimen preparation and immunohistochemical analysis
The clinical NSCLC specimens were purchased from Shanghai Outdo Biotch CO., Ltd (Shanghai, China). Sections of 32 specimens were analyzed by immunohistochemistry (IHC) using indicated antibodies as anti-NOX4 (ab133303), anti-CD206 (ab64693), and anti-CD68 (ab955) from Abcam. The images were captured using the AxioVision Rel.4.6 computerized image analysis system (Carl Zeiss). The immunostaining degree was determined based on both the proportion of positively stained tumor cells and the intensity of staining. The proportion of tumor cells was scored as follows: 0 (no positive tumor cells), 1 (< 10% positive tumor cells), 2 (10–35% positive tumor cells), 3 (35–75% positive tumor cells), and 4 (> 75% positive tumor cells). The intensity of staining was graded according to the criteria: 1 (no staining); 2 (weak staining), 3 (moderate staining), and 4 (strong staining). The staining index (SI) was calculated as staining intensity score × proportion of positive tumor cells. The indicated protein expression was determined by SI, scored as 0, 2, 3, 4, 6, 8, 9, 12, and 16. Samples with SI ≥ 8 were determined as high expression and samples with SI< 8 were determined as low expression.
2.3. Reagents
The NOX4 inhibitor GKT137831 (#S7171), the ROS scavager acetylcysteine (NAC, #S1623), the PI3K inhibitor LY294002 (#S1105), the JNK inhibitor JNK-IN-8 (#S4901) and the JNK inhibitor SP600125 (#S1460) were purchased from Selleckchem. VEGFR-3 Fc chimera recombinant protein (Flt-4/Fc) (#P35916) was purchased from R&D Systems. The mouse EGF neutralizing antibody (#AF2028), the human CCL7 neutralizing antibody (#MAB282), IL8 neutralizing antibody (#MAB208) and CSF-1 neutralizing antibody (#AF216) were R&D Systems. Cell culture reagents were obtained from Invitrogen. All other reagents were from Sigma unless stated otherwise.
2.4. NSCLC cell lines, plasmids, and transfection
A549 and Calu-1 cell lines, originally purchased from ATCC, were incubated at 37 °C in an atmosphere of 5% CO2 in DMEM supplemented with 10% FBS, penicillin-streptomycin and glutamine (2 mM). Cells were transfected with 100 nM of a control shRNA, two individual shRNA against NOX4 or a pCMV-NOX4 cDNA plasmid with Lipofectamine 2000 (Invitrogen, 11668–019) overnight according to manufacturer's instructions according to our previous study [4]. The NOX4 shRNA sequences used in present study were as follows: shRNA1: AGAGTATCACTACCTCCACCAGATGTTGG (sense), and shRNA2: AACCTCTTCTTTGTCTTCTACATGCTGCT (sense). The scramble shRNA sequence is UAGCGACUAAACACAUCAATT (sense).
2.5. Preparation of conditioned medium (CM)
The preparation protocol of CM of cancer cells (A549 and Calu-1) was according to a previous study with minor modifications [13]. Briefly, cancer cells (5 × 104/well) were seeded in a 12-well plate for 24 h. Then, the supernatants were harvested for treatment of macrophages. For induction of M2 polarization of macrophages, CM was replaced once after 48 h.
2.6. In vitro assay migration and M2 polarization of macrophages
Murine peritoneal macrophages were obtained according to another study [14]. The animal experiment was approved by the Animal handling and procedures were approved by the Guangdong Animal Center (No. GDPU20170235). In brief, female BALB/c mice were intraperitoneally injected each with 1 ml of 6% starch-broth solution. After 3 days, the mice were sacrificed and intraperitoneally injected each with 5 ml of PBS, massaging the mice abdomen gently for 3 min. The peritoneal fluid was pulled out and centrifugated at 1500 rpm for 8 min. The cells were collected, washed twice with PBS, re-suspended into 24-well culture plates with the cell density of 2 × 105 cells/well, and cultured with 1 ml of RPMI-1640 containing 10% FBS.
Macrophage migration was assayed using the Falcon TM Cell Culture Inserts containing polycarbonate membranes with pore sizes of 8 µm. Macrophages were seeded (1 × 105 cells/well) in the upper chamber of a transwell and placed it on the 24-well plate. The CM of A549 or Calu-1 cells were added into the lower chamber. After 10 h, the cell suspension in the upper chamber was aspirated, and the upper surface of the filter was carefully cleaned with cotton plugs. Macrophages were stained with crystal violet and images from five representative fields of each membrane were captured. The migratory cells within the lower chamber were counted and statistically analyzed. The evaluation method of M1 or M2 polarization of macrophages was according a previous report [15], as mannose receptor CD206 (an M2 macrophage marker) was detected by western blotting and levels of M1 cytokines (IL 12 and IL23), and the M2 cytokine (IL10) were determined by ELISA.
2.7. Western blotting
Western blotting protocol was according to our previous report [4]. Cells were lysed in RIPA buffer (50 mM Tris–HCl/pH 7.4, 1% NP‐40, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, 1 mM okadaic acid and 1 mg/ml aprotinin, leupeptin and pepstatin). Each sample (25 mg protein) was prepared for electrophoresis running on 10% SDS/PAGE gel and then transferred onto PVDF membranes (Millipore). After blocking the membranes with 5% fat free milk in TBST (50 mM Tris/pH 7.5, containing 0.15 M NaCl and 0.05% Tween‐20) for 1 h at room temperature, the membranes were probed with primary antibodies as follows: NOX4 (ab133303), CD206 (ab64693) and β-Tubulin (ab6046) purchased from Abcam at 4 °C overnight. All the primary antibodies were diluted with 5% BSA to 1: 1000. After washing, the blots were incubated with the secondary antibody was goat anti-rabbit IgG (#SA00001–2, Proteintech) for 1 h at room temperature. The secondary antibody was diluted with 5% BSA to 1: 5000. The bands in the membrane were visualized and analyzed by UVP BioImaging systems.
2.8. Cytokine antibody array
The profiles of cytokines secreted by A549 and Calu-1 cells were detected with the culture supernatants using a Human cytokine Array (QAH-CAA-2000-1, RayBiotech, Norcross, GA) according to the manufacturer's instructions. The cytokines with significant differences in expression were screened out.
2.9. ELISA
The supernatants of macrophage or NSCLC cell culture were centrifuged for 15 min at 1000×g to remove cell components before ELISA. Mice macrophage IL-10, IL-12, and IL-23 were measured using commercial ELISA kits (#M1000B, #M1270 and #M2300, respectively) from R&D Systems according to the manufacturer's instructions. Mice macrophage HB-EGF was determined by the ELISA kit (#SEB479Mu) from Cloud-Clone Corp. NSCLC cell CCL7, IL8, CSF-1 and VEGF-C were measured using commercial ELISA kits (#ELH-MCP3-1, #ELH-IL8-1, #ELH-MCSF-1 and #ELH-VEGFC-1, respectively) from Raybiotec according to the manufacturer's instructions. The levels of phospho-JNK (T183/Y185) and total JNK were assayed by the ELISA kit (#PEL-JNK-T183-T-1) from Raybiotec according to the manufacturer's instructions.
2.10. Measurement of H2O2 production
Extracellular steady-state generation of H2O2 was determined by Amplex Red® hydrogen peroxide assay kit (Invitrogen). After each treatment, cellular H2O2 production was determined using the substrate 10-acetyl-3,7-dihydroxyphenoxazine for horseradish peroxidase (HRP) that enables selective detection of H2O2. In the presence of peroxidase, this reagent reacts with H2O2 to produce resorufin. Resorufin fluorescence was assayed in the plate reader with excitation at 530 nm and emission at 590 nm at 37 °C.
