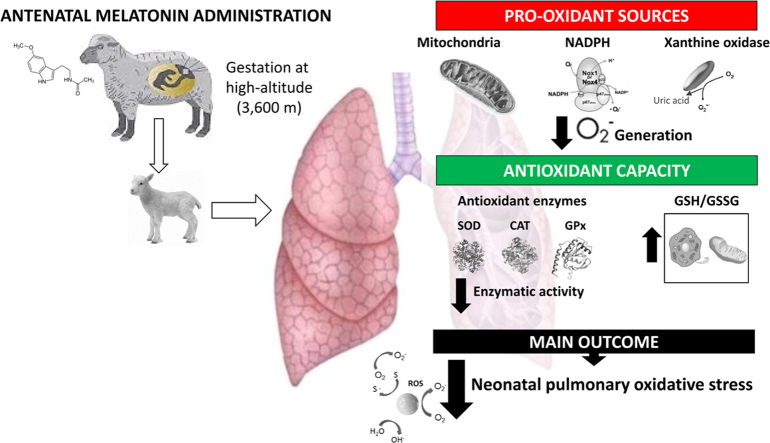
Abstract
Chronic hypobaric hypoxia during fetal and neonatal life induces neonatal pulmonary hypertension. Hypoxia and oxidative stress are driving this condition, which implies an increase generation of reactive oxygen species (ROS) and/or decreased antioxidant capacity. Melatonin has antioxidant properties that decrease oxidative stress and improves pulmonary vascular function when administered postnatally. However, the effects of an antenatal treatment with melatonin in the neonatal pulmonary function and oxidative status are unknown. Therefore, we hypothesized that an antenatal therapy with melatonin improves the pulmonary arterial pressure and antioxidant status in high altitude pulmonary hypertensive neonates. Twelve ewes were bred at high altitude (3600 m); 6 of them were used as a control group (vehicle 1.4% ethanol) and 6 as a melatonin treated group (10 mg d-1 melatonin in vehicle). Treatments were given once daily during the last third of gestation (100–150 days). Lambs were born and raised with their mothers until 12 days old, and neonatal pulmonary arterial pressure and resistance, plasma antioxidant capacity and the lung oxidative status were determined. Furthermore, we measured the pulmonary expression and activity for the antioxidant enzymes superoxide dismutase, catalase and glutathione peroxidase, and the oxidative stress markers 8-isoprostanes, 4HNE and nitrotyrosine. Finally, we assessed pulmonary pro-oxidant sources by the expression and function of NADPH oxidase, mitochondria and xanthine oxidase. Melatonin decreased the birth weight. However, melatonin enhanced the plasma antioxidant capacity and decreased the pulmonary antioxidant activity, associated with a diminished oxidative stress during postnatal life. Interestingly, melatonin also decreased ROS generation at the main pro-oxidant sources. Our findings suggest that antenatal administration of melatonin programs an enhanced antioxidant/pro-oxidant status, modulating ROS sources in the postnatal lung.
Keywords: Oxidative stress, Antioxidant system, Neonatal pulmonary circulation, Chronic hypoxia, Pulmonary pro-oxidant capacity, DOHaD
Graphical abstract
1. Introduction
More than 140 million people worldwide inhabit above 2500 m of altitude, a geographical condition that challenges pulmonary physiology under hypobaric hypoxia [1], [2]. The chronic exposure to hypoxia during pregnancy at high altitude induces important cardiovascular responses, with an increased placental and pulmonary vasoconstriction in the pregnant mother and her fetus, resulting in a intrauterine growth restricted neonate [3], [4], [5]. Furthermore, the postnatal pulmonary vasoconstriction derives in an enhanced cardiac afterload, with an elevated pulmonary arterial pressure and pathological pulmonary vascular and right ventricle remodelling [6], [7]. These changes are key features for pulmonary hypertension of the neonate (PHN), a condition considered a failure in the fetal to neonatal pulmonary vascular transition associated with high mortality and morbidity in both low and high-altitude conditions [5], [8], [9]. One of the causal mechanisms involved in the pathophysiology of PHN is oxidative stress, which implies an imbalance between an increased free radicals generation and/or decreased antioxidant capacity [10]. Hence, reactive oxygen species (ROS) excess is an important mediator of the hypoxic vascular constriction and endothelial dysfunction in the small pulmonary arteries [11], [12], [13], [14]. The major sources involved in the oxidative stress determination at the vascular level are the mitochondria, NADPH oxidase, xanthine oxidase, and uncoupled endothelial nitric oxide synthase (eNOS) [15].
Hypoxia is likely to increase the generation of ROS by several means. During hypoxia, the mitochondrial electron uncoupling at complexes I and III of the electron transport chain, enhances the superoxide anion (O2•-) generation. As superoxide production depends on electron donation from reduced components of the mitochondrial electron transport chain (flavin in Complex I, QH· in Complex III) [12], anything that shifts these components towards a reduced state would enhance O2•- generation by accumulation of electrons upstream complex IV, thereby promoting superoxide formation with subsequent exit through an anion channel in the inner mitochondrial membrane [16].
The net rate of superoxide or hydrogen peroxide generation depends on the substrates present and the antioxidant systems active in the matrix and cytosol. In muscle mitochondria, there are four sites that determine O2•- generation, two in complex I and one each in complexes II and III. Specific suppressors of two sites have been identified, the outer ubiquinone-binding site in complex III (site IIIQo) and the site in complex I active during reverse electron transport (site IQ) [17]. These suppressors prevent superoxide/hydrogen peroxide production from a specific site without affecting oxidative phosphorylation, making them excellent tools to investigate the status of the sites in redox signaling. In addition, sites IIIQo and IQ have important roles in redox signaling (e.g. hypoxic signaling and ER-stress) and in causing oxidative damage in a variety of biological contexts [17], [18].
Another enzymatic source of O2•- are the NADPH oxidases (Noxs) family, which includes Nox1, Nox3, Nox4 and Nox5. Importantly, Noxs might be the main sources for the ROS generation in the vascular smooth muscle cells [19], endothelial cells [20] and fibroblasts [21]. Accumulating evidence indicates that ROS derived from the Nox enzymes play an important role in the pulmonary vasoconstriction response to hypoxia, determining detrimental effects at vascular and cardiac level [15]. Another important source of O2•- is xanthine oxidase (XO) in the vascular cells. XO catalyzes the last two steps of purine metabolism by sequential hydroxylation of hypoxanthine to produce xanthine and uric acid, a process where oxygen is reduced to O2•- [22]. The enzyme can exist in two forms (oxidase and dehydrogenase), differing mainly in their substrate specificity. Dehydrogenase preferably uses NAD+ as electron acceptor, while oxidase only reduces the molecular oxygen. The oxidase-dehydrogenase conversion occurs by proteolytic cleavage or by oxidation of thiol groups associated with an oxidative environment at the cellular level, becoming reversible by the electron-donation of molecular oxygen, thereby producing hydrogen peroxide (H2O2) and O2•- [23].
A dominant mechanism of endothelial dysfunction associated with oxidative stress is the nitric oxide bioavailability diminution by O2•- with the formation of peroxynitrite [24]. Reactive oxygen species may also uncouple eNOS due to oxidation of tetrahydrobiopterin (BH4) to dihydrobiopterin (BH2) [15]. Moreover, the oxidant tone favours sGC oxidation and increases the endogenous eNOS inhibitor, ADMA, particularly in hypoxic conditions [25].
All of the above markedly reduce the function of the nitrergic vasodilatatory pathway in the utero-placental and pulmonary circulation [3], [15]. Considering that hypoxia and oxidative stress has been proved as determinants in the development of intrauterine growth restriction (IUGR) and PHN, several antioxidant treatments have been proposed in perinatal medicine [26], [27], [28], [29], where melatonin has important beneficial properties compared with other antioxidants [13], [30], [31], [32], [33]. Its potent antioxidant properties are based on a direct scavenger action, the upregulation of antioxidant enzymes, and the ability to downregulate pro-oxidant enzymes [34], [35], [36]. Interestingly, neither the fetus nor the neonate are capable of producing endogenous melatonin [37], [38]. However, during gestation, the fetal blood melatonin comes from the mother as it freely crosses the placenta [38].
Here, we propose an antenatal treatment with melatonin aiming to attenuate the oxidative stress occurrence in chronically hypoxic neonatal lambs. Therefore, we hypothesized that a maternal treatment with oral melatonin during the last third of gestation to pregnant ewes, improves vascular pulmonary function, enhances the antioxidant capacity, decreases ROS generation and diminishes oxidative stress in the lung of high-altitude pulmonary hypertensive lambs.
2. Materials and methods
All animal care, procedures and experimentation were approved by the Ethics Committee of the Faculty of Medicine, University of Chile (protocol CBA#0398 FMUCH), and were conducted in accordance with the ARRIVE guidelines and carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments, or the NIH Guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
2.1. Animals
Twelve ewes (Ovis aries) were bred, maintained and gave birth to 12 lambs under chronic hypobaric hypoxia (Putre, 3.600 m). The pregnant ewes received the same amount of alfalfa as percentage of their body weight. Six ewes conformed a control group (CN, 5 ml vehicle 1.4% ethanol) and 6 the melatonin treated group (MM, 10 mg d-1 melatonin in 5 ml ethanol 1.4%). Treatments were given to the pregnant ewes, daily at dusk (18:00 h, oral), in the last third of gestation (100–150 d), until delivery. We obtained blood samples at the end of gestation in the morning (10:00) and at night (22:00) from the pregnant ewes.
Lambs were born without attendance and were raised with their mothers in a group pen. Daily neonatal weight and biparietal diameter (BPD) were recorded from birth until 12 days old.
The pregnant ewes and the lambs with their mothers were maintained in a roofed shed, equivalent to shade illuminated under clear blue sky, which can get to 20,000 lx at midday. At that time of the year (June-July), the sky is clear almost every day, and daylight photoperiod is about 11 h day/13 h night. The International Center for Andean Studies (INCAS) is a much insulated place away from city lights, therefore animals were only exposed to daylight. For the experimental period, we took care of not turning on the lights near the animal facility during the night, except for the moment of plasma sampling where we used mild intensity flashlights.
