Abstract
Of all cases of idiopathic steroid-sensitive nephrotic syndrome (NS) in children, 40%–75% cases need long-term continuous steroids and/or other immunosuppressants to maintain remission, the effects of which on growth and renal function remain an issue of concern. The study aimed at exploring the safety and efficacy of mycophenolate mofetil (MMF) as a remission-maintaining agent in children with a diagnosis of frequent relapsing or steroid-dependent NS (FRNS/SDNS) requiring continuous medication for at least 1 year. Thirty-two children thus included received MMF (1000–1200 mg/m2/day) for 7 months along with tapering doses of oral prednisolone if it was being given from before with an attempt at tapering at 0.25 mg/kg/month ultimately stopping it altogether. Individuals were followed up for at least 5 more months after stopping MMF. Out of 32 children, 26 had SDNS and 6 had FRNS with male:female ratio being 2.2:1. The mean standard deviation (± SD) age of onset of disease was 2.72 ± 1.3 years and that entry to the study was 7.17 ± 2.2 years. Significant fall in number of relapses was observed following the introduction of MMF (110 in pre-MMF12 month period vs. 52 in post-MMF 12 months [p = 0.002]). The mean relapse rate/year/patient also decreased from 3.43 ± 1.26 to 1.62 ± 1.14 after entry in the study. Significant reduction of the cumulative dose of steroid regarding mean ± SD of mg/kg/year was also found following the introduction of MMF (190.9 ± 47.81 vs. 119.09 ± 60.09 [p = 0.001]). MMF is an efficacious agent in maintaining remission and reducing steroid requirement in children with FRNS and SDNS.
Keywords: Children, mycophenolate mofetil, nephrotic syndrome
Introduction
Nephrotic syndrome (NS) is a common condition in children, 95% of which are idiopathic or primary. Eighty percent of these children respond to oral steroids and are thus called steroid-sensitive NS (SSNS). Of all the steroid-sensitive episodes, 25%–40% are infrequent relapsers who remain steroid free for prolonged duration of time. However, the rest are either frequent relapsers NS (FRNS) or steroid-dependent NS (SDNS) who need long-term steroids putting them at risk of various side effects such as cushingoid features, hypertension, growth failure, and even emotional problems.[1,2] In such cases, the aim of the therapy remains to maintain remission with some alternative drugs as steroid-sparing agents such as levamisole and cyclophosphamide cyclosporine which again have the possibility of undesirable effects. Mycophenolate mofetil (MMF), which initially was tried as an immunomodulator in transplant medicine, is gradually evolving as a new therapeutic agent for childhood NS, especially as a steroid-sparing agent for prevention of relapses.
This study was done with an objective to determine the safety and efficacy of MMF in maintaining remission and reducing the number of relapses in childhood idiopathic NS both during and after the period of therapy.
Materials and Methods
All children attending a tertiary care teaching hospital in Kolkata between 2 and 12 years of age given a diagnosis of idiopathic SDNS/FRNS and were on continuous medication for at least 12 months to prevent relapses were considered for inclusion in the study. Institutional Ethics Committee clearance was obtained. Written informed consent was taken from the guardians of the patients included in the study. Assent was also taken from children who were more than 7 years old. Frequent relapse was defined as four or more relapses in any 1-year duration. Steroid dependence was defined as 2 or more consecutive relapses while tapering or within 14 days of stopping the steroids.[1] However, children not in complete remission on presentation, with estimated glomerular filtration rate <50 ML/MIN/1.73 m2, prior administration of MMF for more than 15 days, absolute neutrophil count (ANC) <2000/mm3, and those with comorbidities such as asthma and symptomatic tuberculosis were excluded from the study. Cases where detailed previous records were not available or with irregular history of compliance were also excluded from the study.
