Keywords: nerve regeneration, postnatal, calpeptin, learning and memory, hippocampus, spine development, SCOP, AKT, mTOR, neural regeneration
Abstract
Our previous studies showed that the early use of calpain inhibitors reduces calpain activity in multiple brain regions, and that postnatal treatment with calpeptin may lead to cerebellar motor dysfunction. However, it remains unclear whether postnatal calpeptin application affects hippocampus-related behaviors. In this study, Sprague-Dawley rats were purchased from the Animal Center of Anhui Medical University of China. For the experiments in the adult stage, rats were intraperitoneally injected with calpeptin, 2 mg/kg, once a day, on postnatal days 7–14. Then on postnatal day 60, the Morris water maze test was used to evaluate spatial learning and memory abilities. The open field test was carried out to assess anxiety-like activities. Phalloidin staining was performed to observe synaptic morphology in the hippocampus. Immunohistochemistry was used to count the number of NeuN-positive cells in the hippocampal CA1 region. DiI was applied to label dendritic spines. Calpeptin administration impaired spatial memory, caused anxiety-like behavior in adulthood, reduced the number and area of apical dendritic spines, and decreased actin polymerization in the hippocampus, but did not affect the number of NeuN-positive cells in the hippocampal CA1 region. For the neonatal experiments, neonatal rats were intraperitoneally injected with calpeptin, 2 mg/kg, on postnatal days 7 and 8. Western blot assay was performed to analyze the protein levels of Akt, Erk, p-Akt, p-Erk1/2, Erk1/2, SCOP, PTEN, mTOR, p-mTOR, CREB and p-CREB in the hippocampus. SCOP expression was increased, and the phosphorylation levels of Akt, mTOR and CREB were reduced in the hippocampus. These findings show that calpeptin administration after birth affects synaptic development in neonatal rats by inhibiting the Akt/mTOR signaling pathway, thereby perturbing hippocampal function. Therefore, calpeptin administration after birth is a risk factor for neurodevelopmental defects.
Chinese Library Classification No. R453; R363; R741
Introduction
Neurodevelopment consists of prenatal and postnatal development. Neuronal differentiation and migration preferentially happen in the prenatal phase. Postnatal development is critical for synaptic formation and connection (Andreae and Burrone, 2014). As reported, synaptic malfunction is a major reason for behavioral changes, including memory impairment, anxiety, and depression (Han and Tian, 2005; Sindi and Dodd, 2015). The second week after birth is a critical period of postnatal development in rodents (Tashiro et al., 2007). Environmental stressors, such as maternal separation, or neurotoxin exposure in this period perturb neurodevelopment, causing neuropsychiatric disorders (Skilbeck et al., 2018). A typical example is early maternal separation, which causes memory and neurobehavioral deficits (Vetulani, 2013). However, the underlying mechanisms are still not completely known.
Calpains are important calcium-dependent proteases (Stalker et al., 2003). Two subtypes of calpains are widely found in brain tissue, namely calpain-1 and calpain-2 (Prangsaengtong et al., 2012). Most studies on calpains have focused on their negative role in pathological conditions, i.e., calpain overactivation resulting in neurodegenerative diseases (Ma, 2013; Cheng et al., 2018). However, their positive role in neurodevelopment has recently been recognized. Using transgenic mice, Amini et al. (2013) demonstrated that calpain knockout in the central nervous system alters dendritic growth and impairs memory. Calpain-1 silencing causes cerebellar ataxia (Wang et al., 2016). Furthermore, Zhu et al. (2015b, 2017) reported that calpain-1 silencing impairs hippocampal synaptic plasticity. Together, these studies demonstrate that calpain is required for neurodevelopment, as well as for synaptic plasticity (Briz and Baudry, 2016). Our previous studies also showed that the early use of calpain inhibitors reduces calpain activity in multiple brain regions, and that postnatal application of calpeptin causes cerebellar motor dysfunction (Li et al., 2017a). However, whether postnatal calpeptin application affects hippocampus-related behaviors and the underlying mechanisms are not known. In this study, postnatal rats were administered calpeptin, and the behavioral changes in the adult stage were assessed.
Materials and Methods
Animals
Adult specific-pathogen-free Sprague-Dawley rats aged two months old and weighing 200–250 g (two males and five females) were obtained from the Animal Center of Anhui Medical University, China (license No. SCXY (Wan) 2014-0009). All rats were bred in a temperature-controlled environment (25°C) with a standard 12-hour light/dark cycle and allowed free access to food and water. All experimental procedures were approved by Animals Ethics Committee of Anhui University of Chinese Medicine, China (approval No. AUCM-2015-0901). The pups were kept with their dams throughout the experiment. Control and experimental pups were obtained randomly from the same litters in each experiment.
The experiment was divided into postnatal and adult phases. In the experiments in the postnatal phase, 42 pups (half female, half male) were randomly assigned to the calpeptin and control groups (half female, half male), and were intraperitoneally administered calpeptin (2 mg/kg, 100 μL; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and saline (100 μL), respectively, on postnatal day 7 (P7) and P8 (n = 5 per group) (Figure 1A). Pups were sacrificed after anesthesia with isoflurane (RWD Life Science, Shenzhen, China), and the hippocampus was isolated for biochemical experiments 2 hours after the last injection of calpeptin. For the experiments in the adult phase (Figure 1B), 32 pups were randomly divided into calpeptin and control groups and intraperitoneally administered calpeptin (2 mg/kg) and saline (100 μL), respectively, from P7 to P14 (one injection per day). The open field test, Morris water maze (MWM) test, and NeuN, DiI and actin polymerization assays (n = 8 per group) were performed on P60.