2.11. Cell growth evaluation and clonogenic survival assay
The protocols used for MTT assay (detection of cell proliferation/viability) and clonogenic survival assay were all according to our previous study with some minor modifications [5]. Mice macrophages were incubated with the CM of A549 cells for 72 h. After treatment, cells were washed three times with RPMI-1640 and cultured in this medium for 48 h. After 48-h serum starvation, cells were collected and used as M2-polarized macrophages, and the medium was collected, added with 10% FBS and prepared as M2 macrophage-conditioned medium (M2-CM). For evaluation of A549 cell growth, 3 × 103 cells in 100 μl of M2-CM were seeded in 96-well and incubated for 72 h. Then, MTT was added to each well (with a final concentration of 0.5 mg/ml). After incubation at 37 °C for 4 h, the plates were centrifuged at 450×g for 5 mins. Untransformed MTT was removed by aspiration, and formazan crystals were dissolved in dimethyl suloxide (150 μl/well) quantified spectrophotometrically at 563 nm. In addition, A549 cells were plated in 6-well plates (1 × 103 cells per well) and cultured for 14 days with or without M2-CM incubation. The colonies were stained with 1% crystal violet for 20 mins after fixation with 10% formaldehyde for 15 mins. M2-CM was replaced every two days.
2.12. Xenografted tumor model
The animal experiment was approved by the Animal handling and procedures were approved by the Guangdong Animal Center (No. GDPU20170235). A549 cells (approximately 2 × 106) with or without NOX4 overexpression were subcutaneously inoculated into the right flank of 6-week-old BALB/C nude mice. Treatments were initiated when tumors reached 80–100 mm3 and included: saline, GKT137831 (60 mg/kg per day, by gavage for 10 days), NAC (7 mg/ml) given in the drinking water for the length of the experiment, each group = 5. Tumor sizes were calculated with the formula: (mm3) = (L×W2) × 0.5. The tumor volume was measured every other day. The student's t-test (two-tailed) was used to compare statistical significance between two groups in animal work.
2.13. Flow cytometry analysis of F4/80 and CD206 in xenografted A549 tumors
Animals were lethally anesthetized by intraperitoneal injection of ketamine/xylazine. Tissues were homogenized and incubated for 45 min at 37 °C in an enzymatic cocktail (1 ml per 10 mg tissue) containing 488 U/ml collagenase I (Sigma cat # C0130), 230 U/ml collagenase XI (Sigma, #C7657), 125 U/ml hyaluronidase (Sigma, #H3506) and 60 U/ml DNase I (Sigma, #D4527) in an enzymatic digestion buffer containing PBS and 20 mM HEPES. Tissues were then mashed through a 70-μm cell strainer to create a suspension. Cells were counted, diluted to 1 × 106 cells per 100 μl and incubated with antibodies (F4/80 FITC-conjugated antibody from Abcam and CD206 PE-conjugated Antibody from R&D Systems). The samples were analyzed with a FACS (Beckman Coulter, CA).
2.14. Statistical analysis
Statistical analysis was evaluated by Student's t-test for simple comparisons between two groups using GraphPad Prism5. The relationship between NOX4 expression and clinicopathologic characteristic was analyzed using the chi-square test. Data are expressed as mean ± S.D. P value of < 0.05 was considered statistically significant.
3. Results
3.1. Clinical relevance of high levels of NOX4 with chemotaxis and M2 macrophage markers in NSCLC
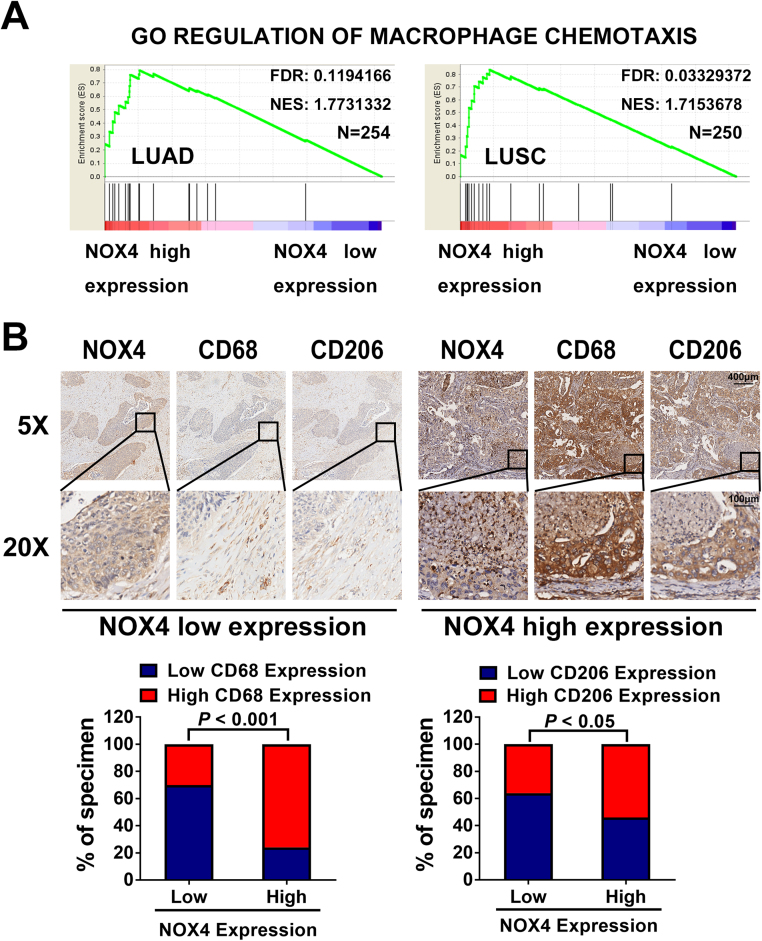
To explore the influence of NOX4 on stromal macrophages, we first performed Gene Set Enrichment Analysis (GSEA) using data from the TCGA and found that the chemotaxis pathway was identified to be closely related with NOX4 expression in lung adenocarcinoma (LUAD) patients and lung squamous carcinoma (LUSC) patients (Fig. 1A). To further delineate the correlation of NOX4 and stromal macrophages, we stained 32 NSCLC specimens with antibodies against NOX4, the human macrophage marker CD68, as well as the M2 macrophage markers CD206. As shown in Fig. 1B, correlation studies revealed that NOX4 levels were strongly correlated with expression levels of CD68 (p < 0.001) and CD206 (p < 0.05). These results strongly suggest NOX4 in NSCLC cells plays an important role in macrophage recruitment and M2 polarization.
Fig. 1.
Clinical relevance of high levels of NOX4 with chemotaxis and M2 macrophage markers in NSCLC. (A) A gene set enrichment analysis (GSEA) was performed with the permutation number of 1000 per run with the TCGA LUAD or LUSC data set using Broad Institute GSEA software. Displayed are the enrichment results of NOX4 with macrophage chemotaxis signature genes. (B) Immunohistochemical analysis of 32 human NSCLC stained with NOX4, CD68 and CD206 antibodies. Representative photographs of cancers based on the expression level of NOX4 in two groups are shown (low; n = 15, high; n = 17).
