Abstract
Mitophagy is activated by a number of stimuli, including hypoxia, energy stress, and increased oxidative phosphorylation activity. Mitophagy is associated with oxidative stress conditions and central neurodegenerative diseases. Proper regulation of mitophagy is crucial for maintaining homeostasis; conversely, inadequate removal of mitochondria through mitophagy leads to the generation of oxidative species, including reactive oxygen species and reactive nitrogen species, resulting in various neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis. These diseases are most prevalent in older adults whose bodies fail to maintain proper mitophagic functions to combat oxidative species. As mitophagy is essential for normal body function, by targeting mitophagic pathways we can improve these disease conditions. The search for effective remedies to treat these disease conditions is an ongoing process, which is why more studies are needed. Additionally, more relevant studies could help establish therapeutic conditions, which are currently in high demand. In this review, we discuss how mitophagy plays a significant role in homeostasis and how its dysregulation causes neurodegeneration. We also discuss how combating oxidative species and targeting mitophagy can help treat these neurodegenerative diseases.
Keywords: nerve regeneration, mitophagy, central nervous system, Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis, oxidative species, reactive oxygen species, reactive nitrogen species
Introduction
Mitophagy is a term that was introduced by Le Masters in 2005 to describe the selective removal of mitochondria by autophagy (Lemasters, 2005). The degradation of mitochondria by mitophagy is especially important in cellular metabolism in which mitochondria play an essential role. The removal of dysfunctional and elderly mitochondria is essential for cell survival (Wallace, 2005). Additionally, neuronal cells are dependent on mitochondrial function, whereas its dysfunction is associated with neurodegenerative diseases. Disturbed mitochondrial function makes neurons especially sensitive to a wide variety of insults such as oxidative stress and bioenergetic defects. Thus, mitochondrial defects can greatly affect neuronal fate (Palomo and Manfredi, 2015).
Mitochondria are considered the main intracellular source of reactive oxygen species (ROS), which they produce during oxidative phosphorylation within all mammalian cells (Dai et al., 2014). ROS and reactive nitrogen species (RNS) play crucial roles in maintaining normal cellular behavior when regulated properly (Finkel and Holbrook, 2000). When ROS and RNS levels are excessive in terms of normal cellular requirements, it causes molecular damage and cellular debilitation. Higher levels of ROS may oxidize cellular constituents such as lipids, proteins and deoxyribonucleic acid (DNA), which interferes with cellular integrity (Finkel and Holbrook, 2000).
A previous study that used a mouse model of Purkinje cell degeneration demonstrated that altered mitophagy can cause excessive neuronal cell death, which was observed in the cerebellum. These results suggested that both uncontrollable mitophagy and inadequate mitophagy produce harmful effects (Kamat et al., 2014). Reduced autophagic function is believed to be responsible for many neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS). Therefore, mitochondria were recently considered a potential therapeutic drug target (Kamat et al., 2014).
In this review, we briefly discuss mitophagy and its involvement in the central nervous system (CNS) (i.e., AD, PD, HD, and ALS) and how these disease conditions occur when normal mitophagic function is compromised. By proper regulation of mitophagic pathways, the body can avoid harmful oxidative species, such as ROS and RNS, and harmful neurodegenerative diseases. Thus, by targeting these pathways, we can gain more knowledge about the therapeutic options to mitigate neurodegenerative disease conditions. Database search strategy is shown in Additional file 1.
Database search strategy- Mitophagy links oxidative stress conditions and neurodegenerative diseases
| Serial No. | Article title | Eligibility criteria | Keywords/ Key terms | Publication date/Year | Database | Publishing language |
|---|---|---|---|---|---|---|
| 1. | Getting ready for building: signaling and autophagosome biogenesis | A review that discusses recent progress in our understanding of autophagosome biogenesis | Atgs, autophagosome,biogenesis, autophagy, MTOR signaling | July 15, 2014 | Google scholar | English |
| 2. | Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease | A report that discusses age-dependent onset and progressive course of these neurodegenerative diseases | Neurodegenerative diseases, Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD) and progressive supranuclear palsy (PSP), oxidative damage, superoxide dismutase (SOD1) | 2000 | Google scholar | English |
| 3. | Cannabinoids for treatment of Alzheimer’s disease: Moving toward the clinic | A review that discusses the polyvalent properties of cannabinoid compounds for the treatment of AD, which together encourage progress toward a clinical trial. | Alzheimer’s disease (AD), cannabinoid, β-amyloid peptide, oxidative stress | March 5, 2014 | Google scholar | English |
| 4. | Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons | An article that discusses persistent mitochondrial fission may play a causal role in NO-mediated neurotoxicity | Mitochondria, nitric oxide (NO), autophagy, Dynamin related protein 1, mitochondrial fission | July 27, 2006 | Google scholar | English |
| 5. | Potential compensatory responses through autophagic/lysosomal pathways in neurodegenerative diseases | An article that discusses positive modulation of protein degradation processes represents a strategy to promote clearance of toxic accumulations and to slow the synaptopathogenesis | Protein degradation, protein accumulation, age-related neurodegenerative disorders, synaptopathogenesis | March 22, 2006 | Google scholar | English |
| 6. | Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress 1, 2 | A review summarizes current knowledge on phospholipid peroxidation and protein oxidation in AD brain, one potential cause of this oxidative stress, and consequences of Aβ-induced lipid peroxidation and protein oxidation in AD brain. | Amyloid β-peptide (Aβ), Alzheimer’s disease (AD), free radical oxidative stress, phospholipid peroxidation , oxidation, lipid peroxidation | June 1, 2002 | Google scholar | English |
| 7. | Pathways to mitochondrial dysfunction in ALS pathogenesis | An article that describes the genetic and mechanistic evidence that make dysfunction of mitochondria a candidate major player in this process. | Mitochondria, Amyotrophic Lateral Sclerosis, upper and lower motor neurons, neurodegenerative disease | February 19, 2017 | Google scholar | English |
| 8. | Multiple pathways for mitophagy: a neurodegenerative conundrum for Parkinson’s disease | An review that discusses role of mitophagy in modulating neuronal vulnerability in Parkinson’s spectrum (PD/PDD/DLB) and other neurodegenerative diseases. | Mitochondria, autophagy, neurodegeneration, mitophagy, Parkinson’s disease,dementia,dementia with Lewy bodies,Parkinson’s disease | 2018 | Google scholar | English |
| 9. | LC3 binds externalized cardiolipin on injured mitochondria to signal mitophagy in neurons: implications for Parkinson disease | An article that discusses fine-tune the mitochondrial recycling response | Mitophagy, Parkinson, cardiolipin, rotenone, MAP1-LC3, neurons, 6-hydroxydopamine, cargo recognition, autophagy, neurodegenerative diseases | November 26, 2013 | Google scholar | English |
| 10. | Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells | This article discusses redistribution of cardiolipin serves as an ‘eat-me’ signal for the elimination of damaged mitochondria from neuronal cells. | Mitochondria, macroautophagy, cardiolipin, mitophagy, neuronal cells | September 15,2013 | Google scholar | English |
| 11. | Beclin 1-independent pathway of damage-induced mitophagy and autophagic stress: implications for neuro-degeneration and cell death | Discusses about Beclin 1 may serve to prevent harmful overactivation of autophagy | Macroautophagy, neuronal cell death, neurodegeneration, autophagy, autophagy proteins, Lewy body diseases, autophagic stress | November 1, 2007 | Google scholar | English |
| 12. | Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission | Discusses about PINK1 and Parkin may cooperate through different mechanisms to maintain mitochondrial homeostasis | Mitochondrial dysregulation, Parkinson’s disease, PTEN-induced kinase 1 (PINK1), familial parkinsonism, neuropsychiatric disorders, mitochondrial fragmentation, RNAi knockdown | March 10, 2009 | Google scholar | English |
| 13. | Mitochondrial oxidative stress in aging and healthspan | A review that focuses on mitochondrial protective drugs, such as the mitochondrial antioxidants MitoQ, SkQ1, and the mitochondrial protective peptide SS-31 | Mitochondria,oxidative stress, aging, healthspan | May 1, 2014 | Google scholar | English |
| 14. | TDP-43 interacts with mitochondrial proteins critical for mitophagy and mitochondrial dynamics. | Discusses TDP-43 processing may contribute to metabolism and mitochondrial function | TDP-43, APP/PS1, PHB2, mitophagy, MFN2 mitochondria, PMPCA | June 21, 2018 | Google scholar | English |
| 15. | Mitochondria at the neuronal presynapse in health and disease | Importance of presynaptic mitochondria in maintaining neuronal homeostasis and how dysfunctional presynaptic mitochondria might contribute to the development of disease. | Synapses, mitochondria, neuronal homeostasis | January 19, 2018 | Google scholar | English |
