Keywords: nerve regeneration, spinal cord injury, tau, injury severity, outcome, cerebrospinal fluid, serum, biomarker, Basso, Beattie, Bresnahan locomotor rating scale, neural regeneration
Abstract
Tau protein, a microtubule-associated protein, has a high specific expression in neurons and axons. Because traumatic spinal cord injury mainly affects neurons and axons, we speculated that tau protein may be a promising biomarker to reflect the degree of spinal cord injury and prognosis of motor function. In this study, 160 female Sprague-Dawley rats were randomly divided into a sham group, and mild, moderate, and severe spinal cord injury groups. A laminectomy was performed at the T8 level to expose the spinal cord in all groups. A contusion lesion was made with the NYU-MASCIS impactor by dropping a 10 g rod from heights of 12.5 mm (mild), 25 mm (moderate) and 50 mm (severe) upon the exposed dorsal surface of the spinal cord. Tau protein levels were measured in serum and cerebrospinal fluid samples at 1, 6, 12, 24 hours, 3, 7, 14 and 28 days after operation. Locomotor function of all rats was assessed using the Basso, Beattie and Bresnahan locomotor rating scale. Tau protein concentration in the three spinal cord injury groups (both in serum and cerebrospinal fluid) rapidly increased and peaked at 12 hours after spinal cord injury. Statistically significant positive linear correlations were found between tau protein level and spinal cord injury severity in the three spinal cord injury groups, and between the tau protein level and Basso, Beattie, and Bresnahan locomotor rating scale scores. The tau protein level at 12 hours in the three spinal cord injury groups was negatively correlated with Basso, Beattie, and Bresnahan locomotor rating scale scores at 28 days (serum: r = −0.94; cerebrospinal fluid: r = −0.95). Our data suggest that tau protein levels in serum and cerebrospinal fluid might be a promising biomarker for predicting the severity and functional outcome of traumatic spinal cord injury.
Chinese Library Classification No. R446; R364
Introduction
Traumatic spinal cord injury (SCI) is one of the major causes of death and disabilities among all traumas. The reported incidence of SCI ranges from 9.2 to 246 cases per million (Jazayeri et al., 2015). Between 1993 and 2012, the incidence of acute traumatic SCI in the United States remained relatively stable; however, the total number of cases increased (Jain et al., 2015). To date, much effort has been made to evaluate the severity and the potential of recovery in patients with traumatic SCI; however, a reliable method for evaluating the severity and predicting the outcome following traumatic SCI, especially in the acute stages, has still not been achieved (Krishna et al., 2014; Silva et al., 2014). The current evaluation of traumatic SCI severity is still predominantly limited to neurological evaluation and imaging studies (Cheran et al., 2011; Pouw et al., 2014; Yokobori et al., 2015), which are often imprecise due to the unstable conditions of patients (such as the phenomenon of spinal shock) and the artifacts of metal implants after spinal operations. The limitation of current evaluation methods is also an obstacle for the development of new treatments for SCI patients (Hulme et al., 2017). Therefore, it would be beneficial and necessary to supplement current methods of evaluation with chemical biomarkers that reliably quantify traumatic SCI severity.
Tau protein is a microtubule-associated protein that is primarily localized in neurons (Breuzard et al., 2013). Tau protein has been shown to be a promising biomarker for axonal injury, because this protein binds to axonal microtubules and forms axonal microtubule bundles (Caprelli et al., 2017). Numerous studies have reported that tau protein concentrations in cerebrospinal fluid (CSF) and serum can serve as a biological marker for injury severity of the central nervous system, such as in traumatic brain injury (Liliang et al., 2010a, b; Magnoni et al., 2012), cerebral stroke (Bitsch et al., 2002; Wunderlich et al., 2006), Alzheimer’s disease (Lewczuk et al., 2004; Tatebe et al., 2017; Mukaetova-Ladinska et al., 2018), and other central nervous system diseases (Brettschneider et al., 2005; Buongiorno et al., 2011). However, only one previous study has evaluated the relationship between tau protein levels and injury severity in traumatic SCI (Yokobori et al., 2015). Following traumatic SCI, neuronal cell death at the injury site is likely to cause a release of intracellular microtubule binding proteins, such as tau, into the extracellular space, where they are transported by convective bulk flow to CSF and peripheral blood. Recently, only one study in dogs with intervertebral disc herniation has reported that CSF tau levels were positively associated with the severity of spinal cord damage and may serve as a biomarker for severity of intervertebral disc herniation (Roerig et al., 2013).
This study attempted to measure tau protein levels in serum and CSF in rats with traumatic SCI. Our aims were to determine whether: (1) tau protein is detectable in serum and CSF samples of traumatic SCI, and (2) the tau protein level reflects the severity of the injury.
Materials and Methods
Animals
One-hundred sixty female Sprague-Dawley rats, aged 8–9 months and weighing 230–280 g, were purchased from Beijing Experimental Animal Center of China (animal license No. SYXK (Jing) 2015-0046). All rats were housed in a climate-controlled barrier facility with 12-hour light/dark cycles at 24 ± 2°C, and allowed free access to food and water for a period of at least 1 week prior to the experimental procedures. All protocols were reviewed and approved by the Ethics Committee of Southwest Hospital, China (approval No. SWH20160126) on August 22, 2016. All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996).
All rats were randomly and equally divided into four groups: sham group, mild SCI group, moderate SCI group and severe SCI group. Each group was further subdivided according to eight time points (1, 6, 12, 24 hours, 3, 7, 14 and 28 days after operation) to collect CSF and peripheral blood samples (n = 5 for each subgroup at each time point).