3.2. NOX4 promotes migration and M2 polarization of macrophages
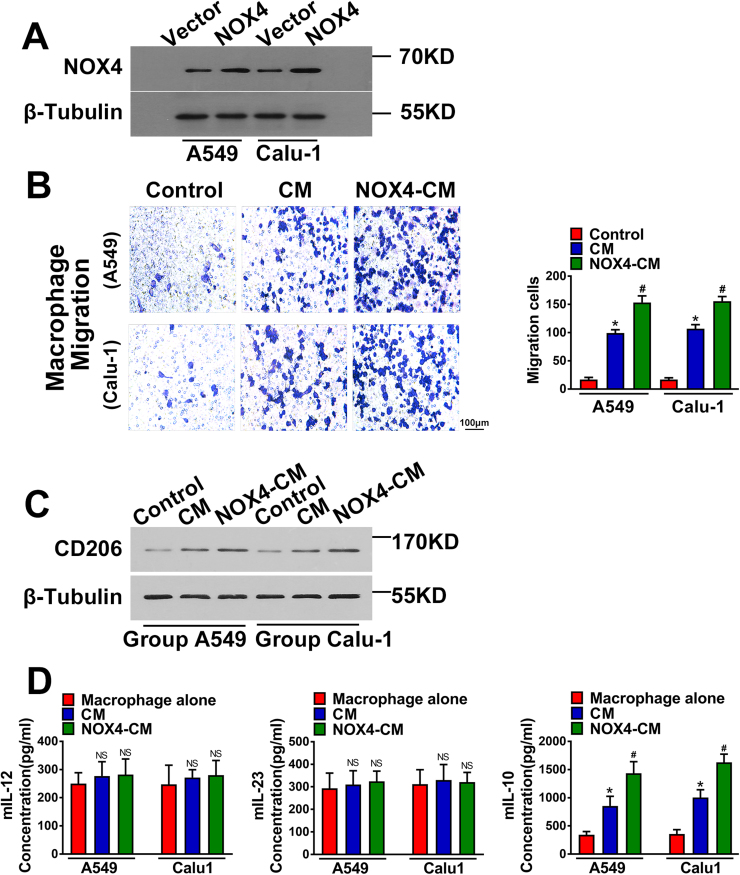
Fig. 2A showed that after transfection with NOX4 plasmid, NSCLC cell lines (A549 and Calu-1) exhibited much stronger NOX4 expression than vector control cells. Then, NOX4-overexpressing A549 and Calu-1 cells (A549-NOX4 and Calu-1-NOX4) and vector control cells (5 × 104/well) were seeded in a 12-well plate and cultured in medium containing 1% FBS for 24 h for harvest of the supernatants as the CM of cancer cells.
Fig. 2.
NOX4 promotes migration and M2 polarization of macrophages. (A) Western blotting analyses of NOX4 expression in transfected NSCLC cell lines. The data represent three independent experiments. *Compared with control, p < 0.05. (B) The CM of tumor cells and the CM of NOX4-overexpressing NSCLC cells promoted the macrophage migration. The data represent three independent experiments. *Compared with control, p < 0.05. #Compared with CM, p < 0.05. (C) Western blotting analyses of CD206 expression in macrophages cultured alone, macrophages cultured with CM of empty vector NSCLC cells and macrophages cultured with CM of NOX4-overexpression NSCLC cells. The data represent three independent experiments. *Compared with control, p < 0.05. #Compared with CM, p < 0.05. (D) The protein secretions of mIL-12, mIL-23 and mIL-10 of macrophage cells cultured with CM of NSCLC cells or CM of NOX4-overexpression NSCLC cells, assayed by ELISA. The data represent three independent experiments. *Compared with control, p < 0.05. #Compared with CM, p < 0.05.
To determine the effect of NOX4 on macrophage migration, murine peritoneal macrophages were seeded into the upper of the transwell chambers and treated by CM of cancer cells for 10 h. The results showed that the migration of macrophages treated by A549-vector-CM and Calu-1-vector-CM was increased compared with the control group. Furthermore, A549-NOX4-CM and Calu-1-NOX4-CM strikingly increased macrophage migration compared with CM of A549-vector and Calu-1-vector cells (Fig. 2B).
We next determined whether there is a relationship between NOX4 and macrophage polarization. The detection methods of M2 polarization of macrophages were according to a previous report [15], as CD206 (an M2 macrophage marker) was detected by western blotting and levels of M1 cytokines (IL12 and IL23), and the M2 cytokine (IL10) were determined by ELISA. The results indicated that after 3-day treatment of macrophages, A549-vector-CM and Calu-1-vector-CM increased CD206 expression, and A549-NOX4-CM and Calu-1-NOX4-CM could further enhance CD206 expression in macrophages compared with vector-CM of A549 and Calu-1 cells (Fig. 2C). Additionally, we found that the levels of IL12 and IL23 were not changed among different groups, however, the CM of NOX4-overexpressing A549 and Calu-1 cells could significantly promote IL10 production in macrophages compared with that of vector control cells (Fig. 2D).
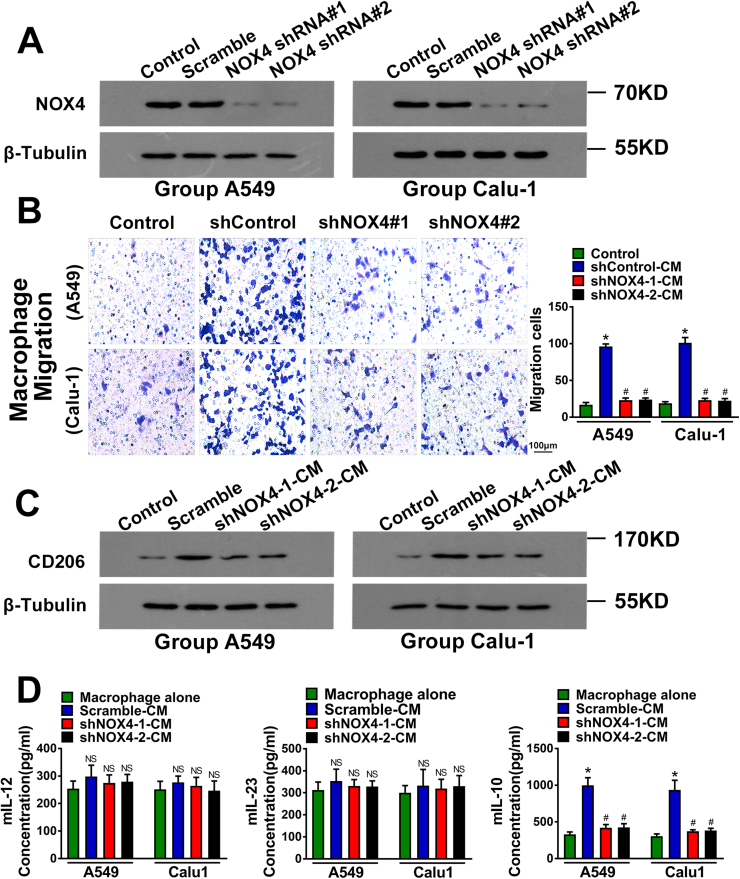
To further confirm that NOX4 regulates macrophage migration and M2 polarization, we silenced NOX4 expression in A549 and Calu-1 cells using two individual shRNAs against NOX4 and the silencing efficiency was determined by western blotting (Fig. 3A). The CM of shRNA control A549 and Calu-1 cells increased macrophage migration (Fig. 3B), CD206 expression (Fig. 3C) and IL10 production in macrophages (Fig. 3D). The CM of NOX4-depleted A549 and Calu-1 cells could efficiently reverse the effects of CM of their parental control cells (Figs. 3B-3D). The levels of IL12 and IL23 in macrophages were not statistically different among these groups.
Fig. 3.
NOX4 promotes migration and M2 polarization of macrophages. (A) Western blotting analyses of NOX4 expression in wild type, scramble control and shRNA transfected NSCLC cell lines. The data represent three independent experiments. *Compared with control, p < 0.05. #Compared with CM, p < 0.05. (B) Migration of macrophages cultured with the CM of NOX4-silencing NSCLC cells. The data represent three independent experiments. *Compared with control, p < 0.05. #Compared with scramble control CM, p < 0.05. (C) Western blotting analyses of CD206 expression in macrophages cultured alone, macrophages cultured with CM of scramble control NSCLC cells and macrophages cultured with CM of shRNA transfected NSCLC cells. The data represent three independent experiments. *Compared with control, p < 0.05. #Compared with CM, p < 0.05. (D) The protein secretions of mIL-12, mIL-23 and mIL-10 of macrophage cells cultured with CM of NOX4-silence NSCLC cells, assayed by ELISA. The data represent three independent experiments. *Compared with control, p < 0.05. #Compared with scramble control CM, p < 0.05.
