Abstract
Background
Mechanical ventilation is life-saving for patients with acute respiratory insufficiency. In a German prevalence study, 13.6% of patients in intensive care units received mechanical ventilation for more than 12 hours; 20% of these patients received mechanical ventilation as treatment for acute respiratory distress syndrome (ARDS). The new S3 guideline is the first to contain recommendations for the entire process of treatment in these groups of patients (indications, ventilation modes/parameters, accompanying measures, treatments for refractory impairment of gas exchange, weaning, and follow-up care).
Methods
This guideline was developed according to the GRADE methods. Pertinent publications were identified by a systematic search of the literature, the quality of the evidence was evaluated, a risk/benefit assessment was conducted, and recommendations were issued by interdisciplinary consensus.
Results
Mechanical ventilation is recommended as primary treatment for patients with severe ARDS. In other patient groups, non-invasive ventilation can lower mortality. If mechanical ventilation is needed, ventilation modes allowing spontaneous breathing seem beneficial (quality of evidence [QoE]: very low). Protective ventilation (high positive end-expiratory pressure, low tidal volume, limited peak pressure) improve the survival of ARDS patients (QoE: high). If a severe impairment of gas exchange is present, prone positioning lessens mortality (QoE: high). Veno-venous extracorporeal membrane oxygenation (vvECMO) has not unequivocally been shown to improve survival. Early mobilization and weaning protocols can shorten the duration of ventilation (QoE: moderate).
Conclusion
Recommendations for patients undergoing mechanical ventilation include lung-protective ventilation, early spontaneous breathing and mobilization, weaning protocols, and, for those with severe impairment of gas exchange, prone positioning. It is further recommended that patients with ARDS and refractory impairment of gas exchange should be transferred to an ARDS/ECMO center, where extracorporeal methods should be applied only after application of all other therapeutic options.
Mechanical ventilation through an endotracheal tube or tracheostomy is an essential treatment for patients with acute respiratory insufficiency of any cause and is one of the major types of apparatus-assisted treatment in intensive care units. In a German prevalence study, 13.6% of patients in intensive care units received mechanical ventilation for more than 12 hours; 20% of these patients received mechanical ventilation as treatment for acute respiratory distress syndrome (ARDS) (1).
Although many clinical trials of mechanical ventilation have been conducted, there has not yet been any comprehensive clinical practice guideline based on a systematic review and assessment of the pertinent literature.
Mechanically ventilated patients now receive care of variable quality. For example, simple measures that improve survival, such as limiting the tidal volume and peak inspiratory pressure, are clinically implemented in only approximately two-thirds of patients with ARDS (2). At the same time, extracorporeal gas-exchange methods have become easier to use through technical improvements and are now being used more commonly and sometimes indiscriminately, in the absence of adequate scientific evidence for the specific clinical use (3).
The new S3 guideline is a source of evidence-based information and an aid to clinical decision-making on mechanical ventilation and extracorporeal techniques in patients with acute respiratory insufficiency. The text of the guideline is practically organized, along the lines of the course of treatment of such patients in the intensive care unit: the indications for mechanical ventilation and the alternatives to it (if applicable) are discussed first, followed by the choice of a ventilation mode, the setting of ventilation parameters, accompanying measures, the treatment of refractory impairment of gas exchange, weaning off the ventilator, and follow-up care after mechanical ventilation has been discontinued.
The present article summarizes the new S3 guideline and contains its key recommendations. The complete guideline is available for no charge on the web portal of the Association of the Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, AWMF) (www.awmf.org/leitlinien/detail/ll/001–021.html).
Method
The guideline group, which received methodological support from the AWMF, comprised 59 delegates of 21 scientific medical societies from Germany, Austria, and Switzerland, representing all of the medical disciplines and professions that make up the treatment team in an intensive care unit; patient representatives were included as well (ebox). Over the four-year period of guideline development, all group members provided statements of their conflicts of interest, which were then evaluated. Members with relevant conflicts of interest abstained from voting on the corresponding recommendations. The conflicts of interest are described in detail in the guideline report.
eBOX. Composition of the guideline group—collaborators*.