Group assignment and model establishment
The graded contusion models of SCI were performed using the NYU-MASCIS (New York University-Multicenter Animal Spinal Cord Injury Study) impactor according to previous methods (Agrawal et al., 2010). Briefly, rats were anesthetized via intraperitoneal injection of 10% chloralhydrate (3.0 mL/kg), and the vertebral column of the rats was exposed. A laminectomy was performed at the T8 level to expose the cord. A contusion lesion was made with the NYU-MASCIS impactor by dropping a 10 g rod from heights of 12.5 mm (mild SCI group), 25 mm (moderate SCI group) and 50 mm (severe SCI group) upon the exposed dorsal surface of the spinal cord. Rats in the sham group received laminectomy only. Paralysis of the lower limbs was observed along with tail swinging and spasms. These responses confirmed successful establishment of the model. After operation, rats were placed back into their cages with heating pads, and were closely observed until they were conscious. As a prophylactic for infections, penicillin (200,000 unit/animal/d) was subcutaneously given for 3 consecutive days after operation. Saline was subcutaneously injected immediately after lesioning and then daily for 7 days. Food and water were provided ad libitum. Post-operative care included manual expression of bladders twice a day until a reflex pattern of emptying the bladder was established.
Behavioral scores
Locomotor functions of all rats were assessed using the Basso, Beattie, and Bresnahan locomotor rating scale (Basso et al., 1995), a 21-point scale to assess and analyze the hind limb movements of a rat in an open field, at 1, 6, 12, 24 hours, and 3, 7, 14 and 28 days after operation. The Basso, Beattie, Bresnahan scale ranges from 0 to 21, where 0 = complete paralysis and 21 = normal. Two investigators who were blinded to treatment assignment assessed the motor function of the rats at each time point.
Sample collection
CSF and peripheral blood samples were immediately collected from all four groups at each time point while the rats were terminally anesthetized using an overdose of 10% chloralhydrate. CSF was collected from the cisterna magna as described previously (Shapiro et al., 2012). The 27 G needle syringe was inserted into the cisterna magna through the occipital membrane, and approximately 50 μL of CSF was collected. CSF samples were centrifuged at 2500 r/min at −4°C for 15 minutes to remove cellular debris. Immediately after CSF collection, 3 mL of blood was collected by cardiac puncture as described previously (Kim et al., 2014; Zhang et al., 2016). To harvest cell-free serum, the blood samples were drawn into a tube containing clot activator. After standing upright at 37°C for 30 minutes, the blood samples were centrifuged at 1500 r/min and 4°C for 15 minutes and the supernatant was collected as the serum component. The serum component and CSF were immediately frozen in liquid nitrogen and stored at −80°C until further testing.
Enzyme-linked immunosorbent assay (ELISA)
Concentration of serum and CSF tau protein was measured using the commercial Rat pτ (phospho-Tau protein) ELISA kit (BioSource, Camarillo, CA, USA) according to the manufacturer’s instructions. The minimum detectable dose of tau protein was 12 pg/mL. The detection methods were as follows: standards or test samples were added to the wells, incubated and washed to remove unbound proteins. An anti-tau-horseradish peroxidase-conjugated detector antibody was then added, incubated and unbound conjugate was washed away. An enzymatic reaction was produced through the addition of 3,3′,5′-tetramethylbenzidine substrate, which was catalyzed by horseradish peroxidase generating a blue color product that changes to yellow after adding acidic stop solution. The absorbance value was measured at 450 nm using a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). The concentration of tau in the samples was then determined by comparing the optical density of the samples to the standard curve.
Statistical analysis
Data, expressed as the mean ± SD, were analyzed with SPSS 19.0 software (IBM, Armonk, NY, USA). Results were evaluated by one-way analysis of variance followed by Bonferroni’s post hoc test. The relationship between variables was determined by the Spearman or Pearson correlation coefficient method. A value of P < 0.05 was considered statistically significant.
Results
Tau protein levels in serum and CSF
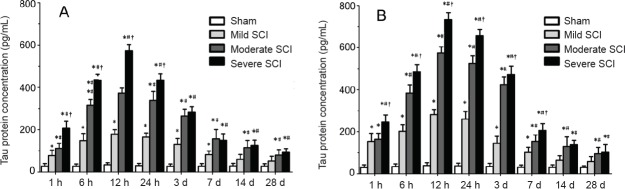
As shown in Figure 1, the tau protein level in serum and CSF was slightly increased in the sham group at 12 hours after SCI compared with 1 hour after SCI. However, there was no significant difference in comparison with the rest of the time points. In all SCI rats, the levels of serum and CSF tau protein were increased at 1 hour to 6 hours after SCI, reached a peak at 12 hours, and then slowly decreased. However, tau protein levels in serum and CSF were relatively high for a period both in the moderate and severe SCI groups. Significant correlations were detected between CSF tau protein levels and serum tau levels at each time point in SCI rats (Table 1).
Figure 1.
Tau protein levels in serum and cerebrospinal fluid of rats following SCI.
(A) Serum tau protein levels; (B) cerebrospinal fluid tau protein levels. *P < 0.05, vs. sham group; #P < 0.05, vs. mild SCI group; †P < 0.05, vs. moderate SCI group. Data are expressed as the mean ± SD (n = 5; one-way analysis of variance followed by Bonferroni’s post hoc test). SCI: Spinal cord injury; h: hour(s); d: days.
Table 1.