2.2. In vivo experiments
All lambs were instrumented at 2 days old for daily hemodynamic monitoring as described previously [13], [39], [40], [41]. Briefly, lambs were anaesthetized with a ketamine–xylazine association (10/0.04 mg/kg I.M.) and a Swan–Ganz catheter was placed in the pulmonary artery [13], [39], [40], [41]. The animals recovered and the proximal end of the cathether was kept in a scarf attached to the animal’s neck. At 3, 7 and 12 days of age, we assessed pulmonary arterial pressure (PAP), cardiac output (CO) and pulmonary vascular resistance (PVR) in both groups of lambs, recorded by a data acquisition system (Powerlab 8/30, ADInstruments). At 12 days postnatal, lambs underwent euthanasia with an overdose of sodium thiopentone (100 mg/kg, slow I.V. infusion), and pulmonary tissue samples from the medial lobe of the right lung were frozen and kept in liquid nitrogen [42].
2.3. In vitro experiments
2.3.1. Melatonin plasma levels
Maternal blood samples were taken at the end of gestation (147–149 days of gestation). Furthermore, neonatal blood samples were obtained at 3, 7 and 12 days old. Blood was immediately mixed with EDTA (1 mg/ml) and centrifuged at 5000 ×g for 5 min to obtain plasma. Samples were obtained at 10:00 and 22:00 h to assess melatonin rhythmicity. Plasma levels of melatonin were determined by radio immunoassay as previously described [13], [42], using melatonin antiserum (Guildhay Antisera Ltd., Guildford, Surrey, UK) and [O-methyl-3H]-labeled melatonin (85 Ci mmol/L; Amersham Biosciences AB, Uppsala, Sweden) as a tracer.
2.3.2. Total antioxidant capacity
Antioxidant status of the neonates was assessed by the ferric reducing ability of plasma (FRAP) at days 3, 7 and 12 of age. Due to the potential effects of plasma melatonin on the plasma reducing capacity, we determined FRAP at 10:00 and at 22:00 each day. Briefly, the FRAP solution was prepared by mixing 300 mM of acetate buffer (pH 3.6), 10 mM of 2,4,6-tri(2-pyridyl)-1,3,5-triazine (TPTZ) solution, and 20 mM of FeCl3·6H2O in a 10:1:1 ratio and by subsequent heating of the resultant mixture to 37 °C. The reaction mixture was composed of 750 µl FRAP solution, 75 µl H2O and 25 µl of the sample and then incubated at 25 °C in darkness for 30 min, and the absorbance was read at 593 nm. A standard curve ranging from 50 μM to 1.5 mM of FeSO4 was prepared for the quantitative determination of FeSO4 as mM Fe2+ and FeSO4 equivalents (eq) produced in the samples [43]. This method is based on the capacity of the plasma to reduce Fe3+ to Fe2+ by transferring an electron; this is interpreted as antioxidant capacity as, this is a mechanism used to stabilize a free radical. Fe2+ is chelated by TPTZ (TPTZ-Fe(2+)) and the complex is colorimetrically detected. Results are expressed as Fe2+ equivalent by interpolation and its disruption in the presence of chelating agents, measured in Fe2+ concentration, often used to measure the antioxidant capacity of fluids.
2.3.3. Protein expression
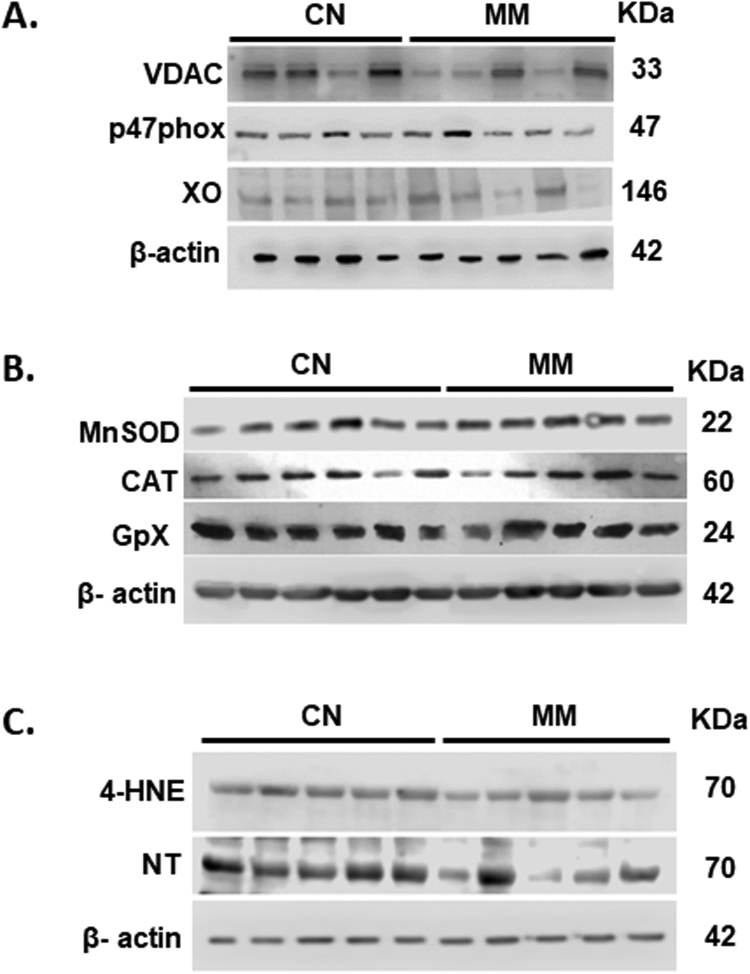
Protein expression of SOD2, CAT, GPx1,VDAC, p47-phox, Xantine Oxidase and β-actin was determined in total lung lysates by immunoblot with specific antibodies (anti-Mn-SOD, Millipore, 06-984; anti-CAT, Abcam Laboratories, ab1877; anti-GPx1, Abcam Laboratories, ab22604; anti-VDAC, Millipore, AB10527; anti-p47-phox, Sigma Aldrich, SAB45028110; anti-Xantine oxidase, Abcam, ab125133 and anti-β-actin, AC-15, Thermo Fisher Scientific, MA1-91399, respectively) as described previously [13]. The signals obtained on immunoblot determinations were scanned and quantified by densitometric analysis with a chemoluminescence detection device (Odyssey Imaging System, Li-Cor Biosciences).
2.3.4. Antioxidant enzyme activities
Activity of antioxidant enzymes in lung tissue homogenate was measured using the Superoxide Dismutase (SOD) Activity Assay Kit (K335-100, Biovision), OxiSelect Catalase Activity Assay Kit (STA-341, Cell Biolabs Inc.) and the Glutathione Peroxidase Assay Kit (703102, Cayman Chemical Company, Ann Arbor, MI), according to the manufacturers’ guidelines. Sample protein concentration was used for normalization purposes [44].
2.3.5. Oxidative and nitrosative stress markers
The oxidative and nitrosative stress markers 4-HNE and NT, were also measured by Western blot with specific antibodies (anti-4-HNE, Abcam Laboratories ab46545; anti-NT, Millipore 05-233). Furthermore, 8-Isoprostanes concentration in lung homogenates was determined with a specific enzyme immunoassay (EIA) kit following manufacturer recommendations (Cayman Chemical, Ann Arbor, MI) [13] and uric acid was measured using the uric acid assay kit quantichron™ (DIUA-250) (BioAssay Systems, Hayward, CA 94545, USA).
2.3.6. Redox status in the lung
The redox status in lung tissue was assessed by a fluorometric method in order to measure oxidized (GSSG) and reduced glutathione (GSH). Lung tissue was homogenized on ice using a Polytron homogenizer. The solution used for homogenization consisted of 250 mM sucrose, 1 mM EDTA, 10 mM triethanolamine, adjusted to pH 7.8. The total homogenate was centrifuged at 4 °C at 10,000 ×g for 30 min to separate cytosolic fraction and the pellet was resuspended in 0.6 M sorbitol, 50 mM Tris–HCl, pH 7.5 for the preparation of mitochondrial extracts. The o-phthalaldehyde (OPT) was used as a fluorescent reagent. This method assesses the reaction of GSH with OPT at pH 8 and of GSSG with OPT at pH 12; GSH can be complexed to N-ethylmaleimide to prevent interference of GSH with the measurement of GSSG [45]. The GSH⁄GSSG ratio was then calculated for total cytosolic fractions and for isolated mitochondria.
2.3.7. Pulmonary pro-oxidants sources in the lung
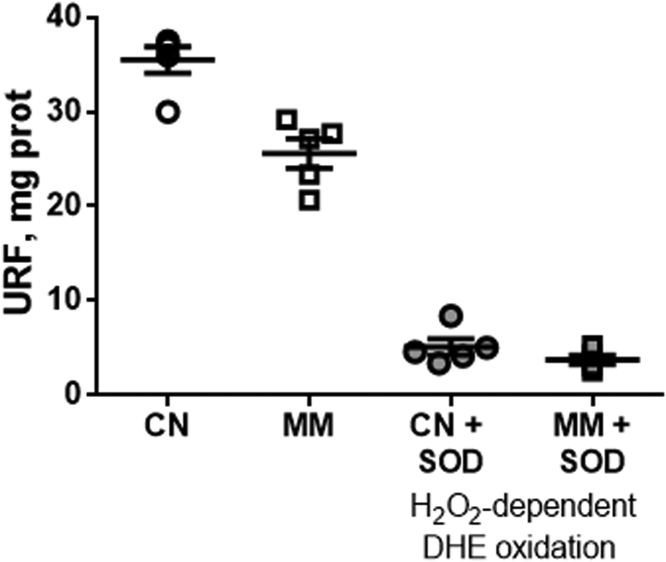
Mitochondrial extract was prepared from pulmonary tissue on ice using chilled buffers. Lung tissue from each sheep was homogenized individually in 25 volumes of a buffer composed of 70 mM sucrose, 210 mM mannitol, 5 mM HEPES, 1 mM EGTA and 0.5% (w/v) fatty acid-free BSA, pH 7.2, at 4 °C using a Teflon dounce homogenizer for 10 strokes. This homogenate was centrifuged at 1000 ×g for 10 min at 4 °C and the supernatant was collected and centrifuged at 12,000 ×g for 10 min. Then the pellet was washed, centrifuged at 10,000 ×g for 10 min and resuspended in a buffer containing 70 mM sucrose, 210 mM mannitol and 5 mM HEPES, pH 7.2, at 4 °C. This preparation was used immediately for assessing the mitochondrial superoxide production. The protein content was measured (Pierce BCA Protein Assay Kit, Thermo Scientific, Rockford, IL USA). Superoxide anion production was assessed by measuring the oxidation of 10 μM Dihydroethdium (DHE; 470Excitation/590Emission) for 10 min at 37 °C with the mitochondria preparation (20–50 μg of protein). Mitochondria was assessed at State 1, a steady rate of respiration at basal condition under substrate starvation. This limits the O2•- production by the addition of exogenous substrate that potentiates the complex activities and promotes their artifactual individual contribution to the mitochondrial oxidative status. In order to evidence the specificity of DHE for O2•-, a control incubating the samples with SOD 100 U/ml for 10 min was also carried out (data not shown) [46]. In the presence of SOD, the DHE oxidation was only 10–15%, indicating that under this experimental condition the oxidation of DHE induced by H2O2 was marginal (raw data of the DHE oxidation is presented in Suplemmentary Fig. 1S). The H2O2-independent DHE oxidation was calculated as URFDHE oxidation - URFDHE oxidation in the presence of SOD.