Taken together, the above results clearly support the critical role of NOX4 in NSCLC cells in inducing macrophage migration and M2 polarization.
3.3. NOX4 promotes macrophage migration and M2 polarization via induction of various cytokine production in NSCLC cells through ROS/PI3K signaling
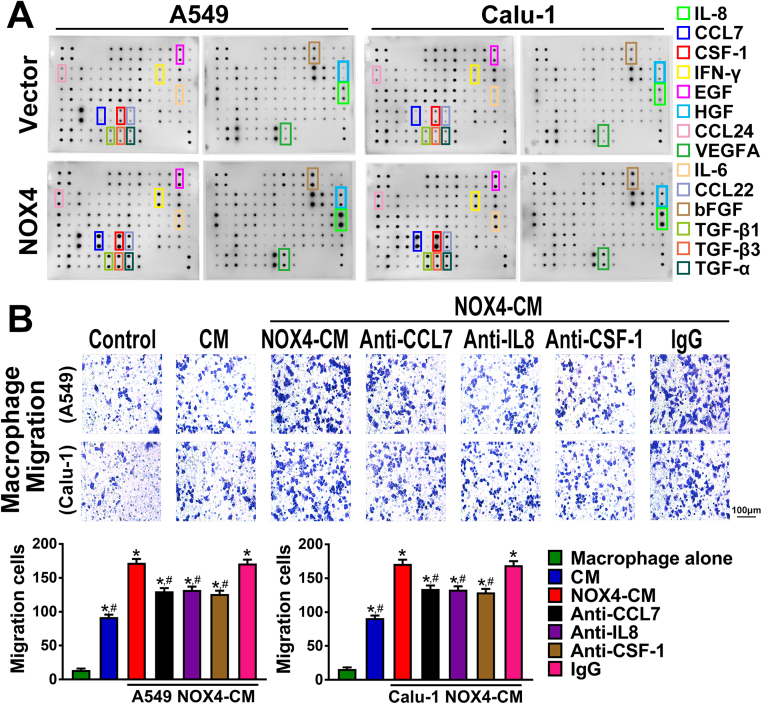
To explore the mechanism for NOX4-mediated macrophage attraction and M2 polarization, we sought to determine the effect of NOX4 on cytokine expression in NSCLC cells through cytokine screening using Raybio Human Cytokine antibody array. As shown in Fig. 4A, several upregulated cytokines or chemokines in NOX4-overexpressed A549 and Calu-1 cells were identified, and among these factors, CCL7, IL8 and CSF-1 were identically most upregulated in A549 and Calu-1 cells.
Fig. 4.
NOX4 promotes macrophage migration and M2 polarization. (A) Cytokine array of the CM of the NSCLC cells and NOX4-overexpressed NSCLC cells. (B) The CM of NOX4-overexpression NSCLC cell were treated with anti-CCL7 (20 μg/ml), anti-IL8 (20 μg/ml) or anti-CSF-1 (20 μg/ml). Macrophage migration was detected by migration assay. The data represent three independent experiments. *Compared with control, p < 0.05. #Compared with NOX4-overexpression CM, p < 0.05.
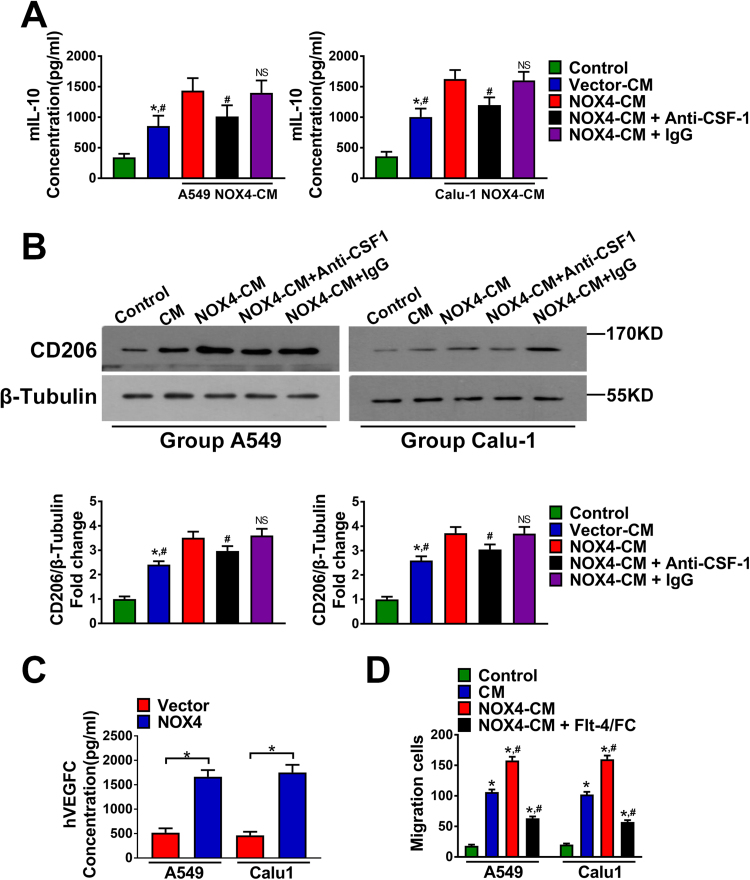
The cytokines CCL7, IL8 and CSF-1 have been confirmed to have macrophage chemotactic function and CSF-1 is a classical inducer of M2 polarization of macrophages [16], [17]. As shown in Fig. 4B, addition of anti-CCL7 (20 μg/ml), anti-IL8 (20 μg/ml) or anti-CSF-1 (20 μg/ml) to the CM of A549-NOX4 and Calu-1-NOX4 cells could partly abrogate the enhancement effect of NOX4 on macrophage migration. Then, we explored the possible involvement of CSF-1 in NOX4-mediated macrophage M2 polarization. The results shown in Fig. 5A and Fig. 5B revealed that after 3-day treatment with A549-vector-CM or Calu-1-vector-CM, macrophages exhibited increased IL10 production and CD206 expression. Furthermore, the CM of NOX4-overexpressing A549 and Calu-1 cells could significantly promote IL10 production and CD206 expression in macrophages compared with that of vector control cells. Addition of anti-CSF-1 (20 μg/ml) to the CM of A549-NOX4 and Calu-1-NOX4 cells partly blocked the enhancement effect of NOX4 on IL10 production and CD206 expression in macrophages.
Fig. 5.
NOX4 promotes macrophage migration and M2 polarization. (A) The protein secretions of mIL-10 of macrophage cells cultured alone, macrophage cells cultured with CM of empty vector NSCLC cells, assayed by ELISA. The data represent three independent experiments. *Compared with control, p < 0.05. #Compared with NOX4-CM, p < 0.05. (B) Western blotting analyses of CD206 expression in macrophages cultured alone, macrophages cultured with CM of empty vector NSCLC cells, macrophages cultured with CM of NOX4-overexpression NSCLC cells and macrophages cultured with NOX4-CM+Anti-CSF or NOX4-CM+IgG. The data represent three independent experiments. *Compared with control, p < 0.05. #Compared with NOX4-CM, p < 0.05. (C) The protein secretions of VEGF-C of empty vector NSCLC cells and NOX4-overexpression NSCLC cells, assayed by ELISA. The data represent three independent experiments. *Compared with empty vector, p < 0.05. (D) Migration of macrophage cells cultured with NOX4-CM (pretreated with Flt-4/Fc (100 ng/ml)). The data represent three independent experiments. *Compared with control, p < 0.05. #Compared with CM, p < 0.05.
Our recent study revealed that paracrine VEGF-C signaling from NSCLC cells promotes macrophage recruitment [18], therefore, we next sought to determine whether paracrine VEGF-C is also involved in NOX4-mediated macrophage attraction. Fig. 5C indicated that NOX4 overexpression obviously increased VEGF-C production in A549 and Calu-1 cells. Recombinant Flt-4/Fc specifically neutralizes the secreted form of VEGF-C [19], and is therefore utilized to interrupt VEGF-C signaling. Additional treatment of Flt-4/Fc (100 ng/ml) could also partially reverse the enhancement effect of NOX4 on macrophage migration (Fig. 5D).