The demographic, clinical, treatment and investigational details of the included patients were documented in a predesigned pro forma. It was a prospective, interventional study taking each patients’ retrospective data for the last 12 months into account, each patient serving as his/her own control in the preintervention period. MMF was started at the dose of 1000–1200 mg/m2/day (maximum of 1 g twice daily) in two divided doses till 24 weeks. Then, it was reduced by 50% every 2 weeks with the aim to stop it at the end of 28 weeks. Oral prednisolone was continued if it was being given from before with an attempt at tapering at 0.25 mg/kg/month ultimately stopping it altogether. Any relapse during the study period was managed according to the recommendation of Indian Pediatric Nephrology Study Group.[2] The patients were closely monitored both as regards their blood counts, renal functions, and other adverse effects. Children were followed up routinely every 2 months (more frequently if required) till at least 5 months of stopping MMF. The total duration of follow up was thus 12 months, 7 months on MMF, and next 5 months after withdrawal of MMF. The guardians were trained to monitor urinary protein at home and to maintain a written record. They were asked to report immediately if there were signs of relapse or urine protein became ≥+++ or there were any other significant health problem. The dosage of MMF was reduced to 800 mg/m2 when MMF related toxicity was suspected. The dosage was further reduced, or it was completely stopped if the problem persisted.
The outcome variables were measured regarding relapse rates and cumulative dose of steroids required to maintain remission before and after intervention. More than two relapses during the study period were considered as treatment failure, and alternative drugs were considered for those cases.
Statistical analysis
The data were entered into MS-Excel and then analyzed with Statistica version 8 (Tulsa, Oklahoma: StatSoft Inc., 2007) and MedCalc version 11.6 (Mariakerke, Belgium: MedCalc Software, 2011) statistical software. Key proportions have been expressed with 95% confidence interval values. Paired t-test was used for comparison of numerical variables with the patients acting as their own control. Value of p < 0.05 was taken to be statistically significant.
Results
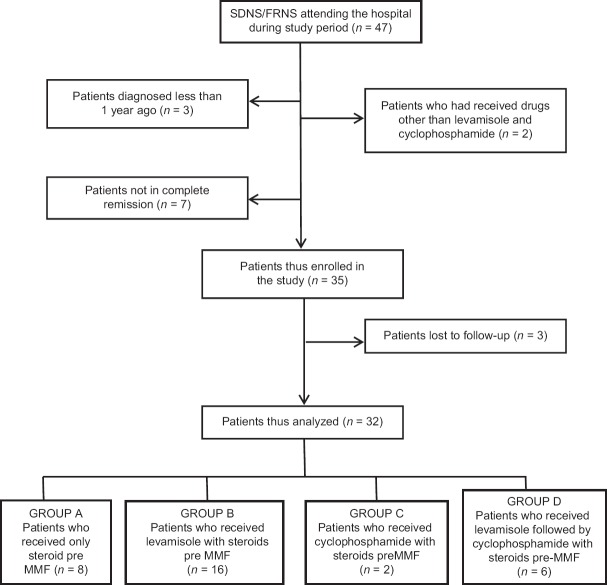
Out of a total of 47 children with SDNS/FRNS attending our hospital during the study, 35 were included in the study [Figure 1]. The records of 32 children were analyzed as 3 were lost to follow-up. Of these, 26 were SDNS and 6 FRNS [Table 1]. The cohort consisted of 22 (69%) boys and 10 (31%) girls. The mean standard deviation (±SD) age of onset of NS was 2.72 ± 1.3 years (range 1.5–4.5 years) that of diagnosis of FRNS/SDNS was 4.9 ± 1.6 years (range 3.0–7.0 years) and the mean (±SD) age of entry to the study was 7.17 ± 2.2 years (range 4.8–9.3 years). Children were receiving continuous immunosuppressant for 1.8 ± 0.6 years (mean ± SD) before entering the study phase. While 8 had been on only low-dose steroids, 16 had received levamisole with steroids, 2 children had got a course of oral cyclophosphamide, and 6 had received both levamisole and cyclophosphamide in a sequential manner with steroids. The mean dosage of MMF used was 1131.56 ± 49.6 mmg/m2/day (range 1041–1198 mg/m2/day).
Figure 1.
Patient flow
Table 1.
