Abstract
Multiple protocols have been devised to generate cerebral organoids that recapitulate features of the developing human brain, including the presence of a large, multi-layered, cortical-like neuronal zone. However, the central question is whether these organoids truly present mature, functional neurons and astrocytes, which may qualify the system for in-depth molecular neuroscience studies focused at neuronal and synaptic functions. Here, we demonstrate that cerebral organoids derived under optimal differentiation conditions exhibit mature, fully functional neurons and astrocytes, as validated by immunohistological, gene expression, and electrophysiological, analyses. Neurons in cerebral organoids showed gene expression profiles and electrophysiological properties similar to those reported for fetal human brain. These important findings indicate that cerebral organoids recapitulate the developing human brain and may enhance use of cerebral organoids in modeling human brain development or investigating neural deficits that underlie neurodevelopmental and neuropsychiatric conditions, such as autism or intellectual disorders.
Keywords: cerebral organoids, human brain, stem cells, neurons, astrocytes, neurodevelopmental disorders, neuropsychiatric disorders, autism
Human cerebral organoids have recently emerged as the state-of-the-art technology to model human brain development in three-dimensions (3D) and represent a system that can best recapitulate in vitro the in-vivo-developing human brain for studies of human-specific brain development and neurodevelopmental or neuropsychiatric disorders. Most of studies thus far have focused on aspects such as neurogenesis and neural stem cell functions or radial glial development and functions, but the use of organoids in in-depth neuroscience studies, such as neuronal and synaptic functions and their link to neurodevelopmental disorders, has been delayed, due at least in part to uncertainty about whether these models indeed present fully functional, or “mature”, neurons and astroglial cells that support mature neuronal functions. In this focused article, we will briefly discuss recent findings in the field over the past ~2–3 years, and their impact on advancing the cerebral organoid model system.
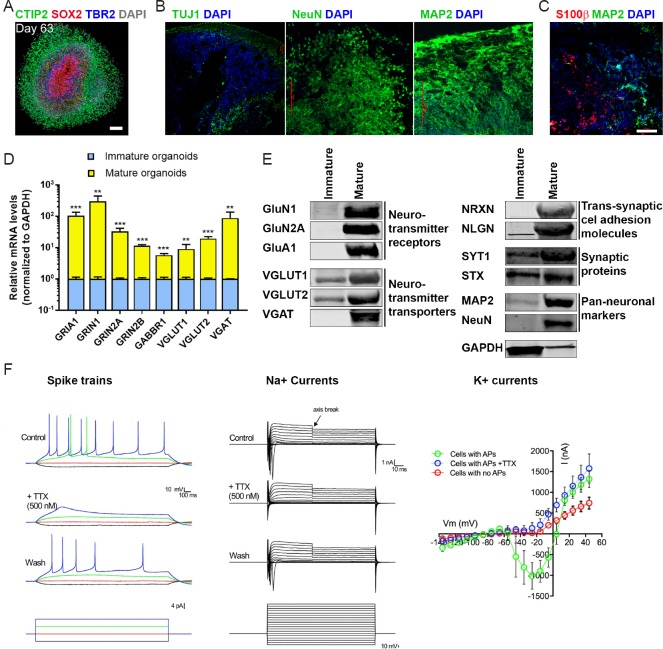
Various protocols have been developed, over the past 10 years, to generate cortical neurons or cerebral organoids from pluripotent stem cells, including the use of patterning factors to enhance neuronal fate specification of stem cells and/or through the self-organizing ability of pluripotent stem cells into 3D tissues (Eiraku et al., 2008; Gaspard et al., 2008; Chambers et al., 2009; Mariani et al., 2012; Shi et al., 2012; Kadoshima et al., 2013; Lancaster et al., 2013; Paşca et al., 2015; Qian et al., 2016). Qian et al. (2016) attempted to build upon a previous, intrinsic self-organization-based, protocol (Lancaster et al., 2013) and to improve it by using a miniaturized spinning bioreactor that allows homogenous distribution of nutrients during organoid growth in the culture. They were able to generate 3D organoid tissues that show radial distribution of layers, and cell types similar to the developing human neocortex (Qian et al., 2016) (Figure 1A). Recently we have used H1 human embryonic stem cell (ESC) line to derive further optimized cerebral organoids, and built upon several previously reported neuronal differentiation (2D and 3D) methods (Eiraku et al., 2008; Gaspard et al., 2008; Chambers et al., 2009; Mariani et al., 2012; Shi et al., 