| 16. | AMBRA1-mediated mitophagy counteracts oxidative stress and apoptosis induced by neurotoxicity in human neuroblastoma SH-SY5Y cells. | Important role in limiting ROS-induced dopaminergic cell death, and the utmost potential to prevent PD or other neurodegenerative diseases associated with mitochondrial oxidative stress | Parkinson’s disease (PD), Oxidative stress, autophagy of mitochondria, cell homeostasis, neurodegenerative diseases | April 18, 2018 | Google scholar | English |
| 17. | Mechanism and medical implications of mammalian autophagy. | Discusses about deregulation of autophagy in the context of various human pathologies, including cancer and neurodegeneration, and its modulation has considerable potential as a therapeutic approach. | Autophagy, cellular stress, catabolic process, cytoprotective functions, cancer, neurodegeneration | April 4, 2018 | Google scholar | English |
| 18. | Mitochondria, calcium-dependent neuronal death and neurodegenerative disease | Possible roles of cell type-specific calcium signaling mechanisms in defining the pathological phenotype of each of these major diseases and review central mechanisms of calcium-dependent mitochondrial-mediated cell death. | Mitochondria, intracellular calcium, neurodegenerative disease, glutamate excitotoxicity | May 22, 2012 | Google scholar | English |
| 19. | PINK1/Parkin-mediated mitophagy in mammalian cells | Discusses about how PINK1 activates Parkin in response to mitochondrial malfunction, how Parkin localizes specifically to impaired mitochondria, and how ubiquitination and deubiquitination regulate PINK1/Parkin-mediated mitophagy. | Mitophagy, parkin, PINK1, ubiquitination, deubiquitination, mitochondria | April, 2015 | Google scholar | English |
| 20. | Oxidants, oxidative stress and the biology of ageing | Describes that the appropriate and inappropriate production of oxidants, together with the ability of organisms to respond to oxidative stress, is intricately connected to ageing and life span. | Reactive oxygen species, oxidative stress, ageing and life span, metabolites | November 9, 2000 | Google scholar | English |
| 21. | Mitophagy in neurodegeneration and aging | Overview of mitophagy pathways and discuss the role of reduced mitophagy in neurodegeneration | Mitochondrial dysfunction, Parkinson’s disease, Alzheimer’s disease, proteolysis, mitophagy, autophagy, homeostasis | October, 2017 | Google scholar | English |
| 22. | Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson’s disease | This article discusses the mechanisms and effects of oxidative stress, the emerging concept of the impact of environmental toxins, and a possible neuroprotective role of the antioxidant astaxanthin in various neurodegenerative disorders with particular emphasis in Parkinson’s disease | Parkinson’s disease, oxidative stress, signaling pathways, PINK1, MPTP, Astaxanthin | February 13, 2014 | Google scholar | English |
| 23. | Deconstructing mitochondrial dysfunction in Alzheimer disease | This article summarizes the novel protocols for the generation of neurons by reprogramming or direct transdifferentiation, which offer useful tools to achieve this result | mitochondrial damage, Alzheimer’s disease, mitochondrial-targeted antioxidant | 2013 | Google scholar | English |
| 24. | The PINK1/Parkin-mediated mitophagy is compromised by PD-associated mutations | Importance of compromised PINK1 kinase activity, reduced binding of PINK1 to Parkin leads to failure in Parkin mitochondrial translocation, resulting in the accumulation of damaged mitochondria, which may contribute to disease pathogenesis | Mitochondrial dysfunction, neurodegenerative diseases, mitophagy, macroautophagy,damaged mitochondria | October 1, 2010 | Google scholar | English |
| 25. | Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment | Highlights a new role for MPP in PINK1 import and mitochondrial quality control via the PINK1–Parkin pathway | Mitochondria, mitophagy, Parkinson’s disease, PINK1, proteases | February 21, 2012 | Google scholar | English |
| 26. | An over-oxidized form of superoxide dismutase found in sporadic amyotrophic lateral sclerosis with bulbar onset shares a toxic mechanism with mutant SOD1 | Demonstrates the existence of an iper-oxidized SOD1 with toxic properties in patient-derived cells and identifies a common SOD1-dependent toxicity between mutant SOD1-linked familial ALS and a subset of sporadic ALS, providing an opportunity to develop biomarkers to subclassify ALS and devise SOD1-based therapies that go beyond the small group of patients with mutant SOD1. | Superoxide dismutase, amyotrophic lateral sclerosis, posttranslational modifications, mitochondria | March 27, 2012 | Google scholar | English |
| 27. | Targeting the unfolded protein response in disease. | Discusses recent advances in the design of novel compounds and therapeutic strategies to manipulate levels of ER stress in disease. | Unfolded proteins, endoplasmic reticulum (ER), cellular adaptation, apoptosis, neurodegenerative disorders | August 30, 2013 | Google scholar | English |
| 28. | Full-length TDP-43 and its C-terminal fragments activate mitophagy in NSC34 cell line | Discusses about human TDP-43 and its C-terminal fragments may cause mitochondrial dysfunction and enhance mitophagy. | Amyotrophic lateral sclerosis, TDP-43, Mitochondrial dysfunction, Mitophagy | November 21, 2012 | Google scholar | English |
| 29. | Functional impairment in Miro degradation and mitophagy is a shared feature in familial and sporadic Parkinson’s disease | Reveals that prolonged retention of Miro, and the downstream consequences that ensue, may constitute a central component of PD pathogenesis. | Homeostasis, oxidative stress, outer mitochondrial membrane, induced pluripotent stem cell, mitophagy, Parkinson’s disease | December 1, 2016 | Google scholar | English |
| 30. | Loss of axonal mitochondria promotes tau-mediat-ed neurodegeneration and Alzheimer’s disease–related tau phosphorylation via PAR-1 | Loss of axonal mitochondria may play an important role in tau phosphorylation and toxicity in the pathogenesis of AD | Alzheimer’s disease (AD), Tau phosphorylation, neurodegeneration, axonal mitochondria | August 30, 2012 | Google scholar | English |
| 31. | Mitofusin 2 protects cerebellar granule neurons against injury-induced cell death | Highlights a signaling role for Mfn2 in the regulation of apoptosis that extends beyond its role in mitochondrial fusion | Mitofusin 2 (Mfn2), nervous system, neuronal injury, oxidative stress, apoptosis, mitochondrial fusion | May 30, 2007 | Google scholar | English |
| 32. | PGC-1α, mitochondrial dysfunction, and Huntington’s disease | Discusses the role of PGC-1α in mitochondrial dysfunction in HD and its potential as a therapeutic target to cure HD. | Mitochondria, energy metabolism, calcium buffering, reactive oxygen species, neurodegeneration, mitochondrial biogenesis | September, 2013 | Google scholar | English |
| 33. | ALS: astrocytes move in as deadly neighbors | Discusses non-neuronal cells contribute to ALS pathogenesis | Amyotrophic lateral sclerosis, motor neurons, astrocytes, superoxide dismutase, motor neuron death | May 1, 2007 | Google scholar | English |
| 34. | DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders | Augmenting DJ-1 activity might provide novel approaches to treating chronic neurodegenerative illnesses such as Parkinson’s disease and acute damage such as stroke | DJ-1 redox signaling neurodegeneration Parkinson’s disease free radicals | November 15, 2009 | Google scholar | English |
| 35. | Autophagy of mitochondria: a promising therapeutic target for neurodegenerative disease | Explores new approaches that can prevent mitochondrial dysfunction, improve neurodegenerative etiology, and also offer possible cures to the aforementioned neurodegenerative diseases. | Autophagy, mitophagy, neurodegeneration, oxidative stress | May 8, 2014 | Google scholar | English |
| 36. | Understanding miro GTPases: implications in the treatment of neurodegenerative disorders. | Potential human Miros hold as novel therapeutic targets for the treatment of such disease. | Miro GTPase, atypical GTPase, neurodegenerative diseases, amyotropic lateral sclerosis | February 6, 2018 | Google scholar | English |
| 37. | PINK1-induced mitophagy promotes neuroprotection in Huntington’s disease | Mitophagy is altered in the presence of mHtt and that increasing PINK1/Parkin mitochondrial quality control pathway may improve mitochondrial integrity and neuroprotection in HD | Huntington’s disease (HD), huntingtin gene, mitochondria, PTEN-induced putative kinase 1 (PINK1), neuroprotection | January 22, 2015 | Google scholar | English |
| 38. | PINK1 signaling in mitochondrial homeostasis and in aging | Cellular protection could be critical for developing treatments to prevent and control excessive progression of neurodegenerative disorders. | Mitochondrial dysfunction, Parkinson’s disease, oxidative stress, neurodegenerative disorders, mitophagy | December 12, 2016 | Google scholar | English |
| 39. | Nix restores mitophagy and mito-chondrial function to protect against PINK1/Parkin-related Parkinson’s disease | Demonstrate that Nix can serve as an alternative mediator of mitophagy to maintain mitochondrial turnover, identifying Nix as a promising target for neuroprotective treatment in PINK1/Parkin-related PD. | Parkinson’s disease (PD), mitophagy, dysfunctional mitochondria, Nip3-like protein X (Nix) | March 10, 2017 | Google scholar | English |
| 40. | Inhibition of au-tophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury | Autophagy plays an essential role in triggering neuronal death execution after hypoxia/ischemia injury and Atg7 represents an attractive therapeutictarget for minimizing the neurological deficits associated with H/I brain injury | Brain injury, cognitive and motor dysfunction, gene essential, autophagy, caspase-3 | February, 2008 | Google scholar | English |