Correlation of tau protein levels (pg/mL) in cerebrospinal fluid and serum in SCI rats (Pearson correlation analysis)
| 1 hour | 6 hours | 12 hours | 24 hours | 3 days | 7 days | 14 days | 28 days | |
|---|---|---|---|---|---|---|---|---|
| r | 0.83 | 0.97 | 0.98 | 0.98 | 0.96 | 0.81 | 0.77 | 0.51 |
| P | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | 0.02 |
The data are analyzed from the four groups (sham group, mild SCI group, moderate SCI group and severe SCI group, n = 20). SCI: Spinal cord injury.
Basso, Beattie, and Bresnahan locomotor rating scale scores
SCI caused complete paralysis of both lower extremities in all rats 12 hours post surgery, with a Basso, Beattie, and Bresnahan locomotor rating scale score of 0–1. Over the 4-week period, a gradual recovery was observed in all SCI groups (Figure 2). Significant motor functional improvement was detected in the mild SCI group compared with moderate and severe SCI groups at 24 hours following SCI (P < 0.05). The Basso, Beattie, and Bresnahan locomotor rating scale scores were negatively correlated with SCI severity at 24 hours, 3, 7, 14 and 28 days after SCI (Table 2).
Figure 2.
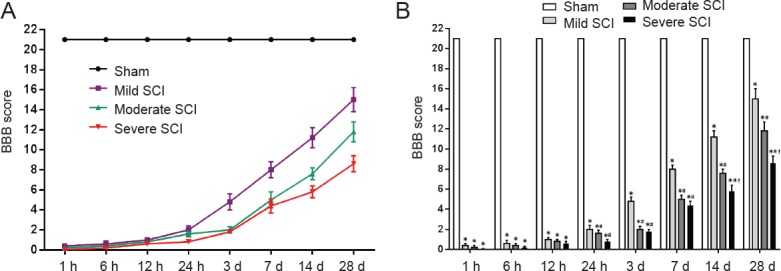
Temporal changes in BBB scores of rats following SCI.
(A) Changes of BBB score over time; (B) Comparison of BBB score among groups. *P < 0.05, vs. sham group; #P < 0.05, vs. mild SCI group; †P < 0.05, vs. moderate SCI group. Data are expressed as the mean ± SD (n = 5; one-way analysis of variance followed by Bonferroni’s post hoc test). BBB: Basso, Beattie, and Bresnahan locomotor rating scale; SCI: spinal cord injury; h: hour(s); d: days.
Table 2.
Correlation between SCI severity (mild, moderate, severe) and BBB scores (Spearman correlation analysis)
| 1 hour | 6 hours | 12 hours | 24 hours | 3 days | 7 days | 14 days | 28 days | |
|---|---|---|---|---|---|---|---|---|
| rs | –0.41 | –0.45 | –0.46 | –0.83 | –0.89 | –0.91 | –0.97 | –0.97 |
| P | 0.13 | 0.07 | 0.13 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
Two variables of the correlation analysis were: Basso, Beattie, and Bresnahan locomotor rating scale (BBB) scores and SCI severity (sham = 1; mild = 2; moderate = 3; severe = 4), respectively. All groups were involved in the analysis (n = 20).
Correlation of tau protein levels and SCI severity
A statistically significant, negative linear correlation was found between the concentration of serum tau protein and SCI severity at each time point; the same relationship was also found between CSF tau protein level and SCI severity (Table 3).
Table 3.
Correlation between tau protein levels and SCI severity (mild, moderate, severe; Spearman correlation analysis)
| 1 hour | 6 hours | 12 hours | 24 hours | 3 days | 7 days | 14 days | 28 days | ||
|---|---|---|---|---|---|---|---|---|---|
| Serum | rs | 0.92 | 0.97 | 0.97 | 0.97 | 0.89 | 0.86 | 0.83 | 0.78 |
| P | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | |
| Cerebrospinal fluid | rs | 0.87 | 0.97 | 0.97 | 0.97 | 0.92 | 0.93 | 0.87 | 0.78 |
| P | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
Two variables of the correlation analysis were: Basso, Beattie, and Bresnahan locomotor rating scale score and SCI severity (sham = 1; mild = 2; moderate = 3; severe = 4), respectively. All groups were involved in the analysis (n = 20). SCI: Spinal cord injury.
Correlation of tau protein levels and Basso, Beattie, and Bresnahan locomotor rating scale scores
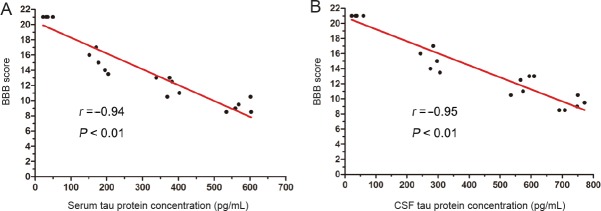
The concentrations of serum tau protein were negatively correlated with the Basso, Beattie, and Bresnahan locomotor rating scale scores at each time point except 1, 6 and 12 hours, and the same relationship was also found between the CSF tau protein level and SCI severity (Table 4). We also analyzed the relationship between the peak concentration of serum and CSF tau protein at 12 hours and the Basso, Beattie, and Bresnahan locomotor rating scale scores at 28 days. Results showed a significant negative correlation in CSF tau protein levels with 28 days’ Basso, Beattie, and Bresnahan locomotor rating scale scores (r = −0.95, P < 0.01); similar results also were found with serum tau protein level (r = −0.94, P < 0.01) (Figure 3).
Table 4.