Figure 1.
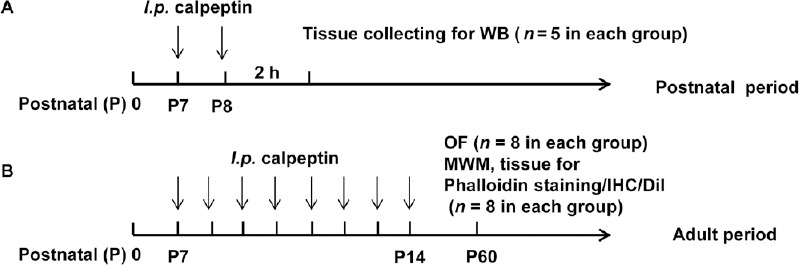
Experimental procedure.
(A) Experiments in the postnatal period. (B) Experiments in the adult period. WB: Western blot assay; MWM: Morris water maze; IHC: immunohistochemistry; h: hours; i.p.: intraperitoneally.
MWM test
The navigation experiment was performed for 5 days. The rats were randomly placed in one of the four quadrants of the MWM apparatus (Shanghai Xinruan Information Technology Co., Ltd., Shanghai, China). The latency to find the platform was recorded and averaged. If the rat did not find the platform in 60 seconds, it was led to the platform and the escape latency was counted as 60 seconds. Then, 24 hours after the navigation experiment, the MWM spatial exploration task was conducted. The rats were placed in the contralateral quadrant and the time spent in the platform quadrant was recorded by Ugo Basile software (Gemonio, Varese, Italy).
Open-field test
Adult rats were tested in an open field. In a quiet environment, the rats were put in the center of the box (40 cm × 40 cm × 65 cm). The behaviors were captured by a video camera. All experiments were carried out in a fixed time period of 5 minutes. After that, the box was cleaned with 70% alcohol. The border and central distances were analyzed by SUPER MAZE software (Shanghai Xinruan Information Technology Co. Ltd.).
Phalloidin staining
Actin polymerization in apical dendrites was analyzed after MWM by phalloidin staining (Kaech et al., 1997). The rats were sacrificed, and the hippocampus was isolated. Phalloidin-rhodamine dye was applied to the hippocampal CA1 dendrites in ventral hippocampal slices. After incubating for 30 minutes, the slices were fixed in 4% paraformaldehyde for 24 hours at 4°C. The slices were cryoprotected in 30% sucrose and cut into 20-μm-thick frozen sections. The images were taken on a confocal microscope (F1000; Olympus, Tokyo, Japan) using Z-axis scanning (thickness: 0.5 μm). The number and area of the spines were analyzed using ImageJ v2.1.4.7 software (National Institutes of Health, Bethesda, MD, USA) after the 3D image was obtained using the “Z Project” function.
Immunohistochemistry
Pyramidal neurons in the CA1 region were detected by counting NeuN-positive cells as described previously (Zhu et al., 2015a; Li et al., 2017c). Briefly, hippocampal slices from adult rats were fixed in 4% paraformaldehyde for 1 hour, cryoprotected in 30% sucrose for 1 hour at 4°C, and sectioned on a freezing microtome (20 μm). The slides were blocked with goat serum, incubated with primary antibody (rabbit anti-NeuN; 1:500; Millipore, Shanghai, China) overnight at 4°C, washed three times (15 minutes each) in phosphate-buffered saline (PBS), and incubated in Alexa Fluor 488 goat anti-mouse IgG (Life Technologies, Boston, MA, USA) for 2 hours at room temperature. The images were taken under a confocal microscope (F1000; Olympus, Tokyo, Japan).
DiI labeling procedure
The hippocampus was isolated. Dendritic spines were stained with DiI (Molecular Probes, Eugene, OR, USA) as previously described (Zhu et al., 2015a). Briefly, the hippocampal slices were labeled with DiI and fixed with 4% paraformaldehyde. Afterwards, the slices were cryoprotected in 30% sucrose for 1 hour at 4°C and sectioned on a freezing microtome (20 μm). Dendritic spines in the CA1 region that belonged to a different neuron were imaged using a confocal laser scanning microscope (FV1000; Olympus). Serial stack images with a step size of 0.5 μm were collected, and then projected to reconstruct a 3D image. Hippocampal slices from eight animals in each group were stained with DiI. For the analysis, at least one hundred dendrites in each image were imaged, and the average value was obtained with ImageJ software (National Institutes of Health).