2012; Kadoshima et al., 2013; Lancaster et al., 2013; Paşca et al., 2015; Qian et al., 2016), and compared the outcomes of various methods. We noticed that by eliminating many of the factors employed by previous protocols (e.g., dual SMAD inhibitors), we can obtain more optimal organoids, with less inter-batch and intra-batch variability (Yakoub and Sadek, 2018). These optimized cerebral organoids exhibited a robust, thick neuronal zone (that ranged from ~30-50 neuronal cell layers in mature organoids at ~2.5- to 3-month age). These optimal cerebral organoids exhibited neurons that stained positive for general neuronal markers such as TUJ1 (neuron-specific class III β-tubulin), NeuN (neuronal nuclei) and MAP2 (microtubule-associated protein 2) (Figure 1B), and mature astroglial cells that stained positive for GFAP (glial fibrillary acidic protein) and S100β (S100 calcium-binding protein-β) (Figure 1C). To probe for neuronal maturity, we performed gene expression analyses on organoids via quantitative polymerase chain reaction (qPCR) (Figure 1D) and immunoblotting (Figure 1E) assays to determine mRNA and protein expression levels of important genes for neuronal and synaptic functions. We found that optimal organoids showed upregulation of the neurotransmitter receptor gene that are highly expressed in the human brain, such as the glutamate, AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor GluA1, and the NMDA (N-methyl-D-aspartate) receptors GluN1, GluN2A and GluN2B, or the γ-amino butyric acid (GABA) receptor GABA-B receptor 1. Interestingly, protein expression of the NMDA (N-methyl-D-aspartic acid) receptors is a hallmark of neuronal maturity during fetal human brain development (beyond 23 weeks of gestation): while immature neurons derived from early-stage (< 22 post-gestational week-old) fetal human brain, as well as mature neurons derived from later-stage (> 23 weeks post-gestation) human brain displayed mRNA expression of the NMDA receptor subunits 1 and 2A, only mature neurons showed protein expression of the receptors (Eugenin et al., 2011). Moreover, upregulation of the neurotransmitter transporters such as the VGLUT (vesicular glutamate transporter) 1 and 2 or VGAT (vesicular GABA transporter), or of the important synaptic proteins such as synaptotagmin 1 (SYT1) or syntaxin (STX) was also observed in mature organoids (Figure 1F). Additionally, upregulation of essential trans-synaptic cell-adhesion molecules neurexin (NRXN) and its cognate binding partner, neuroligin (NLGN) was observed in optimal cerebral organoids (Figure 1E). In the human brain, expression of NRXN isoforms is significantly upregulated between gestational weeks 18–20 (Jenkins et al., 2016), suggesting that these cerebral organoids express mature neurons reminiscent of mid-gestational human brain, as was also suggested by other studies (Watanabe et al., 2017). Moreover, single-cell RNA-seq analysis on cells dissociated from cerebral organoids or human fetal brain tissue revealed great similarity between organoids and the human brain in terms of gene expression programs and the corticogenesis and neurogenesis programs (Ritter et al., 2001; Camp et al., 2015; Bagasrawala et al., 2017). Altogether, these results indicate that organoids recapitulate the gene expression patterns of the developing human brain, and suggest that organoids can be used to study human corticogenesis in vitro (Camp et al., 2015).
Figure 1.
Optimal cerebral organoids recapitulate the structure, gene expression profiles and electrophysiological properties of the human brain.