Correlation between tau protein levels (pg/mL) and Basso, Beattie, and Bresnahan rating scale score (Pearson correlation analysis)
| 1 hour | 6 hours | 12 hours | 24 hours | 3 days | 7 days | 14 days | 28 days | ||
|---|---|---|---|---|---|---|---|---|---|
| Serum | r | –0.13 | –0.25 | –0.55 | –0.81 | –0.87 | –0.84 | –0.82 | –0.85 |
| P | 0.95 | 0.78 | 0.13 | 0.02 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | |
| Cerebrospinal fluid | r | –0.15 | –0.31 | –0.69 | –0.82 | –0.81 | –0.85 | –0.81 | –0.71 |
| P | 0.87 | 0.71 | 0.03 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
All groups were involved in the analysis (n = 20).
Figure 3.
Relationship between peak concentration of tau protein at 12 hours and BBB score at 28 days post injury.
All groups were involved in the correlation analysis (n = 20). (A, B) Pearson correlation analysis showed a significant negative correlation between serum tau protein levels and 28 days’ BBB score (A), and between CSF tau protein level and 28 days’ BBB score (B). BBB: Basso, Beattie, and Bresnahan locomotor rating scale; CSF: cerebrospinal fluid.
Discussion
In this study, a standardized rat injury model with NYU-MASCIS impactor was used to evaluate the relationship between tau protein level and SCI severity. This allowed us to define the severity of injury (mild, moderate, and severe) as described previously (Agrawal et al., 2010). A significant positive correlation was found between tau protein levels (both in CSF and serum) and measures of severity in rats with traumatic SCI.
The pathophysiology of traumatic SCI includes primary and secondary injuries to the spinal cord (Zhang et al., 2018). The primary injury is mainly due to the mechanical force to the spinal cord, which results in the disruption of axons. The injury then leads to a cascade of biological events, described as ‘‘secondary injury’’, which occurs over the course of minutes to weeks, and leads to further neurological damage (Wen et al., 2016; Rong et al., 2017; Fan et al., 2018). The severity of the injury is thought to be largely dependent on the extent of neuronal damage, including the primary and second injuries to the spinal cord. The microtubule-associated protein tau is a cytoskeletal protein expressed primarily in the central nervous system, where it stabilizes microtubules and regulates microtubule assemblies (Caprelli et al., 2017; Guo et al., 2017; Josephs, 2017; Sotiropoulos et al., 2017). Therefore, this investigation was designed to evaluate whether tau protein level in CSF or serum reflects the severity of traumatic SCI.
To our knowledge, this is the first report investigating the dynamic changes of the tau protein level in a traumatic SCI model. In our study, we found that tau protein level (both in serum and CSF) was increased significantly after SCI. The peak concentration occurred at 12 hours and remained at a relatively high level for 3 days. Similarly, in a model of rat traumatic brain injury, the tau protein level was significantly increased after injury, and peaked as early as 1 hour (Liliang et al., 2010b). Consistent with many other studies of biomarkers (Wang et al., 2016; Wu et al., 2016), our results also showed that the tau protein level in CSF was significantly higher than that in serum; furthermore, the level of serum tau protein was positively correlated with CSF tau protein level.
The Basso, Beattie, and Bresnahan locomotor rating scale has been widely used to evaluate the behavioral outcome after SCI (Duan et al., 2018; Fu et al., 2018; Ko et al., 2018). In our study, behavioral outcomes measured by Basso, Beattie, Bresnahan score were similar to those in previous studies (Yahata et al., 2016; Gu et al., 2017; Rink et al., 2018). Mean Basso, Beattie, and Bresnahan locomotor rating scale scores showed a significant difference between different severity groups at the same time point after SCI, and Basso, Beattie, Bresnahan scores were negatively correlated with SCI severity at 7, 14 and 28 days after SCI. Furthermore, a negative correlation was found between the levels of tau protein and Basso, Beattie, and Bresnahan locomotor rating scale scores after traumatic SCI. Thus, a higher tau protein concentration in serum or CSF at 12 hours suggested worse locomotor function outcome at 28 days. In the current study, the tau protein level both in serum and CSF was remarkably correlated with SCI severity at 28 days after injury. This association would be helpful to evaluate the severity of SCI, especially in the early phase of injury. Similarly, in a study in dogs with SCI by intervertebral disc herniation, CSF tau protein concentrations showed significant differences between different severities of intervertebral disc herniation (Roerig et al., 2013).
Neuronal cell injury or death is likely to cause a release of intracellular microtubule binding proteins, such as tau, into the extracellular space, where they are transported by convective bulk flow to CSF (Segal, 1993; Zetterberg, 2017). Previous studies have reported that biomarkers, such as S100-β and serum microRNAs, may have potential to aid the evaluation or diagnosis of SCI (Cao et al., 2008; Hachisuka et al., 2014; Kuhle et al., 2015; Sabour et al., 2017; Tigchelaar et al., 2017). However, because these markers are not involved in structural components of neurons, they do not directly reflect the functional status of the cells. Further, because of their low specificity in patients with multiple traumas, these markers seem to be inadequate for diagnosis and outcome prediction (Ydens et al., 2017).