NADPH oxidase activity was determined based on the rate of NADPH consumption monitored at 340 nm at 37 °C. The assay was performed on a solution containing 50 mM phosphate buffer, pH 7.0, 1 mM EDTA, 150 mM sucrose and tissue homogenate (2–10 μg of protein). The enzymatic reaction was initiated by adding 0.1 mM NADPH. NADPH activity was calculated as μmoles of NADPH oxidized/mg protein/min. Only a slight oxidation of NADPH could be detected in the presence of 100 μM apocynin, an inhibitor of NADPH oxidase (2.35 μmoles NADPH oxidized/mg protein/min) [46].
Uric acid levels in the lung tissue were assessed using the uric acid assay kit from BioAssay Systems (Hayward, CA) as described by the manufacturer. Approximately 5 µl of supernatant were reacted with 200 µl of assay solution for 30 min at room temperature. Absorbance was read at 590 nm. A uric acid standard was prepared, and concentrations were calculated based on this standard [47].
2.4. Statistical analyses
All data were expressed as means ± SEM. The Shapiro-Wilk test was used to assess normality of data and results were compared statistically by a one-way ANOVA on ranks and Dunn´s test, or a Mann-Whitney test, as appropriate. In addition, to assess the association of the antenatal treatment with the neonatal effects, all neonatal variables were correlated with nocturnal maternal levels of melatonin, and analyzed by a Pearson test. Significant differences were accepted when P ≤ 0.05 (Prism 5.0; GraphPad Software).
3. Results
3.1. Maternal and neonatal melatonin in plasma
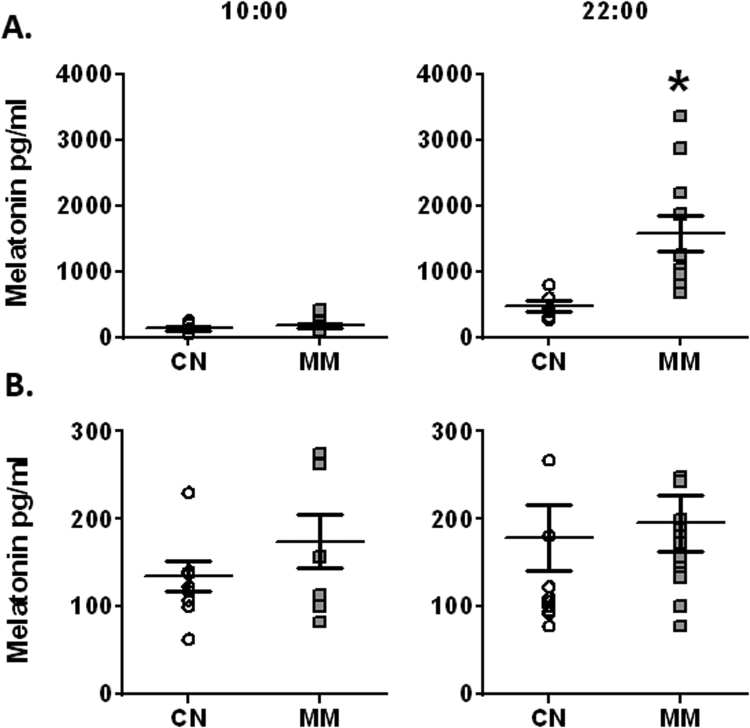
Melatonin plasma levels during the morning were similar between CN and MM groups in pregnant ewes. However, melatonin levels in ewes from the MM group were markedly increased at night-time, relative to CN group (Fig. 1A). Furthermore, melatonin levels of neonates did not change either between day and night, or along postnatal life. Therefore, we plotted the melatonin levels in one graph (Fig. 1B).
Fig. 1.
Melatonin plasma levels in maternal and neonatal sheep. Melatonin treatment was given to the pregnant ewes in the last third of gestation. (A) Maternal samples were obtained at term gestation and (B) neonatal samples were obtained and averaged at 3, 7 and 12 days old. Values are means ± SEM, determined in blood samples taken at 10:00 and 22:00. Groups are control (CN, open circles, n = 6) and antenatal melatonin treated (MM, grey squares, n = 6). Significant differences (P ≤ 0.05): * vs. CN.
3.2. Neonatal birth weight and growth
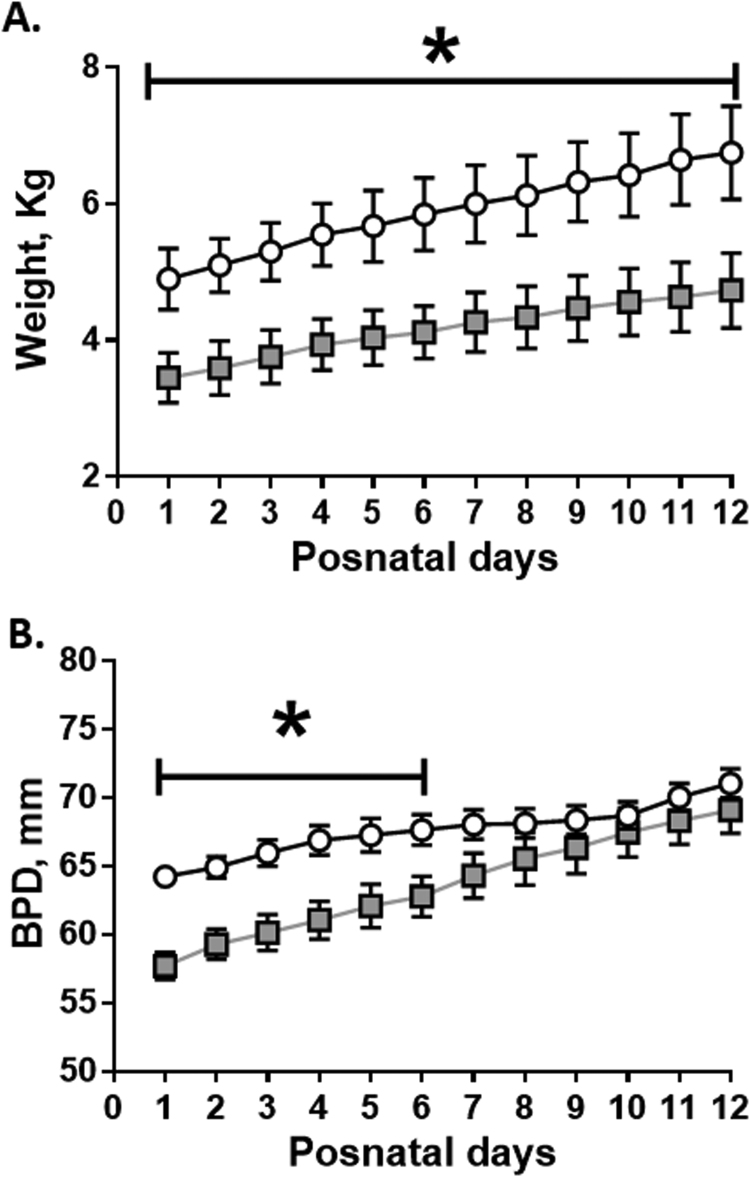
Birth weight was significantly decreased in the melatonin treated group compared to CN (CN: 4.90 ± 0.44 vs MM: 3.45 ± 0.36 kg). Both groups of neonates increased their weight during postnatal life, while this difference was maintained (Fig. 2A).
Fig. 2.
Postnatal biometry in neonatal sheep. Daily measurements for weight (A) and biparietal diameter (DBP, B) during neonatal period. Values are means ± SEM. Groups are control (CN, open circles, n = 6) and antenatal melatonin treated (MM, grey squares, n = 6) lambs. Significant differences (P ≤ 0.05): * vs. CN at equivalent days.
Furthermore, birth biparietal diameter was decreased in MM realtive to CN. However, the intrauterine treated animals caught-up the BPD growth at one week old, compared to CN animals (Fig. 2B).
3.3. Neonatal biometry and cardiopulmonary variables
Mean pulmonary arterial pressure (PAP), cardiac output (CO) and pulmonary artery vascular resistance (PVR) were similar between groups at 3, 7 and 12 days old. In addition, both groups of animals tended to decreased these variables with time, with a significant fall in PAP and CO at 12 days old (Table 1).
Table 1.
Cardiopulmonary variables in neonatal sheep. Values are means ± SEM, for pulmonary arterial pressure (PAP), cardiac output (CO) and pulmonary vascular resistance (PVR) recorded in 3, 7 and 12 days old lambs. Groups are control (CN, n = 6) and antenatal melatonin treated (MM, n = 6). Significant differences (P ≤ 0.05): † vs. Day 3.
|
Day 3 |
Day 7 |
Day 12 |
||||
|---|---|---|---|---|---|---|
| CN | MM | CN | MM | CN | MM | |
| mPAP (mmHg) | 36.1 ± 2.7 | 31.6 ± 2.3 | 31.2 ± 3.6 | 30.2 ± 2.0 | 26.4 ± 1.7† | 24.3 ± 2.6† |
| CO (ml min−1 Kg−1) | 383 ± 39 | 407 ± 26 | 377 ± 32 | 362 ± 20 | 314 ± 19† | 317 ± 15† |
| PVR (mmHg ml−1 min−1 Kg−1) | 0.083 ± 0.009 | 0.095 ± 0.009 | 0.084 ± 0.006 | 0.081 ± 0.008 | 0.082 ± 0.008 | 0.072 ± 0.007 |
3.4. Neonatal antioxidant capacity of plasma
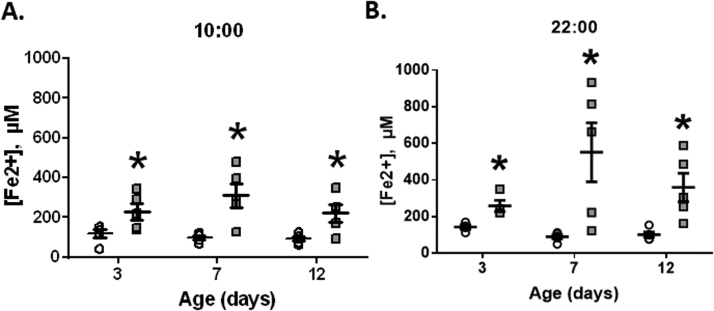
FRAP (Fe2+) levels showed a significant increase in the treated group (MM) relative to the control group, both during day and night-time, along the postnatal period (Fig. 3). The association between neonatal FRAP and nocturnal melatonin levels in the pregnant ewes did not show any significant correlation (data not shown).