(A) An example organoid on day 63 post-differentiation immunohistochemically stained, showing a multi-layer structure encompassing SOX2+ neural progenitor cells, TBR2+ intermediate progenitor cells, and CTIP2+ neurons; scale bar: 100 μm; adapted, with permission, from Qian et al. (2016). (B) Three sections from H1-derived organoids were stained with TUJ1, NeuN or MAP2, and DAPI and imaged using confocal microscopy; scale bar: 100 μm; adapted, with permission, from Yakoub and Sadek (2018). (C) An organoid section was stained with S100β and MAP2 and imaged using confocal microscopy; scale bar: 100 μm; adapted, with permission, from Yakoub and Sadek (2018). (D) qPCR analysis of mature-neuron markers in organoids on day 35 post-differentiation (“mature”), compared to the day-0 (“immature”) stage. Relative mRNA levels were calculated and normalized to the housekeeping gene GAPDH; error bars: SD; **P < 0.01, ***P < 0.001 (Student’s t-test). (E) Immunoblotting analysis of mature-neuron markers in 44-day old (“mature”), relative to day 0 (“immature”), organoids; adapted, with permission, from Yakoub and Sadek (2018). (F) Electrophysiological recordings from organoid slices. Left panel shows representative spike trains upon current stimulation in the absence (control) or presence of the sodium channel blocker TTX (n = 12 out of 24 cells showed action potentials (APs)). Middle panel shows TTX-sensitive sodium currents. Right panel shows potassium currents in the cells without or with APs, or with APs in presence of TTX. The left, middle, and right panels were adapted, with permission, from Watanabe et al. (2017). SOX2: Sex determining region Y-box 2; TBR2: T-box brain protein 2; DAPI: 4′,6-diamidino-2-phenylindole; TUJ1: neuron-specific class III β-tubulin; MAP2: microtubule-associated protein 2; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; SD: standard deviation; TTX: tetrodotoxin; CTIP2: (chicken ovalbumin upstream promoter transcription factor)-interacting protein 2; GluN1: glutamate ionotropic receptor NMDA-type subunit 1 (encoded by the gene GRIN1); GluN2A: glutamate ionotropic receptor NMDA-type subunit 2A (encoded by the gene GRIN2A); GluA1: glutamate ionotropic receptor AMPA-type subunit 1 (encoded by the gene GRIA1); VGLUT1: vesicular glutamate transporter 1; VGLUT2: vesicular glutamate transporter 2; VGAT: vesicular γ-amino butyric acid transporter; NRXN: neurexin; NLGN: neuroligin; SYT1: synaptotagmin 1; STX: syntaxin; S100β: S100 calcium-binding protein-β.
The robust array of neuronal maturation markers tested strongly suggested that organoids can generate mature neurons. In another study, Matsui and colleagues found that cerebral organoids derived from H9 human ESC line at 6-month age of differentiation exhibited VGLUT1-positive excitatory neurons and GAD67 (glutamate decarboxylase 67-kDa)-positive inhibitory neurons, in addition to MBP (myelin-basic protein)-positive mature oligodendrocytes (Matsui et al., 2018). In contrast, organoids derived from HUES8 human ESC line and an induced pluripotent stem cell (iPSC) line under conditions of dual SMAD inhibition via a combination of activin/TGF (transforming growth factor) β inhibitor (SB431542) and BMP (bone morphogenetic protein) inhibitor (LDN193189) showed no presence of GFAP-positive astroglial cells (Rigamonti et al., 2016). Neurons dissociated from these organoids, however, showed dendritic synapsin 1-positive and GluR (glutamate receptor)-1-positive punctae, suggestive of synapses, in addition to voltage-gated Na+ and K+ currents, spontaneous action potentials (APs) and post-synaptic currents as measured by patch-clamp electrophysiological recordings. However, presence of astrocytes is important for regulation of synaptic function in vivo, and thus the absence of astrocytes in this case may reduce the advantage of an organoid model over 2D systems, the former being expected to harbor multiple cell types that regulate neuronal maturity and synaptic functions in vivo.