Western blot assay
Hippocampi were isolated and lysed as previously described (Xu et al., 2018; Zhu et al., 2018). The protein samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis for 75 minutes at 120 V and transferred onto a nitrocellulose membrane for 2 hours at 300 mA. The membrane was blocked with 5% skim milk for 2 hours at room temperature and incubated with the following primary antibodies overnight at 4°C: protein kinase B (Akt) (1:1000; rabbit mAb; Cell Signaling Technology, Boston, MA, USA), p-Akt (1:1000; rabbit mAb; Cell Signaling Technology), p-Erk1/2 (1:1000; rabbit mAb; Cell Signaling Technology), Erk1/2 (1:1000; rabbit mAb; Cell Signaling Technology), suprachiasmatic nucleus circadian oscillatory protein (SCOP) (1:500; rabbit mAb; Santa Cruz Biotechnology), phosphatase and tensin homolog (PTEN) (1:1000; rabbit mAb; Cell Signaling Technology), mammalian target of rapamycin (mTOR) (1:500; mouse mAb; Santa Cruz Biotechnology), p-mTOR (1:500; mouse mAb; Santa Cruz Biotechnology), cAMP response element-binding protein (CREB) (1:500; mouse mAb; Santa Cruz Biotechnology), p-CREB (1:500; mouse mAb; Santa Cruz Biotechnology), β-actin (1:1000; mouse mAb; ZsBio, Beijing, China) and GAPDH (1:1000; mouse mAb; BIOSS, Beijing, China). After washing in PBS, the secondary antibody (1:1000; goat anti-mouse or rabbit; ZsBio) was incubated with the membrane for 2 hours at room temperature. The phosphorylated proteins were normalized to the total levels, while other proteins were normalized to β-actin or GAPDH. Chemiluminescent substrate detection reagent was used to visualize immunoreactive bands, and the target band was analyzed with ImageJ software v2.1.4.7 (National Institutes of Health).
Statistical analysis
The data are presented as the mean ± SEM. Statistical analysis was performed using GraphPad Prism 6.0 statistical software (GraphPad Software, Inc., La Jolla, CA, USA). Significant differences between two groups were analyzed by independent samples t-test. A value of P < 0.05 was considered statistically significant.
Results
Postnatal administration of calpeptin impairs memory in rats
As shown in Figure 2A, the latency to the platform in the calpeptin group was not statistically different from that in the control group (P > 0.05). By contrast, the time spent in the target quadrant was significantly less in the calpeptin group compared with control group (P < 0.05; Figure 2B & C). There was no significant difference in swimming time or the number of crossings of the original platform location among the first, third and fourth quadrants. These results suggest that postnatal injection of calpeptin impairs spatial memory in rats.
Figure 2.
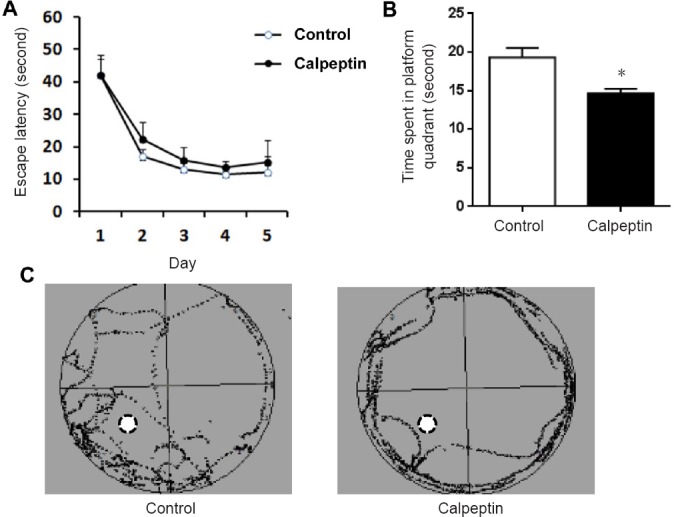
Calpeptin administration impairs memory in adult rats as assessed with the Morris water maze test.
(A) Escape latency in the learning session. (B) The time spent in the platform quadrant. Data are presented as the mean ± SEM (n = 8; independent samples t-test). *P < 0.05, vs. control group. (C) Representative traces of control and calpeptin-treated rats.
Postnatal administration of calpeptin increases anxiety-like activity
As shown in Figure 3, the ratio of border and central distances was significantly higher in the calpeptin group compared with the control group (P < 0.001). The total distance was not significantly different between the two groups (P > 0.05).
Figure 3.
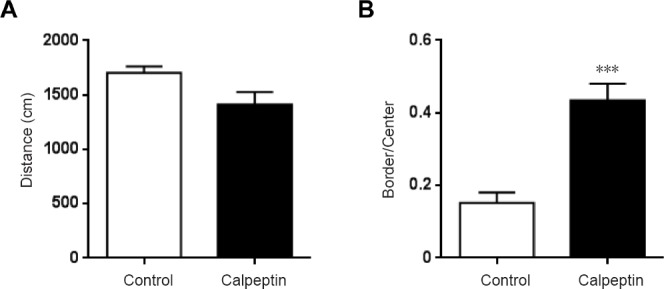
Calpeptin administration increases anxiety-like activity in adult rats as assessed with the open-field test.
(A) Total distance in the open field represents the motor function of the rats. (B) The ratio of the times in the border and center represents anxiety-like activity. Data are presented as the mean ± SEM (n = 8; independent samples t-test). ***P < 0.001, vs. control group.
Postnatal administration of calpeptin reduces the number of apical dendritic spines and actin polymerization
As shown in Figures 4 & 5, pyramidal neurons in the hippocampal CA1 region (Figure 4A), apical dendritic spines and actin polymerization (Figure 4A) were detected by NeuN, DiI and phalloidin staining. Compared with the control group, pyramidal neurons in the hippocampal CA1 region were not altered in the adult rats in the calpeptin group (Figure 4B). By contrast, spine numbers and area in apical dendrites were reduced in rats in the calpeptin group compared with the control group (P < 0.05; Figure 4C–E). Compared with the control, actin polymerization in the hippocampal CA1 region was also significantly reduced after calpeptin injection (P < 0.05; Figure 5).