| 41. | Homeostatic levels of p62 control cy-toplasmic inclusion body formation in autophagy-deficient mice | Highlight the unexpected role of homeostatic level of p62, which is regulated by autophagy, in controlling intracellular inclusion body formation, and indicate that the pathologic process associated with autophagic deficiency is cell-type specific. | Autophagy, cytoplasmic protein, neurodegeneration, protein aggregates, genetic ablation, inclusion body | December 14, 2007 | Google scholar | English |
| 42. | Mitochondria and mitophagy: The yin and yang of cell death control | The importance of mitochondria and mitophagy in cardiovascular health and disease and provide a review of our current understanding of how these processes are regulated. | Apoptosis, autophagy, mitochondria, p53, Parkin, phosphatase and tensin homolog–induced putative kinase 1 | 2012 | PubMed | English |
| 43. | Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin | The association of PINK1 with the TOM complex allows rapid reimport of PINK1 to rescue repolarized mitochondria from mitophagy, and discount mitochondrial-specific factors for Parkin translocation and activation. | Mitochondria, mitophagy, peroxisomes, ubiquitin ligase, translocase of the outer membrane (TOM) | February 14, 2012 | Google scholar | English |
| 44. | Lysosomal proteol-ysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations | Defective lysosomal proteolysis represents a basis for pathogenic protein accumulations and neuronal cell death in AD and suggests previously unidentified therapeutic targets. | Macroautophagy, Alzheimer’s disease, presenilin-1, proteolysis, autophagosome, autolysosome acidification, cathepsin | June 25, 2010 | Google scholar | English |
| 45. | Autophagy in neurodegeneration: Two sides of the same coin | The two sides of autophagy will be discussed in the context of several neurodegenerative diseases. | Autophagy;cell death;cell survival;neurodegeneration | June 30, 2009 | Google scholar | English |
| 46. | Basal mitophagy is widespread in Drosophila but minimally affected by loss of Pink1 or parkin | Pink1 and parkin are not essential for bulk basal mitophagy in Drosophila | Parkinson’s disease, stress-induced mitophagy, basal mitophagy, dopaminergic neurons | March 2, 2018 | Google scholar | English |
| 47. | Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging | Mitophagy may play a key role in retarding accumulation of somatic mutations of mtDNA with aging. | Autophagy, autophagosomes, mitochondria, outer membrane protein | March 29, 2005 | Google scholar | English |
| 48. | Pink1 protects cortical neurons from thapsigargin-induced oxidative stress and neuronal apoptosis | Neuronal protective role of Pink1 against oxidative stress and afford rationale for developing new strategy to the therapy of neurodegenerative diseases. | Apoptosis, neurogenesis, neurodegeneration, oxidative stress, endoplasmic reticulum, antioxidant gene | February 1, 2015 | Google scholar | English |
| 49. | Rapamycin attenuates mitochondrial dysfunction via activation of mitophagy in experimental ischemic stroke | Rapamycin treatment attenuates mitochondrial dysfunction following cerebral ischemia, which is linked to enhanced mitophagy. | Brain ischemia, mitochondria function, mitophagy, rapamycin | February 7, 2014 | Google Scholar | English |
| 50. | Structural insights into the recognition of phosphorylated FUNDC1 by LC3B in mitophagy | Reversible phosphorylation modification of mitophagy receptors may be a switch for selective mitophagy | Microtubule-associated protein light chain 3 beta, Fun14 domain-containing protein 1, mitophagy, phosphorylation | October 18, 2016 | Google scholar | English |
| 51. | Abnormal mitochon-drial transport and morphology are common pathological denominators in SOD1 and TDP43 ALS mouse models | Manifestation of mitochondrial abnormalities between the two mouse models of familial ALS imply that different molecular mechanisms may be involved. | Amyotrophic lateral sclerosis, mitochondrial transport, mitochondria, sciatic nerve | October 23, 2013 | Google scholar | English |
| 52. | Sigma-1 receptor in motoneuron disease. In: Sigma receptors: their role in disease and as therapeutic targets | The multi-functional nature of the Sigma-1R represents an attractive target for treating aspects of ALS and other motoneuron diseases | Sigma-1 receptor, motorneuron disease, amyotropic lateral sclerosis, etipathology | March 18, 2017 | Google Scholar | English |
| 53. | Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease | Inefficient engulfment of cytosolic components by autophagosomes is responsible for their slower turnover, functional decay and accumulation inside HD cells. | Autophagy, cellular homeostasis, macroautophagy, autophagosomes, cytosolic components | April 11, 2010 | Google scholar | English |
| 54. | Basal mitophagy occurs independently of PINK1 in mouse tissues of high metabolic demand | Orchestrating mammalian mitochondrial integrity in a context-dependent fashion, and this has profound implications for our molecular understanding of vertebrate mitophagy | Mitophagy, Parkinson’s disease, dopaminergic neurons, mammalian mitophagy | February 6, 2018 | Google scholar | English |
| 55. | The mitochondrial intramembrane protease PARL cleaves human Pink1 to regulate Pink1 trafficking | Two Parkinson’s disease-causing mutations decrease the processing of Pink1 by PARL, with attendant implications for pathogenesis. | Intramembrane proteolysis, Parkinson’s disease, mitophagy, mitochondrial integrity | March 23, 2011 | Google scholar | English |
| 56. | Autophagosomes in GFP-LC3 transgenic mice | GFP-LC3 transgenic mice and describe here how we determine the occurrence of autophagy in vivo using this mouse model. | Autophagsome, GFP, green fluorescent protein, LC3, Atg8 | 2008 | Google scholar | English |
| 57. | Parkinson’s disease proteins: novel mitochondrial targets for cardioprotection | The role of these PD proteins in the heart and explore their potential as novel mitochondrial targets for cardioprotection | Coronary heart disease, Parkinson’s disease,myocardial ischaemia-reperfusion injury, mitochondria ischaemic preconditioning | December, 2015 | Google scholar | English |
| 58. | Pathogenic role of BECN1/Beclin 1 in the development of amyotrophic lateral sclerosis | Dual role of BECN1 in ALS and depict a complex scenario in terms of predicting the effects of manipulating autophagy in a disease context | ALS, autophagy, Beclin 1, neurodegenerative disease, SOD1 | May 12, 2014 | Google scholar | English |
| 59. | Loss of Miro1-directed mitochondrial movement results in a novel murine model for neuron disease | Defects in mitochondrial motility and distribution are sufficient to cause neurological disease | Calcium-binding mitochondrial Rho, mitochondrial respiration, Miro GTPase | August 18, 2014 | Google scholar | English |
| 60. | Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study | Neuroprotecive functions of autophagy | Lysosomes, neurodegeneration, amyloid, apoptosis, necrosis | February 1, 2005 | Google scholar | English |
| 61. | Nix is a selective autophagy receptor for mitochondrial clearance | Nix functions as an autophagy receptor, which mediates mitochondrial clearance after mitochondrial damage and during erythrocyte differentiation | GABARAP, LC3, mitophagy, Nix, selective autophagy | December 11, 2009 | Google Scholar | English |
| 62. | Pathology of protein synthesis and degradation systems in ALS | The main morphological abnormalities detected in the anterior horn cells of ALS patients | Protein synthesis, pathomechanisms, autophagic systems, ubiquitin-proteasomal | March 21, 2010 | Google scholar | English |
| 63. | Exploring new pathways of neurode-generation in ALS: the role of mitochondria quality control | Since ALS motor neurons progressively accumulate damaged mitochondria, it is plausible that the MQC is ineffective or overwhelmed by excessive workload imposed by the chronic and extensive mitochondrial damage. | ALS, mitochondria, mitophagy, SOD1, Parkin, p62 | May 14, 2015 | Google scholar | English |
| 64. | The autophagy-re-lated protein beclin1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice | Beclin 1 deficiency disrupts neuronal autophagy, modulates APP metabolism, and promotes neurodegeneration in mice and that increasing beclin 1 levels may have therapeutic potential in AD. | Autophagy, neurodegeneration, AD, amyloid-β, APP metabolism | May 22, 2008 | Google scholar | English |
| 65. | The roles of PINK1, Parkin, and mitochondrial fidelity in Parkinson’s disease | PINK1 and Parkin play within cells, their molecular mechanisms of action, and the pathophysiological consequences of their loss. | Parkinson’s disease, parkinsonism, Parkin, mitochondria, E3 ubiquitin ligase, membrane proteins | January 21, 2015 | Google scholar | English |
| 66. | Mutations in PINK1 and Parkin impair ubiquitination of Mitofusins in human fibroblasts | UPS is involved in mitofusin degradation. | Parkinson’s disease (PD), Mitofusins, mitochondrial stress, Mitofusin degradation | March 8, 2011 | Google scholar | English |
| 67. | HTT/Huntingtin in selective autophagy and Huntington disease: A foe or a friend within? | Role of HTT/Huntingtin in selective autophagy | aggrephagy, cargo recognition, Huntingtin, Huntington disease, lipophagy, mitophagy, MTORC1, nonselective autophagy, selective autophagy, SQSTM1/p62, ULK1 | May 18, 2015 | Google scholar | English |
| 68. | A rational mechanism for combination treatment of Huntington’s disease using lithium and rapamycin | Rational combination treatment approach in vivo by showing greater protection against neurodegeneration in an HD fly model with TOR inhibition and lithium, or in HD flies treated with rapamycin and lithium, compared with either pathway alone | Huntington’s disease, mammalian target of rapamycin, glycogen synthase kinase-3b | October 6, 2007 | Google scholar | English |