Coordinating group
Dr. med. Falk Fichtner, DGAI
Prof. Dr. med. Onnen Moerer, DGAI
PD Dr. med. Sven Laudi, DGAI
Prof. Dr. med. Steffen Weber-Carstens, DGAI
Prof. Dr. med. Udo Kaisers, DGAI
Author group for guideline texts
Prof. Dr. Michael Adamzik*, DGAI
PD Dr. Andreas Bauer*, DGAI
Prof. Dr. Thomas Bein*, DGAI
Prof. Dr. Christoph Benk*, DGfK
Dr. Dirk Buchwald*, DGfK
Prof. Dr. Iris Chaberny*, DGHM
Prof. Dr. Maria Deja*, DGAI
Dr. Sandra Delis*, DGPalliativmedizin
Prof. Dr. Rolf Dembinski*, DGAI
Rolf Dubb*, DGF
Prof. Dr. Björn Ellger*, DGAI
Dr. Falk Fichtner, DGAI
Prof. Dr. Helmut Frohnhofen*, DGG
Prof. Dr. Marcelo Gama de Abreu*, DGAI
Prof. Dr. Christoph Haberthür*, SGI
Prof. Dr. Marcus Hennersdorf*, DGK
Prof. Dr. Uwe Janssens*, DGK
Prof. Dr. Udo Kaisers, DGAI
Prof. Dr. Christian Karagiannidis*, DGP
Prof. Dr. Stefan Klotz*, DGTHG
Prof. Dr. Stefan Kluge*, DGIIN
PD Dr. Sven Laudi, DGAI
Prof. Dr. Klaus Markstaller*, ÖGARI
Prof. Dr. Frauke Mattner*, DGHM
Prof. Dr. Onnen Moerer, DGAI
Prof. Dr. Ralf Muellenbach*, DGAI
Dr. Anika Müller*, DGAI
PD Dr. Thomas Müller*, DGP
Prof. Dr. Wolfgang Müllges*, DGN und DGNI
Prof. Dr. Peter Neumann*, DGAI
Dr. Jan-Oliver Neumann*, DGNC
Prof. Dr. Thomas Nicolai*, DGKJ und DGNI
Prof. Dr. Christian Putensen*, DGAI
Prof. Dr. Michael Quintel*, DGAI
Prof. Dr. Maximilian Ragaller*, DGAI
Prof. Dr. Rolf Rossaint*, DGAI
Dr. med. Dirk Schädler*, DGAI
PD Dr. Thomas Schaible*, DGNI und DGKJ
Prof. Dr. Bernd Schönhofer*, DIVI
Dr. Dierk Schreiter*, DGC
Dr. Christian Seeber*, DGAI
Prof. Dr. Steffen Weber-Carstens, DGAI
Dr. Björn Weiss*, DGAI
Prof. Dr. Hermann Wrigge*, DGAI
Further members of the guideline group
Enrico Bock, DGF
Prof. Dr. Udo Boeken*, DGTHG
Uta Brückner*, ZVK
Ron Fantl*, DGP
Prof. Dr. Marius Hoeper*, DGP
Prof. Dr. Konstantin Mayer*, DGIIN
Prof. Dr. Erich Kilger*, DGAI
PD Dr. Ludwig Ney*, DGAI
Prof. Dr. Michael Pfeifer, DGIM
Dr. Simone Rosseau*, DGIIN
Reina Tholen*, ZVK
Dorothea Stanic*, Sepsishilfe e.V.