Figure 4.
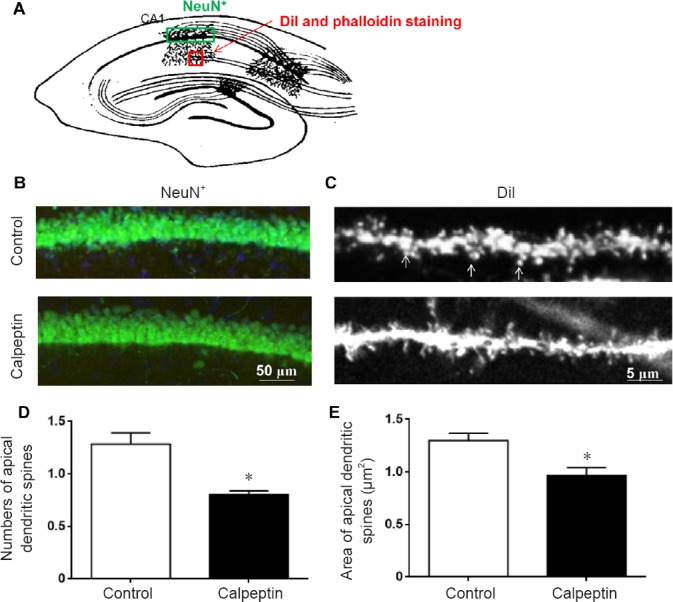
Calpeptin administration reduces the number and area of apical dendritic spines, but does not alter pyramidal neurons in the CA1 region in adult animals (60 days).
(A) Regions of NeuN staining (green) and DiI/phalloidin staining (red). (B) Representative images of NeuN staining. Calpeptin injection did not affect pyramidal cell numbers in the CA1 region. (C) DiI staining of the apical spine of the dendrite in the hippocampal CA1. Scale bars: 50 μm in B, 5 μm in C. (D) Numbers of apical dendritic spines were reduced in adult rats following calpeptin treatment. (E) Area of apical dendritic spines was reduced in adult rats following calpeptin treatment. Data are presented as the mean ± SEM (n = 8; independent samples t-test). *P < 0.05, vs. control group.
Figure 5.
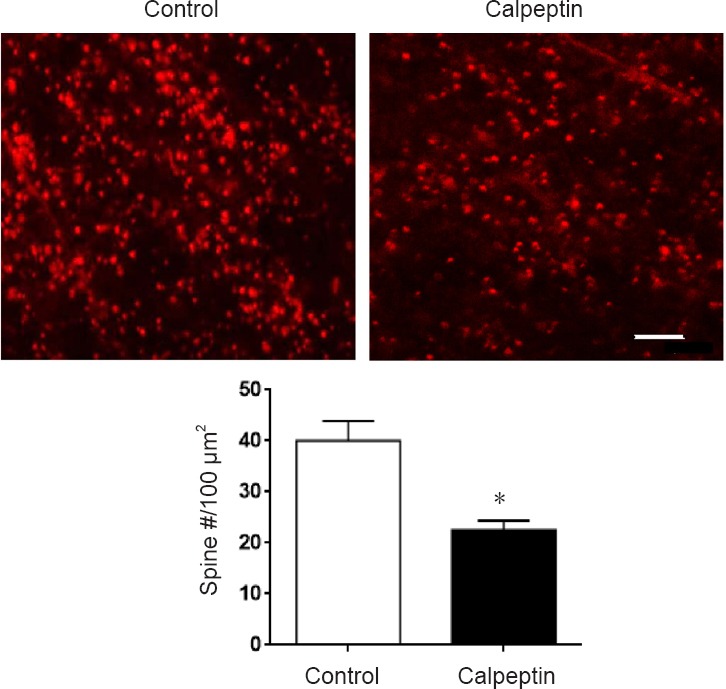
Calpeptin administration reduces actin polymerization in dendrites in the CA1 region in adult rats (60 days).
Upper panel: Representative images of phalloidin staining, where red represents actin polymerization. Scale bar: 5.5 μm. Lower panel: Quantification of phalloidin-positive spines. Actin polymerization was significantly reduced after calpeptin injection. Data are presented as the mean ± SEM (independent samples t-test). *P < 0.05, vs. control.
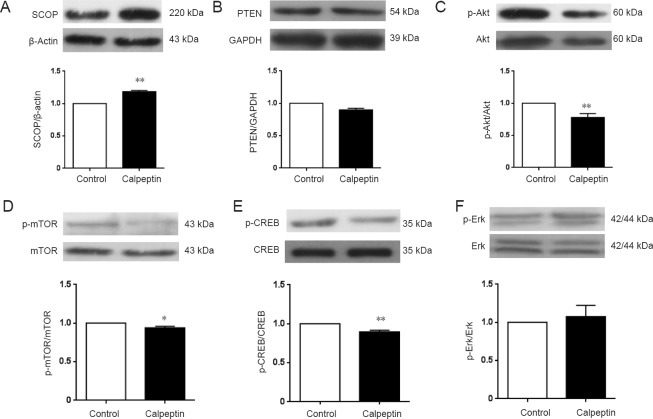
Postnatal administration of calpeptin inhibits the SCOP-Akt pathway
We next sought to clarify the mechanisms underlying the behavioral changes and neurodevelopmental impairment. The Akt-mTOR pathway is critical for protein synthesis and spine development (Wang et al., 2015; Zhu et al., 2015b). Because spine development was perturbed in calpeptin-treated rats, we examined spine-related protein expression in the postnatal period. After calpeptin injection, SCOP expression in the hippocampus was significantly increased compared with the control group (P < 0.01; Figure 6A), while calpeptin injection did not affect PTEN expression, another substrate of calpain (P > 0.05; Figure 6B).