Fig. 3.
Ferric Reducing Ability of Plasma (FRAP) in neonatal sheep. Neonatal blood samples for FRAP determination were obtained at 10:00 (A) and 22:00 (B) during days 3, 7 and 12 days of age. Values are means ± SEM. Groups are control (CN, open circles, n = 6) and antenatal melatonin treated (MM, grey squares, n = 6) lambs. Significant differences (P ≤ 0.05): * vs. CN at equivalent days.
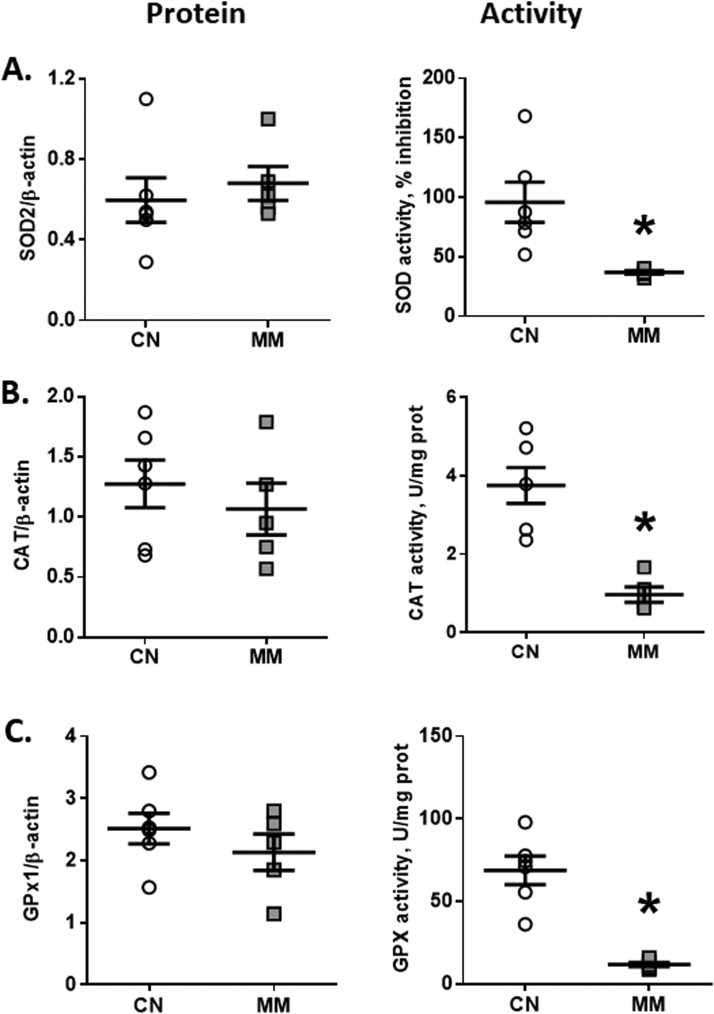
3.5. Antioxidant enzymes function in the lung
The antioxidant enzymes were analyzed for their protein expression and activity in lung tissue. Protein expression of SOD2, CAT and GPX1 were similar between groups (Fig. 4A–C). However, melatonin treatment induced a marked decrease in the activity of SOD, CAT and GPX antioxidant enzymes in lung tissue relative to CN group (Fig. 4A–C). The relationship between neonatal antioxidant enzymes function and nocturnal melatonin levels in the pregnant ewes did not show any significant correlation (data not shown).
Fig. 4.
Enzymatic antioxidant function in neonatal lung. Neonatal lung samples were obtained at 12 days old for protein expression and activity of superoxide dismutase (SOD, A), catalase (CAT, B) and glutathione peroxidase (GPx, C). Values are means ± SEM. Groups are control (CN, open circles, n = 5) and antenatal melatonin treated (MM, grey squares, n = 5) lambs. Significant differences (P ≤ 0.05): * vs. CN.
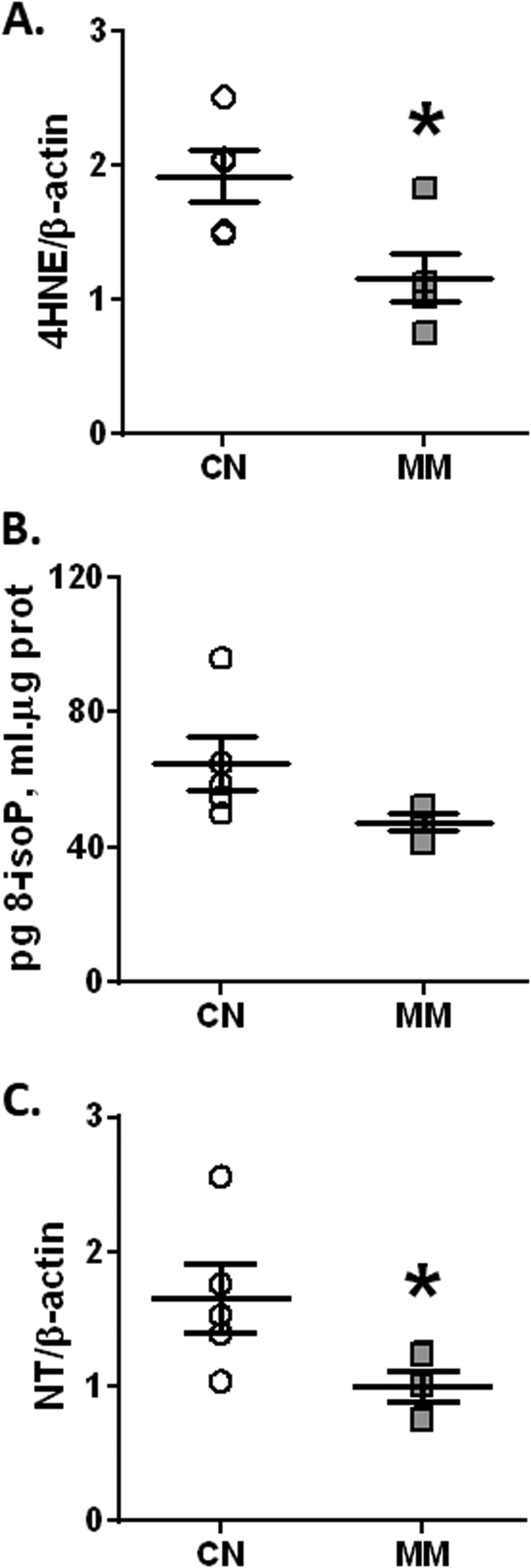
3.6. Pulmonary oxidative stress
MM neonates showed a significant reduction in the oxidative stress marker, 4HNE (Fig. 5A) and the nitrosative stress marker, NT (Fig. 5C) relative to the control group. However, 8-isoprostanes showed similar levels in both groups (P = 0.1094; Fig. 5B). The association between these oxidative stress markers and nocturnal melatonin levels in the pregnant ewes did not show any significant correlation (data not shown).
Fig. 5.
Oxidative stress markers in neonatal lung. Neonatal lung samples were obtained at 12 days old for 4-HNE 70KDa (A), 8-isoprostanes (B) and NT 85KDa (C) determination. Values are means ± SEM. Groups are control (CN, open circles, n = 5) and antenatal melatonin treated (MM, grey squares, n = 5) lambs. Significant differences (P ≤ 0.05): * vs. CN.
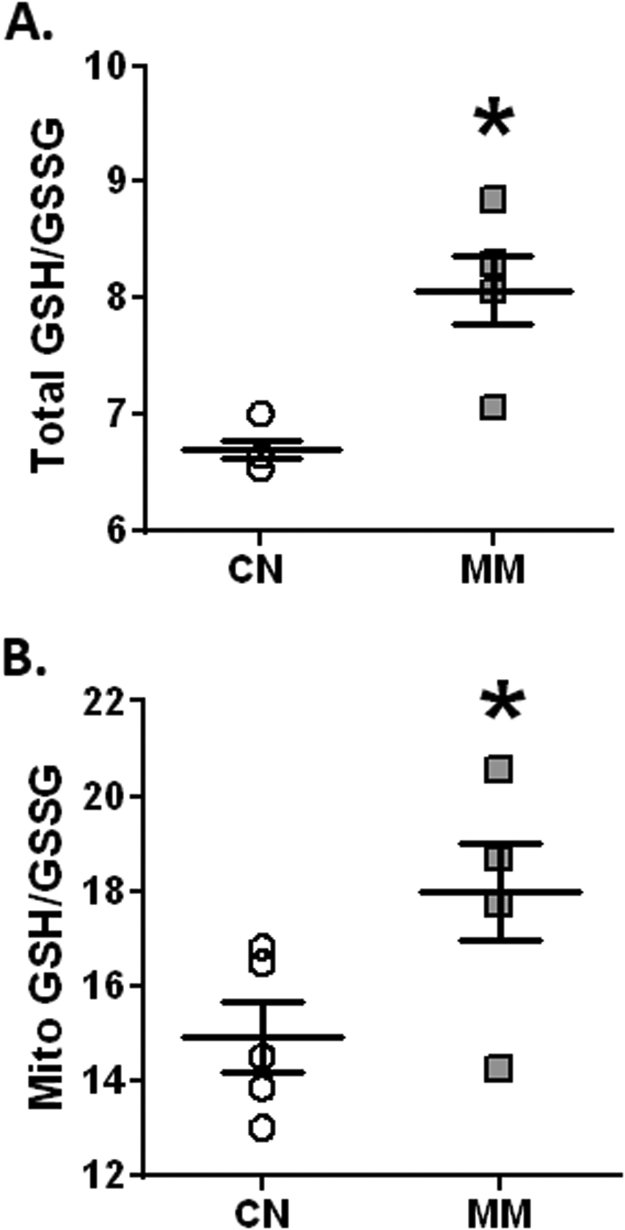
3.7. Pulmonary reduced/oxidized glutathione
Melatonin markedly increased GSH at citoplasmatic (CN, 11.23 ± 2.71 vs. MM, 21.33 ± 3.23 uM) and mitochondrial (CN 33.25 ± 8.83 vs. MM 43.50 ± 7.72 uM) compartments. In addition, melatonin increased GSSG at cytoplasmatic level (CN 1.69 ± 0.08 vs. MM 2.24 ± 0.08 uM) without variations at mithocondrial level (CN 2.18 ± 0.75 vs, MM 2.36 ± 0.95 uM), relative to controls. Therefore, antenatal melatonin induced higher values of pulmonary GSH/GSSG ratio, both at cytoplasmic (Fig. 6A) and mitochondrial (Fig. 6B) compartments. However, the association between pulmonary reduced/oxidized glutathione and maternal nocturnal melatonin levels did not show any significant correlation (data not shown).