In the present study, tau protein was identified both in serum and CSF as a promising biomarker for evaluating the severity of SCI in the acute phase. The levels of tau protein both in serum and CSF were higher with increasing severity of SCI. Although the tau protein level was lower in serum compared with CSF at the same time points, a positive correlation was found between them. When a neuron is severely injured, tau protein is released into the extracellular space through the damaged membrane (Lee et al., 2018). Because the blood-brain barrier was probably damaged at the same time of traumatic SCI, tau protein likely flowed into the blood as well (Caprelli et al., 2018). Tau protein directly reflects neuronal injury because tau protein is primarily localized in axons (Qi et al., 2016). Therefore, we believe that the tau protein level may be a perfect biomarker for evaluating the severity of SCI. Monitoring tau protein level in serum may have a profound use for the prediction of neuronal functional outcomes after SCI. As tau protein contributes to microtubule assembly and stabilization in axons, further research will focus on the possibility of using a microtubule stabilizer to prevent abnormal tau release from healthy neurons and to improve its function after traumatic SCI.
There were certain limitations to our study. First, although our study suggests that tau has potential as a biomarker of SCI, further studies in patients with varying degrees of SCI are needed. The use of such biomarkers in clinical trials may accelerate the development of novel therapeutic approaches of SCI. Second, it is worth mentioning that in recent years, immune cells have been shown to play an increasingly important role in the pathophysiology of brain and SCI (Feng et al., 2016; Barbagallo et al., 2017; Li et al., 2017). Recent studies have found that mice show a strong inflammatory response accompanied by activation of glial cells and involvement of necroptosis signaling following chronic ischemic injury (Yang et al., 2017; Cruz et al., 2018). Furthermore, inflammatory cytokines expression levels are markedly increased in brain ischemic injury (Xu et al., 2016; Chen et al., 2017). Further studies are needed to explore whether associations exist between immune cells and tau protein following central nervous system damage.
Taken together, there is clearly an urgent need to innovate more sensitive and reliable biomarkers in the acute stages of SCI, because successful management of SCI necessitates an appropriate diagnostic standard for the acute stages after injury. To our knowledge, this is the first study to investigate the relationship between tau protein level and injury severity in rats with traumatic SCI. Our preliminary data showed that tau protein level (both in serum and CSF) was positively correlated with the injury severity and negatively correlated with the locomotor outcome. Therefore, we suggest that tau protein level may be a perfect biomarker for evaluating the severity of SCI. Further studies in patients are warranted to increase the evidence for tau protein as an injury severity marker.
Additional file: Open peer review report 1 (62.9KB, pdf) .
Acknowledgments:
The authors thank Dr. Fan-Xing Meng and Jia Liu from Department of Orthopedics, Chinese PLA Beijing Army General Hospital, China for providing an experimental space and facilities. The authors also thank Professor. Hui-Wen Ma from Chongqing University Cancer Hospital, China for revising the manuscript and optimizing the figures.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This study was supported by the National Natural Science Foundation of China, No.81671211 (to HLL), 81672251 (to HLL). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: All protocols were approved by the Experimental Animal Ethics Committee of Southwest Hospital, China (approval No. SWH20160126) on August 22, 2016. All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Pio Conti, University of Chieti, Italy.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81671211, 81672251 (both to HLL).
P-Reviewer: Conti P; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: McCollum L, Haase R, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Agrawal G, Kerr C, Thakor NV, All AH. Characterization of graded multicenter animal spinal cord injury study contusion spinal cord injury using somatosensory-evoked potentials. Spine. 2010;35:1122–1127. doi: 10.1097/BRS.0b013e3181be5fa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbagallo M, Vitaliti G, Greco F, Pavone P, Matin N, Panta G, Lubrano R, Falsaperla R. Idiopathic intracranial hypertension in a paediatric population: a retrospective observational study on epidemiology, symptoms and treatment. J Biol Regul Homeost Agents. 2017;31:195–200. [PubMed] [Google Scholar]
- 3.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 4.Bitsch A, Horn C, Kemmling Y, Seipelt M, Hellenbrand U, Stiefel M, Ciesielczyk B, Cepek L, Bahn E, Ratzka P, Prange H, Otto M. Serum tau protein level as a marker of axonal damage in acute ischemic stroke. Eur Neurol. 2002;47:45–51. doi: 10.1159/000047946. [DOI] [PubMed] [Google Scholar]