Overall comparison of the cumulative dose of oral steroid pre- and post-mycophenolate mofetil intervention
| Cumulative steroid (mg/kg/year) | Before MMF | After MMF |
|---|---|---|
| Range | 110-274 | 42-242.5 |
| Mean | 190.9±47.81 | 119.09±60.09 |
| Median | 188 | 105 |
| IQR | 165.5-218 | 86.5-149.3 |
IQR: Interquartile ratio, MMF: Mycophenolate mofetil
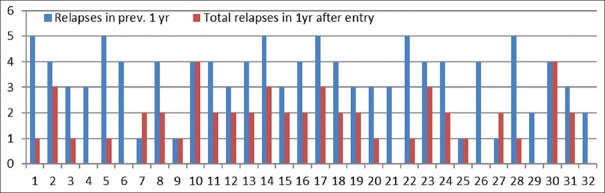
Renal biopsy done in eight patients revealed minimal change disease in 7 and IgM nephropathy in 1. Significant fall in a number of relapses was observed following the introduction of MMF (110 in pre-MMF12-month period versus. 52 in post-MMF 12 months [p = 0.002]). Figure 2 shows the comparison of relapses/year in individual patients before and after entering the study. By applying WILCOXON nonparametric test, the mean relapse rate/year/patient before entry was 3.43 ± 1.26 (range 1–5, Interquartile ratio 3–4) whereas it was 1.62 ± 1.14 (range 1–4, interquartile ratio 1–2) after entry in the study.
Figure 2.
Comparison of number of relapses in individual patients 1 year pre- and post-intervention
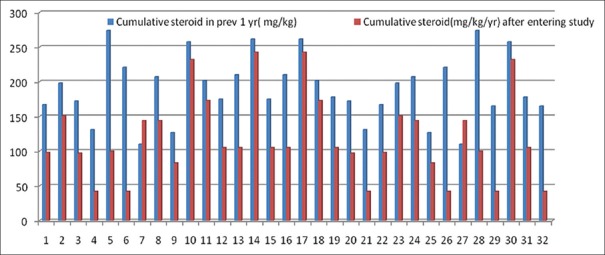
Significant reduction of cumulative dose [Table 1] of steroid ranging mean ± SD of mg/kg/year was also found following introduction of MMF (190.9 ± 47.81 vs. 119.09 ± 60.09 [p = 0.001]) [Table 1]. Figure 3 shows a comparison of cumulative dose of steroid in pre- and post-MMF12 month period.
Figure 3.
Comparison of the cumulative dose of steroid in an individual patient in mg/kg/year pre- and post-intervention
Two patients complained of pain abdomen within 2 weeks of starting MMF. However, the symptoms gradually subsided on reducing the dose to 800 mg/m2/day. None of the patients showed any significant alteration of blood counts and none required withdrawal or stoppage of MMF because of side effects.
Children were classified into four groups based on the medications received in the year before starting MMF [Table 2]. It was seen that MMF fared significantly better in all the groups both in reducing the number of relapses and also the cumulative dose of steroids. Test of significance could not be done in Group C due to few subjects (n = 2). These two children in Group C had one episode of relapse each both in pre-intervention, and postintervention period, however, there were no relapses while on MMF. Both the relapses occurred after withdrawal of MMF during the 5-month drug-free follow-up period. These two patients also required much less cumulative dose of steroid after administration of MMF compared to when they got cyclophosphamide.
Table 2.
Comparison of 4 groups regarding number of relapses and cumulative dose of steroid
| Parameters analysed | Group A | Group B | Group C | Group D | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-MMF (n=8) | Post-MMF (n=8) | P/r value (95% CI) | Pre-MMF (n=16) | Post-MMF (n=16) | P/r value (95% CI) | Pre-MMF (n=2) | Post-MMF (n=2) | P/r value | Pre-MMF (n=6) | Post-MMF (n=6) | P/r value (95% CI) | |
| Total | 34 | 9 | 26 | 22 | 1 | 1 | 13 | 10 | ||||
| Relapses (n) | ||||||||||||
| Mean±SD relapse/patient/year | 4.25±0.5 | 2.25±1.2 | 0.007/−0.66 (0.73-3.26) | 3.00±1.2 | 1.38±1.1 | 0.000/−0.11 (0.36-0.85) | 1.00 | 1.00 | _ | 3.83±1.5 | 1.67±1.4 | 0.006/−0.66 (0.95-3.39) |
| Range of number of relapse | 4-5 | 1-4 | 1-5 | 0-3 | 1 | 1 | 1-5 | 0-3 | ||||
| Mean±SD cumulative steroid dose (mg/kg/year) | 235.85±32.7 | 15,250±57.9 | 0.009/0.04 (28.6-138.0) | 162.02±27.1 | 98.00±38.80 | 0.000/0.16 (40.78-87.26) | 126.70 | 83.00 | _ | 229.77±25.3 | 142.83±89.7 | 0.034/0.71 (9.51-164.34) |
| Range of cumulative steroid dose | 201.8-274.0 | 100-232 | 110-198 | 42-151.2 | 115.1-138.3 | 68.0-98.0 | _ | 207.1-261.5 | 42.5-242.2 | |||
| Side effects | Hypertension-3 Growth failure-1 Cushingoid facies-4 |
Pain abdomen-1 | Leukopenia-2 Flu-like illness-2 Hypertension-5 |