| 69. | The interplay between mitochondria and autophagy and its role in the aging process | Mitochondrial function and autophagy with particular focus on their crosstalk and its possible implication in the aging process | Aging, autophagy, C. elegans, diseases, mitochondria, mitophagy, hormesis | August, 2014 | Google scholar | English |
| 70. | Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases | The immune function of both glial cells and neurons, and the roles they play in regulating inflammatory processes and maintaining homeostasis of the CNS. | Microglia, astrocyte, neuron, neuroinflammation, innate immunity, adaptive immunity | July 2, 2012 | Google scholar | English |
| 71. | Protein turnover differences between neurons and other cells | Revealed some surprising differences in the ways that neurons regulate protein turnover compared with non-neuronal cells, which we discuss further in this article. | Huntington disease, autophagy, neurodegeneration, rapamycin, everolimus, LC3 | October, 2009 | Google scholar | English |
| 72. | Decreased glutathione ac-celerates neurological deficit and mitochondrial pathology in familial ALS-linked hSOD1 G93A mice model | The potential difference in the molecular pathways by which different hSOD1 mutants generate disease | Amyotrophic lateral sclerosis,Glutathione, GCLM, Mitochondria | September, 2011 | Google scholar | English |
| 73. | Resveratrol protects neuronal-like cells expressing mutant Huntingtin from dopamine toxicity by rescuing ATG4-mediated autophagosome formation | Mechanistic explanation of the neuroprotective activity of Resveratrol and support its inclusion in a therapeutic regimen to slow down HD progression. | Huntington, Parkinson, dopaminergic neurons, autophagy, anti-oxidant neurodegeneration | July, 2018 | Google scholar | English |
| 74. | Mitochondria and cancer: Warburgaddressed | The increased ROS mutagenizes nuclear proto-oncogenes (initiation) and drives nuclear replication (promotion), resulting in cancer. Therefore, hexokinase II and mitochondrial ROS may be useful alternate targets for cancer therapeutics. | Oxidative phosphorylation, reactive oxygen species, glycolytic metabolism | 2005 | Google scholar | English |
| 75. | Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer’s disease | Mitophagy pathway may become a new targeted therapy to attenuate neuronal damage induced by AD. | Autophagy, oxidative stress, apoptosis, 3-MA, Aβ1-42 | January 5, 2018 | Google Scholar | English |
| 76. | ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-de-pendent mitochondrial degradation by autophagy. | ROS-induced mitochondrial damage may be an important upstream activator of mitophagy. | neurodegenerative disorders, mitophagy, mitochondrial morphology, KillerRed, live-cell imaging, reactive oxygen species, SOD2, PARK2/PARKIN, PINK1 | August 14, 2012 | Google scholar | English |
| 77. | Motor cortex glutathione deficit in ALS measured in vivo with the J-editing technique. | Discrepancy is attributed to small but opposite changes in NAA and tCr in ALS that, as a ratio, resulted in a statistically significant group difference, further suggesting caution in using tCr as an internal reference under pathological conditions. | Magnetic resonance spectroscopy,amyotrophic lateral sclerosis,glutathione, oxidative stress, neurodegeneration, biomarker | June 6, 2014 | Google scholar | English |
| 78. | Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane | Parkin regulates degradation of outer and inner mitochondrial membrane proteins differently through proteasome-and mitophagy-dependent pathways. | Autophagy, Electron microscopy (EM), Parkinson’s disease, proteasome, mitophagy, parkin | March 18, 2011 | Google scholar | English |
| 79. | Miro1 deficiency in amyotrophic lateral sclerosis | Miro1 deficiency in ALS patients and ALS animal models and suggest glutamate excitotoxicity as a likely cause of Miro1 deficiency. | Amyotrophic lateral sclerosis, Miro1, spinalcord, lutamate excitotoxicity | May 26, 2015 | Google scholar | English |
| 80. | Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia | Mitochondrial autophagy is an adaptive metabolic response which is necessary to prevent increased levels of reactive oxygen species and cell death. | Autophagy, cytoplasmic organelles, Beclin-1, reactive oxygen species | February 15, 2008 | Googlescholar | English |
| 81. | Autophagy and mitophagy in cellular damage control. | Mitophagy are described in the context of bioenergetic dysfunction. | Neurodegeneration, alpha-synuclein,lysosomes, fission, fusion, reactive species, cellular bioenergetics pharmacological agents | 2013 | Google scholar | English |
Mitophagy
Mitophagy is induced by oxidative stress. Direct production of mitochondrial ROS by using a mitochondrial-targeted photosensitizer can also induce mitophagy (Wang et al., 2012). The induction of autophagy results in the recruitment of autophagy-related genes (Atgs) to a particular subcellular location, termed the phagophore assembly site, and nucleation of an isolation membrane that forms a cup-shaped structure, termed a phagophore (Figure 1). Eventual elongation of the curved isolation membrane results in expansion of the phagophore into a sphere around a portion of the cytosol. The isolation membrane subsequently seals into a double-membraned vesicle termed the autophagosome (Figure 1), trapping the engulfed cytosolic material as autophagic cargo (Dikic and Elazar, 2018). Previous studies that utilized the autophagosome indicator green fluorescent-protein-light chain 3 (GFP-LC3) in vitro and in vivo demonstrated that autophagy is eminently maintained in neurons (Mizushima and Kuma, 2008). More recent studies have also revealed the distinctions of basal autophagy between non-neuronal and neuronal cells (Tsvetkov et al., 2009). For example, GFP-LC3-positive autophagosomes were rarely observed in normal neurons, as huge aggregations of autophagic vacuoles were observed under disease conditions (Lee, 2009).
Figure 1.
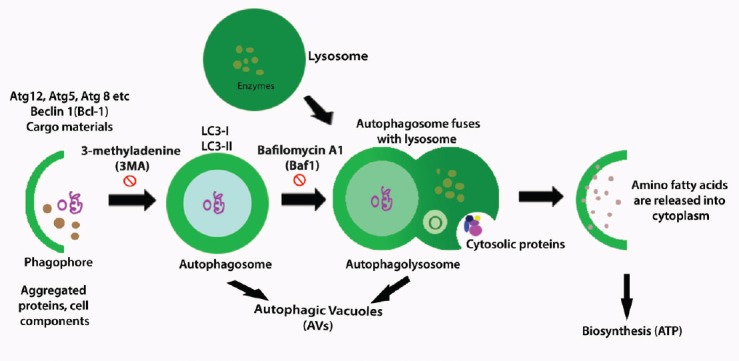
General process of autophagy.
At the begining of this process, cup-shaped phagophore is formed around the folded or aggregated proteins and with other cellular components, this is called nucleation. In the first step, the autophagic proteins (Atgs) such as Atg12, Atg 5, Atg 8, Beclin-1 (Bcl-1) and cargo materials are brought about through the ubiquitin-like conjugation systems Atg12-Atg5-Atg16L and Atg8 (LC3)-phosphatidylethanolamine (PE). In the second step, the expansion and maturation of the cup-shaped structure become rounded one and form autophagolysosomes which are double membraned vesicles with presence of LC3-I, LC3-II where the 3-methyladenine (3-MA) plays an inhibitatory role. In the third step, with an inhibitiory effect of bafilomycin A1 (Baf1), autophagosome is fused with lysosome and form single membraned autophagolysosome and this step is called fusion and autophagic vacuoles (AVs) and cytosolic proteins are seen. In the last step, the degradation of the autophagolysosome, with hyrolytic enzymes contributes to degradation of sequestered material, release of amino or fatty acids, and maintaineance of biogenesis.
After clearance of most Atgs and delivery along microtubules to the lysosome, the outer membrane of the autophagosome fuses with the lysosomal membrane to form an autophagolysosome (Figure 1). This fusion results in the release of a single-membrane autophagic body into the lysosomal lumen, followed by degradation of the autophagic body together with its cargo by the autolysosomal hydrolytic milieu (Abada and Elazar, 2014). Another study using mutant mice, in which Atg5 or Atg7 gene, specifically in the brain was deleted, showed the importance of basal autophagy in neurons (Komatsu et al., 2007). In the mutant mice, neurons lacked Atg5 or Atg7, and animals experienced continuous neurodegeneration (Koike et al., 2008). According to another experiment, rapamycin, which is involved in autophagy induction, conferred protection in animal models of neurodegenerative diseases (Sarkar et al., 2008).
Additionally, NIX, which is also known as beclin-2 (BCL2)/adenovirus E1B 19 kDa protein-interacting protein 3-like (BNIP3L), is transcriptionally upregulated in the period of reticulocyte maturation to erythrocytes. NIX/BNIP3L interacts directly with LC3B or Golgi-associated ATPase Enhancer of 16 kDa (GATE-16) via an LC3-interacting region (LIR), hence mediating the sequestration of NIX-expressing mitochondria via the growing phagophore (Novak et al., 2010). Interestingly, hypoxia also mediates the expression of NIX/BNIP3L and a related BH3 protein, BHIP3, demonstrating similar receptor-induced mitophagy mechanisms in injury-mediated mitophagy. Post-translational modifications also play a major role in mitophagy, permitting a more rapid response to hypoxic stress, as observed for the mitophagy receptor Fun14 domain-containing protein 1 (FUNDC1) (Lv et al., 2017).
Mitophagy is a selective form of autophagy that removes dysfunctional mitochondria and their harmful byproducts and oxidative species to help maintain homeostasis.
PINK1/PARKIN Pathway in the Regulation of Mitophagy
There are several pathways through which mitochondria are targeted for degeneration at the autophagosome; however, PTEN-induced putative kinase protein 1 (PINK1)/cytosolic E3 ubiquitin ligase PARKIN (PINK1/PARKIN-induced mitophagy is the most well-understood pathway regarding the maintenance of mitochondrial homeostasis in degenerative diseases (Kitagishi et al., 2017). PINK1 is a mitochondrial-targeted serine/threonine kinase that plays a protective role against mitochondrial dysfunction and apoptosis with mitochondrial quality control by activating PINK1/PARKIN-induced mitophagy (Figure 2) (Fivenson et al., 2017). The significance of PINK1 in the mitochondria is needed in cell-protective characteristics for combating oxidative stress (Eiyama and Okamoto, 2015). The role of PINK1 has been well-documented in neurodegenerative and aging-related diseases (Li and Hu, 2015).
Figure 2.
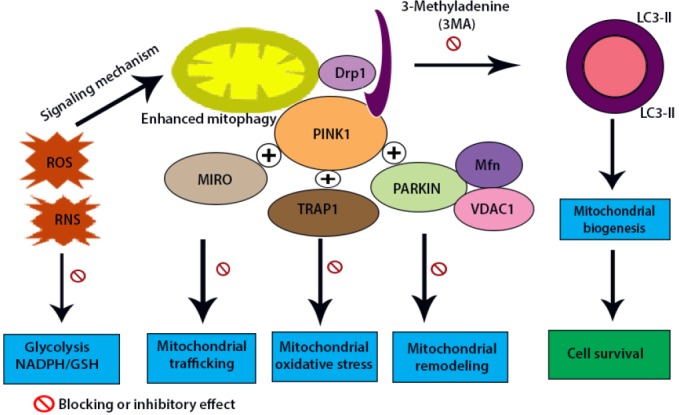
Protective roles of mitophagy.