In addition to the immunohistological studies and gene expression analyses, multiple studies have further confirmed the maturity of neurons in organoids using electrophysiological recordings on neurons isolated from organoids or organoid slices. For example, via a modified protocol (Kadoshima et al., 2013) and using human ESC (H9 or UCLA1/U1) or iPSC lines, Watanabe et al. (2017) performed electrophysiological recordings on slices of ~3 month-old organoids and could detect TTX (tetrodotoxin)-sensitive spike trains upon current stimulation in half of the recorded neurons and corresponding Na+ and K+ currents (Figure 1F), similar to what was previously shown for forebrain organoids derived by Qian et al. (2016) under conditions of dual SMAD inhibition (dorsomorphin + A-83), followed by treatment with GSK (glycogen synthase kinase) 3β inhibitor (CHIR99021) and SMAD inhibitor (SB-431542). Quite interestingly, measuring the intrinsic properties (membrane potential, membrane resistance, capacitance, and peak Na+ and K+ currents) of neurons in the organoids, these properties (except for the Na+ and K+ currents’ peak amplitudes, which were higher in organoids) were found to be strikingly similar between organoids and cortical plate neurons in slices of gestational week 16–22 human fetal cortex (Moore et al., 2009; Watanabe et al., 2017).
While most cortical organoids exhibited glutamatergic and GABAergic neurons, other neuron subtypes were observed in specific brain region-like organoids. For example, Jo et al. (2016) derived midbrain-like organoids from H1 and H9 human ESC lines via embryoid body (EB) induction under conditions of dual-SMAD inhibition by Noggin and SB431542 and Wnt activation by CHIR99021, and then patterning the EBs into a mesencephalic fate by shh (sonic hedgehog) and FGF (fibroblast growth factor) 8 treatment, and finally growing the organoids in presence of BDNF (brain-derived neurotrophic factor), GDNF (glial cell-derived neurotrophic factor), ascorbic acid and cAMP (cyclic adenosine monophosphate). In these organoids, dopaminergic neurons that were assessed to be mature by immunohistological and electrophysiological analyses were obtained. Similarly, other groups have adopted similar approaches to derive midbrain organoids that expressed mature, electrophysiologically active, dopaminergic neurons (Tieng et al., 2014; Monzel et al., 2017).
Astrocytes dissociated from 45-day-old cerebral organoids derived from human ESC BR-1 or H9 lines expressed most astrocytic markers and strongly enhanced neurite outgrowth and neuronal survival when co-cultured with mouse embryo cortical neurons (Dezonne et al., 2017). Studies by Paşca et al. (2015) and Sloan et al. (2017) also showed that astrocytes isolated from iPSC-derived cortical spheroids cultured over substantially long periods of time (~17–20 months) exhibit signs of functional maturity and exhibited upregulation of mature-astrocyte genes, e.g., AQP4, RANBP3L, IGFBP7, similar to those in human brain primary astroglial cells. Collectively, there is a strong evidence that mature, functional neurons and astroglia could be achieved in optimally derived organoids; however protocol modifications, choice of the pluripotent cell line used for organoid derivation and the analysis time points significantly affect the outcome.
Successful derivation of organoids containing mature neurons, astrocytes and glial cells strongly suggests that organoids can be used to model human neurodevelopmental and neurological diseases, or even, arguably, neurodegenerative disorders. They can serve as a reasonable complement to in-vivo animal models. For example, patient-derived iPSCs could be easily obtained and differentiated into 3D neural tissue or organoids of the same genetic makeup as the patient. These patient-derived organoids can be investigated for disease-related phenotypes, CRISPR gene-edited and investigated for the effect of correcting a disease-associated mutation on the disease phenotype, or used in testing new therapeutic drug candidates. However, a notable caveat to modeling certain neurological disorders, such as neurodegeneration and aging-associated conditions, in organoids is that organoids, being in-vitro, and embryonic-like, tissues are not usually exposed to micro-environmental cues that could induce these diseases in vivo, or to aging-associated (epigenetic or environmental) factors that may contribute to the development of certain age-related neurodegenerative conditions (Lou and Leung, 2018). Despite these reservations, a recent study was able to model hereditary spastic paraplegia using organoids derived from patient’s iPSCs with mutant SPG11 (spastic paraplegia 11) gene. Mutant organoids displayed accumulation of gangliosides in the lysosomes of the organoids’ peripheral-layer neurons. However, the long-term effect of such accumulation on survival of the neurons was not studied (Boutry et al., 2018). These results further support the possibility of modeling various neurological disease types in organoids.