Nil | Alopecia and nail discoloration-1 | Nausea and pain abdomen -1 | Rash-2 Cushingoid facies-2 Leukopenia-1 |
Nil | ||||
Group A: Cases who received only steroid pre-MMF, Group B: Cases who received steroid and levamisole pre-MMF, Group C: Cases who received steroid followed by cyclophosphamide pre-MMF, Group D: Cases who received steroid followed by levamisole and cyclophosphamide pre-MMF. As there are only two patients in group C statistical calculation could not be done. MMF: Mycophenolate mofetil, CI: Confidence interval, SD: Standard deviation
Discussion
MMF is the 2-morpholinoethyl ester of mycophenolic acid (MPA). It is a prodrug that is rapidly hydrolyzed to the active drug, mycophenolate acid (MPA), a selective, noncompetetive and reversible inhibitor of inosine monophosphate dehydrogenase, an important enzyme in the de novo pathway of guanine synthesis. Besides inhibiting both B and T lymphocytes, MPA is shown to inhibit vascular smooth muscle cell and mesangial cell proliferation, to be a selective inhibitor of inducible nitric oxide synthase and to induce apoptosis in activated T cells. One or any combination of these functions may be accountable for amelioration of inflammation and/or structural remodeling characteristic of the glomerular disease. The principal toxicities of MMF are gastrointestinal and hematologic which include leukopenia, diarrhea, and vomiting.[11]
It was initially used as an immunosuppressant for prevention of organ transplant rejection.[12,13,14,15,16] However, afterward the role of MMF as a steroid-sparing alternative in idiopathic NS has also been explored. It has generally been used when the frequency of relapses remain unacceptable despite prolonged steroids, levamisole, cyclophosphamide, and even cyclosporine.
The findings of few previous studies using MMF in NS are summarized in Table 3. In most of the studies, MMF has been shown to result in statistically significant reduction in frequency of relapses as well as the cumulative dose of steroid required, irrespective of the previous alternative drugs used.[3,5,6,7,9,17,18] Our results were also comparable. Some studies have also been published from India and other Asian countries starting from the year 2003, which also show favorable response to MMF with minimal side effects.[4,8,10] It should, however, be noted that there is considerable non-uniformity in both MMF dosage and duration and other drugs used both pre MMF and with MMF. The dosage of MMF has been calculated both regarding body weight and body surface area, and it has varied from 600 mg/m2/day to 1200 mg/m2/day and from 25 mg/kg to 40 mg/kg. In addition, there seems to be wide variation in duration of therapy, from 3 months to 7 years. The previous studies have not shown any significant variation of outcome regarding efficacy and side effects with the dosage of MMF given per day. However, the studies involving MMF for longer duration give an indication that MMF is more efficacious in maintaining remission if the duration of therapy is increased from months to several years.[8] Afzal et al. concluded that giving MMF for more than 12 months is more efficacious than 6 months. Treatment continuation beyond 12 months resulted in sustained steroid sparing and reduced need for alternative treatments while maintaining low relapse rates.[8] However, the implications of increasing the duration of therapy on possible long-term complications remain to explored by more robust long-term follow-up studies. Our study included MMF usage for 6 months with benefits in maintaining remission. Search for a systematic review to find out more robust evidence yielded a Cochrane study, which included 32 published studies involving 1443 children regarding the use of nonsteroidal immunosuppressants in SSNS. This review showed an overall beneficial effect of MMF.[19]
Table 3.