Fig. 6.
Reduced/oxidized glutathione in neonatal lung. Neonatal lung samples were obtained at 12 days old for the total (A) and mitochondrial (B) levels of the GSH/GSSG ratio determinations. Values are means ± SEM. Groups are control (CN, open circles, n = 6) and antenatal melatonin treated (MM, grey squares, n = 6) lambs. Significant differences (P ≤ 0.05): *vs. CN.
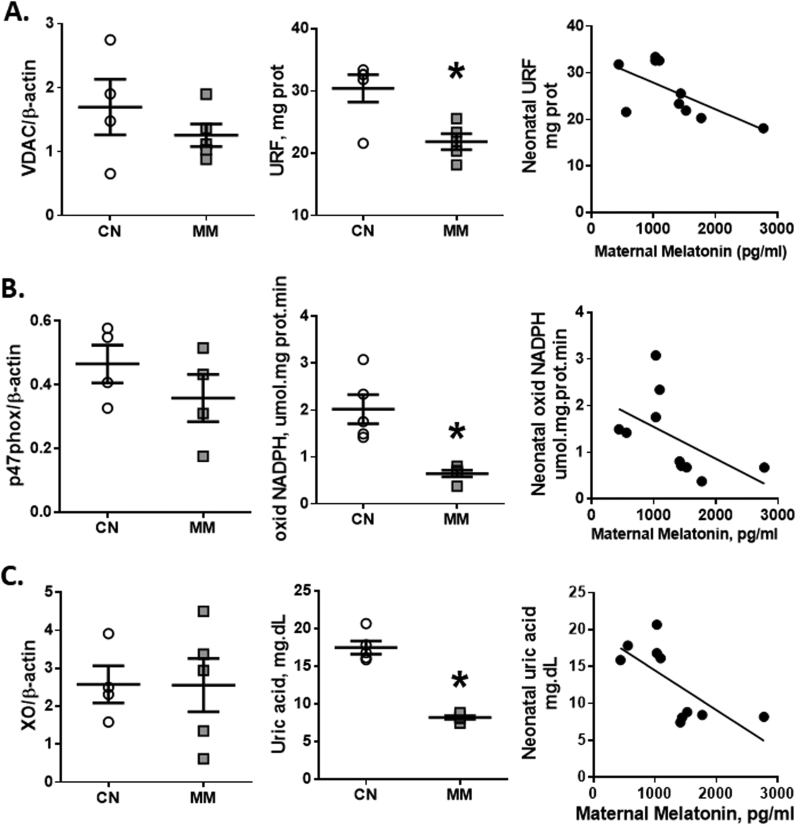
3.8. Pulmonary pro-oxidants sources
The estimated mitochondrial population by the Voltage-dependent anion channels (VDAC) did not change between the analyzed groups. However, the mitochondrial O2•- production from lung tissue (URF) showed a marked decrease in MM relative to CN (Fig. 7A). In addition, the amount of total NADPH quantified as the expression of the p47-phox subunit was similar between groups (Fig. 7B); however the activity of NADPH oxidase was decreased in the MM group when compared to the CN group (Fig. 7B). Finally, the expression of xanthine oxidase did not change between analyzed groups (Fig. 7C) although uric acid, a marker of xanthine oxidase activity, decreased in the lung tissue of the animals treated with melatonin (Fig. 7C). Furthermore, the O2•- generation from the three evaluated sources have a significant inverse correlation with melatonin (Fig. 7).
Fig. 7.
Cellular pro-oxidants sources in neonatal lung. Neonatal lung samples were obtained at 12 days old for the source expression (left column), ROS generation (central column) and the correlation between maternal melatonin and neonatal ROS generation (right column) from the mitochondria (A), NADPH oxidase (B) and xanthine oxidase (C). Values are means ± SEM. Groups are control (CN, open circles, n = 5) and antenatal melatonin treated (MM, grey squares, n = 5) lambs. Significant differences (P ≤ 0.05): *vs. CN.
4. Discussion
The results of this study showed that maternal melatonin administration during the last third of gestation modulates the antioxidant capacity and pro-oxidant sources, leading to decreased oxidative stress in the newborn under chronic hypoxia. However, this treatment enhanced fetal growth restriction and did not have any clinical cardiopulmonary effect on postnatal life. Pregnancy at high altitude is associated with increased oxidative stress in the mother and the placenta, being of utmost importance for intrauterine fetal development, growth and programming [3], [48], [49], [50]. Although we did not measure maternal and fetal arterial PO2 in these animals, we have shown previously that pregnant ewes and the IUGR fetal sheep at 3600 m are markedly hypoxemic relative to lowland animals [4].
The administration regimen of melatonin at 10 mg/day to the pregnant ewe reinforces the plasma antioxidant capacity of newborns and decreased oxidative and nitrosative stress markers, measured even after 12 postnatal days without any treatment. This evidence supports that antenatal administration of melatonin regulates the postnatal REDOX status. In addition, these findings are consistent with the fact that antioxidants during development may program some postnatal cellular functions [49], [50], [51]. The main mechanisms by which melatonin may influence the REDOX status at prenatal and postnatal level, are the reinforcement of the antioxidant defense system [52] or the attenuation of ROS generation at the pro-oxidant sources. In our study, prenatal administration of melatonin did not change the expression of antioxidant enzymes SOD, GSH-Px and CAT, but it decreased the activity of these enzymes relative to the control group. Melatonin has a dual role of regulating nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2) gene induction by acetylation and recruiting the basal transcriptional machinery to the promoter region of Nrf2-related genes, inducing the expression of antioxidant enzymes [52], [53], [54]. This could happen while melatonin was administered, however the oxidative stress detection was carried out at 2 weeks old. Therefore, we speculate that upon reaching the steady state of Nrf2 induction, the proteasomic degradation was enhanced during the postnatal days due to a drop in the pro-oxidant state at the cellular level, thereby decreasing the induction and expression of antioxidant enzymes [55]. Interestingly, melatonin induces the expression of antioxidant enzymes and decreased ROS production in previous studies with postnatal models [13], [56]; however, these enzymes may became inactive due to the pro-oxidant transitory status of the cell [57], [58].
On the other hand, the administration of melatonin achieved an increase GSH/GSSG ratio at cytosolic and mitochondrial levels. Such an effect may be explained by a melatonin-induced expression of the enzymes responsible for glutathione synthesis such as gamma glutamyl transpeptidase (γ-GGT) [59], [60], [61] or in the mechanisms of glutathione recycling by increasing the expression of the enzyme glutathione reductase (GSR) [61]. The latter mechanisms increase reduced glutathione bioavailability, which is responsible for the maintenance of the cellular redox state decreasing oxidative stress.
The most novel finding of this work is the long-lasting regulatory mechanisms exerted by melatonin on the main ROS sources at the pulmonary level involved in the pathophysiology of PHN. These sources are mitochondria, NADPH oxidases and xanthine oxidase [12], which were similar in expression but markedly reduced their activities with the melatonin treatment. Chronic hypoxia determines an incomplete reduction process on the oxygen final acceptor at the mitochondrial electron transport chain [62]. It seems that ubisemiquinone (UQ) could be responsible for ROS generation both at complex I and III level [63]. As well as being able to generate O2•- and H2O2, mitochondria are themselves susceptible to be damaged by these compounds [64], therefore melatonin might be protecting the mitochondria. In fact, melatonin can be selectively taken up by mitochondria and acts as a powerful antioxidant, regulating the mitochondrial bioenergetic function, improving ATP synthesis and decreasing ROS formation [65], [66]. To the best of our knowledge, there are no studies that describe melatonin effects on pulmonary mitochondrial function. However, it has been recently shown that neuronal mitochondria are able to produce melatonin, which upon binding to its melatonin type 1 (MT1) receptor on the mitochondrial membrane inhibits cytochrome c release, caspase activation, and apoptosis [67]. Nevertheless, it seems that this protective effect is tissue specific, as melatonin drives ATP synthesis [65] and increases angiogenesis in gastric endothelial cells [68]. The latter effects of melatonin on mitochondria are not only protective against oxidative stress, but also seems that melatonin is regulating mitochondrial function. In our study the prenatal administration of melatonin effectively modulates the production of ROS by decreasing the amount of superoxide anion generated by the mitochondria, but maintaining the mitochondrial population measured by VDAC expression. The potential underlying mechanism is that melatonin, is able to diffuse towards the mitochondria and reacts with free radicals generated by the decoupling of the respiratory chain in the mitochondrial crest (scavenger) [64]. Furthermore, melatonin may induce at the mitochondrial level the increase of GSH [59], therefore enhancing the mitochondrial antioxidant capacity. These effects were expressed in higher levels of GSH in both cytoplasmatic and mitochondrial samples, respect to control values. More interestingly, is the fact that this effect lasted in the lambs even after being born and raised without postnatal melatonin administration. Therefore, our study clearly supports melatonin as a mitochondria-targeted antioxidant, particularly relevant in diseases related with oxidative stress and mitochondrial dysfunction [69]. Further studies will need to address the mechanisms determining this mitochondrial regulation.