Interestingly, genetically engineered ESCs may provide a precious tool to tracing a particular neuronal subtype functions and activity or contribution to a neural network in a cerebral organoid model. For example, Pacini et al. (2017) introduced into mouse ESCs a Tph (tryptophan hydroxylase) 2GFP reporter, which expresses GFP (green fluorescent protein) upon differentiation of these ESCs into serotonergic neurons in vitro. This approach successfully traced in real-time serotonergic neuronal development and activity (Pacini et al., 2017), similar to what was done in mouse (Migliarini et al., 2013), thus provided valuable, similar to in vivo, insights into human-specific brain development.
Moreover, multiple groups have attempted to assemble distinct neural networks in vitro using the organoid model. This approach has provided unprecedented insights into neural circuitry development in humans and their aberrations in neurodevelopmental disorders. Recently, a cerebral organoid fusion method was introduced, whereby EBs were differentially patterned into dorsal or ventral forebrain organoids. These dorsal-ventrally patterned organoids were then cocultured together in matrigel droplets and allowed to grow further (Bagley et al., 2017). These ‘stitched’ organoids were found to recapitulate a dorso-ventral axis and displayed GABAergic interneuron migration along their dorsoventral axis tracked in real time using time-lapse microscopy. Similarly, another group assembled a neural circuit involving glutamatergic and GABAergic neurons by fusing differentially patterned pallial-like cortical spheroids (rich in glutamatergic neurons) with subpallial-like spheroids (rich in GABAergic interneurons). This has allowed monitoring of interneuron migration through the fused spheroids, and demonstrated deficits in interneuron migratory saltations in organoids derived from the neurodevelopmental disorder Timothy syndrome (Birey et al., 2017). Fusing cortical and medial ganglionic eminence organoids also produced electrophysiologically functional neurons and neural networks, and enabled the study of interneuron migration during human brain development via organoids (Xiang et al., 2017).
Equally important is the regenerative potential of organoids, as stem-cell-derived (2D or 3D) neural transplants are now being investigated as potential therapeutic interventions for certain brain disorders and injuries. Interestingly, iPSC-derived neural progenitors could be transplanted into the spinal cord of spinally injured syngeneic minipigs without the need for immunosuppression. Encouragingly, the resulting graft showed long-term survival and neuronal and glial differentiation (Strnadel et al., 2018). Cerebral organoids grafted into mouse brain also showed successful integration, including formation of extensive graft-host synaptic networks, differentiation and neuronal maturation (Mansour et al., 2018). While these results are promising, there remain significant limitations and challenges (Wang, 2018). For example, in vitro-derived cerebral organoids, recapitulate embryonic, rather than adult, stages of brain development, anatomically and functionally (Lancaster et al., 2013), questioning the possibility of using these existing organoids in regenerative therapies. Moreover, how an organoid graft might repair neural structures or functions specifically in a degenerate or injured region of a host’s brain, without influencing other regions seems a significant challenge and plausible question for future studies. Overall, the findings that cerebral organoids derived under optimal conditions display mature, functional neurons and astrocytes pave the way for significant advances in molecular neuroscience, and disease modeling, studies, and open the door for potential therapeutic and regenerative interventions based on cerebral organoids.
Footnotes
Conflicts of interest: None declared.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by the author before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed by two independent reviewers.
Open peer reviewers: Attilio Marino, Istituto Italiano di Tecnologia, Italy; Anonymous (reviewer 2).