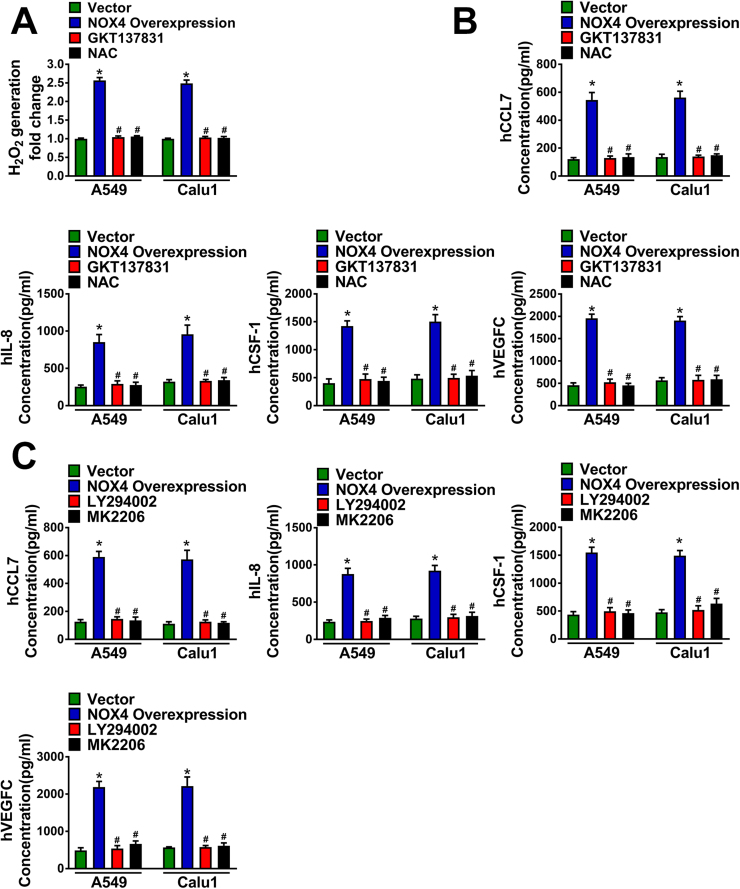
Our previous studies indicate that NOX4 can stimulate ROS/PI3K signaling in NSCLC cells [4], therefore, we speculated whether NOX4 induces the expression of CCL7, IL8, CSF-1 and VEGF-C dependent on ROS/PI3K signaling. Fig. 6A showed that NOX4 overexpression increased H2O2 production approximately 2.5 fold than vector control, and this effect was blocked by either NOX4 inhibitor GKT137831 (20 μM) or ROS scavenger NAC (2.5 mM) treatment for 24 h with continuing administration throughout the experiment. Fig. 6B showed that either administration of NOX4 inhibitor GKT137831 (20 μM) or treatment of cells with ROS scavenger NAC (2.5 mM) could efficiently block the effect of NOX4 overexpression on the production of CCL7, IL8, CSF-1 and VEGF-C. Besides, pretreatment with PI3K specific inhibitor LY294002 (20 μM) or Akt inhibitor MK2206 (10 μM) for 1 h could also compromise the effect of NOX4 on these cytokine production (Fig. 6C).
Fig. 6.
NOX4 promotes macrophage migration and M2 polarization. (A) Production of H2O2 of empty vector NSCLC cells, NOX4-overexpression NSCLC cells and NOX4-overexpression NSCLC cells treated with GKT137831 (20 μM) or NAC (2.5 mM). (B) The protein secretions of hCCL7, hIL-8, hCSF-1 and VEGFC of empty vector NSCLC cells, NOX4-overexpression NSCLC cells and NOX4-overexpression NSCLC cells treated with GKT137831 (20 μM) or NAC (2.5 mM), assayed by ELISA. *Compared with empty vector, p < 0.05. #Compared with NOX4-overexpression, p < 0.05. (C) The protein secretions of hCCL7, hIL-8, hCSF-1 and VEGFC of empty vector NSCLC cells, NOX4-overexpression NSCLC cells and NOX4-overexpression NSCLC cells treated with LY294002 (20 μM) or MK2206 (10 μM), assayed by ELISA. The data represent three independent experiments. *Compared with empty vector, p < 0.05. #Compared with NOX4-overexpression, p < 0.05.
These data together strongly suggest that NOX4 in NSCLC cells mediates macrophage chemotaxis and M2 polarization through stimulating expression of various cytokines, including CCL7, IL8, CSF-1 and VEGF-C, via ROS/PI3K signaling.
3.4. NOX4-induced HB-EGF secretion from NSCLC cells-educated macrophages mediates NSCLC cell growth in vitro
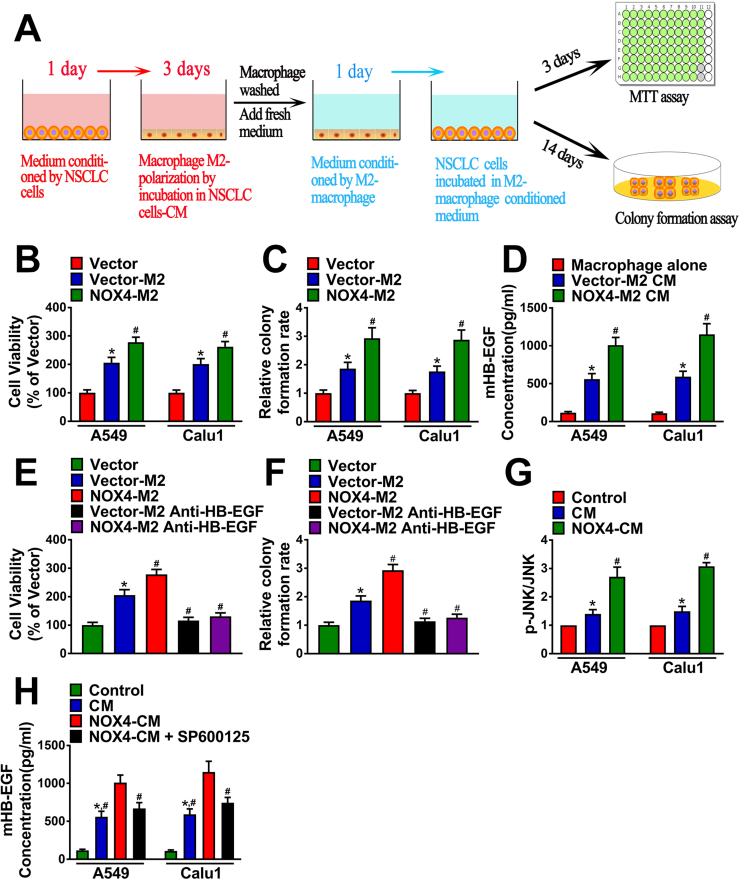
In order to define whether tumoral NOX4-induced M2 polarization of macrophages promotes NSCLC cell growth in vitro, we first treated macrophages with the CM of A549-vector cells or the CM of A549-NOX4 cells for 3 days to obtain M2 macrophages (vector-M2 macrophages and NOX4-M2 macrophages, respectively). Then, the M2-CM was added to A549 cells for 3 days or 14 days to detect the short-term or long-term growth of A549 cells determined by MTT or colony formation assay, respectively. M2-CM was replaced every two days. The schematic diagram of the experimental procedure was displayed in Fig. 7A.
Fig. 7.