Reactive oxygen species such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) acts as a signaling mechanism to induce autophagy or mitophagy which has a role in bioenergetic pathway and protective roles in cell survival. On the other hand, an increase in mitochondrial fusion protects mitochondria through mitophagy. The PTEN-induced putative kinase protein 1 (PINK1) of mitochondria is destabilized by presenilin rhomboid like (PARL) and cytosolic E3 ubiquitin ligase PARKIN (PARKIN) ubiquitinates mitofusion (Mfn), voltage dependent protein channel 1 (VDAC1) etc. that results in elevated mitophagy. PINK1 with different proteins such as mitochondrial RhoGTase (MIRO), tumor necrosis factor receptor associated protein 1 (TRAP1), PARKIN combined with Mfn and voltage dependent anion channel 1 (VDAC1) has different inhibitory roles such as mitochondrial trafficking and mitochondrial remodeling. Clearance of damaged mitochondria by mitophagy enhances mitochondrial biogenesis and increases the rate of cell survival.
The cytosolic E3 ubiquitin ligase PARKIN and mitochondrial PINK1 have been implicated in the abnormal expression of genes associated with a recessive form of Parkinsonism (Schiavi and Ventura, 2014). However, the engagement of these proteins in the pathogenesis of PD remains unclear. Previous studies in Drosophila melanogaster have demonstrated that PINK1 and PARKIN function in the same genetic pathway to maintain mitochondrial network integrity (Greene et al., 2012). In healthy mitochondria, PINK1 is imported via the translocase complexes of the outer and inner mitochondrial membranes. PINK1 is then degraded by various proteases, such as mitochondria-processing protease (MPP+), the inner membrane presenilin-related rhomboid-like protease (Meissner et al., 2011). Following mitochondrial depolarization, PINK1 is translocated to the inner mitochondrial membrane, degraded, and sustained on the mitochondrial membrane (Lazarou et al., 2012). The aggregation of PINK on the mitochondrial surface induces mitophagy by volunteering PARKIN to degrade mitochondria via a mechanism that is not completely understood. Hence, PINK1 likely acts as a sensor for degraded mitochondria. Translocation of PARKIN to damaged mitochondria has been shown to weaken PINK1 function (Geisler et al., 2010).
As a consequence of its translocation, PARKIN ubiquitylates outer mitochondrial membrane proteins. Another adaptor molecule, such as p62, is then engaged to mitochondria to induce mitophagy. The mitochondrial fusion proteins mitofusion 1 (Mfn1) and mitofusion 2 (Mfn2) have been recognized as substrates of PARKIN (Rakovic et al., 2011), as illustrated in Figure 2. PARKIN hinders mitochondrial fusion via the degeneration of mitofusions, thus isolating damaged mitochondria from the healthy mitochondrial membrane proteins, such as the voltage-dependent anion channel (VADC), the mitochondrial RhoGTase (MIRO) 1 (Figure 2) and constituents of the mitochondrial translocase complex (TOM70, TOM40 and TOM20) (Yoshii et al., 2011). It is important to note that mitochondrial mobility is strongly maintained by the mitochondrial MIROs. MIRO1 and MIRO2 are both GTPases. MIRO function is essential for neuronal health: knockout of Miro1 in mice is lethal in the early postnatal period (Devine and Kittler, 2018).
Recessive mutations in PINK1 and PARKIN can cause PD and lead to a failure of mitophagy, causing mitochondrial damage (Kahle et al., 2009) and contributing to disease pathogenesis. Mitochondrial fission is also important for the function of neurons: dominant-negative dynamin-associated protein 1 (Drp1) mutation can cause a lethal infantile neurodegenerative phenotype. Drp1 knockout mice revealed embryonic lethality characterized by aberrant development of the brain and failure of synapse formation (Dagda et al., 2009).
Mitophagy and Neurodegeneration Diseases
The maintenance of mitochondrial physiology is essential for the nervous system because a disorder causes oxidative damage and many neurodegeneration diseases.
Alzheimer’s disease
AD is a chronic neurodegenerative disease characterized by the extracellular accumulation of β-amyloid (Aβ) (Wang et al., 2018). As the current therapies have limited effectiviness against AD, there is an urgent need for more research efforts concentrated at developing new agents for preventing the disease process (Aso and Ferrer, 2014).
ROS-mediated injury is observed in AD brains, and higher levels of malondialdehyde (MA) and 4-hydroxy nonenal (4-HNE) are observed in the brain (Figure 3) and cerebrospinal fluid of AD patients compared to controls (Butterfield and Lauderback, 2002). Transactive response DNA-binding protein of 43 kDa (TDP-43) pathology may be present as a comorbidity in approximately 20–50% of sporadic AD cases (Di et al., 2018). A recent study showed that resveratrol weakened Aβ1–42-induced cell death and significantly increased mitophagy (i.e., increased the acidic vesicular organelle number, LC3-II/LC3-I ratio, Parkin and Beclin-1 (Bcl-1) expression and LC3 and TOMM20 co-localization in Aβ1–42-treated PC12 cells) (Wang et al., 2018).
Figure 3.
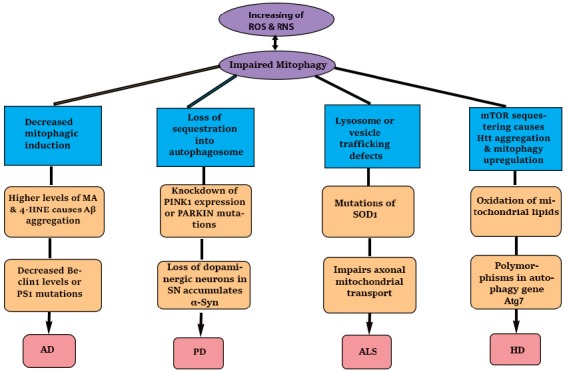
Correlation of mitophagy and neurodegenerative diseases.
Upregulation or downregulation of autophagic and mitophagic function has a role in the development of many neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Hungtington’s disease (HD), and amyotrophic lateral sclerosis (ALS). AD is caused by decreased mitophagic induction which causes higher levels of malondehyde (MA), 4-hydroxynonela (4-HNE), and Beclin-1 (Bcl-1), and increases amyloid beta (Aβ) aggregation and presenilin (PS1) mutations. PD is caused by loss of sequestration into autophagosome which causes loss of dopaminergic neurons in substantia nigra (SN) as well as knockdown of PINK1 expression or PARKIN mutations. ALS is caused by decreased lysosome or vesicle trafficking defects that result in formation of SOD1 formation and inpair axonal mitochondrial transport. HD is caused by mechanistic target of rapamycin (mTOR) sequestering into huntingtin protein (Htt) aggregates and oxidation of mitochondrial lipids which inhibits signaling that results in upregulation of mitophagy and polymorphism of autophagy related protein 7 (Atg7). It is shown that the dysfunction of mitochondria is responsible for generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) to cause malfunction in mitophagy which is vice versa.
Ultrastructural analysis revealed extensive dystrophy of virtually all neurites in the vicinity of and within β-amyloid deposits. There is also marked aggregation of vacuoles (mostly autophagic vacuoles [AVs] and smaller numbers of condensed mitochondria). The numbers of AVs in neuritic processes of AD brains have exceeded the incidence of AVs in cell bodies, although the AV numbers in neuronal Perikarya were also remarkably increased in AD (Nixon et al., 2005). On the other hand, AD may cause improper clearance of autophagosomes that contain both amyloid precursor protein (APP) and its processing enzymes, thus increasing the propensity to produce toxic Aβ peptides (Figure 3) (Butler et al., 2006). Aβ is transported to mitochondria where it interacts with mitochondrial proteins, causing an increase in ROS production, excess accumulation of mitochondrial Ca2+ and mitochondrial fragmentation, a decrease in the number of functionally active mitochondria and, ultimately, neuronal damage (Duchen, 2012). In an APP transgenic mouse model, the down- or up-regulation of Bcl-1 enhanced or decreased, respectively, extracellular Aβ aggregation and neurodegeneration, highlighting the importance of mitophagy in AD-associated pathology. Furthermore, a correlation between flavin adenine dinucleotide (FAD) and autophagy was currently noted, reporting that autophagy needs functional presenilin (PS-1) for lysosomal maturation, which is altered by Alzheimer-related presenilin 1 (PS-1) mutations (Lee et al., 2010). Hence, PS-1 alterations may indirectly affect mitochondrial function by impairing its recycling by mitophagy (García-Escudero et al., 2013).
An increasing number of studies have investigated the defensive aspect of mitophagy in various harmful situations, such as coenzyme Q10 (CoQ10) inadequacy, hypoxia (Zhang et al., 2008), and exposure to rotenone, thereby making mitophagy an appropriate target for therapeutic mediation. Similarly, injection of lentivirus-infected Bcl-1 in a mouse model of spinocerebellar ataxia type 3 (Machado-Joseph disease) elevated motor function and subsequently decreased protein accumulation (Hetz et al., 2013). Consistent with these results, haploinsufficiency of Bcl-1 promoted the advancement of experimental AD in vivo (Pickford et al., 2008). These phenomena were accompanied by the aggregation of p62, diminished levels of LC3-II and a modified equality between monomeric and oligomeric components of mutant superoxide dismutase 1 (SOD1) in the spinal cord (Nassif et al., 2014).
Using transgenic Drosophila expressing human tau, Iijima-Ando et al. (2012) demonstrated that RNAi-mediated Drosophila Miro (dMiro) knockdown enhanced human tau phosphorylation at the AD-related site Ser262 (phopgo-tau), resulting in enhanced levels of active PAR-1 and increased tau-mediated neurodegeneration. Moreover, knockdown of Miro generated late-onset neurodegeneration in the fly brain, an effect that was suppressed by knockdown of Drosophila tau or PAR-1 (Kay et al., 2018). Surprisingly, the heterozygous Miro mutation (miro[Sd32]) has been connected to mitochondrial mislocalization and the amyloid-β 42 (Aβ42)-mediated onset of AD symptoms in an attenuated fly model (Kay et al., 2018).