P-Reviewer: Marino A; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Bagasrawala I, Memi F, N VR, Zecevic N. N-methyl d-aspartate receptor expression patterns in the human fetal cerebral cortex. Cereb Cortex. 2017;27:5041–5053. doi: 10.1093/cercor/bhw289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagley JA, Reumann D, Bian S, Levi-Strauss J, Knoblich JA. Fused cerebral organoids model interactions between brain regions. Nat Methods. 2017;14:743–751. doi: 10.1038/nmeth.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, O’Rourke NA, Steinmetz LM, Bernstein JA, Hallmayer J, Huguenard JR, Paşca SP. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–59. doi: 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutry M, Branchu J, Lustremant C, Pujol C, Pernelle J, Matusiak R, Seyer A, Poirel M, Chu-Van E, Pierga A, Dobrenis K, Puech JP, Caillaud C, Durr A, Brice A, Colsch B, Mochel F, El Hachimi KH, Stevanin G, Darios F. Inhibition of lysosome membrane recycling causes accumulation of gangliosides that contribute to neurodegeneration. Cell Rep. 2018;23:3813–3826. doi: 10.1016/j.celrep.2018.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camp JG, Badsha F, Florio M, Kanton S, Gerber T, Wilsch-Bräuninger M, Lewitus E, Sykes A, Hevers W, Lancaster M, Knoblich JA, Lachmann R, Pääbo S, Huttner WB, Treutlein B. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci U S A. 2015;112:15672–15677. doi: 10.1073/pnas.1520760112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dezonne RS, Sartore RC, Nascimento JM, Saia-Cereda VM, Romao LF, Alves-Leon SV, de Souza JM, Martins-de-Souza D, Rehen SK, Gomes FC. Derivation of functional human astrocytes from cerebral organoids. Sci Rep. 2017;7:45091. doi: 10.1038/srep45091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Eugenin EA, King JE, Hazleton JE, Major EO, Bennett MV, Zukin RS, Berman JW. Differences in NMDA receptor expression during human development determine the response of neurons to HIV-tat-mediated neurotoxicity. Neurotox Res. 2011;19:138–148. doi: 10.1007/s12640-010-9150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann SN, Gaillard A, Vanderhaeghen P. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins AK, Paterson C, Wang Y, Hyde TM, Kleinman JE, Law AJ. Neurexin 1 (NRXN1) splice isoform expression during human neocortical development and aging. Mol Psychiatry. 2016;21:701–706. doi: 10.1038/mp.2015.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran HD, Göke J, Tan ZY, Saw TY, Tan C-P, Lokman H, Lee Y, Kim D, Ko HS, Kim SO, Park JH, Cho NJ, Hyde TM, Kleinman JE, Shin JH, Weinberger DR, et al. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell. 2016;19:248–257. doi: 10.1016/j.stem.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A. 2013;110:20284–20289. doi: 10.1073/pnas.1315710110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lou YR, Leung AW. Next generation organoids for biomedical research and applications. Biotechnol Adv. 2018;36:132–149. doi: 10.1016/j.biotechadv.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Mansour AA, Goncalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, Gage FH. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018;36:432–441. doi: 10.1038/nbt.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mariani J, Simonini MV, Palejev D, Tomasini L, Coppola G, Szekely AM, Horvath TL, Vaccarino FM. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109:12770–12775. doi: 10.1073/pnas.1202944109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsui TK, Matsubayashi M, Sakaguchi YM, Hayashi RK, Zheng C, Sugie K, Hasegawa M, Nakagawa T, Mori E. Six-month cultured cerebral organoids from human ES cells contain matured neural cells. Neurosci Lett. 2018;670:75–82. doi: 10.1016/j.neulet.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 19.Migliarini S, Pacini G, Pelosi B, Lunardi G, Pasqualetti M. Lack of brain serotonin affects postnatal development and serotonergic neuronal circuitry formation. Mol Psychiatry. 2013;18:1106–1118. doi: 10.1038/mp.2012.128. [DOI] [PubMed] [Google Scholar]