Prof. Dr. Roman Ullrich*, ÖGARI
Monika Veit*, BdO
Wolfgang Veit*, BdO
Prof. Dr. Norbert Weiler* DGAI
Prof. Dr. Karl Werdan*, DGK
Methodological support
Dr. Monika Nothacker, AWMF: advice and support of the entire project
PD Dr. Helmuth Sitter*, AWMF: conductance and moderation of the 3rd consensus conference
Outside experts for the literature search and evaluation
Dr. Christiane Hofmann*, Universitätsbibliothek Leipzig
Dr. Astrid Vieler*, Universitätsbibliothek Leipzig
Dr. Annegret Franke*, Zentrum für Klinische Studien Leipzig
Participating scientific medical societies
Main issuing society:
German Society for Anesthesiology and Intensive Care Medicine
Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin (DGAI)
Participating scientific medical societies and organizations:
German Interdisciplinary Association for Intensive Care and Emergency Medicine
Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin (DIVI)
Swiss Society for Intensive Care Medicine
Schweizerische Gesellschaft für Intensivmedizin (SGI)
Austrian Society for Anesthesiology, Resuscitation, and Intensive Care Medicine
Österreichische Gesellschaft für Anästhesiologie, Reanimation und Intensivmedizin (ÖGARI)
German Society for Internal Medicine
Deutsche Gesellschaft für Innere Medizin (DGIM)
German Society for Pulmonology and Respiratory Medicine
Deutsche Gesellschaft für Pneumologie und Beatmungsmedizin (DGP)
German Society for Cardiology and Cardiovascular Research
Deutsche Gesellschaft für Kardiologie – Herz- und Kreislaufforschung (DGK)
German Society for Intensive Care Medicine and Emergency Medicine in Internal Medicine
Deutsche Gesellschaft für Internistische Intensivmedizin und Notfallmedizin (DGIIN)
German Surgical Society
Deutsche Gesellschaft für Chirurgie (DGCH)
German Society for Thoracic, Cardiac, and Vascular Surgery
Deutsche Gesellschaft für Thorax-, Herz- und Gefäßchirurgie (DGTHG)
German Society for Neurosurgery
Deutsche Gesellschaft für Neurochirurgie (DGNC)
German Neurological Society
Deutsche Gesellschaft für Neurologie (DGN)
German Society for Neurointensive Care and Emergency Medicine
Deutsche Gesellschaft für Neurointensiv- und Notfallmedizin (DGNI)
German Society for Pediatrics and Adolescent Medicine
Deutsche Gesellschaft für Kinder- und Jugendmedizin (DGKJ)
German Society for Neonatology and Pediatric Intensive Care Medicine
Deutsche Gesellschaft für Neonatologie und pädiatrische Intensivmedizin (DGNPI)
German Geriatric Society
Deutsche Gesellschaft für Geriatrie (DGG)
German Society for Palliative Care
Deutsche Gesellschaft für Palliativmedizin (DGP)
German Society for Hygiene and Microbiology
Deutsche Gesellschaft für Hygiene und Mikrobiologie (DGHM)
German Society for Specialized Nursing and Auxiliary Services
Deutsche Gesellschaft für Fachkrankenpflege und Funktionsdienste (DGF)
German Physiotherapy Association—Central Association of Physiotherapists
Deutscher Verband für Physiotherapie - Zentralverband der Physiotherapeuten/Krankengymnasten (ZVK)
German Society for Cardiotechnology
Deutsche Gesellschaft für Kardiotechnik (DGfK)
German Sepsis Aid
Deutsche Sepsis-Hilfe
German National Association of Organ Transplant Recipients
Bundesverband der Organtransplantierten (BDO)
Methodological support
Association of the German Medical Scientific Societies
Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF)
The guideline was developed according to the GRADE methods (Grading of Recommendations, Assessment, Development and Evaluation) (4).
The systematic literature search was carried out in PubMed, Embase, Cochrane, and international guideline databases (publication dates: any time up to December 2014 for the main search, any time up to June 2016 for the search update [only meta-analyses and randomized, controlled trials]).