Figure 6.
Calpeptin administration attenuates SCOP-Akt pathway activity in the hippocampus during the postnatal period.
(A) SCOP expression in the calpeptin group was elevated. (B) PTEN expression in the calpeptin group was not affected. (C) p-Akt/Akt was reduced after calpeptin administration. (D) p-mTOR/mTOR was reduced after calpeptin administration. (E) p-CREB/CREB was reduced after calpeptin administration. (F) p-Erk/Erk was not affected by calpeptin administration. Data are presented as the mean ± SEM of the optical density (n = 5; independent samples t-test). *P < 0.05, **P < 0.01, vs. control group. SCOP: Suprachiasmatic nucleus circadian oscillatory protein; PTEN: phosphatase and tensin homolog; CREB: cAMP response element-binding protein; p-CREB: phosphorylated cAMP response element-binding protein.
As shown in Figure 6C–F, compared with the control group, p-Akt levels were significantly reduced after calpeptin injection (P < 0.05). There was no significant difference in p-Erk levels between the control and calpeptin groups (P > 0.05). In addition, p-mTOR and p-CREB levels were also significantly reduced after calpeptin injection compared with the control group (P < 0.05 or P < 0.01).
Discussion
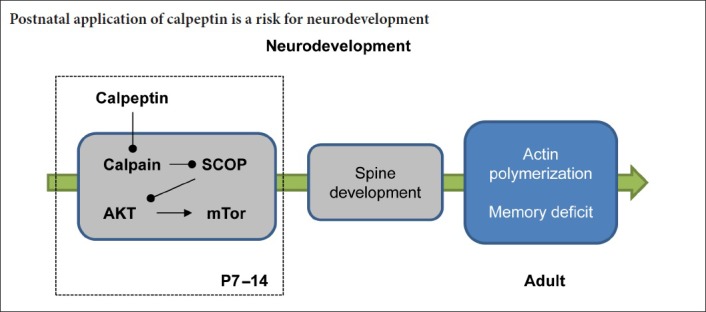
Here, our results show that postnatal calpeptin administration impairs spatial memory and causes anxiety-like behavior. Furthermore, calpeptin injection promoted SCOP accumulation, which reduced Akt/mTOR-mediated spine formation. These findings provide insight into the mechanisms underlying early stress-induced neurodevelopmental deficits.
Acquisition function relies on a series of precisely-timed neurodevelopmental events. Perturbation of neuronal network organization during critical neurodevelopmental periods can have severe and long-lasting effects in the adult phase (Kuchibhotla et al., 2008). Moreover, a balance of synaptic excitatory and inhibitory activity is critically important for neurodevelopment (Gatto and Broadie, 2010; Tatti et al., 2017). Maternal separation causes malfunction of γ-aminobutyric acid receptors during development (Furukawa et al., 2017; Skilbeck et al., 2018). Glutamate signaling plays a key role in brain development, promoting neuronal progenitor propagation and migration, as well as dendritic outgrowth and axonal elongation, and synapse formation and connection. Fast excitatory neurotransmission in the postnatal period is predominantly modulated by the N-methyl-D-aspartate (NMDA) receptor. Calpain has been proposed to be the major effector connecting NMDA receptors with downstream functional changes (Baudry and Bi, 2016). Therefore, inhibition of calpain activity should reduce excitatory synaptic transmission, consistent with our present observations. Postnatal calpeptin administration produced symptoms similar to those in animals exposed to early life stress (Vetulani, 2013).
In our previous study, calpeptin reduced calpain activity in several brain regions, especially the cerebellum, and caused cerebellum-related motor abnormalities (Li et al., 2017d). In contrast to its effect on the cerebellum, calpeptin did not trigger cell loss in the hippocampus. Calpeptin blocks both calpain-1 and calpain-2. It can pass through the blood-brain barrier and distributes in various brain areas. Thus, calpeptin is considered an effective inhibitor of calpain activity in the nervous system (D’Orsi et al., 2012; Samantaray et al., 2015). Although calpain transgenic animals show a variety of genetic functional deficits, such as decline of memory and cerebellar dysfunction (Zhu et al., 2015b, 2017; Wang et al., 2016), the role of calpain in postnatal development, especially in the hippocampus, has not been reported. Our current study shows for the first time that application of a calpain inhibitor during the postnatal period perturbs neurodevelopment.
The morphology of the hippocampus was not impaired in the rats following early calpeptin administration in the CA1 region. In fact, calpain 4 conditional knockout mice also display grossly normal brain morphology and cell arrangement. Therefore, we examined apical dendritic spines and actin polymerization in the CA1 region. Consistent with a previous study (Fan et al., 2011), spine development was impaired in rats given early calpeptin administration. Actin polymerization is required for the activation of synapses (Huang et al., 2007) and memory (Baudry et al., 2012). Here, a substantial reduction in actin polymerization was found in calpeptin-treated rats.