NOX4-induced HB-EGF secretion from NSCLC cells-educated macrophages mediates NSCLC cell growthin vitro. (A) The schematic diagram of the MTT assay and colony formation assay procedure. NSCLC cells were cultured with CM of M2 type macrophages. Cell viability (B) and Colony formation ability (C) was measured. The data represent three independent experiments. *Compared with empty vector, p < 0.05. #Compared with Vector-M2, p < 0.05. (D) HB-EGF production in culture medium of macrophages, Vector-M2 CM and NOX4-M2 CM. The data represent three independent experiments. *Compared with macrophage alone, p < 0.05. #Compared with Vector-M2 CM, p < 0.05. NSCLC cells were cultured with Vector-M2 CM or NOX4-M2 CM. Cell viability (E) and Colony formation ability (F) was measured. The data represent three independent experiments. *Compared with empty vector, p < 0.05. #Compared with Vector M2, p < 0.05. (G) The level of phosphorylated JNK of macrophages alone and macrophages cultured with CM of empty vector NSCLC cells or CM of NOX4-overexpression NSCLC cells, assayed by ELISA. The data represent three independent experiments. *Compared with control, p < 0.05. #Compared with CM, p < 0.05. (H) HB-EGF production in culture medium of macrophages, Vector-M2 CM, NOX4-M2 CM and NOX4-M2 CM + SP600125. The data represent three independent experiments. *Compared with control, p < 0.05. #Compared with NOX4-M2 CM, p < 0.05.
Fig. 7B showed that treatment with the CM of vector-M2 macrophages increased A549 cell growth approximately by 2 folds determined by MTT assay after 3 days, and the CM of NOX4-M2 macrophages could further promote A549 cell proliferation by nearly 2.8 folds compared with control. The similar trends were also shown by the data obtained from the colony formation assay after 14-day treatment (Fig. 7C).
M2 macrophages confer cancer cell growth advantage via release of growth factors in vitro [20], and release of HB-EGF has been confirmed to be critically involved in such effect [21], [22]. Therefore, we first assayed HB-EGF production in culture medium of macrophages and M2-CM. The results revealed that M0 macrophages released HB-EGF rarely, however, the level of HB-EGF in the CM of vector-M2 macrophages was strikingly increased and was further enhanced in the CM of NOX4-M2 macrophages (Fig. 7D). Next, we sought to determine the role of HB-EGF in M2 macrophage-mediated A549 cell growth. Fig. 7E showed that when added into the CM of vector-M2 macrophages and NOX4-M2 macrophages, the HB-EGF neutralizing antibody (20 μg/ml) could obviously reverse the promoting effect of M2-CM on A549 cell proliferation determined by MTT assay. The similar trends were also shown by the data obtained from the colony formation assay after 14-day treatment (Fig. 7F).
As the JNK signaling has been confirmed to play a critical role in M2 polarization of macrophages [23], we next determined whether tumoral NOX4-induced HB-EGF production in macrophages via the JNK signaling. Fig. 7H showed that after treatment of macrophages with the CM of A549-vector cells for 12 h, the level of phosphorylated JNK (p-JNK) in macrophages was markedly increased, while the total level of JNK was not affected assayed by ELISA. The CM of A549-NOX4 cells could even stimulating JNK activity compared with the CM of A549-vector cells. As shown in Fig. 7H, the enhancement effect of tumoral NOX4 on HB-EGF production in macrophages was obviously blocked by pretreatment of macrophages with the JNK inhibitor SP600125 (20 μM) for 2 h.
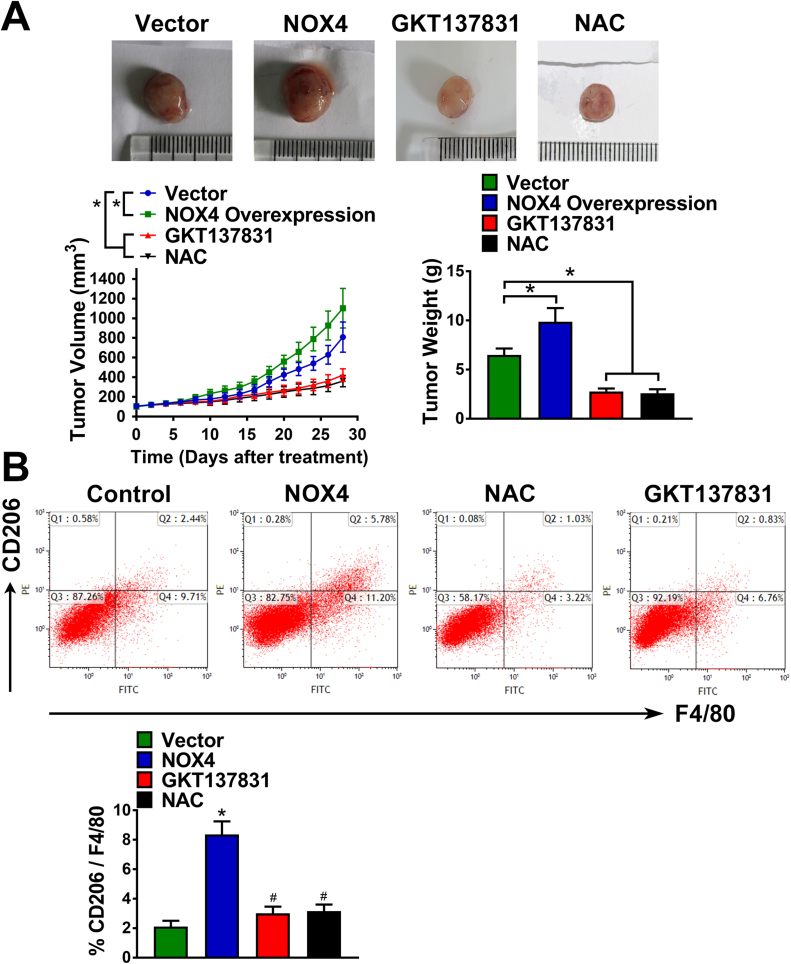
3.5. NOX4 enhances M2-polarized macrophage infiltration and induces NSCLC cell growth in vivo
To extend our in vitro observations, we investigated whether tumoral NOX4 could regulate induce infiltration and M2 polarization of macrophages, and thus facilitating NSCLC cell growth in vivo. Fig. 8A showed that compared with the vector control group, NOX4 overexpression could significantly promote A549 tumor growth, accompanied by the increased percentage of total (F4/80+) and M2 (CD206+) macrophages determined by flow cytometry assay (Fig. 8B). Besides, inhibition of NOX4 or scavenging of ROS by GKT137831 or NAC administration could suppressed A549 tumor growth, and reduced the percentage of total (F4/80+) and M2 (CD206+) macrophages in the tumor tissues.
Fig. 8.
NOX4 enhances M2-polarized macrophage infiltration and induces NSCLC cell growthin vivo. (A) Nude mice (5 per group) were injected with vector or NOX4-overexpressed A549 cells. Once tumor size reached 80–100 mm3, nude mice were treated with saline, NAC (7 mg/ml) or GKT137831 (60 mg/kg) through drinking water for the length of the experiment. Tumors’ weight and volume were measured every other day for indicated 28-day period. (B) The percentage of total (F4/80 +) and M2 (CD206 +) macrophages in the tumor tissues were assayed by flow cytometry. *Compared with vector, p < 0.05. #Compared with NOX4, p < 0.05.
Taken together, these results intensely suggest that tumoral NOX4-mediated ROS promote macrophage recruitment and M2 polarization, and resultantly leads to tumor growth in vivo.
4. Discussion
Overproduction of ROS by NOXs results in oxidant stress and tumor progression [24]. Tumoral NOXs display many functions in cancer including promoting proliferation, apoptosis resistance, angiogenesis, glycolysis, EMT and metastasis [25], [26], [27], [28]. NOXs may be closely involved in inflammatory cancer microenvironment wherein oxidant species act as positive signaling intermediates for tumor growth [29], however, the interaction between tumoral microenvironment and NOXs remains still largely undentified. The present study found that tumoral NOX4 promotes macrophage recruitment, as well as induces M2 polarization of macrophages, thus enhancing NSCLC cell proliferation both in vitro and in vivo. Therefore, our study uncovers a novel function of NOX4 that affects cancer progression via action on microenvironmental TAM. However, diverse cancer tissues exhibit different expression phenotype of NOX isoforms, whether other NOXs in specific circumstance also have similar function in remodeling of macrophage microenvironment needs further exploration.