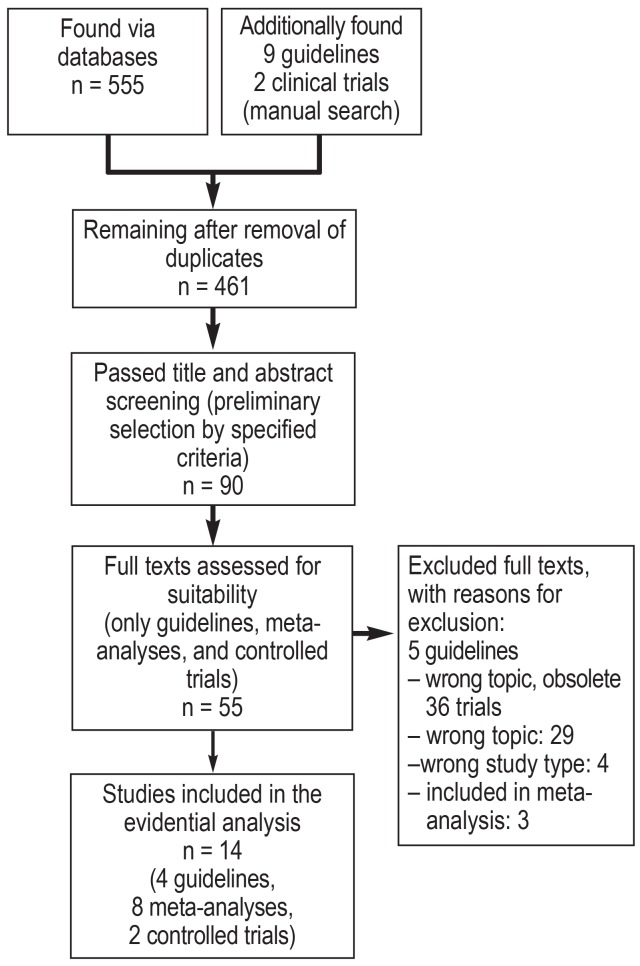
Publications were selected by topic according to uniform criteria, the selected studies were sorted by type, and a full-text database encompassing approximately 3500 studies was created (as per the PRISMA scheme, cf. eFigure).
eFigure.
Flowchart of the literature search and selection for the creation of the S3 guideline on mechanical ventilation and extracorporeal techniques in the treatment of acute respiratory insufficiency—example: Chapter 4.1 (PEEP).
In the literature assessment, national and international guidelines were examined, and relevant content of high-quality guidelines was adopted. Next, current meta-analyses were analyzed and evaluated. In the absence of evaluable meta-analyses, randomized and controlled trials (RCT) and studies that provided evidence of lesser quality were analyzed, evaluated, and summarized in evidence tables. Finally, the literature on each question was summarized using evidence profiles and then qualitatively assessed (for the five categories of quality of evidence [QoE], see eTable 1) (5).
eTable 1. The assessment of evidence quality and the determination of recommendation strengths according to GRADE (5, 6).
| Evidence quality (EQ) | |
| Symbol | Assessment |
| ++++ |
High-quality evidence Description: “[…] Further research is very unlikely to change our confidence in the estimate of effect […]” |
| +++ |
Moderate-quality evidence Description: “[…] Higher-quality research may well have an important impact on our confidence in the estimate of effect and may change the estimate […]” |
| ++ |
Low-quality evidence Description: “[…] Further research is likely to have an important impact on our confidence in the estimate of effect and may well change the estimate […]” |
| + |
Very low-quality evidence Description: “[…] The observed effect is fraught with great uncertainty […]” |
| Expert consensus | No relevant evidence |
| Recommendation strength | |
| Formulation | Assessment |
| X is recommended X is not recommended |
Strong recommendation for/against Description: “[…] strong confidence in the relation between desired and undesired effects or strong predominance of desired or undesired effects […]” |
| It is suggested that X It is suggested that not-X |
Weak recommendation for/against Description: “[…] weak confidence in the relation between desired and undesired effects or weak predominance of desired or undesired effects […]” |
| No recommendation for or against X can be given |
No recommendation Description: “[…] no confidence in the relation between desired and undesired effects or no predominance of desired or undesired effects […]” |
GRADE, Grading of Recommendations, Assessment, Development and Evaluation
The recommendations found in the guideline are thus based on information from a total of 297 evaluated publications.