Calpain may play multiple roles in various biological processes by degrading different substrates. For example, calpain was reported to degrade SCOP, and regulate the activity of Akt and other kinases in learning and memory (Smith and Dodd, 2007). In a previous study, we reported that 1-methyl-4-phenylpyridinium (MPP+) activates calpain, which degrades protein tyrosine phosphatase enzymes, thereby impairing 3,5-dihydroxyphenylglycine-induced long-term depression (Li et al., 2017b). SCOP protein is a high molecular weight polypeptide containing a pleckstrin homology domain, leucine-rich repeat, protein phosphatase 2C-like domain, glutamine-rich region, and a PDZ-binding domain. As reported, SCOP protein is highly expressed in the nervous system (Shimizu et al., 2010). The overexpression of SCOP protein in the hippocampus impairs learning and memory (Shimizu et al., 2007). In the current study, we found that early application of calpeptin results in the accumulation of SCOP, whereas another key substrate of calpain, PTEN, was unaffected. These results suggest that calpain-mediated degradation of SCOP may play an important role in hippocampal neurodevelopment. Protein kinases are also indispensable for the formation of synaptic connections (Cuesto et al., 2011; Hojo et al., 2015). For example, early inhibition of Akt inhibits hippocampal neuronal spine development (Wang et al., 2015; Zhu et al., 2015b). In our study, calpain inhibition attenuated the SCOP-Akt signaling pathway, thereby affecting the development of neurons and spines.
mTOR is an integrator of signaling pathways that plays a key role in the hippocampal CA3–CA1 region (Xu, 2011; Komatsuzaki et al., 2012). It has been reported that mTOR-dependent protein synthesis induced by brain-derived neurotrophic factor is associated with calpain-2 (Briz et al., 2013). Here, we found that early administration of calpeptin decreases p-mTOR levels. Therefore, it is likely that calpeptin inhibits calpain-2 activity to exert its function. It is well known that mTOR phosphorylation can be induced by p-Akt, thereby initiating a protein synthesis cascade that regulates neuronal synaptic development. The accumulation of SCOP caused by calpeptin inhibits Akt phosphorylation and inhibits protein synthesis via the mTOR pathway. We also found here that CREB phosphorylation was robustly decreased by calpeptin. CREB is a transcription factor that influences gene transcription, which might be required for spine formation. Moreover, CREB is a regulatory target of the protein kinase Akt (Li et al., 2011). Therefore, early administration of calpeptin likely interrupts protein synthesis and spine development, thereby causing behavioral changes.
This study provides novel insight into the function of calpain in brain development. However, there are some limitations. For example, additional calpain inhibitors should be tested to confirm the results. Furthermore, the underlying mechanisms need to be investigated in greater detail. Ultimately, clinical studies are needed to assess the effects of calpain inhibition on neurodevelopment in humans.
In summary, postnatal administration of calpeptin impaired memory and produced an anxiety-like phenotype in rats. The mechanism is associated with the impairment of Akt-mTOR-regulated spine development.
Additional file: Open peer review reports 1 (103KB, pdf) , 2 (105KB, pdf) .
Footnotes
Conflicts of interest: None.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81601181, 81673716 (to GZ); the Natural Science Foundation of Anhui Province of China, No. 1808085J15 (to GZ); and the Natural Science Research Project of Anhui Province of China, No. KJ2016A417 (to GZ). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: The study was approved by the Ethics Committee of Anhui University of Chinese Medicine, China (approval No. AUCM-2015-0901).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Chong Gao, University of Hong Kong, China; Sumana Chakravarty, CSIR-Indian Institute of Chemical Technology (IICT), India.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81601181, 81673716 (to GZ); the Natural Science Foundation of Anhui Province of China, No. 1808085J15 (to GZ); and the Natural Science Research Project of Anhui Province of China, No. KJ2016A417 (to GZ).