- 5.Brettschneider J, Maier M, Arda S, Claus A, Sussmuth SD, Kassubek J, Tumani H. Tau protein level in cerebrospinal fluid is increased in patients with early multiple sclerosis. Mult Scler. 2005;11:261–265. doi: 10.1191/1352458505ms1159oa. [DOI] [PubMed] [Google Scholar]
- 6.Breuzard G, Hubert P, Nouar R, De Bessa T, Devred F, Barbier P, Sturgis JN, Peyrot V. Molecular mechanisms of Tau binding to microtubules and its role in microtubule dynamics in live cells. J Cell Sci. 2013;126:2810–2819. doi: 10.1242/jcs.120832. [DOI] [PubMed] [Google Scholar]
- 7.Buongiorno M, Compta Y, Marti MJ. Amyloid-beta and tau biomarkers in Parkinson’s disease-dementia. J Neurol Sci. 2011;310:25–30. doi: 10.1016/j.jns.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 8.Cao F, Yang XF, Liu WG, Hu WW, Li G, Zheng XJ, Shen F, Zhao XQ, Lv ST. Elevation of neuron-specific enolase and S-100beta protein level in experimental acute spinal cord injury. J Clin Neurosci. 2008;15:541–544. doi: 10.1016/j.jocn.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Caprelli MT, Mothe AJ, Tator CH. CNS injury: posttranslational modification of the tau protein as a biomarker. Neuroscientist. 2017 doi: 10.1177/1073858417742125. doi: 10.1177/1073858417742125. [DOI] [PubMed] [Google Scholar]
- 10.Caprelli MT, Mothe AJ, Tator CH. Hyperphosphorylated Tau as a novel biomarker for traumatic axonal injury in the spinal cord. J Neurotrauma. 2018;35:1929–1941. doi: 10.1089/neu.2017.5495. [DOI] [PubMed] [Google Scholar]
- 11.Chen CX, Huang J, Tu GQ, Lu JT, Xie X, Zhao B, Wu M, Shi QJ, Fang SH, Wei EQ, Zhang WP, Lu YB. NAMPT inhibitor protects ischemic neuronal injury in rat brain via anti-neuroinflammation. Neuroscience. 2017;356:193–206. doi: 10.1016/j.neuroscience.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Cheran S, Shanmuganathan K, Zhuo J, Mirvis SE, Aarabi B, Alexander MT, Gullapalli RP. Correlation of MR diffusion tensor imaging parameters with ASIA motor scores in hemorrhagic and nonhemorrhagic acute spinal cord injury. J Neurotrauma. 2011;28:1881–1892. doi: 10.1089/neu.2010.1741. [DOI] [PubMed] [Google Scholar]
- 13.Cruz SA, Qin Z, Stewart AFR, Chen HH. Dabrafenib, an inhibitor of RIP3 kinase-dependent necroptosis, reduces ischemic brain injury. Neural Regen Res. 2018;13:252–256. doi: 10.4103/1673-5374.226394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan HQ, Wu QL, Yao X, Fan BY, Shi HY, Zhao CX, Zhang Y, Li B, Sun C, Kong XH, Zhou XF, Feng SQ. Nafamostat mesilate attenuates inflammation and apoptosis and promotes locomotor recovery after spinal cord injury. CNS Neurosci Ther. 2018;24:429–438. doi: 10.1111/cns.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan YD, Zhu ML, Geng D, Zhou K, Du GJ, Wang ZL. The study on pathological mechanism and solution method for spinal cord ischemia reperfusion injury. Eur Rev Med Pharmacol Sci. 2018;22:4063–4068. doi: 10.26355/eurrev_201807_15394. [DOI] [PubMed] [Google Scholar]
- 16.Feng HL, Dang HZ, Fan H, Chen XP, Rao YX, Ren Y, Yang JD, Shi J, Wang PW, Tian JZ. Curcumin ameliorates insulin signalling pathway in brain of Alzheimer’s disease transgenic mice. Int J Immunopathol Pharmacol. 2016;29:734–741. doi: 10.1177/0394632016659494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu H, Hu D, Zhang L, Shen X, Tang P. Efficacy of oligodendrocyte progenitor cell transplantation in rat models with traumatic thoracic spinal cord injury: a systematic review and meta-analysis. J Neurotrauma. 2018 doi: 10.1089/neu.2017.5606. doi: 10.1089/neu.2017.5606. [DOI] [PubMed] [Google Scholar]
- 18.Gu M, Gao Z, Li X, Guo L, Lu T, Li Y, He X. Conditioned medium of olfactory ensheathing cells promotes the functional recovery and axonal regeneration after contusive spinal cord injury. Brain Res. 2017;1654:43–54. doi: 10.1016/j.brainres.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Guo T, Noble W, Hanger DP. Roles of tau protein in health and disease. Acta Neuropathol. 2017;133:665–704. doi: 10.1007/s00401-017-1707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hachisuka S, Kamei N, Ujigo S, Miyaki S, Yasunaga Y, Ochi M. Circulating microRNAs as biomarkers for evaluating the severity of acute spinal cord injury. Spinal Cord. 2014;52:596–600. doi: 10.1038/sc.2014.86. [DOI] [PubMed] [Google Scholar]
- 21.Hulme CH, Brown SJ, Fuller HR, Riddell J, Osman A, Chowdhury J, Kumar N, Johnson WE, Wright KT. The developing landscape of diagnostic and prognostic biomarkers for spinal cord injury in cerebrospinal fluid and blood. Spinal Cord. 2017;55:114–125. doi: 10.1038/sc.2016.174. [DOI] [PubMed] [Google Scholar]
- 22.Jain NB, Ayers GD, Peterson EN, Harris MB, Morse L, O’Connor KC, Garshick E. Traumatic spinal cord injury in the United States, 1993-2012. JAMA. 2015;313:2236–2243. doi: 10.1001/jama.2015.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jazayeri SB, Beygi S, Shokraneh F, Hagen EM, Rahimi-Movaghar V. Incidence of traumatic spinal cord injury worldwide: a systematic review. Eur Spine J. 2015;24:905–918. doi: 10.1007/s00586-014-3424-6. [DOI] [PubMed] [Google Scholar]