AD is most prevalent in the elderly. It is defined by the accumulation of Aβ plaques and occurs when the normal mitophagic functions of the body are decreased; conversely, it produces ROS, which acts as an initiator of AD.
Parkinson’s disease
PD is the second most common progressive disorder of the CNS and is caused by a continuous loss of dopaminergic neurons (Tian et al., 2012). In dopaminergic neurons of the substantia nigra (SN), PD proteins such as Parkin, PINK1, DJ-1, and leucine-rich repeat serine/threonine-protein kinase 2 (LARRK2) as well as α-synucelin, play important roles in preventing cell death by maintaining normal mitochondrial function, protecting against oxidative stress, mediating mitophagy, and preventing apoptosis (Mukherjee et al., 2015). In addition to defective mitochondrial clearance, knockdown of PINK1 (Figure 3) also causes mitochondrial fragmentation followed by the activation of mitophagy (Dagda et al., 2009). A previous study also showed that oxidative stress is one of the most common causes of PD (Gaki and Papavassiliou, 2014).
Damaged mitochondria can also hinder movement via the PINK1-PARKIN-mediated degradation of MIRO1. MIRO1 turnover on degraded mitochondria is altered in fibroblasts from individuals with PD-related E3 ubiquitin protein ligase PARKIN (PARK2) mutations (Pickrell and Youle, 2015). The PD-related protein named Leucine-rich repeat kinase 2 (LRRK2) was recently shown to bind to MIRO1, inducing its degradation. A pathogenic mutation in LRRK2 impairs such binding, delaying the arrest and eventual removal of degraded mitochondria (Hsieh et al., 2016).
In a Drosophila PD model with loss of PINK1 function, weakened dMiro function improved the degenerative phenotype (as demonstrated in PINK1-mutant DA neurons). This result indicates a role for mitochondrial transport and Miro in PINK1-related PD pathogenesis (Pickrell and Youle, 2015), an idea further supported by the profound effects observed in altered PINK1 function or the transportation of axonal mitochondria in Drosophila larval motor neurons or mammalian hippocampal neurons (Kay et al., 2018).
Lee et al. (2018) reported that transgenic Drosophila melanogaster expressing fluorescent mitophagy affected PINK1/PARKIN mutations on basal mitophagy under physiological conditions. The author also showed that PINK1 and PARKIN are not essential for bulk basal mitophagy in Drosophila. More importantly, this is the first work to visualize mitophagy in fly models. The degree of/extent to which PINK1-triggered mitophagy is essential for mitochondrial quality control in the mammalian brain and the extent to which its deviated regulation is responsible for PD pathogenesis remain unclear (Chu, 2018). By contrast, a complementary study demonstrated the effect of PINK1 on the mito-QC reporter system in PINK1 knockout mice (McWilliams et al., 2018). The same study also showed that basal mitophagy is unaffected by the loss of PINK1 in most tissues (Lee et al., 2018).
Cardiolipin in mitochondria is redistributed to the surface of degraded mitochondria, where it engages LC3 to assist in the generation of autophagosomes centered on mitochondria termed mitosomes (Chu et al., 2013). In cardiolipin-mediated mitophagy, a cargo-targeting mechanism does not require PINK1 aggregation or PARKIN association with the mitochondria (Chu et al., 2013). Another study revealed that the Atg32 system in yeast cells, the association of LIR proteins such as BNIP3, BNIP3L/NIX, sequestosome 1 (SQSTM1), or FUNDC1, and the PINK1-PARKIN2/PARKIN pathway, which is defined by two proteins, are genetically linked to PD (Chu et al., 2014). However, according to another study, PINK1 along with PARKIN is not needed for receptor-induced mitophagy. A concurrent study reported that NIX compensated for the dysfunction of PINK1 or PARKIN in fibroblasts from PD patients (Koentjoro et al., 2017).
In general, defects in the formation of autophagosomes cause impaired mitophagy, which causes PARKIN mutations that further result in neurodegenerative disorders, such as PD (Figure 3). Moreover, AVs were recently observed in an experimental neurodegenerative model and in dying striatal neurons in PD; however, information on the extent to which autophagy is associated with neurodegeneration and its pathogenic significance is limited (Nixon et al., 2005).
In PD, the accumulation of α-synucelin in the SN, which results in excessive ROS, eventually impairs the normal mitophagic pathways to regulate the redox balance and homeostasis (Gaki and Papavassiliou, 2014).
Huntington’s disease
Motor deficits in HD patients are related to abnormal dopamine neurotransmission in the striatum (Vidoni et al., 2017). In HD, mitochondrial ROS production and oxidation of mitochondrial lipids play important roles in mitophagy (Johri et al., 2013).
In addition, it has been delineated that nitric oxide increases mitochondrial fission in neurons, initiating neuronal loss in a mouse model of stroke (Barsoum et al., 2006). In contrast, exhibition of Mfn or a dominant-negative Drp1 mutant in cultured neurons is defensive against oxidative insults. Apart from these, mechanistic target of rapamycin sequestration causes the aggregation of Huntington protein (Htt), which results in the upregulation of autophagy or polymorphisms in the autophagy-related gene Atg7 that further causes HD (Barsoum et al., 2006; Jahani-Asl et al., 2007) (Figure 3). Oxidative damage is found in the plasma of HD patients, HD postmortem brain tissue, lymphoblasts and cerebrospinal fluid (Khalil et al., 2015). In HD, degradation by autophagy is poorly understood, but the alterations in mitochondrial fission/fusion are likely to interfere with mitophagy, leading to the aggregation of degraded mitochondria in the cytoplasm. Martinez-Vicerte et al. (2010) showed that autophagosomes had a defect in cargo recognition that affects organelle sequestration by inducing autophagy, which may explain improper mitochondrial aggregation in HD cells. It was recently demonstrated that Htt is immensely associated with mitophagy by serving as a frame for both sequestosome 1 (SQSTM1/p62) and the autophagy-inducing kinase, UNC-51-like kinase-1 (ULK1), supporting the involvement of mutant Htt in these processes (Rui et al., 2015). In another study, dopamine-induced oxidative stress triggered apoptotic cell death in dopaminergic neuroblastoma SH-SY5Y cells that hyper-express mutant PolyQ Htt (PolyQ-Htt) protein. Dopamine toxicity was accompanied by impaired autophagy clearance of PolyQ-Htt aggregates. Dopamine also affected the stability and function of ATG4, a redox-sensitive cysteine protein associated with the process of LC3, a main step in autophagosome formation. Resveratrol, a dietary polyphenol with anti-oxidant and pro-autophagic characteristics, has demonstrated neuroprotective potential in HD (Vidoni et al., 2018).
Mitochondrial ROS plays an important role in the generation of HD, and abnormal ROS production imparts mitophagic dysregulation and fails to maintain the normal redox balance, resulting in impaired homeostasis.
Amyotrophic lateral sclerosis
ALS is a neurodegenerative disease affecting the spinal cord and brain motor neurons that ultimately leads to paralysis and early death (Mancuso and Navarro, 2017). Motor neuron death is caused by the dysfunction of mitochondria by directing them toward calcium-mediated excitotoxicity, by stimulating ROS generation and initiating the intrinsic apoptotic pathway (Julien, 2007). The particular mechanism of ALS is still under investigation because it is associated with cells other than neuronal cells. However, many lines of evidence suggest that huge amounts of autophagosomes and increased amounts of autophagic proteins and their activation are harmful for the survival of motor neurons. An increase in the LC3II macroautophagy marker protein and a decreased amount of phosphorylated mechanistic target of rapamycin-positive motor neurons revealed impaired mitophagy related to the loss of motor neurons in ALS (Okamoto et al., 2010). Various studies have reported dysfunctional Miro in ALS patients or animal models of the disease, including a report of significantly lower levels of Miro1 present in spinal cord samples of ALS patients (Zhang et al., 2015).
Mitochondrial fission and fusion hamper mitophagic clearance, which may also be affected by mutant SOD1 (Figure 3) (Albers and Beal, 2000). Glutathione (GSH) is a free radical scavenger tripeptide and acts as a main regulator of the intracellular redox state. GSH levels were lower in the motor cortex of ALS patients than those in the control volunteers (Weiduschat et al., 2014), and decreased levels of GSH result in neurological deficits and promoted the progression of mitochondrial pathology in the mutant SOD1 ALS mouse model (Vargas et al., 2011). Mutant SOD1 has been reported to impart Parkin-dependent degradation of MIRO1, which may explain the mitochondrial trafficking defect (Devine and Kittler, 2018). The same study also described Miro1-knockout mice, which exhibited upper motor neuron degeneration (Nguyen et al., 2014).
The expression of mutant TDP-43 in a motor neuron-like cell line induced oxidative species, mitochondrial disorder, and the accumulation of nuclear factor protein 2 (Nrf2), a modulator of oxidative species in a yeast model. TDP-43-expressing cells displayed increased markers of oxidative stress (Guareschi et al., 2012). Additionally, mitochondrial disorder was noticed, together with oxidative damage, as well as the induction of mitophagy in the mouse motor neuron-like cell line (NSC34) expressing wild-type or mutant TDP-43, representing a pathology resembling ALS. Moreover, motor neurons from these mice displayed cytoplasmic TDP-43-positive inclusions (Hong et al., 2012). In conclusion, lysosome or vesicle trafficking defects result in mutations in dynactin, which result in impaired mitophagy and ALS (Figure 3).