P-Reviewers: Gao C, Chakravarty S; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Patel B, Haase R, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Amini M, Ma CL, Farazifard R, Zhu G, Zhang Y, Vanderluit J, Zoltewicz JS, Hage F, Savitt JM, Lagace DC, Slack RS, Beique JC, Baudry M, Greer PA, Bergeron R, Park DS. Conditional disruption of calpain in the CNS alters dendrite morphology, impairs LTP, and promotes neuronal survival following injury. J Neurosci. 2013;33:5773–5784. doi: 10.1523/JNEUROSCI.4247-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreae LC, Burrone J. The role of neuronal activity and transmitter release on synapse formation. Curr Opin Neurobiol. 2014;27:47–52. doi: 10.1016/j.conb.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baudry M, Bi X. Calpain-1 and Calpain-2: The Yin and Yang of synaptic plasticity and neurodegeneration. Trends Neurosci. 2016;39:235–245. doi: 10.1016/j.tins.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baudry M, Kramar E, Xu X, Zadran H, Moreno S, Lynch G, Gall C, Bi X. Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiol Dis. 2012;47:210–215. doi: 10.1016/j.nbd.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briz V, Baudry M. Calpains: master regulators of synaptic plasticity. Neuroscientist. 2016 doi: 10.1177/1073858416649178. doi: 101177/1073858416649178. [DOI] [PubMed] [Google Scholar]
- 6.Briz V, Hsu YT, Li Y, Lee E, Bi X, Baudry M. Calpain-2-mediated PTEN degradation contributes to BDNF-induced stimulation of dendritic protein synthesis. J Neurosci. 2013;33:4317–4328. doi: 10.1523/JNEUROSCI.4907-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng SY, Wang SC, Lei M, Wang Z, Xiong K. Regulatory role of calpain in neuronal death. Neural Regen Res. 2018;13:556–562. doi: 10.4103/1673-5374.228762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuesto G, Enriquez-Barreto L, Carames C, Cantarero M, Gasull X, Sandi C, Ferrús A, Acebes Á, Morales M. Phosphoinositide-3-kinase activation controls synaptogenesis and spinogenesis in hippocampal neurons. J Neurosci. 2011;31:2721–2733. doi: 10.1523/JNEUROSCI.4477-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Orsi B, Bonner H, Tuffy LP, Dussmann H, Woods I, Courtney MJ, Ward MW, Prehn JH. Calpains are downstream effectors of bax-dependent excitotoxic apoptosis. J Neurosci. 2012;32:1847–1858. doi: 10.1523/JNEUROSCI.2345-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan Y, Tang X, Vitriol E, Chen G, Zheng JQ. Actin capping protein is required for dendritic spine development and synapse formation. J Neurosci. 2011;31:10228–10233. doi: 10.1523/JNEUROSCI.0115-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furukawa M, Tsukahara T, Tomita K, Iwai H, Sonomura T, Miyawaki S, Sato T. Neonatal maternal separation delays the GABA excitatory-to-inhibitory functional switch by inhibiting KCC2 expression. Biochem Biophys Res Commun. 2017;493:1243–1249. doi: 10.1016/j.bbrc.2017.09.143. [DOI] [PubMed] [Google Scholar]
- 12.Gatto CL, Broadie K. Genetic controls balancing excitatory and inhibitory synaptogenesis in neurodevelopmental disorder models. Front Synaptic Neurosci. 2010;2:4. doi: 10.3389/fnsyn.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han L, Tian X. Dynamic-average characteristics of late wave of auditory evoked potentials for synaptic malfunction rat model. Conf Proc IEEE Eng Med Biol Soc. 2005;4:3664–3667. doi: 10.1109/IEMBS.2005.1617277. [DOI] [PubMed] [Google Scholar]
- 14.Hojo Y, Munetomo A, Mukai H, Ikeda M, Sato R, Hatanaka Y, Murakami G, Komatsuzaki Y, Kimoto T, Kawato S. Estradiol rapidly modulates spinogenesis in hippocampal dentate gyrus: Involvement of kinase networks. Horm Behav. 2015;74:149–156. doi: 10.1016/j.yhbeh.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Huang F, Chotiner JK, Steward O. Actin polymerization and ERK phosphorylation are required for Arc/Arg3.1 mRNA targeting to activated synaptic sites on dendrites. J Neurosci. 2007;27:9054–9067. doi: 10.1523/JNEUROSCI.2410-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaech S, Fischer M, Doll T, Matus A. Isoform specificity in the relationship of actin to dendritic spines. J Neurosci. 1997;17:9565–9572. doi: 10.1523/JNEUROSCI.17-24-09565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komatsuzaki Y, Hatanaka Y, Murakami G, Mukai H, Hojo Y, Saito M, Kimoto T, Kawato S. Corticosterone induces rapid spinogenesis via synaptic glucocorticoid receptors and kinase networks in hippocampus. PLoS One. 2012;7:e34124. doi: 10.1371/journal.pone.0034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Yang S, Zhu G. Postnatal calpain inhibition elicits cerebellar cell death and motor dysfunction. Oncotarget. 2017a;8:87997–88007. doi: 10.18632/oncotarget.21324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Chen H, Wu S, Cheng Y, Li Q, Wang J, Zhu G. MPP(+) inhibits mGluR1/5-mediated long-term depression in mouse hippocampus by calpain activation. Eur J Pharmacol. 2017b;795:22–27. doi: 10.1016/j.ejphar.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 21.Li JP, Lu W, Yang L, Xie MX, He X, Pan AH. Neurons in the hippocampus of chemobrain versus non-chemotherapy brain. Zhongguo Zuzhi Gongcheng Yanjiu. 2017c;21:4523–4528. [Google Scholar]