NADPH oxidase (Nox) is present at plasma membranes in endothelial cells, smooth muscle and fibroblasts, where it releases O2•- that serves as a precursor for additional ROS, including hydrogen peroxide, hydroxyl radical, peroxynitrite and other oxidants [70]. This seems to be a main contribution to the pro-oxidant imbalance presented in pulmonary hypertension [17]. Recently, it has been shown that melatonin normalizes the increased Nox activity in the kidney of diabetic rats, accompanied by considerably lowered expression and diminished membrane distribution of the regulatory subunits, phospho-p47(phox) and p67(phox) [71]. Furthermore, it has been postulated that melatonin could impair the assembly of NADPH oxidase, by inhibiting the phosphorylation of the p47phox subunit via a PI3K/Akt-dependent signaling pathway [72]. This blocks the translocation of p47phox and p67phox subunit to the membrane, down-regulates the binding of p47phox to gp91phox, and impairs the assembly of NADPH oxidase [70]. Therefore, melatonin has been shown to decrease NADPH activation and consequently ROS generation. These effects may result in cardioprotection against myocardial injuries induced by chronic hypoxia as shown in rats [73]. Similarly to our study, Yeung and colleagues found not only that Nox activity was decreased, but as well the levels of expression of the antioxidant enzymes SOD and CAT [73].
Furthermore, xanthine oxidase is the enzyme that catalyzes the conversion of hypoxathine to xanthine and xanthine to uric acid with concomitant generation of anion superoxide [74]. Previous studies have shown that the expression of the xanthine oxidase enzyme is regulated by hypoxia, increasing its expression in the lung [75]. Melatonin treated newborns decreased uric acid concentration, suggests a reversal of this effect. As we did not have any difference in XO protein expression, we proposed that melatonin decreased xanthine oxidase activity, which was confirmed in this study. As far as the authors are aware, only one study has seen this effect on rat testis [76], however no mechanism has been proposed by which melatonin can decrease XO activity, even in the long-term. Furthermore, a handful of studies have searched for the XO role during development in cardiovascular function [77], [78], with impact in peripheral and central vascular function. However, further studies need to be done to precisely define the mechanisms determining the long lasting effects of melatonin on decreasing XO activity.
All of the neonatal findings are associated with an antenatal treatment, up to 2 weeks after birth. Therefore, antenatal melatonin must be programming the different determinants of the pulmonary redox status. Several studies have based the developmental programming on epigenetic changes, referring to DNA methylation, histone modification and RNA interference, among others as regulator of gene silencing or expression [79]. For instance, melatonin might inhibit DNA methyltransferases (DNMT) and histone deacetylase (HDAC) [80], [81].
Until now, few studies have proposed the mechanisms under which melatonin may be programming future gene expression and vascular function under development. However, there are reports that maternal melatonin during pregnancy effectively protect the fetal development and birth. For example, in a continuous melatonin deficiency in a pregnant rat model, offspring developed intrauterine growth retardation, which was prevented by maternal melatonin treatment [82]. Similarly, maternal melatonin regulates fetal organogenesis and functions, such as adrenal gland, that are critical for the successful adaptation of the neonate to extra-uterine environment [83]. Furthermore, some studies show that both light/dark environmental cues [84] or melatonin treatment [85] turns on/off microRNA programs that control the REDOX state of cells after many complete proliferative cycles, favoring a longlasting cardioprotective state. In addition, melatonin treatment affects oocytes DNA methylation pattern, reversing the effects of oocyte aging [86]. Thus, although we did not find differential protein expression for the antioxidant enzymes, a recent study in lambs showed that melatonin decreased total DNA methylation of SOD1, GPx4 and CAT, favoring the antioxidant capacity [87]. The melatonin-induced epigenetic regulation must be acting in the pulmonary vasculature of the treated lambs, promoting a balanced REDOX state.
The DOHaD concept offers the potential reprogramming strategy to shift the treatment from adulthood to early life, long before clinical disease appears. In fact, the developmental window of our melatonin intervention was effective for reprogramming the pulmonary redox status towards a diminished pro-oxidant activity.
This study supports our recently published data, where we showed that antenatal melatonin induce a further decrease in birth weight in these high-altitude sheep [42]. A novel study in knock-out mice may partially explain this issue, where a melatonin antenatal treatment was effective in increasing birth weight in IUGR wild-type mice, whereas it did not improve birth weight in nitric oxide synthase knockout (eNOS-/-) and the placental specific Igf2 knockout (P0 + /-) mice. We speculate then that the high-altitude sheep may have an NO or IGF2 impaired expression that prevented the melatonin effects on birth weight. Both of these knockout mice show placental complications, increased oxidative stress and IUGR [88].
In this study, we give further evidence that melatonin might be considered for antenatal treatment in complicated pregnancies as it decreases oxidative stress, programming the future neonatal pulmonary oxidative tone. Consistent with our work, the long-term effects of antenatal melatonin has been recently described as neuroprotective, diminishing brain damage and neurodevelopmental impairments in a preterm and fetal brain injury models, presumably due to a decreased inflammation burst [89], [90].
Chronic non-communicable diseases (NCDs) can originate from perinatal sub-optimal conditions, a concept known as developmental origins of health and disease (DOHaD). Several intrauterine conditions have been shown to program cellular and organ functions that augment the risk of developing NCDs, such as undernutrition, overnutrition, hypoxia and oxidative stress among others. This concept offers the “reprogramming” strategy to shift the treatment from adulthood to early life, before clinical disease is evident [48]. Antenatal melatonin treatment has been proposed as one of the strategies to revert intrauterine complications but as well, to reprogram the developing individual [91], [92]. Melatonin may act on developmental programming by reinforcement of the antioxidant capacity, NO function modulation and reversal of pathologic epigenetic modifications, offering possibilities of correcting gestational malprogramming. However, further research is needed to uncover the involved mechanisms, possible affected signaling pathways and clinical translation.
5. Conclusion
The night-time administration of melatonin during the last third of gestation in high-altitude ewes decreased the main pro-oxidant ROS sources at the cellular level, reducing oxidative stress and reinforcing the antioxidant status at the pulmonary level in newborns with PHN. Although some potential adverse effects of melatonin has been described in this model, we consider that the capacity of antenatal melatonin to modulate postnatal oxidative balance and further pulmonary function merits more research in this area. Our findings add a novel long-lasting ability of melatonin, supporting its use as a therapeutic agent for neonatal diseases that coexist with chronic hypoxia and oxidative stress, as proposed by some researchers.
Acknowledgements
We are very grateful to Mr. Carlos Brito and Mr. Gabino Llusco for their excellent technical assistance. This work was funded by the National Fund for Scientific and Technological Development (FONDECYT) grants no 1110595, 1140647, 11130232, 1151119, and CONICYT/FONDAP #15150012.
Acknowledgments
Declarations of interest
none.
Conflict of interests
None to declare.
Author contributions
AG-C, MV, RLC, CC-P, JCC, GE, RVR, AJLL and EAH conceived and designed the experiments. AG-C, MV, CC-P, RLC and EAH collected, analyzed and interpreted the experimental data. AGC and EAH drafted the article, and all authors revised it critically and approved the final version.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2019.101128.
Appendix A. Supplementary material
Fig. S1.
Hydrogen peroxide dependent DHE oxidation. Samples were preincubated with SOD 100 U/ml for 10 min to evaluate the oxidation of DHE induced by H2O2. Groups are control (CN, open circles, n = 5), control samples preincubate with SOD (CN + SOD, grey circles, N = 5), antenatal melatonin treated (MM, open squares, n = 5), and antenatal melatonin-treated samples with SOD (MM + SOD, grey squares, n = 5).
Fig. S2.
Inmunoblots images. Analyzed immunoblots images for antioxidant proteins (A, Fig. 4), oxidative stress markers (B, Fig. 5) and pro-oxidant sources (C, Fig. 7) in neonatal lung samples.