In a mouse model of motor neuron disease, full-length TDP-43 increased the involvement of mitochondria and blocked the TDP-43/mitochondria interaction, ameliorating mitochondrial TDP-43-interacting partners including VDAC1 and prohibitin 2 (PHB2), a vital mitophagy receptor (Davis et al., 2018). Mutant SOD1 impairs mitochondrial retrograde axonal transport (Magrané et al., 2013) along with mitochondrial network fragmentation, indicating the induction of mitophagy (Carrì et al., 2017).
Based on this review, we conclude that the loss of motor neurons and breakdown of the redox balance cause ALS in which ROS are an important component.
Conclusion and Future Perspectives
Mitophagy can prevent damaged mitochondrial aggregation and induce protective actions against cell demise. Clearing of degraded and aged mitochondria is an essential process for neuron survival. Focal mitophagy eradicates degraded mitochondria and decreases ROS-induced neuronal death (Kubli and Gustafsson, 2012). Li et al. (2014) demonstrated that rapamycin enhanced mitophagy, as evidenced by the increase in LC3-II and Bcl-1 expression in the mitochondria as well as p62 translocation to the mitochondria. Rapamycin decreased infarct volume, thus improving neurological outcomes, and decreased mitochondrial dysfunction compared with control animals. However, the mechanism by which rapamycin increases mitophagy should be further investigated (Li et al., 2014). In addition to 3-MA, other phosphoinositide 3-kinase (PI3K) inhibitors, such as bafilomycin and chloroquine, alter vascular and lysosomal pH and inhibit autophagosomal-lysosomal fusion, and E64 and pepstatin A prevent lysosomal protease activities. The prevention of autophagy usually leads to increased cell death; however, in some cases, autophagy leads to cytotoxicity. Investigating compounds that modulate autophagy and mitophagy will aid in the treatment of various diseases caused by oxidative protein modification aggregation within the cells (Zhang, 2013). It has been demonstrated in the abovementioned studies that mitophagy plays an important role in the course of neurodegenerative diseases by combating ROS in diseases such as AD, PD, HD, and ALS. We believe that by investigating different molecules that induce or inhibit mitophagy in vivo and in vitro, we can develop neuroprotective drugs.
Additional files:
Additional file 1: Database search strategy.
Additional file 2: Open peer review report 1 (94.7KB, pdf) .
Footnotes
Conflicts of interest: The authors reported no potential conflict of interests.
Financial support: This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning, No. 2018R1C1B5029745 (to HJC), 2011-0030072 (to YH), 2018R1D1A1B07040282 (to JJ), and 2018R1A2B6001123 (to NYJ).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Ivan Fernandez-Vega, Hospital Universitario Central de Asturias, Spain.
Funding: This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning, No. 2018R1C1B5029745 (to HJC), 2011-0030072 (to YH), 2018R1D1A1B07040282 (to JJ), and 2018R1A2B6001123 (to NYJ).
P-Reviewer: Fernandez-Vega I; C-Editor: Zhao M; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Liu XL
References
- 1.Abada A, Elazar Z. Getting ready for building: signaling and autophagosome biogenesis. EMBO Rep. 2014;15:839–852. doi: 10.15252/embr.201439076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albers DS, Beal MF. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J Neural Transm Suppl. 2000;59:133–154. doi: 10.1007/978-3-7091-6781-6_16. [DOI] [PubMed] [Google Scholar]
- 3.Aso E, Ferrer I. Cannabinoids for treatment of Alzheimer’s disease: Moving toward the clinic. Front Pharmacol. 2014;5:37. doi: 10.3389/fphar.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Gräber S, Kovacs I, Lee WD, Waggoner J, Cui J. Nitric oxide‐induced mitochondrial fission is regulated by dynamin‐related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler D, Nixon RA, Bahr BA. Potential compensatory responses through autophagic/lysosomal pathways in neurodegenerative diseases. Autophagy. 2006;2:234–237. doi: 10.4161/auto.2729. [DOI] [PubMed] [Google Scholar]
- 6.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress 1, 2. Free Radic Biol Med. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 7.Carrì MT, D’Ambrosi N, Cozzolino M. Pathways to mitochondrial dysfunction in ALS pathogenesis. Biochem Biophys Res Commun. 2017;483:1187–1193. doi: 10.1016/j.bbrc.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 8.Chu CT. Multiple pathways for mitophagy: A neurodegenerative conundrum for Parkinson’s disease. Neurosci Lett. 2018 doi: 10.1016/j.neulet.2018.04.004. pii: S0304-3940(18)30259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu CT, Bayir H, Kagan VE. LC3 binds externalized cardiolipin on injured mitochondria to signal mitophagy in neurons: Implications for Parkinson disease. Autophagy. 2014;10:376–378. doi: 10.4161/auto.27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15:1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu CT, Zhu J, Dagda R. Beclin 1-independent pathway of damage-induced mitophagy and autophagic stress: Implications for neurodegeneration and cell death. Autophagy. 2007;3:663–666. doi: 10.4161/auto.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dagda RK, Cherra SJ, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis SA, Itaman S, Khalid-Janney CM, Sherard JA, Dowell JA, Cairns NJ, Gitcho MA. TDP-43 interacts with mitochondrial proteins critical for mitophagy and mitochondrial dynamics. Neurosci Lett. 2018;678:8–15. doi: 10.1016/j.neulet.2018.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devine MJ, Kittler JT. Mitochondria at the neuronal presynapse in health and disease. Nat Rev Neurosci. 2018;19:63–80. doi: 10.1038/nrn.2017.170. [DOI] [PubMed] [Google Scholar]
- 16.Di RA, D’Acunzo P, Simula L, Campello S, Strappazzon F, Cecconi F. AMBRA1-mediated mitophagy counteracts oxidative stress and apoptosis induced by neurotoxicity in human neuroblastoma SH-SY5Y cells. Front Cell Neurosci. 2018;12:92. doi: 10.3389/fncel.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 18.Duchen MR. Mitochondria, calcium-dependent neuronal death and neurodegenerative disease. Pflugers Arch Eur J Physiol. 2012;464:111–121. doi: 10.1007/s00424-012-1112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eiyama A, Okamoto K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol. 2015;33:95–101. doi: 10.1016/j.ceb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 21.Fivenson EM, Lautrup S, Sun N, Scheibye-Knudsen M, Stevnsner T, Nilsen H, Bohr VA, Fang EF. Mitophagy in neurodegeneration and aging. Neurochem Int. 2017;109:202–209. doi: 10.1016/j.neuint.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaki GS, Papavassiliou AG. Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson’s disease. Neuromolecular Med. 2014;16:217–230. doi: 10.1007/s12017-014-8294-x. [DOI] [PubMed] [Google Scholar]
- 23.García-Escudero V, Martín-Maestro P, Perry G, Avila J. Deconstructing mitochondrial dysfunction in Alzheimer disease. Oxid Med Cell Longev. 2013;2013:162152. doi: 10.1155/2013/162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geisler S, Holmström KM, Treis A, Skujat D, Weber SS, Fiesel FC, Kahle PJ, Springer W. The PINK1/Parkin-mediated mitophagy is compromised by PD-associated mutations. Autophagy. 2010;6:871–878. doi: 10.4161/auto.6.7.13286. [DOI] [PubMed] [Google Scholar]
- 25.Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, McBride HM, Park DS, Fon EA. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep. 2012;13:378–385. doi: 10.1038/embor.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guareschi S, Cova E, Cereda C, Ceroni M, Donetti E, Bosco DA, Trotti D, Pasinelli P. An over-oxidized form of superoxide dismutase found in sporadic amyotrophic lateral sclerosis with bulbar onset shares a toxic mechanism with mutant SOD1. Proc Natl Acad Sci U S A. 2012;109:5074–5079. doi: 10.1073/pnas.1115402109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12:703–719. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- 28.Hong K, Li Y, Duan W, Guo Y, Jiang H, Li W, Li C. Full-length TDP-43 and its C-terminal fragments activate mitophagy in NSC34 cell line. Neurosci Lett. 2012;530:144–149. doi: 10.1016/j.neulet.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh CH, Shaltouki A, Gonzalez AE, da Cruz AB, Burbulla LF, Lawrence ES, Schüle B, Krainc D, Palmer TD, Wang X. Functional impairment in Miro degradation and mitophagy is a shared feature in familial and sporadic Parkinson’s disease. Cell Stem Cell. 2016;19:709–724. doi: 10.1016/j.stem.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iijima-Ando K, Sekiya M, Maruko-Otake A, Ohtake Y, Suzuki E, Lu B, Iijima KM. Loss of axonal mitochondria promotes tau-mediated neurodegeneration and Alzheimer’s disease–related tau phosphorylation via PAR-1. PLoS Genet. 2012;8:e1002918. doi: 10.1371/journal.pgen.1002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jahani-Asl A, Cheung ECC, Neuspiel M, MacLaurin JG, Fortin A, Park DS, McBride HM, Slack RS. Mitofusin 2 protects cerebellar granule neurons against injury-induced cell death. J Biol Chem. 2007;282:23788–23798. doi: 10.1074/jbc.M703812200. [DOI] [PubMed] [Google Scholar]
- 32.Johri A, Chandra A, Beal MF. PGC-1α, mitochondrial dysfunction, and Huntington’s disease. Free Radic Biol Med. 2013;62:37–46. doi: 10.1016/j.freeradbiomed.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Julien JP. ALS: astrocytes move in as deadly neighbors. Nat Neurosci. 2007;10:535–537. doi: 10.1038/nn0507-535. [DOI] [PubMed] [Google Scholar]