Summary of results of previous publications and comparison with the present series
| Author/year/place of publication | Number of patients/age range (months) | Dose/duration of MMF | Summary of previous therapy | Duration of follow-up | Outcome | Adverse effects | Overall impression |
|---|---|---|---|---|---|---|---|
| Barletta et al./2003/USA/retrospective[3] | 14/41-190 months | 800-1200/m2/day for 12months | SRNS -cyclosporine -dependent-10 Steroid with cyclophosphamide-4 |
NA | Number of relapses reduced from 2.85±0.4 to 1.07±0.3 (p<0.01) | Diarrhea, malaise, and splenomegaly in 2 | Reduction in frequency and severity of relapses. Helps in reducing steroids and cyclosporine exposure |
| Bagga et al./2003/India/prospective[4] | 19/3-11 years | 29 (21-33 mg/kg/day)/for 11.8 month | prednisolone 19, levamisole 16 cyclophosphamide 15 | 17 months | Number of relapses 6.6/year to 2/year (p<0.0001). | Nil | MMF appears to be a promising intervention in children with SDNS |
| Novak et al./2005/USA/retrospective[5] | 21/2-17 years | 600 mg/m2 bdfor 1.0±0.5 years | Steroids | NA | 0.80±0.41 to 0.47±0.43 relapses per month (p<0.02) | Minor diarrhea-1 | About 40% reduction of relapse rate without side effects |
| Ulinski et al./2005/France/prospective[6] | 9/3.3-15.7 years | Up to 1 g/1.73 m2 bd for 261±183 days | Steroids and cyclosporine | Median follow-up 261 days | SDNS remained in remission/no significant effect on SRNS | No adverse events | Could be used both as a steroid-sparing and cyclosporine -sparing agent Better growth velocity, BP profile, and physical appearance |
| Hogg et al./2006/USA/Multicentric prospective/open label[7] | 33/2-15 year | 600 mg/m2 twice daily (maximum 1 g twice daily) for 6 month | Only steroid | 18-30 months | One episode of relapse every 2 months before MMF to one every 14.7 months after MMF | ANC <300/mm3-1 Mild decrease in ANC-4 gastritis needing H2 blocker-1 |
MMF is effective for maintaining remission if treated for at least 6 months Low incidence of adverse events |
| Afzal et al./2007/India/prospective[8] | 42/32-187 month | 26.5 (16.6-31.3) mg/kg for 14.3 (6-45) months | Steroid 42 Levamisole 35 Cyclophosphamide 37 |
6-45 months | Mean 6-monthly relapse rates decreased from 3.0 episodes before therapy to 0.9 episodes in the first 6 months, 0.7 in the next 6 months, and 0.3 in those treated longer than 12 months (p<0.0001) | Nil | MMF when given longer than 6 months has more efficacy without significant adverse effects |
| Baudouin et al./2012/France/phase II Bayesian trial[13] | 24/2.8-14.4 years | MMF (1200 mg/m2/day for 12 months | Cyclophosphamide 100% Levamisole + cyclophosphamide 67% |
12 months | Cumulative dose of steroid pre- and post-MMF from 459 to 264 mg/m2/month (p<0.001) | No significant side effect | MMF reduces relapse rate and steroid dose in children with SDNS and should be proposed before cyclosporine and cyclophosphamide |
| Gellermann et al./2013/Germany/prospective randomized multicenter open-label crossover trial[9] | 60 divided into 2 groups 0f 30 each for cross over/9-16 years | 1000-1200 mg/m2/day titrated to trough MPA level 1.5-2.5 µg/ml/12 months | Levamisole, cyclophosphamide, cyclosporine, MMF | 12 months each before and after crossover | 1st year-MMF versus cyclosporine relapse rate (mean 1.10 vs. 0.24; p=0.03), 2nd year - MMF versus cyclosporine (0.40 vs. 0.20; p=0.14) |
52 reports of adverse effects from 20 patients out of which 2 had severe respiratory infection | MMF increased cystatin clearance, estimated GFR, and hemoglobin levels increased compared with cyclosporine MMF is inferior to cyclosporine in preventing relapses but may be a less nephrotoxic agent |