- 20.Monzel AS, Smits LM, Hemmer K, Hachi S, Moreno EL, van Wuellen T, Jarazo J, Walter J, Brüggemann I, Boussaad I, Berger E, Fleming RMT, Bolognin S, Schwamborn JC. Derivation of human midbrain-specific organoids from neuroepithelial stem cells. Stem Cell Rep. 2017;8:1144–1154. doi: 10.1016/j.stemcr.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore AR, Filipovic R, Mo Z, Rasband MN, Zecevic N, Antic SD. Electrical excitability of early neurons in the human cerebral cortex during the second trimester of gestation. Cereb Cortex. 2009;19:1795–1805. doi: 10.1093/cercor/bhn206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacini G, Marino A, Migliarini S, Brilli E, Pelosi B, Maddaloni G, Pratelli M, Pellegrino M, Ferrari A, Pasqualetti M. A Tph2GFP reporter stem cell line to model in vitro and in vivo serotonergic neuron development and function. ACS Chem Neurosci. 2017;8:1043–1052. doi: 10.1021/acschemneuro.6b00403. [DOI] [PubMed] [Google Scholar]
- 23.Paşca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park JY, O’Rourke NA, Nguyen KD, Smith SJ, Huguenard JR, Geschwind DH, Barres BA, Paşca SP. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 2015;12:671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rigamonti A, Repetti GG, Sun C, Price FD, Reny DC, Rapino F, Weisinger K, Benkler C, Peterson QP, Davidow LS, Hansson EM, Rubin LL. Large-scale production of mature neurons from human pluripotent stem cells in a three-dimensional suspension culture system. Stem Cell Reports. 2016;6:993–1008. doi: 10.1016/j.stemcr.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritter LM, Unis AS, Meador-Woodruff JH. Ontogeny of ionotropic glutamate receptor expression in human fetal brain. Brain Res Dev Brain Res. 2001;127:123–133. doi: 10.1016/s0165-3806(01)00126-2. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477–486, S1. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, Caneda C, Reimer R, Quake SR, Barres BA, Paşca SP. Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron. 2017;95:779–790.e776. doi: 10.1016/j.neuron.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strnadel J, Carromeu C, Bardy C, Navarro M, Platoshyn O, Glud AN, Marsala S, Kafka J, Miyanohara A, Kato T, Tadokoro T, Hefferan MP, Kamizato K, Yoshizumi T, Juhas S, Juhasova J, Ho CS, Kheradmand T, Chen P, Bohaciakova D, et al. Survival of syngeneic and allogeneic iPSC–derived neural precursors after spinal grafting in minipigs. Sci Transl Med. 2018;10:eaam6651. doi: 10.1126/scitranslmed.aam6651. [DOI] [PubMed] [Google Scholar]
- 30.Tieng V, Stoppini L, Villy S, Fathi M, Dubois-Dauphin M, Krause KH. Engineering of midbrain organoids containing long-lived dopaminergic neurons. Stem Cells Dev. 2014;23:1535–1547. doi: 10.1089/scd.2013.0442. [DOI] [PubMed] [Google Scholar]
- 31.Wang H. Modeling neurological diseases with human brain organoids. Front Synaptic Neurosci. 2018;10:15. doi: 10.3389/fnsyn.2018.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe M, Buth JE, Vishlaghi N, de la Torre-Ubieta L, Taxidis J, Khakh BS, Coppola G, Pearson CA, Yamauchi K, Gong D, Dai X, Damoiseaux R, Aliyari R, Liebscher S, Schenke-Layland K, Caneda C, Huang EJ, Zhang Y, Cheng G, Geschwind DH, et al. Self-organized cerebral organoids with human-specific features predict effective drugs to combat zika virus infection. Cell Rep. 2017;21:517–532. doi: 10.1016/j.celrep.2017.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang Y, Tanaka Y, Patterson B, Kang YJ, Govindaiah G, Roselaar N, Cakir B, Kim KY, Lombroso AP, Hwang SM, Zhong M, Stanley EG, Elefanty AG, Naegele JR, Lee SH, Weissman SM, Park IH. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell. 2017;21:383–398.e7. doi: 10.1016/j.stem.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yakoub AM, Sadek M. Development and characterization of human cerebral organoids: an optimized protocol. Cell Transplant. 2018;27:393–406. doi: 10.1177/0963689717752946. [DOI] [PMC free article] [PubMed] [Google Scholar]