This study clearly revealed that tumoral NOX4-mediated ROS production induced macrophage migration and M2 polarization. Immunohistochemical analysis of clinical specimens confirmed the positive correlation of NOX4 and CD68 or CD206. What are the mediators for macrophage chemotaxis and M2 polarization induced by NOX4 in cancer cells? Cancer cells kidnap macrophages commonly through release of various cytokines and chemokines [16]. For many types of cancer cells, NOX4-induced ROS stimulates various inflammatory cytokine expression [30], [31], [32]. Therefore, we speculated whether tumoral NOX4 regulated microenvironmental macrophages via cytokine production and paracrine manner. Via the cytokine antibody array assay, we found that NOX4 overexpression mostly stimulated the production and release of CCL7, IL8 and CSF-1, which are classical regulators of macrophage chemotaxis and M2 polarization. Blockade of the function of these cytokines could efficiently counteract NOX4 effect on macrophages. Specifically, we occasionally found that NOX4 upregulated VEGF-C production in NSCLC cells, which was confirmed to possess macrophage chemotaxis function in our previous study [18], and showed that VEGF-C was also involved in NOX4-induced macrophage migration.
The PI3K/Akt pathway acts as a pivotal determinant of cell biology and disease progression including cancer and aging, which is the vital downstream of NOX-induced ROS [33]. Besides, PI3K/Akt signaling controls autocrine and paracrine cytokine production and proliferative loops in cancer [34]. This study revealed that inhibition of PI3K/Akt activity blocked the effect of NOX4 on CCL7, IL8, CSF-1 and VEGF-C production in NSCLC cells. Therefore, our work also strongly suggests that PI3K/Akt activation in NSCLC cells indirectly influences microenvironmental macrophages, and provides new information in PI3K/Akt signaling-mediated cancer progression.
Our previous study has confirmed the positive regulation of NOX-induced ROS in PI3K/Akt activity in NSCLC cells [4]. Many transcriptional factors, including NF-κB and AP-1, are the downstream effectors that mediate cancer progression [35]. It is of great significance to reveal that which transcriptional factors are responsible for ROS/PI3K/Akt signaling-mediated NOX4-stimulated production of CCL7, IL8, CSF-1 and VEGF-C in NSCLC cells, as the findings will identify the novel roles of the potential transcriptional factors. However, much work needs to be done in the future study.
Our in vitro results showed that tumoral NOX4-educated M2 macrophages expressed and released HB-EGF, which was critically involved in TAM-mediated cancer cell growth [22]. Further study found that blockade of HB-EGF function reversed the enhancement effect of M2 macrophage on NSCLC growth. JNK pathway activation has been found to mediate IL4-induced M2 polarization of macrophages [23] and stimulate autocrine production of HB-EGF [36] in cancer cells. In the present study, we found that tumoral NOX4-educated macrophages exhibited elevated activity of JNK, and inhibition of JNK in M2 macrophages suppressed HB-EGF production. Therefore, our data support that M2 macrophages educated by tumoral NOX4 promotes NSCLC cell growth via JNK/HB-EGF axis.
We further assessed the effect of NOX4 on NSCLC cell proliferation and in tumor microenvironment in vivo experiment. NOX4 in cancer cells significantly enhanced the xenograft tumor growth as well as the recruitment of macrophages and M2 polarization. Elimination of ROS by NAC or inhibition of NOX4 activity by GKT137831 suppressed tumor growth accompanied by reduction in the percentage of total (F4/80+) and M2 (CD206+) macrophages in the tumor tissues. It was noted that GKT137831 has great effectiveness on the treatment of liver fibrosis and diabetic nephropathy with moderate toxicity by inhibiting NOX4 activity [37], [38]. Therefore, the present study also suggests that GKT137831 has potential clinical implications as a management for NSCLC for indirect interference of microenvironmental macrophages.
In summary, here we demonstrated that tumoral NOX4 recruits macrophages and induced M2 polarization via ROS/PI3K/Akt signaling-dependent various cytokine production, thus promoting NSCLC cell growth through JNK activation in macrophages and resultant HB-EGF release. Therefore, tumoral NOX4 may be of great value for the microenvironmental intervention treatment of NSCLC.
Acknowledgements
This work was supported by the project of the new star of Zhujiang Science and Technology (No. 201710010001), the National Natural Science Foundation of China (No. 81672836), the Open Project funded by Key laboratory of Carcinogenesis and Translational Research, Ministry of Education/Beijing (No. 2017 Open Project-2), the Projects of Guangzhou Key Laboratory of Construction and Application of New Drug Screening Model Systems (No. 201805010006) and Key Laboratory of New Drug Discovery and Evaluation of Ordinary Universities of Guangdong Province (No. 2017KSYS002). This study was also supported by Guangdong Key Laboratory of Pharmaceutical Bioactive Substances, Guangdong Pharmaceutical University.
Acknowledgments
Conflict of interests
The authors declare that they have no competing interests.
Contributor Information
Luyong Zhang, Email: lyonzhang@yeah.net.
Bing Liu, Email: liubing520@gdpu.edu.cn, liubing52000@163.com.
References
- 1.Kamata T. Roles of Nox1 and other Nox isoforms in cancer development. Cancer Sci. 2009;100:1382–1388. doi: 10.1111/j.1349-7006.2009.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown D.I., Griendling K.K. Nox proteins in signal transduction. Free Radic. Biol. Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skonieczna M., Hejmo T., Poterala-Hejmo A., Cieslar-Pobuda A., Buldak R.J. NADPH oxidases: insights into selected functions and mechanisms of action in cancer and stem cells. Oxid. Med. Cell Longev. 2017;2017:9420539. doi: 10.1155/2017/9420539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C., Lan T., Hou J., Li J., Fang R., Yang Z., Zhang M., Liu J., Liu B. NOX4 promotes non-small cell lung cancer cell proliferation and metastasis through positive feedback regulation of PI3K/Akt signaling. Oncotarget. 2014;5:4392–4405. doi: 10.18632/oncotarget.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng C., Wu Q., Wang J., Yao B., Ma L., Yang Z., Li J., Liu B. NOX4 supports glycolysis and promotes glutamine metabolism in non-small cell lung cancer cells. Free Radic. Biol. Med. 2016;101:236–248. doi: 10.1016/j.freeradbiomed.2016.10.500. [DOI] [PubMed] [Google Scholar]