On the basis of this evidence, the benefits and risks of each therapeutic measure were evaluated and the content and strength of the relevant recommendations were categorized into 3 strengths of evidence (etable 1) (6). Clinical experience, patient preferences, and an estimate of the necessary resources were also taken into account. The strength of each recommendation therefore does not always directly reflect the previously determined quality of the underlying evidence.
The recommendations were voted on within the guideline group individually and in a two-step process by each participating scientific medical society.
In the following paragraphs we present key recommendations that the guideline group considers especially important in terms of achieving a clinical benefit for patients, or avoiding harm to them. We also present recommendations that deviate from the current clinical practice or that address topics that remain controversial.
The definition of acute respiratory insufficiency
Acute respiratory insufficiency has no uniform definition. In awake patients, its leading symptom is dyspnea; impaired consciousness is a further important clinical sign. Blood-gas analysis enables the differentiation of hypoxemia, hypercapnia, and mixed forms, but uniform threshold values and definitions are lacking.
In routine clinical practice, patients are mechanically ventilated when the treating team judges that acute hypoxemic/hypercapnic respiratory insufficiency is present. It is, therefore, suggested that the guideline should be applied, independently of the imprecise definition of acute respiratory insufficiency, whenever the treating team considers a patient to need mechanical ventilation or is contemplating the initiation of an extracorporeal technique.
For the disease entity ARDS, the present guideline follows the Berlin definition (etable 2) (7).
eTable 2. Definition of the acute respiratory distress syndrome (Berlin Definition, 2012) (7).
| Timing | Within one week of a known clinical insult or new or worsening respiratory symptoms |
|
Chest imaging – chest x-ray or chest CT |
Bilateral opacities, not fully explained by effusions, lobar/lung collapse, or nodules |
| Origin of edema | Respiratory failure not fully explained by cardiac failure or fluid overload; need objective assessment (e.g., echocardiography) to exclude hydrostatic edema if no risk factor present |
|
Oxygenation disturbance – at altitudes above 1000m, the correction factor should be calculated as follows: Pa O2 /(FiO2 × barometric pressure [mm Hg]/760) |
Mild: 200 mmHg <paO2/FiO2 ≤ 300 mmHg and PEEP/CPAP ≥ 5 cmH2O* |
| Moderate: 100 mmHg <PaO2/FiO2 ≤ 200 mmHg and PEEP ≥ 5 cmH2O | |
| Severe: PaO2/FiO2 ≤ 100 mmHg und PEEP ≥ 5 cmH2O |
* PEEP/CPAP for mild ARDS also as non-invasive ventilation
ARDS: acute respiratory distress syndrome; Fi O2 : inspiratory oxygen concentration; Pa O2 : arterial partial pressure of oxygen;
PEEP/CPAP: positive end-expiratory pressure / continuous positive airway pressure
Indications for mechanical ventilation
It is recommended that patients with severe ARDS should be treated primarily with invasive mechanical ventilation (expert consensus, weak recommendation). For patients with ARDS, benefits of non-invasive ventilation have not been definitively demonstrated, but harm may be potentially induced (delayed emergency intubation with the risk of hypoxemia).
A trial of non-invasive ventilation is, however, recommended for all other groups of patients with acute respiratory insufficiency (QoE + to ++++; strong/weak recommendations).
In palliative care, the alleviation of dyspnea is a central objective (8). It is recommended that the treating team should ascertain early in the patient’s course whether non-invasive or invasive mechanical ventilation would be consistent with the patient’s wishes (expert consensus, strong recommendation).