- 24.Josephs KA. Current understanding of neurodegenerative diseases associated with the protein Tau. Mayo Clin Proc. 2017;92:1291–1303. doi: 10.1016/j.mayocp.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JE, Lee YJ, Kwak MH, Jun G, Koh EK, Song SH, Seong JE, Kim JW, Kim KB, Kim S, Hwang DY. Metabolomics approach to serum biomarker for loperamide-induced constipation in SD rats. Lab Anim Res. 2014;30:35–43. doi: 10.5625/lar.2014.30.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko WK, Kim SJ, Jo MJ, Choi H, Lee D, Kwon IK, Lee SH, Han IB, Sohn S. Ursodeoxycholic acid inhibits inflammatory responses and promotes functional recovery after spinal cord injury in rats. Mol Neurobiol. 2018 doi: 10.1007/s12035-018-0994-z. doi: 10.1007/s12035-018-0994-z. [DOI] [PubMed] [Google Scholar]
- 27.Krishna V, Andrews H, Varma A, Mintzer J, Kindy MS, Guest J. Spinal cord injury: how can we improve the classification and quantification of its severity and prognosis? J Neurotrauma. 2014;31:215–227. doi: 10.1089/neu.2013.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhle J, Gaiottino J, Leppert D, Petzold A, Bestwick JP, Malaspina A, Lu CH, Dobson R, Disanto G, Norgren N, Nissim A, Kappos L, Hurlbert J, Yong VW, Giovannoni G, Casha S. Serum neurofilament light chain is a biomarker of human spinal cord injury severity and outcome. J Neurol Neurosurg Psychiatry. 2015;86:273–279. doi: 10.1136/jnnp-2013-307454. [DOI] [PubMed] [Google Scholar]
- 29.Lee BG, Leavitt MJ, Bernick CB, Leger GC, Rabinovici G, Banks SJ. A Systematic review of positron emission tomography of tau, amyloid beta, and neuroinflammation in chronic traumatic encephalopathy: the evidence to date. J Neurotrauma. 2018;35:2015–2024. doi: 10.1089/neu.2017.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewczuk P, Esselmann H, Bibl M, Beck G, Maler JM, Otto M, Kornhuber J, Wiltfang J. Tau protein phosphorylated at threonine 181 in CSF as a neurochemical biomarker in Alzheimer’s disease: original data and review of the literature. J Mol Neurosci. 2004;23:115–122. doi: 10.1385/JMN:23:1-2:115. [DOI] [PubMed] [Google Scholar]
- 31.Li Q, Zhao X, Gong DH, Geng YM, Zhang HL, Bi PX. Sleep disorders of acute thalamic stroke and its influence on plasma IL-17. J Biol Regul Homeost Agents. 2017;31:745–751. [PubMed] [Google Scholar]
- 32.Liliang PC, Liang CL, Weng HC, Lu K, Wang KW, Chen HJ, Chuang JH. Tau proteins in serum predict outcome after severe traumatic brain injury. J Surg Res. 2010a;160:302–307. doi: 10.1016/j.jss.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Liliang PC, Liang CL, Lu K, Wang KW, Weng HC, Hsieh CH, Tsai YD, Chen HJ. Relationship between injury severity and serum tau protein levels in traumatic brain injured rats. Resuscitation. 2010b;81:1205–1208. doi: 10.1016/j.resuscitation.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Magnoni S, Esparza TJ, Conte V, Carbonara M, Carrabba G, Holtzman DM, Zipfel GJ, Stocchetti N, Brody DL. Tau elevations in the brain extracellular space correlate with reduced amyloid-beta levels and predict adverse clinical outcomes after severe traumatic brain injury. Brain. 2012;135:1268–1280. doi: 10.1093/brain/awr286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukaetova-Ladinska EB, Abdell-All Z, Andrade J, da Silva JA, Boksha I, Burbaeva G, Kalaria RN, J TOB. Platelet tau protein as a potential peripheral biomarker in Alzheimer’s disease: an explorative study. Curr Alzheimer Res. 2018;15:800–808. doi: 10.2174/1567205015666180404165915. [DOI] [PubMed] [Google Scholar]
- 36.Pouw MH, Kwon BK, Verbeek MM, Vos PE, van Kampen A, Fisher CG, Street J, Paquette SJ, Dvorak MF, Boyd MC, Hosman AJ, van de Meent H. Structural biomarkers in the cerebrospinal fluid within 24 h after a traumatic spinal cord injury: a descriptive analysis of 16 subjects. Spinal Cord. 2014;52:428–433. doi: 10.1038/sc.2014.26. [DOI] [PubMed] [Google Scholar]
- 37.Qi ZP, Wang GX, Xia P, Hou TT, Zhou HL, Wang TJ, Yang XY. Effects of microtubule-associated protein tau expression on neural stem cell migration after spinal cord injury. Neural Regen Res. 2016;11:332–337. doi: 10.4103/1673-5374.177744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rink S, Arnold D, Wohler A, Bendella H, Meyer C, Manthou M, Papamitsou T, Sarikcioglu L, Angelov DN. Recovery after spinal cord injury by modulation of the proteoglycan receptor PTPsigma. Exp Neurol. 2018;309:148–159. doi: 10.1016/j.expneurol.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Roerig A, Carlson R, Tipold A, Stein VM. Cerebrospinal fluid tau protein as a biomarker for severity of spinal cord injury in dogs with intervertebral disc herniation. Vet J. 2013;197:253–258. doi: 10.1016/j.tvjl.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Rong W, Pan YW, Cai X, Song F, Zhao Z, Xiao SH, Zhang C. The mechanism of Naringin-enhanced remyelination after spinal cord injury. Neural Regen Res. 2017;12:470–477. doi: 10.4103/1673-5374.202923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabour H, Latifi S, Soltani Z, Shakeri H, Norouzi Javidan A, Ghodsi SM, Hadian MR, Emami Razavi SH. C-reactive protein as an available biomarker determining mental component of health-related quality of life among individuals with spinal cord injury. J Spinal Cord Med. 2017;40:329–337. doi: 10.1080/10790268.2016.1139771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segal MB. Extracellular and cerebrospinal fluids. J Inherit Metab Dis. 1993;16:617–638. doi: 10.1007/BF00711896. [DOI] [PubMed] [Google Scholar]