| Lim et al./2015/Korea/retrospective comparative[14] | 11/8.55±2.62 years | 40 mg/kg/day for 15.27±3.13 months | cyclosporine.levamisole | 3.4/year to 0.2/year (p=0.003) | Herpezs zoster-1. | MMF comparable in efficacy in reducing relapses to cyclosporine and levamisole with lesser side effects | |
| Iftikhar et al./2011/Pakistan/cross-sectional retrospective[10] | 11/1.6-12.6 years | 800 mg/m2/day to 1200 mg/m2/day | Cyclophosphamide -9 Levamisole 3 Cyclosporin -3 |
Mean relapse rate 4.31+0.87/patient/year to 1.12+0.718/patient/year after starting MMF (p=0.0001) | Brief diarrhea-2 | MMF is an effective drug for SDNS with minimal side effects | |
| Present series/2017/India/prospective on retrospective | 32/4.9-9 years | 1000-1200 mg/m2/day for 24 weeks and reduced and withdrawn in next 4 weeks | Steroid -32 Levamisole - 22 Cyclophosphamide- 8 |
12 months | Mean relapse rate/year/patient before entry was 3.43±1.26 reduced to 1.62±1.14after entry in the study (p=0.002) | Pain abdomen/vomiting-2 | MMF is better than levamisole and cyclophosphamide in maintain remission with lesser side effects |
SRNS: Steroid-resistant nephrotic syndrome, MMF: Mycophenolate mofetil, BP: Blood pressure, ANC: Absolute neutrophil count, GFR: Glomerular filtration rate
None one of the previous studies including ours have tried head-on comparison of the efficacy of MMF with steroids, levamisole, and cyclophosphamide as a remission maintaining agent. In our study, MMF could achieve a statistically significant reduction in both number of relapses and also cumulative dose of steroid (p < 0.05). If this favorable profile of MMF is reflected in larger studies also, then MMF could be considered much earlier in the sequence of immunosuppressants for prevention of relapses in childhood NS.
MMF associated infectious complications and leukopenia from bone marrow suppression appears to be much less than levamisole and cyclophosphamide, which may be a significant advantage of MMF over these drugs. MMF seems to have a satisfactory and acceptable adverse effect profile, at least in the short term, both in previous studies as well as in the present series. None of the patients in our series required the withdrawal of MMF due to unacceptable side effects. Commonly reported side effects have been gastrointestinal or hematological or infectious, but rarely serious enough to need stoppage or withdrawal of MMF. Hogg et al., in a series of 33 patients, reported that only one patient required the withdrawal of MMF due to fall in ANC to <300/mm3, four others had transient mild reduction in ANC not requiring stoppage of MMF.[7] In other studies, the side effects have been relatively minor which subsided after reducing the dosage.[3,5,9] Of course, more studies of longer duration are required to know the incidence of long-term effects, if any.
Limitations
Our study throws light on the efficacy of MMF as a remission maintaining agent from Eastern India. However, it also has its share of limitations regarding limited sample size and shorter duration of follow-up. Owing to logistic issues and the drug being costly, we had to limit the duration of administration to reduce follow-up attrition of patients in our resource-limited settings. Longer duration of administration of MMF and longer duration of follow-up after withdrawal would have given more robust results. In addition, the previous year data were collected retrospectively from patient records. Retrospective data of each patient served as his own control and were used as the comparison arm to minimize variability of clinicoepidemiological profile of MMF and non-MMF arms, the drug administered being the only variable.