The choice of ventilation mode
Many different ventilation modes are available (table), but only a few of them are regularly used in clinical practice. In controlled ventilation, a ventilator performs all of the work of breathing. In assisted ventilation, by definition, the ventilator performs only part of the work; spontaneous breathing is enabled and supported.
Table. Overview of invasive ventilation modes.
| Mode | |||
| Subgroup | Mode | Abbreviation | Control mechanism |
| Controlled ventilation modes | |||
| Volume-controlled ventilation | VCV | Set-point targeting: application of volume-controlled ventilation on the basis of set target variables |
|
| Pressure-controlled ventilation | PCV | Set-point targeting: application of pressure-controlled ventilation on the basis of set target variables |
|
| Pressure-regulated volume control | PRVC | Set-point/adaptive: volume-controlled ventilation with adaptive pressure changes | |
| Ventilation modes that support spontaneous breathing | |||
| Tidal-volume- supporting | Assist-control ventilation | A/C VC-CMVs |
Set-point targeting: application of (patient-triggered) volume-controlled ventilation on the basis of set target variables |
| Pressure-support ventilation, assisted spontaneous breathing, ASB |
PSV | Set-point targeting: spontaneous breathing is assisted by a set amount of pressure support |
|
| Variable pressure support | noisy PSV | Set-point targeting: automatic variation of the level of pressure support |
|
| Minute-volume- supporting | Volume-controlled synchronized intermittent mandatory ventilation | VC-SIMV | Automatic adaptation of peak inspiratory pressure to the attainment of a target tidal volume |
| Presssure-controlled ventilation enabling spontaneous breathing in inspiration and expiration, e.g., biphasic positive airway pressure (BIPAP), airway pressure release ventilation (APRV); synonyms, DuoPAP, BI-LEVEL, BI-VENT, etc. | APRV BIPAP |
Set-point targeting: time-regulated, pressure-controlled ventilation enabling spontaneous breathing |
|
| Pressure-controlled synchronized intermittent mandatory ventilation | PC-SIMV | Automatic adaptation of the tidal volume to the attainment of a target airway pressure | |
| Adaptive | Adaptive support ventilation | ASV | Adaptive targeting or optimal targeting: variable pressure-controlled or pressure-supported ventilation depending on pulmonary mechanics and the work of breathing |
| Intellivent-ASV | Intellivent-ASV | Adaptive targeting or intelligent targeting: combination of ASV with additional treatment approaches |
|
| Neurally adjusted ventilatory assist | NAVA | Adaptive targeting or servo-targeting: applied ventilation pressure proportional to respiratory effort, measured by the electrical activation of the diaphragm |
|
| SmartCare/PS | SmartCare/PS | Automatic adaptation of pressure support with the goal of keeping the patient within a target comfort zone | |
| Proportional assist ventilation and proportional assist ventilation plus, also known as proportional pressure support, PPS |
PAV and PAV+ | Automatic adaptation of the level of support and the performance of the ventilator according to demand or respiratory effort | |
| Hybrid ventilation modes | |||
| Intermittent mandatory ventilation with pressure-support ventilation (IMV+PSV), intermittent mandatory ventilation with automatic tube compensation (IMV+ATC), biphasic positive airway pressure with pressure-support ventilation (BIPAP+PSV), biphasic positive airway pressure with automatic tube compensation (BIPAP+ATC), pressure-support ventilation with automatic tube compensation (PSV+ATC) and proportional assist ventilation with automatic tube compensation (PAV+ATC) | |||
| Special ventilation modes | |||
| High-frequency oscillation ventilation | HFOV | High-frequency, constant-volume maintenance of a continually high airway pressure | |
When choosing a ventilation mode, one must first ascertain whether spontaneous breathing can be enabled. Excessive sedation is associated with higher long-term mortality (9). In general, therefore, the target of sedation is a patient who is as awake as possible, with intact spontaneous breathing (10). It is recommended that an assisted ventilation mode be initiated early on, in order to enable spontaneous breathing (QoE +; weak recommendation).