- 34.Kahle PJ, Waak J, Gasser T. DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic Biol Med. 2009;47:1354–1361. doi: 10.1016/j.freeradbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Kamat PK, Kalani A, Kyles P, Tyagi SC, Tyagi N. Autophagy of mitochondria: a promising therapeutic target for neurodegenerative disease. Cell Biochem Biophys. 2014;70:707–719. doi: 10.1007/s12013-014-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kay L, Pienaar IS, Cooray R, Black G, Soundararajan M. Understanding miro GTPases: implications in the treatment of neurodegenerative disorders. Mol Neurobiol. 2018;55:7352–7365. doi: 10.1007/s12035-018-0927-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khalil B, El Fissi N, Aouane A, Cabirol-Pol MJ, Rival T, Liévens JC. PINK1-induced mitophagy promotes neuroprotection in Huntington’s disease. Cell Death Dis. 2015;6:e1617. doi: 10.1038/cddis.2014.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitagishi Y, Nakano N, Ogino M, Ichimura M, Minami A, Matsuda S. PINK1 signaling in mitochondrial homeostasis and in aging. Int J Mol Med. 2017;39:3–8. doi: 10.3892/ijmm.2016.2827. [DOI] [PubMed] [Google Scholar]
- 39.Koentjoro B, Park JS, Sue CM. Nix restores mitophagy and mitochondrial function to protect against PINK1/Parkin-related Parkinson’s disease. Sci Rep. 2017;7:44373. doi: 10.1038/srep44373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, Kawahara N, Kuida K, Nagata S, Kominami E. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol. 2008;172:454–469. doi: 10.2353/ajpath.2008.070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komatsu M, Waguri S, Koike M, Sou Y, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 42.Kubli DA, Gustafsson ÅB. Mitochondria and mitophagy: The yin and yang of cell death control. Circ Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lazarou M, Jin SM, Kane LA, Youle RJ. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell. 2012;22:320–333. doi: 10.1016/j.devcel.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JA. Autophagy in neurodegeneration: Two sides of the same coin. BMB Rep. 2009;42:324–330. doi: 10.5483/bmbrep.2009.42.6.324. [DOI] [PubMed] [Google Scholar]
- 45.Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JJ, Sanchez-Martinez A, Zarate AM, Benincá C, Mayor U, Clague MJ, Whitworth AJ. Basal mitophagy is widespread in Drosophila but minimally affected by loss of Pink1 or parkin. J Cell Biol. 2018;217:1613–1622. doi: 10.1083/jcb.201801044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Hu G. Pink1 protects cortical neurons from thapsigargin-induced oxidative stress and neuronal apoptosis. Biosci Rep. 2015;35 doi: 10.1042/BSR20140104. pii: e00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Q, Zhang T, Wang J, Zhang Z, Zhai Y, Yang GY, Sun X. Rapamycin attenuates mitochondrial dysfunction via activation of mitophagy in experimental ischemic stroke. Biochem Biophys Res Commun. 2014;444:182–188. doi: 10.1016/j.bbrc.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 50.Lv M, Wang C, Li F, Peng J, Wen B, Gong Q, Shi Y, Tang Y. Structural insights into the recognition of phosphorylated FUNDC1 by LC3B in mitophagy. Protein Cell. 2017;8:25–38. doi: 10.1007/s13238-016-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magrané J, Cortez C, Gan W-B, Manfredi G. Abnormal mitochondrial transport and morphology are common pathological denominators in SOD1 and TDP43 ALS mouse models. Hum Mol Genet. 2013;23:1413–1424. doi: 10.1093/hmg/ddt528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mancuso R, Navarro X. Smith SB, Su TP. Springer, Cham; 2017. Sigma-1 receptor in motoneuron disease. In: Sigma receptors: their role in disease and as therapeutic targets; pp. 235–254. [DOI] [PubMed] [Google Scholar]
- 53.Martinez-Vicente M, Talloczy Z, Wong E, Tang G, Koga H, Kaushik S, De Vries R, Arias E, Harris S, Sulzer D. Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nat Neurosci. 2010;13:567–576. doi: 10.1038/nn.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McWilliams TG, Prescott AR, Montava-Garriga L, Ball G, Singh F, Barini E, Muqit MMK, Brooks SP, Ganley IG. Basal mitophagy occurs independently of PINK1 in mouse tissues of high metabolic demand. Cell Metab. 2018;27:439–449. doi: 10.1016/j.cmet.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meissner C, Lorenz H, Weihofen A, Selkoe DJ, Lemberg MK. The mitochondrial intramembrane protease PARL cleaves human Pink1 to regulate Pink1 trafficking. J Neurochem. 2011;117:856–867. doi: 10.1111/j.1471-4159.2011.07253.x. [DOI] [PubMed] [Google Scholar]
- 56.Mizushima N, Kuma A. Autophagosomes in GFP-LC3 transgenic mice. Methods Mol Biol. 2008;445:119–124. doi: 10.1007/978-1-59745-157-4_7. [DOI] [PubMed] [Google Scholar]
- 57.Mukherjee UA, Ong SB, Ong SG, Hausenloy DJ. Parkinson’s disease proteins: Novel mitochondrial targets for cardioprotection. Pharmacol Ther. 2015;156:34–43. doi: 10.1016/j.pharmthera.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nassif M, Valenzuela V, Rojas-Rivera D, Vidal R, Matus S, Castillo K, Fuentealba Y, Kroemer G, Levine B, Hetz C. Pathogenic role of BECN1/Beclin 1 in the development of amyotrophic lateral sclerosis. Autophagy. 2014;10:1256–1271. doi: 10.4161/auto.28784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen TT, Oh SS, Weaver D, Lewandowska A, Maxfield D, Schuler MH, Smith NK, Macfarlane J, Saunders G, Palmer CA. Loss of Miro1-directed mitochondrial movement results in a novel murine model for neuron disease. Proc Natl Acad Sci U S A. 2014;111:E3631–3640. doi: 10.1073/pnas.1402449111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 61.Novak I, Kirkin V, McEwan DG, Zhang J, Wild P, Rozenknop A, Rogov V, Löhr F, Popovic D, Occhipinti A. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okamoto K, Fujita Y, Mizuno Y. Pathology of protein synthesis and degradation systems in ALS. Neuropathology. 2010;30:189–193. doi: 10.1111/j.1440-1789.2009.01088.x. [DOI] [PubMed] [Google Scholar]
- 63.Palomo GM, Manfredi G. Exploring new pathways of neurodegeneration in ALS: The role of mitochondria quality control. Brain Res. 2015;1607:36–46. doi: 10.1016/j.brainres.2014.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pickrell AM, Youle RJ. The roles of PINK1, Parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rakovic A, Grünewald A, Kottwitz J, Brüggemann N, Pramstaller PP, Lohmann K, Klein C. Mutations in PINK1 and Parkin impair ubiquitination of Mitofusins in human fibroblasts. PLoS One. 2011;6:e16746. doi: 10.1371/journal.pone.0016746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rui YN, Xu Z, Patel B, Cuervo AM, Zhang S. HTT/Huntingtin in selective autophagy and Huntington disease: A foe or a friend within? Autophagy. 2015;11:858–860. doi: 10.1080/15548627.2015.1039219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sarkar S, Krishna G, Imarisio S, Saiki S, O’kane CJ, Rubinsztein DC. A rational mechanism for combination treatment of Huntington’s disease using lithium and rapamycin. Hum Mol Genet. 2008;17:170–178. doi: 10.1093/hmg/ddm294. [DOI] [PubMed] [Google Scholar]
- 69.Schiavi A, Ventura N. The interplay between mitochondria and autophagy and its role in the aging process. Exp Gerontol. 2014;56:147–153. doi: 10.1016/j.exger.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 70.Tian L, Ma L, Kaarela T, Li Z. Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. J Neuroinflammation. 2012;9:155. doi: 10.1186/1742-2094-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsvetkov AS, Mitra S, Finkbeiner S. Protein turnover differences between neurons and other cells. Autophagy. 2009;5:1037. doi: 10.4161/auto.5.7.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vargas MR, Johnson DA, Johnson JA. Decreased glutathione accelerates neurological deficit and mitochondrial pathology in familial ALS-linked hSOD1 G93A mice model. Neurobiol Dis. 2011;43:543–551. doi: 10.1016/j.nbd.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vidoni C, Secomandi E, Castiglioni A, Melone MA, Isidoro C. Resveratrol protects neuronal-like cells expressing mutant Huntingtin from dopamine toxicity by rescuing ATG4-mediated autophagosome formation. Neurochem Int. 2018;117:174–187. doi: 10.1016/j.neuint.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 74.Wallace DC. Mitochondria and cancer: Warburg addressed. Cold Spring Harb Symp Quant Biol. 2005;70:363–374. doi: 10.1101/sqb.2005.70.035. [DOI] [PubMed] [Google Scholar]
- 75.Wang H, Jiang T, Li W, Gao N, Zhang T. Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer’s disease. Toxicol Lett. 2018;282:100–108. doi: 10.1016/j.toxlet.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, Nartiss Y, Steipe B, McQuibban GA, Kim PK. ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy. 2012;8:1462–1476. doi: 10.4161/auto.21211. [DOI] [PubMed] [Google Scholar]
- 77.Weiduschat N, Mao X, Hupf J, Armstrong N, Kang G, Lange DJ, Mitsumoto H, Shungu DC. Motor cortex glutathione deficit in ALS measured in vivo with the J-editing technique. Neurosci Lett. 2014;570:102–107. doi: 10.1016/j.neulet.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 78.Yoshii SR, Kishi C, Ishihara N, Mizushima N. Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane. J Biol Chem. 2011;286:19630–19640. doi: 10.1074/jbc.M110.209338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang F, Wang W, Siedlak SL, Liu Y, Liu J, Jiang K, Perry G, Zhu X, Wang X. Miro1 deficiency in amyotrophic lateral sclerosis. Front Aging Neurosci. 2015;7:100. doi: 10.3389/fnagi.2015.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Zhang J. Autophagy and mitophagy in cellular damage control. Redox Biol. 2013;1:19–23. doi: 10.1016/j.redox.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