References
- 1.Moore L.G., Shriver M., Bemis L., Hickler B., Wilson M., Brutsaert T. Maternal adaptation to high-altitude pregnancy: an experiment of nature review. Placenta. 2004;25(Suppl. A):S60–S71. doi: 10.1016/j.placenta.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Herrera E.A., Farías J.G., Ebensperger G., Reyes R.V., Llanos A.J., Castillo R.L. Pharmacological approaches in either intermittent or permanent hypoxia: a tale of two exposures. Pharmacol. Res. 2015;101:94–101. doi: 10.1016/j.phrs.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Herrera E.A., Krause B., Ebensperger G., Reyes R.V., Casanello P., Parra-Cordero M. The placental pursuit for an adequate oxidant balance between the mother and the fetus. Front. Pharmacol. 2014;24(5):149. doi: 10.3389/fphar.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrera E.A., Rojas R.T., Krause B.J., Ebensperger G., Reyes R.V., Giussani D.A. Cardiovascular function in term fetal sheep conceived, gestated and studied in the hypobaric hypoxia of the Andean Altiplano. J. Physiol. 2016;594(5):1231–1245. doi: 10.1113/JP271110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keyes L.E., Armaza J.F., Niermeyer S., Vargas E., Young D.A., Moore L.G. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr. Res. 2003;54:20–25. doi: 10.1203/01.PDR.0000069846.64389.DC. [DOI] [PubMed] [Google Scholar]
- 6.Basnyat B., Murdoch D.R. High-altitude illness. Lancet. 2003;361:1967–1974. doi: 10.1016/S0140-6736(03)13591-X. [DOI] [PubMed] [Google Scholar]
- 7.Peñaloza D., Arias Stella J. The heart and pulmonary circulation at high altitudes: ealthy highlanders and chronic mountain sickness. Circulation. 2007;115(9):1132–1146. doi: 10.1161/CIRCULATIONAHA.106.624544. [DOI] [PubMed] [Google Scholar]
- 8.Lakshminrusimha S., Saugstad O.D. The fetal circulation, pathophysiology of hypoxemic respiratory failure and pulmonary hypertension in neonates, and the role of oxygen therapy. J. Perinatol. 2016;36(Suppl. 2):S3–S11. doi: 10.1038/jp.2016.43. [DOI] [PubMed] [Google Scholar]
- 9.Walsh-Sukys M.C., Tyson J.E., Wright L.L., Bauer C.R., Korones S.B., Stevenson D.K. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105:14–20. doi: 10.1542/peds.105.1.14. [DOI] [PubMed] [Google Scholar]
- 10.Sies H. Oxidative stress: oxidants and antioxidants. Exp. Physiol. 1997;82(2):291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y., Raj J.U. Regulation of the pulmonary circulation in the fetus and newborn. Physiol. Rev. 2010;90(4):1291–1335. doi: 10.1152/physrev.00032.2009. [DOI] [PubMed] [Google Scholar]
- 12.Sylvester J.T., Shimoda L.A., Aaronson P.I., Ward J.P. Hypoxic pulmonary vasoconstriction. Physiol. Rev. 2012;92(1):367–520. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres F., González-Candia A., Montt C., Ebensperger G., Chubretovic M., Serón-Ferré M. Melatonin reduces oxidative stress and improves vascular function in pulmonary hypertensive newborn sheep. J. Pineal Res. 2015;58(3):362–373. doi: 10.1111/jpi.12222. [DOI] [PubMed] [Google Scholar]
- 14.Sommer N., Strielkov L., Pak O., Weissman N. Oxygen sensing and signal transduction in hypoxic pulmonary vasoconstriction. Eur. Respir. J. 2015;47:288–303. doi: 10.1183/13993003.00945-2015. [DOI] [PubMed] [Google Scholar]
- 15.Tabima D.M., Frizzell S., Gladwin M.T. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radic. Biol. Med. 2012;52(9):1970–1986. doi: 10.1016/j.freeradbiomed.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Misra H.P., Fridovich I. The univalent reduction of oxygen by reduced flavins and quinones. J. Biol. Chem. 1972;247:188–192. [PubMed] [Google Scholar]
- 17.Brand M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Munro D., Treberg J.R. A radical shift in perspective: mitochondria as regulators of reactive oxygen species. J. Exp. Biol. 2017;220(Pt 7):1170–1180. doi: 10.1242/jeb.132142. [DOI] [PubMed] [Google Scholar]
- 19.Konior A., Schramm A., Czesnikiewicz-Guzik M., Guzik T.J. NADPH oxidases in vascular pathology. Antioxid. Redox Signal. 2014;20(17):2794–2814. doi: 10.1089/ars.2013.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatterjee A., Black S.M., Catravas J.D. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vasc. Pharmacol. 2008;49:134–140. doi: 10.1016/j.vph.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagano P.J., Clark J.K., Cifuentes-Pagano M.E., Clark S.M., Callis G.M., Quinn M.T. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement byangiotensin II. PNAS. 1997;94:14483–14488. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doehner W., Landmesser U. Xanthine oxidase and uric acid in cardiovascular disease: clinical impact and therapeutic options. Semin. Nephrol. 2011;31(5):433–440. doi: 10.1016/j.semnephrol.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Nishino T., Okamoto K., Eger B.T., Pai E.F. Mammalian xanthine oxidoreductase mechanism of transition from xanthine dehydrogenase to xanthine oxidase. FEBS J. 2008;275:3278–3289. doi: 10.1111/j.1742-4658.2008.06489.x. [DOI] [PubMed] [Google Scholar]
- 24.Cai H., Harrison D.G. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 25.Lüneburg N., Siques P., Brito J., Arriaza K., Pena E., Klose H. Long-term chronic intermittent hypobaric hypoxia in rats causes an imbalance in the asymmetric dimethylarginine/nitric oxide pathway and ROS activity: a possible synergistic mechanism for altitude pulmonary hypertension? Pulm. Med. 2016;2016:6578578. doi: 10.1155/2016/6578578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farías J.G., Herrera E.A., Carrasco-Pozo C., Sotomayor-Zárate R., Cruz G., Morales P. Pharmacological models and approaches for pathophysiological conditions associated with hypoxia and oxidative stress. Pharmacol. Ther. 2016;158:1–23. doi: 10.1016/j.pharmthera.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Miller S.L., Wallace E.M., Walker D.W. Antioxidant therapies: a potential role in perinatal medicine. Neuroendocrinology. 2012;96:13–23. doi: 10.1159/000336378. [DOI] [PubMed] [Google Scholar]
- 28.Hobson S.R., Lim R., Wallace E.M. Phase I pilot clinical trial of antenatal maternally administered melatonin to decrease the level of oxidative stress in human pregnancies affected by preeclampsia. Methods Mol. Biol. 2018;1710:335–345. doi: 10.1007/978-1-4939-7498-6_27. [DOI] [PubMed] [Google Scholar]
- 29.Yiallourou S.R., Wallace E.M., Miller S.L., Horne R.S. Effects of intrauterine growth restriction on sleep and the cardiovascular system: the use of melatonin as a potential therapy? Sleep Med. Rev. 2016;26:64–73. doi: 10.1016/j.smrv.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Aversa S., Pellegrino S., Barberi I., Reiter R.J., Gitto E. Potential utility of melatonin as an antioxidant during pregnancy and in the perinatal period. J. Matern. Fetal Neonatal Med. 2012;25:207–221. doi: 10.3109/14767058.2011.573827. [DOI] [PubMed] [Google Scholar]
- 31.Buonocore G., Groenendaal F. Anti-oxidant strategies. Semin. Fetal Neonatal Med. 2007;12(4):287–295. doi: 10.1016/j.siny.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Gitto E., Marseglia L., Manti S., D'Angelo G., Barberi I., Salpietro C. Protective role of melatonin in neonatal diseases. Oxid. Med. Cell. Longev. 2013:980374. doi: 10.1155/2013/980374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan D.X., Hardeland R., Manchester L.C., Poeggeler B., Lopez-Burillo S., Mayo J.C. Mechanistic and comparative studies of melatonin and classic antioxidants in terms of their interactions with the ABTS cation radical. J. Pineal Res. 2003;34(4):249–259. doi: 10.1034/j.1600-079x.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 34.Galano A., Tan D.X., Reiter R.J. On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J. Pineal Res. 2013;54:245–257. doi: 10.1111/jpi.12010. [DOI] [PubMed] [Google Scholar]
- 35.Reiter R.J., Tan D.X., Mayo J.C., Sainz R.M., Leon J., Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003;50:1129–1146. [PubMed] [Google Scholar]
- 36.Rodriguez C., Mayo J.C., Sainz R.M., Antolín I., Herrera F., Martín V. Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 37.Claypool L.E., Wood R.I., Yellon S.M., Foster D.L. The ontogeny of melatonin secretion in the lamb. Endocrinology. 1989;124:2135–2143. doi: 10.1210/endo-124-5-2135. [DOI] [PubMed] [Google Scholar]
- 38.Serón-Ferré M., Torres-Farfán C., Forcelledo M.L., Valenzuela G.J. The development of circadian rhythms in the fetus and neonate. Semin. Perinatol. 2001;25:363–370. doi: 10.1053/sper.2001.29037. [DOI] [PubMed] [Google Scholar]
- 39.Herrera E.A., Pulgar V.M., Riquelme R.A., Sanhueza E.M., Reyes V.R., Ebensperger G. High altitude chronic hypoxia during gestation and after birth modifies cardiovascular responses in newborn sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292(6):R2234–R2240. doi: 10.1152/ajpregu.00909.2006. [DOI] [PubMed] [Google Scholar]
- 40.Herrera E.A., Krause B.J., Ebensperger G., Riquelme R.A., Reyes V.R., Capetillo M. Sildenafil reverses hypoxic pulmonary hipertensión in highland and lowland newborn sheep. Pediatr. Res. 2008;63(2):169–175. doi: 10.1203/PDR.0b013e31815ef71c. [DOI] [PubMed] [Google Scholar]
- 41.Astorga C.A., González-Candia A., Candia A.A., Figueroa E.G., Cañas D., Ebensperger G. Melatonin decreases pulmonary vascular remodelling and oxygen sensitivity in pulmonary hypertensive newborn lambs. Front. Physiol. 2018;9:185. doi: 10.3389/fphys.2018.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.González-Candia A., Veliz M., Araya C., Quezada S., Ebensperger G., Serón-Ferré M. Potential adverse effects of antenatal melatonin as a treatment for intrauterine growth restriction: findings in pregnant sheep. Am. J. Obstet. Gynecol. 2016;215(2):245. doi: 10.1016/j.ajog.2016.02.040. (e1-7) [DOI] [PubMed] [Google Scholar]
- 43.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 44.Peddireddy V., Siva Prasad B., Gundimeda S.D., Penagaluru P.R., Mundluru H.P. Assessment of 8-oxo-7, 8-dihydro-20 -deoxyguanosine and malondi- aldehyde levels as oxidative stress markers and antioxidant status in non-small cell lung cancer. Biomarkers. 2012;17:261–268. doi: 10.3109/1354750X.2012.664169. [DOI] [PubMed] [Google Scholar]