- 6.Jinushi M., Chiba S., Yoshiyama H., Masutomi K., Kinoshita I., Dosaka-Akita H., Yagita H., Takaoka A., Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc. Natl. Acad. Sci. USA. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chanmee T., Ontong P., Konno K., Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackute J., Zemaitis M., Pranys D., Sitkauskiene B., Miliauskas S., Vaitkiene S., Sakalauskas R. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol. 2018;19:3. doi: 10.1186/s12865-018-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridlender Z.G., Kapoor V., Buchlis G., Cheng G., Sun J., Wang L.C., Singhal S., Snyder L.A., Albelda S.M. Monocyte chemoattractant protein-1 blockade inhibits lung cancer tumor growth by altering macrophage phenotype and activating CD8+ cells. Am. J. Respir. Cell Mol. Biol. 2011;44:230–237. doi: 10.1165/rcmb.2010-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genin M., Clement F., Fattaccioli A., Raes M., Michiels C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer. 2015;15:577. doi: 10.1186/s12885-015-1546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mongue-Din H., Patel A.S., Looi Y.H., Grieve D.J., Anilkumar N., Sirker A., Dong X., Brewer A.C., Zhang M., Smith A., Shah A.M. NADPH oxidase-4 driven cardiac macrophage polarization protects Against myocardial infarction-induced remodeling. JACC Basic Transl. Sci. 2017;2:688–698. doi: 10.1016/j.jacbts.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Choksi S., Chen K., Pobezinskaya Y., Linnoila I., Liu Z.G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23:898–914. doi: 10.1038/cr.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko S.Y., Ladanyi A., Lengyel E., Naora H. Expression of the homeobox gene HOXA9 in ovarian cancer induces peritoneal macrophages to acquire an M2 tumor-promoting phenotype. Am. J. Pathol. 2014;184:271–281. doi: 10.1016/j.ajpath.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mou W., Xu Y., Ye Y., Chen S., Li X., Gong K., Liu Y., Chen Y., Li X., Tian Y., Xiang R., Li N. Expression of Sox2 in breast cancer cells promotes the recruitment of M2 macrophages to tumor microenvironment. Cancer Lett. 2015;358:115–123. doi: 10.1016/j.canlet.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Liu C.Y., Xu J.Y., Shi X.Y., Huang W., Ruan T.Y., Xie P., Ding J.L. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab. Investig.: J. Tech. Methods Pathol. 2013;93:844–854. doi: 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- 16.Quatromoni J.G., Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am. J. Transl. Res. 2012;4:376–389. [PMC free article] [PubMed] [Google Scholar]
- 17.Solinas G., Germano G., Mantovani A., Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 18.Deng Y., Yang Y., Yao B., Ma L., Wu Q., Yang Z., Zhang L., Liu B. Paracrine signaling by VEGF-C promotes non-small cell lung cancer cell metastasis via recruitment of tumor-associated macrophages. Exp. Cell Res. 2018;364:208–216. doi: 10.1016/j.yexcr.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Su J.L., Yang P.C., Shih J.Y., Yang C.Y., Wei L.H., Hsieh C.Y., Chou C.H., Jeng Y.M., Wang M.Y., Chang K.J., Hung M.C., Kuo M.L. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell. 2006;9:209–223. doi: 10.1016/j.ccr.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Siveen K.S., Kuttan G. Role of macrophages in tumour progression. Immunol. Lett. 2009;123:97–102. doi: 10.1016/j.imlet.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Carroll M.J., Kapur A., Felder M., Patankar M.S., Kreeger P.K. M2 macrophages induce ovarian cancer cell proliferation via a heparin binding epidermal growth factor/matrix metalloproteinase 9 intercellular feedback loop. Oncotarget. 2016;7:86608–86620. doi: 10.18632/oncotarget.13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rigo A., Gottardi M., Zamo A., Mauri P., Bonifacio M., Krampera M., Damiani E., Pizzolo G., Vinante F. Macrophages may promote cancer growth via a GM-CSF/HB-EGF paracrine loop that is enhanced by CXCL12. Mol. Cancer. 2010;9:273. doi: 10.1186/1476-4598-9-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao J., Hu Y., Li Y., Zhou Q., Lv X. Involvement of JNK signaling in IL4-induced M2 macrophage polarization. Exp. Cell Res. 2017;357:155–162. doi: 10.1016/j.yexcr.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Landry W.D., Cotter T.G. ROS signalling, NADPH oxidases and cancer. Biochem. Soc. Trans. 2014;42:934–938. doi: 10.1042/BST20140060. [DOI] [PubMed] [Google Scholar]
- 25.Tang C.T., Lin X.L., Wu S., Liang Q., Yang L., Gao Y.J., Ge Z.Z. NOX4-driven ROS formation regulates proliferation and apoptosis of gastric cancer cells through the GLI1 pathway. Cell Signal. 2018;46:52–63. doi: 10.1016/j.cellsig.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Vara D., Watt J.M., Fortunato T.M., Mellor H., Burgess M., Wicks K., Mace K., Reeksting S., Lubben A., Wheeler-Jones C.P.D., Pula G. Direct activation of NADPH oxidase 2 by 2-deoxyribose-1-phosphate triggers nuclear factor kappa B-dependent angiogenesis. Antioxid. Redox Signal. 2018;28:110–130. doi: 10.1089/ars.2016.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu W., Hu Y., Chen G., Chen Z., Zhang H., Wang F., Feng L., Pelicano H., Wang H., Keating M.J., Liu J., McKeehan W., Wang H., Luo Y., Huang P. Novel role of NOX in supporting aerobic glycolysis in cancer cells with mitochondrial dysfunction and as a potential target for cancer therapy. PLoS Biol. 2012;10:e1001326. doi: 10.1371/journal.pbio.1001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boudreau H.E., Casterline B.W., Rada B., Korzeniowska A., Leto T.L. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic. Biol. Med. 2012;53:1489–1499. doi: 10.1016/j.freeradbiomed.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Block K., Gorin Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat. Rev. Cancer. 2012;12:627–637. doi: 10.1038/nrc3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzgerald J.P., Nayak B., Shanmugasundaram K., Friedrichs W., Sudarshan S., Eid A.A., DeNapoli T., Parekh D.J., Gorin Y., Block K. Nox4 mediates renal cell carcinoma cell invasion through hypoxia-induced interleukin 6- and 8- production. PLoS One. 2012;7:e30712. doi: 10.1371/journal.pone.0030712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J., Lan T., Zhang C., Zeng C., Hou J., Yang Z., Zhang M., Liu J., Liu B. Reciprocal activation between IL-6/STAT3 and NOX4/Akt signalings promotes proliferation and survival of non-small cell lung cancer cells. Oncotarget. 2015;6:1031–1048. doi: 10.18632/oncotarget.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fletcher E.V., Love-Homan L., Sobhakumari A., Feddersen C.R., Koch A.T., Goel A., Simons A.L. EGFR inhibition induces proinflammatory cytokines via NOX4 in HNSCC. Mol. Cancer Res. 2013;11:1574–1584. doi: 10.1158/1541-7786.MCR-13-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakanishi A., Wada Y., Kitagishi Y., Matsuda S. Link between PI3K/AKT/PTEN Pathway and NOX Proteinin Diseases. Aging Dis. 2014;5:203–211. doi: 10.14336/AD.2014.0500203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatt A.P., Bhende P.M., Sin S.H., Roy D., Dittmer D.P., Damania B. Dual inhibition of PI3K and mTOR inhibits autocrine and paracrine proliferative loops in PI3K/Akt/mTOR-addicted lymphomas. Blood. 2010;115:4455–4463. doi: 10.1182/blood-2009-10-251082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X., Jin B., Huang C. The PI3K/Akt pathway and its downstream transcriptional factors as targets for chemoprevention. Curr. Cancer Drug Targets. 2007;7:305–316. doi: 10.2174/156800907780809741. [DOI] [PubMed] [Google Scholar]
- 36.Caceres M., Tobar N., Guerrero J., Smith P.C., Martinez J. c-jun-NH2JNK mediates invasive potential and EGFR activation by regulating the expression of HB-EGF in a urokinase-stimulated pathway. J. Cell Biochem. 2008;103:986–993. doi: 10.1002/jcb.21469. [DOI] [PubMed] [Google Scholar]
- 37.Aoyama T., Paik Y.H., Watanabe S., Laleu B., Gaggini F., Fioraso-Cartier L., Molango S., Heitz F., Merlot C., Szyndralewiez C., Page P., Brenner D.A. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56:2316–2327. doi: 10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jha J.C., Gray S.P., Barit D., Okabe J., El-Osta A., Namikoshi T., Thallas-Bonke V., Wingler K., Szyndralewiez C., Heitz F., Touyz R.M., Cooper M.E., Schmidt H.H., Jandeleit-Dahm K.A. Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J. Am. Soc. Nephrol. 2014;25:1237–1254. doi: 10.1681/ASN.2013070810. [DOI] [PMC free article] [PubMed] [Google Scholar]