Conclusions
MMF seems to have a favorable efficacy and side effect profile as a steroid-sparing agent in maintaining remission in childhood NS. While there seems to be a general agreement on the efficacy of MMF in preventing the relapses in NS, jury is still out regarding the optimal dosage and duration of MMF.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bagga A, Srivastava RN, editors. Pediatric Nephrology. 5th ed. New Delhi: Jaypee; 2011. Nephrotic syndrome; pp. 195–234. [Google Scholar]
- 2.Bagga A, Ali U, Banerjee S, Kanitkar M, Phadke KD, et al. Indian Pediatric Nephrology Group, Indian Academy of Pediatrics. Management of steroid sensitive nephrotic syndrome: Revised guidelines. Indian Pediatr. 2008;45:203–14. [PubMed] [Google Scholar]
- 3.Barletta GM, Smoyer WE, Bunchman TE, Flynn JT, Kershaw DB. Use of mycophenolate mofetil in steroid-dependent and -resistant nephrotic syndrome. Pediatr Nephrol. 2003;18:833–7. doi: 10.1007/s00467-003-1175-4. [DOI] [PubMed] [Google Scholar]
- 4.Bagga A, Hari P, Moudgil A, Jordan SC. Mycophenolate mofetil and prednisolone therapy in children with steroid-dependent nephrotic syndrome. Am J Kidney Dis. 2003;42:1114–20. doi: 10.1053/j.ajkd.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Novak I, Frank R, Vento S, Vergara M, Gauthier B, Trachtman H, et al. Efficacy of mycophenolate mofetil in pediatric patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2005;20:1265–8. doi: 10.1007/s00467-005-1957-y. [DOI] [PubMed] [Google Scholar]
- 6.Ulinski T, Dubourg L, Saïd MH, Parchoux B, Ranchin B, Cochat P, et al. Switch from cyclosporine A to mycophenolate mofetil in nephrotic children. Pediatr Nephrol. 2005;20:482–5. doi: 10.1007/s00467-004-1778-4. [DOI] [PubMed] [Google Scholar]
- 7.Hogg RJ, Fitzgibbons L, Bruick J, Bunke M, Ault B, Baqi N, et al. Mycophenolate mofetil in children with frequently relapsing nephrotic syndrome: A report from the Southwest Pediatric Nephrology Study Group. Clin J Am Soc Nephrol. 2006;1:1173–8. doi: 10.2215/CJN.00550206. [DOI] [PubMed] [Google Scholar]
- 8.Afzal K, Bagga A, Menon S, Hari P, Jordan SC. Treatment with mycophenolate mofetil and prednisolone for steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2007;22:2059–65. doi: 10.1007/s00467-007-0617-9. [DOI] [PubMed] [Google Scholar]
- 9.Gellermann J, Weber L, Pape L, Tönshoff B, Hoyer P, Querfeld U, et al. Mycophenolate mofetil versus cyclosporin A in children with frequently relapsing nephrotic syndrome. J Am Soc Nephrol. 2013;24:1689–97. doi: 10.1681/ASN.2012121200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iftikhar E, Khan HI, Rabia T, Sheikh SA, Malik A, Rahman NI. Effect of mycophenolate mofetil in steroid dependent and steroid resistant nephrotic syndrome patients in Pakistan. Malays J Paediatri Child Health Online Early. 2011;4:17–21. [Google Scholar]
- 11.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 12.Bansal SB, Saxena V, Pokhariyal S, Gupta P, Kher V, Ahlawat R, et al. Comparison of azathioprine with mycophenolate mofetil in a living donor kidney transplant programme. Indian J Nephrol. 2011;21:258–63. doi: 10.4103/0971-4065.85483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mele TS, Halloran PF. The use of mycophenolate mofetil in transplant recipients. Immunopharmacology. 2000;47:215–45. doi: 10.1016/s0162-3109(00)00190-9. [DOI] [PubMed] [Google Scholar]
- 14.Thervet E, Anglicheau D, Legendre C. Pharmacology of mycophenolate mofetil: Recent data and clinical consequences. Nephrologie. 2001;22:331–7. [PubMed] [Google Scholar]
- 15.Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal transplant mycophenolate mofetil study group. Transplantation. 1995;60:225–32. doi: 10.1097/00007890-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 16.A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation. 1996;61:1029–37. [PubMed] [Google Scholar]
- 17.Baudouin V, Alberti C, Lapeyraque AL, Bensman A, André JL, Broux F, et al. Mycophenolate mofetil for steroid-dependent nephrotic syndrome: A phase II bayesian trial. Pediatr Nephrol. 2012;27:389–96. doi: 10.1007/s00467-011-2006-7. [DOI] [PubMed] [Google Scholar]
- 18.Lim TJ, Kim SH, Kim SY. Efficacy and safety of mycophenolate mofetil in children with steroid dependent nephrotic syndrome. Child Kidney Dis. 2015;19:105–11. [Google Scholar]
- 19.Pravitsitthikul N, Willis NS, Hodson EM, Craig JC. Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Database Syst Rev. 2013;10:CD002290. doi: 10.1002/14651858.CD002290.pub4. [DOI] [PubMed] [Google Scholar]