- 45.Hissin P.J., Hilf R.A. Fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 46.Herrera E.A., Farías J.G., González-Candia A., Short S.E., Carrasco-Pozo C., Castillo R.L. Ω3 supplementation and intermittent hypobaric hypoxia induce cardioprotection enhancing antioxidant mechanisms in adult rats. Mar. Drugs. 2015;13(2):838–860. doi: 10.3390/md13020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whidden M.A., McClung J.M., Falk D.J., Hudson M.B., Smuder A.J., Nelson W.B. Xanthine oxidase contributes to mechanical ventilation-induced diaphragmatic oxidative stress and contractile dysfunction. J. Appl. Physiol. 2009;106:385–394. doi: 10.1152/japplphysiol.91106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanson M.A., Gluckman P.D. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol. Rev. 2014;94(4):1027–1076. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson L.P., Al-Hasan Y. Impact of oxidative stress in fetal programming. J. Pregnancy. 2012:582–748. doi: 10.1155/2012/582748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giussani D.A., Camm E.J., Niu Y., Richter H.G., Blanco C.E., Gottschalk R. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS One. 2012;7(2):e31017. doi: 10.1371/journal.pone.0031017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y.C., Sheen J.M., Tiao M.M., Tain Y.L., Huang L.T. Roles of melatonin in fetal programming in compromised pregnancies. Int. J. Mol. Sci. 2013;14:5380–5401. doi: 10.3390/ijms14035380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Z., Chin Y.E., Zhang D.D. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol. Cell. Biol. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawai Y., Garduño L., Theodore M., Yang J., Arinze I.J. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J. Biol. Chem. 2011;286:7629–7640. doi: 10.1074/jbc.M110.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Korkmaz A., Rosales-Corral S., Reiter R.J. Gene regulation by melatonin linked to epigenetic phenomena. Gene. 2012;503:1–11. doi: 10.1016/j.gene.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 55.Huang Y., Li W., Su Z.Y., Kong A.N. The complexity of the Nrf2 pathway: beyond the antioxidant response. J. Nutr. Biochem. 2015;26(12):1401–1413. doi: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leibowitz A., Volkov A., Voloshin K., Shemesh C., Barshack I., Grossman E. Melatonin prevents kidney injury in a high salt diet-induced hypertension model by decreasing oxidative stress. J. Pineal Res. 2016;60:48–54. doi: 10.1111/jpi.12287. [DOI] [PubMed] [Google Scholar]
- 57.Carrasco-Pozo C., Gotteland M., Castillo R.L., Chen C. 3,4-Dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, protects against pancreatic β-cells dysfunction induced by high cholesterol. Exp. Cell Res. 2015;334(2):270–282. doi: 10.1016/j.yexcr.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 58.Lochner A., Marais E., Huisamen B. Melatonin and cardioprotection against ischaemia/reperfusion injury: what's new? J. Pineal Res. 2018;65(1):e12490. doi: 10.1111/jpi.12490. [DOI] [PubMed] [Google Scholar]
- 59.Urata Y., Honma S., Goto S., Todoroki S., Iida T., Cho S. Melatonin induces gamma-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radic. Biol. Med. 1999;27(7–8):838–847. doi: 10.1016/s0891-5849(99)00131-8. [DOI] [PubMed] [Google Scholar]
- 60.Shih A.Y., Johnson D.A., Wong G., Kraft A.D., Jiang L., Erb H. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J. Neurosci. 2003;23(8):3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Limón-Pacheco J.H., Gonsebatt M.E. The glutathione system and its regulation by neurohormone melatonin in the central nervous system. Cent. Nerv. Syst. Agents Med. Chem. 2010;1(4):287–297. doi: 10.2174/187152410793429683. (10) [DOI] [PubMed] [Google Scholar]
- 62.Zorov D.B., Juhaszova M., Sollott S. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J. Bioenerg. Biomembr. 1999;31:347–366. doi: 10.1023/a:1005427919188. [DOI] [PubMed] [Google Scholar]
- 64.Acuña-Castroviejo D., Martín M., Macías M., Escames G., León J., Khaldy H. Melatonin, mitochondria, and cellular bioenergetics. J. Pineal Res. 2001;30:65–74. doi: 10.1034/j.1600-079x.2001.300201.x. [DOI] [PubMed] [Google Scholar]
- 65.Kleszczyński K., Zillikens D., Fischer T.W. Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (γ-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK) J. Pineal Res. 2016;61(2):187–197. doi: 10.1111/jpi.12338. [DOI] [PubMed] [Google Scholar]
- 66.Sharafati-Chaleshtori R., Shirzad H., Rafieian-Kopaei M., Soltani A. Melatonin and human mitochondrial diseases. J. Res. Med. Sci. 2017;22:2. doi: 10.4103/1735-1995.199092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suofu Y., Li W., Jean-Alphonse F.G., Jia J., Khattar N.K., Li J. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. PNAS USA. 2017;114(38):E7997–E8006. doi: 10.1073/pnas.1705768114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahluwalia A., Brzozowska I.M., Hoa N., Jones M.K., Tarnawski A.S. Melatonin signaling in mitochondria extends beyond neurons and neuroprotection: implications for angiogenesis and cardio/gastroprotection. PNAS USA. 2018;115(9):E1942–E1943. doi: 10.1073/pnas.1722131115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramis M.R., Esteban S., Miralles A., Tan D.X., Reiter R.J. Protective Effects of melatonin and mitochondria-targeted antioxidants against oxidative stress: a review. Curr. Med. Chem. 2015;22(22):2690–2711. doi: 10.2174/0929867322666150619104143. [DOI] [PubMed] [Google Scholar]
- 70.Zhou J., Zhang S., Zhao X. Melatonin impairs NADPH oxidase assembly and decreases superoxide anion production in microglia exposed to amyloid-b1–42. J. Pineal Res. 2008;45:157–165. doi: 10.1111/j.1600-079X.2008.00570.x. [DOI] [PubMed] [Google Scholar]
- 71.Winiarska K., Dzik J.M., Labudda M., Focht D., Sierakowski B., Owczarek A. Melatonin nephroprotective action in Zucker diabetic fatty rats involves its inhibitory effect on NADPH oxidase. J. Pineal Res. 2016;60(1):109–117. doi: 10.1111/jpi.12296. [DOI] [PubMed] [Google Scholar]
- 72.Perisic O., Wilson M.I., Karathanassis D., Bravo J., Pacold M.E., Ellson C.D. The role of phosphoinositides and phosphorylation in regulation of NADPH oxidase. Adv. Enzym. Regul. 2004;44:279–298. doi: 10.1016/j.advenzreg.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 73.Yeung H.M., Hung M.W., Lau C.F., Fung M.L. Cardioprotective effects of melatonin against myocardial injuries induced by chronic intermittent hypoxia in rats. J. Pineal Res. 2015;58(1):12–25. doi: 10.1111/jpi.12190. [DOI] [PubMed] [Google Scholar]
- 74.Hille R., Nishino T. Xanthine oxidase and Xanthine dehydrogenase. FASEB J. 1995;9:995–1003. [PubMed] [Google Scholar]
- 75.Hassoun P.M., Yu F.S., Cote C.G., Zulueta J.J., Sawhney R., Skinner K.A. Upregulation of xanthine oxidase by lipopolysaccharide, interleukin-1 and hypoxia: role in acute lung injury. Am. J. Resp. Crit. Care Med. 1998;158:299–305. doi: 10.1164/ajrccm.158.1.9709116. [DOI] [PubMed] [Google Scholar]
- 76.Sokolovic D., Djordjevic B., Kocic G., Stoimenov T.J., Stanojkovic Z., Sokolovic D.M. The effects of melatonin on oxidative stress parameters and DNA fragmentation in testicular tissue of rats exposed to microwave radiation. Adv. Clin. Exp. Med. 2015;24(3):429–436. doi: 10.17219/acem/43888. [DOI] [PubMed] [Google Scholar]
- 77.Herrera E.A., Kane A.D., Hansell J.A., Thakor A.S., Allison B.J., Niu Y. A role for xanthine oxidase in the control of fetal cardiovascular function in late gestation sheep. J. Physiol. 2012;590(8):1825–1837. doi: 10.1113/jphysiol.2011.224576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kane A.D., Hansell J.A., Herrera E.A., Allison B.J., Niu Y., Brain K.L. Xanthine oxidase and the fetal cardiovascular defence to hypoxia in late gestation ovine pregnancy. J. Physiol. 2014;592(3):475–489. doi: 10.1113/jphysiol.2013.264275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Casanello P., Schneider D., Herrera E.A., Uauy R., Krause B.J. Endothelial heterogeneity in the umbilico-placental unit: dna methylation as an innuendo of epigenetic diversity. Front. Pharmacol. 2014;5:49. doi: 10.3389/fphar.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Korkmaz A., Reiter J. Epigenetic regulation: a new research area for melatonin? J. Pineal Res. 2008;44:41–44. doi: 10.1111/j.1600-079X.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 81.Wu T.H., Kuo H.C., Lin I.C., Chien S.J., Huang L.T., Tain Y.L. Melatonin prevents neonatal dexamethasone induced programmed hypertension: histone deacetylase inhibition. J. Steroid Biochem. Mol. Biol. 2014;144:253–259. doi: 10.1016/j.jsbmb.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 82.Mendez N., Abarzua-Catalan L., Vilches N., Galdames H.A., Spichiger C., Richter H.G. Timed maternal melatonin treatment reverses circadian disruption of the fetal adrenal clock imposed by exposure to constant light. PLoS One. 2012;7(8):e42713. doi: 10.1371/journal.pone.0042713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Torres-Farfan C., Valenzuela F.J., Mondaca M., Valenzuela G.J., Krause B., Herrera E.A. Evidence of a role for melatonin in fetal sheep physiology: direct actions of melatonin on fetal cerebral artery, brown adipose tissue and adrenal gland. J. Physiol. 2008;586(16):4017–4027. doi: 10.1113/jphysiol.2008.154351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marçola M., Lopes-Ramos C.M., Pereira E.P., Cecon E., Fernandes P.A., Tamura E.K. Light/dark environmental cycle imposes a daily profile in the expression of microRNAs in rat CD133(+) cells. J. Cell. Physiol. 2016;231(9):1953–1963. doi: 10.1002/jcp.25300. [DOI] [PubMed] [Google Scholar]
- 85.Ma W., He F., Ding F., Zhang L., Huang Q., Bi C. Pre-treatment with melatonin enhances therapeutic efficacy of cardiac progenitor cells for myocardial infarction. Cell. Physiol. Biochem. 2018;47(3):1287–1298. doi: 10.1159/000490224. [DOI] [PubMed] [Google Scholar]
- 86.Nie J., Xiao P., Wang X., Yang X., Xu H., Lu K. Melatonin prevents deterioration in quality by preserving epigenetic modifications of porcine oocytes after prolonged culture. Aging. 2018;10(12):3897–3909. doi: 10.18632/aging.101680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fang Y., Zhang J., Li Y., Guo X., Li J., Zhong R. Melatonin-induced demethylation of antioxidant genes increases antioxidant capacity through RORα in cumulus cells of prepubertal lambs. Free Radic. Biol. Med. 2018;131:173–183. doi: 10.1016/j.freeradbiomed.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 88.Renshall L.J., Morgan H.L., Moens H., Cansfield D., Finn-Sell S.L., Tropea T. Melatonin increases fetal weight in wild-type mice but not in mouse models of fetal growth restriction. Front. Physiol. 2018;9:1141. doi: 10.3389/fphys.2018.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Castillo-Melendez M., Yawno T., Sutherland A., Jenkin G., Wallace E.M., Miller S.L. Effects of antenatal melatonin treatment on the cerebral vasculature in an ovine model of fetal growth restriction. Dev. Neurosci. 2017;39(1–4):323–337. doi: 10.1159/000471797. [DOI] [PubMed] [Google Scholar]
- 90.Domínguez Rubio A.P., Correa F., Aisemberg J., Dorfman D., Bariani M.V., Rosenstein R.E. Maternal administration of melatonin exerts short- and long-term neuroprotective effects on the offspring from lipopolysaccharide-treated mice. J. Pineal Res. 2017;63(4) doi: 10.1111/jpi.12439. [DOI] [PubMed] [Google Scholar]
- 91.Tain Y.L., Huang L.T., Hsu C.N. Developmental programming of adult disease: Reprogramming by melatonin? Int. J. Mol. Sci. 2017;18(2) doi: 10.3390/ijms18020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Groom K.M., David A.L. The role of aspirin, heparin, and other interventions in the prevention and treatment of fetal growth restriction. Am. J. Obstet. Gynecol. 2018;218(2S):S829–S840. doi: 10.1016/j.ajog.2017.11.565. [DOI] [PubMed] [Google Scholar]