- 22.Li K, Chen Y, Jiang R, Chen D, Wang H, Xiong W, Li D, Liu Z, Li X, Li J, Yuan K. Protective effects of astragaloside IV against ovalbumin-induced allergic rhinitis are mediated by T-box protein expressed in T cells/GATA-3 and forkhead box protein 3/retinoic acid-related orphan nuclear receptor gammat. Mol Med Rep. 2017d;16:1207–1215. doi: 10.3892/mmr.2017.6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li XY, Zhan XR, Liu XM, Wang XC. CREB is a regulatory target for the protein kinase Akt/PKB in the differentiation of pancreatic ductal cells into islet beta-cells mediated by hepatocyte growth factor. Biochem Biophys Res Commun. 2011;404:711–716. doi: 10.1016/j.bbrc.2010.12.048. [DOI] [PubMed] [Google Scholar]
- 24.Ma M. Role of calpains in the injury-induced dysfunction and degeneration of the mammalian axon. Neurobiol Dis. 2013;60:61–79. doi: 10.1016/j.nbd.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prangsaengtong O, Senda K, Doki Y, Park JY, Jo M, Sakurai H, Shibahara N, Saiki I, Koizumi K. Calpain 1 and -2 play opposite roles in cord formation of lymphatic endothelial cells via eNOS regulation. Hum Cell. 2012;25:36–44. doi: 10.1007/s13577-012-0042-7. [DOI] [PubMed] [Google Scholar]
- 26.Samantaray S, Knaryan VH, Shields DC, Cox AA, Haque A, Banik NL. Inhibition of calpain activation protects MPTP-induced nigral and spinal cord neurodegeneration, reduces inflammation, and improves gait dynamics in mice. Mol Neurobiol. 2015;52:1054–1066. doi: 10.1007/s12035-015-9255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu K, Mackenzie SM, Storm DR. SCOP/PHLPP and its functional role in the brain. Mol Biosyst. 2010;6:38–43. doi: 10.1039/b911410f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu K, Phan T, Mansuy IM, Storm DR. Proteolytic degradation of SCOP in the hippocampus contributes to activation of MAP kinase and memory. Cell. 2007;128:1219–1229. doi: 10.1016/j.cell.2006.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sindi IA, Dodd PR. New insights into Alzheimer’s disease pathogenesis: the involvement of neuroligins in synaptic malfunction. Neurodegener Dis Manag. 2015;5:137–145. doi: 10.2217/nmt.14.54. [DOI] [PubMed] [Google Scholar]
- 30.Skilbeck KJ, Johnston GAR, Hinton T. Long-lasting effects of early-life intervention in mice on adulthood behaviour, GABAA receptor subunit expression and synaptic clustering. Pharmacol Res. 2018;128:179–189. doi: 10.1016/j.phrs.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Smith IJ, Dodd SL. Calpain activation causes a proteasome-dependent increase in protein degradation and inhibits the Akt signalling pathway in rat diaphragm muscle. Exp Physiol. 2007;92:561–573. doi: 10.1113/expphysiol.2006.035790. [DOI] [PubMed] [Google Scholar]
- 32.Stalker TJ, Skvarka CB, Scalia R. A novel role for calpains in the endothelial dysfunction of hyperglycemia. FASEB J. 2003;17:1511–1513. doi: 10.1096/fj.02-1213fje. [DOI] [PubMed] [Google Scholar]
- 33.Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tatti R, Haley MS, Swanson OK, Tselha T, Maffei A. Neurophysiology and regulation of the balance between excitation and inhibition in neocortical circuits. Biol Psychiatry. 2017;81:821–831. doi: 10.1016/j.biopsych.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vetulani J. Early maternal separation: a rodent model of depression and a prevailing human condition. Pharmacol Rep. 2013;65:1451–1461. doi: 10.1016/s1734-1140(13)71505-6. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Hersheson J, Lopez D, Hammer M, Liu Y, Lee KH, Pinto V, Seinfeld J, Wiethoff S, Sun J, Amouri R, Hentati F, Baudry N, Tran J, Singleton AB, Coutelier M, Brice A, Stevanin G, Durr A, Bi X, et al. Defects in the CAPN1 gene result in alterations in cerebellar development and cerebellar ataxia in mice and humans. Cell Rep. 2016;16:79–91. doi: 10.1016/j.celrep.2016.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang YL, Li F, Chen X. Pten inhibitor-bpV ameliorates early postnatal propofol exposure-induced memory deficit and impairment of hippocampal LTP. Neurochem Res. 2015;40:1593–1599. doi: 10.1007/s11064-015-1633-y. [DOI] [PubMed] [Google Scholar]
- 38.Xu F. Interaction effects between AMP-activated protein kinase and mammalian target of rapamycin signaling pathways. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2011;15:3775–3777. [Google Scholar]
- 39.Xu W, Cao J, Zhou Y, Wang L, Zhu G. GPR30 activation improves memory and facilitates DHPG-induced LTD in the hippocampal CA3 of middle-aged mice. Neurobiol Learn Mem. 2018;149:10–19. doi: 10.1016/j.nlm.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Zhu G, Wang Y, Li J, Wang J. Chronic treatment with ginsenoside Rg1 promotes memory and hippocampal long-term potentiation in middle-aged mice. Neuroscience. 2015a;292:81–89. doi: 10.1016/j.neuroscience.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 41.Zhu G, Yang S, Xie Z, Wan X. Synaptic modification by L-theanine, a natural constituent in green tea, rescues the impairment of hippocampal long-term potentiation and memory in AD mice. Neuropharmacology. 2018;138:331–340. doi: 10.1016/j.neuropharm.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 42.Zhu G, Liu Y, Wang Y, Bi X, Baudry M. Different patterns of electrical activity lead to long-term potentiation by activating different intracellular pathways. J Neurosci. 2015b;35:621–633. doi: 10.1523/JNEUROSCI.2193-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu G, Briz V, Seinfeld J, Liu Y, Bi X, Baudry M. Calpain-1 deletion impairs mGluR-dependent LTD and fear memory extinction. Sci Rep. 2017;7:42788. doi: 10.1038/srep42788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.