- 43.Shapiro JS, Stiteler M, Wu G, Price EA, Simon AJ, Sankaranarayanan S. Cisterna magna cannulated repeated CSF sampling rat model-effects of a gamma-secretase inhibitor on Abeta levels. J Neurosci Methods. 2012;205:36–44. doi: 10.1016/j.jneumeth.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Sotiropoulos I, Galas MC, Silva JM, Skoulakis E, Wegmann S, Maina MB, Blum D, Sayas CL, Mandelkow EM, Mandelkow E, Spillantini MG, Sousa N, Avila J, Medina M, Mudher A, Buee L. Atypical, non-standard functions of the microtubule associated Tau rotein. Acta Neuropathol Commun. 2017;5:91. doi: 10.1186/s40478-017-0489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tatebe H, Kasai T, Ohmichi T, Kishi Y, Kakeya T, Waragai M, Kondo M, Allsop D, Tokuda T. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Mol Neurodegener. 2017;12:63. doi: 10.1186/s13024-017-0206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tigchelaar S, Streijger F, Sinha S, Flibotte S, Manouchehri N, So K, Shortt K, Okon E, Rizzuto MA, Malenica I, Courtright-Lim A, Eisen A, Keuren-Jensen KV, Nislow C, Kwon BK. Serum microRNAs reflect injury severity in a large animal model of thoracic spinal cord injury. Sci Rep. 2017;7:1376. doi: 10.1038/s41598-017-01299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Li J, Han L, Guo S, Wang L, Xiong Z, Chen Z, Chen W, Liang J. Serum tau protein as a potential biomarker in the assessment of traumatic brain injury. Exp Ther Med. 2016;11:1147–1151. doi: 10.3892/etm.2016.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wen T, Hou J, Wang F, Zhang Y, Zhang T, Sun T. Comparative analysis of molecular mechanism of spinal cord injury with time based on bioinformatics data. Spinal Cord. 2016;54:431–438. doi: 10.1038/sc.2015.171. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Streijger F, Wang Y, Lin G, Christie S, Mac-Thiong JM, Parent S, Bailey CS, Paquette S, Boyd MC, Ailon T, Street J, Fisher CG, Dvorak MF, Kwon BK, Li L. Parallel metabolomic profiling of cerebrospinal fluid and serum for identifying biomarkers of injury severity after acute human spinal cord injury. Sci Rep. 2016;6:38718. doi: 10.1038/srep38718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wunderlich MT, Lins H, Skalej M, Wallesch CW, Goertler M. Neuron-specific enolase and tau protein as neurobiochemical markers of neuronal damage are related to early clinical course and long-term outcome in acute ischemic stroke. Clin Neurol Neurosurg. 2006;108:558–563. doi: 10.1016/j.clineuro.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 52.Xu M, Zhou GM, Wang LH, Zhu L, Liu JM, Wang XD, Li HT, Chen L. Inhibiting high-mobility group box 1 (HMGB1) attenuates inflammatory cytokine expression and neurological deficit in ischemic brain injury following cardiac arrest in rats. Inflammation. 2016;39:1594–1602. doi: 10.1007/s10753-016-0395-2. [DOI] [PubMed] [Google Scholar]
- 53.Yahata K, Kanno H, Ozawa H, Yamaya S, Tateda S, Ito K, Shimokawa H, Itoi E. Low-energy extracorporeal shock wave therapy for promotion of vascular endothelial growth factor expression and angiogenesis and improvement of locomotor and sensory functions after spinal cord injury. J Neurosurg Spine. 2016;25:745–755. doi: 10.3171/2016.4.SPINE15923. [DOI] [PubMed] [Google Scholar]
- 54.Yang XS, Yi TL, Zhang S, Xu ZW, Yu ZQ, Sun HT, Yang C, Tu Y, Cheng SX. Hypoxia-inducible factor-1 alpha is involved in RIP-induced necroptosis caused by in vitro and in vivo ischemic brain injury. Sci Rep. 2017;7:5818. doi: 10.1038/s41598-017-06088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ydens E, Palmers I, Hendrix S, Somers V. The next generation of biomarker research in spinal cord injury. Mol Neurobiol. 2017;54:1482–1499. doi: 10.1007/s12035-016-9757-x. [DOI] [PubMed] [Google Scholar]
- 56.Yokobori S, Zhang Z, Moghieb A, Mondello S, Gajavelli S, Dietrich WD, Bramlett H, Hayes RL, Wang M, Wang KK, Bullock MR. Acute diagnostic biomarkers for spinal cord injury: review of the literature and preliminary research report. World Neurosurg. 2015;83:867–878. doi: 10.1016/j.wneu.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Zetterberg H. Review: Tau in biofluids-relation to pathology, imaging and clinical features. Neuropathol Appl Neurobiol. 2017;43:194–199. doi: 10.1111/nan.12378. [DOI] [PubMed] [Google Scholar]
- 58.Zhang JH, Li Y, Song XB, Ji XH, Sun HN, Wang H, Fu SB, Zhao LJ, Sun DJ. Differential expression of serum proteins in rats subchronically exposed to arsenic identified by iTRAQ-based proteomic technology-14-3-3 zeta protein to serve as a potential biomarker. Toxicol Res (Camb) 2016;5:651–659. doi: 10.1039/c5tx00393h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Shen CL, Dong FL, Zhang RJ, Ge P. Correlation of cytokine levels in the peripheral blood within 24 hours after cervical spinal cord injury with the American Spinal Injury Association impairment scale:a comparative study. Zhongguo Zuzhi Gongcheng Yanjiu. 2018;22:3824–3830. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.