On the other hand, for patients with severe ARDS, a single multicenter RCT showed that 28-day mortality was significantly lowered by muscle relaxation to eliminate spontaneous breathing (cis-atracurium 23.7% vs. control 33.3%) (11). This study was of limited methodological quality, however, and there are risks associated with oversedation and prolonged diaphragmatic inactivity. Therefore, no recommendation can now be given either for or against the enabling of spontaneous breathing in the first 48 hours in patients with severe ARDS (QoE ++; no recommendation grade).
Because of the low quantity and quality of the published research findings, the guideline group could not identify any particular ventilation mode that was more beneficial than others. Nonetheless, to avoid harming patients, it is recommended that high-frequency oscillation ventilation (HFOV) should not be used to treat adult patients with ARDS (QoE ++++; strong recommendation), because the most recent meta-analysis (12) showed no advantage for survival, while a recent large-scale RCT (13) showed a significant rise in in-hospital mortality among patients treated with HFOV compared to conventional protective ventilation (HFOV 47%, control 35%).
Ventilation parameter settings
Positive end-expiratory pressure (PEEP)
PEEP is intended to counteract a decline in functional residual capacity. Its potential major side effects include overexpansion of ventilated portions of the lung, diminished cardiac output, and elevated intracranial pressure.
Two meta-analyses, each of which was based on three multicenter RCTs, showed that ventilation with high PEEP lowers the mortality of ARDS patients, compared to ventilation with low PEEP or conventional ventilation (for mortality in the intensive care unit, 37.6% vs. 56.3%; for in-hospital mortality, 34.1% vs. 39.1% [14, 15]). Thus, in patients with ARDS, ventilation with high PEEP is recommended (QoE ++++; strong recommendation).
The use of ventilatory protocol cards of the ARDS Network (www.ardsnet.org/tools) to determine the PEEP setting is both very well-documented and easily implementable, but these tables take no account of individual respiratory mechanics (QoE ++; weak recommendation).
Inspiratory oxygen concentration (FiO2)
In observational studies on selected groups of patients, hyperoxia was found to be associated with increased mortality (16). At the same time, restrictive FiO2 settings in mechanically ventilated patients were not found to be associated with elevated mortality or organ failure rates (17). It is, therefore, recommended that mechanically ventilated patients should be treated with the lowest possible FiO2 with which a target arterial oxygen saturation (SaO2) of 90–94% or an arterial partial pressure of oxygen (PaO2) of 60–80 mmHg (8.0–10.7 kPa) can be attained (expert consensus, weak recommendation).
Tidal volume
High tidal volumes (Vt) can cause additional ventilator-associated lung damage through overexpansion.
It was found in two meta-analyses (18, 19) that low tidal volumes lower the mortality of ARDS patients (reduction of in-hospital mortality from 43.2% to 34.5% [19]). It is, therefore, recommended that ARDS patients should be ventilated with a Vtnot exceeding 6 mL/kg standard body weight (BW) (QoE +++; strong recommendation).
Likewise, for mechanically ventilated patients without ARDS, meta-analyses have shown positive effects on critical outcome variables (meta-analysis [20]: reduction of postoperative complications from 14.7% [Vt> 8 mL/kg standard BW] to 8.7% [Vt < 8 mL/kg standard BW]).
Ventilation with low tidal volumes is, therefore, recommended for patients without ARDS (Vtin the range of 6–8 mL/kg standard BW) (QoE +++; strong recommendation).
Peak inspiratory pressure
The limitation of peak inspiratory pressure to prevent barotrauma is a component of a lung-protective ventilation strategy. In a meta-analysis (19), reduced mortality was found only in three trials in which the peak inspiratory pressure was greater than 31 cm H2O in the control group (mortality 31.9% with protective ventilation versus 42.6% in the control group). The peak inspiratory pressure for patients with ARDS should therefore not exceed 30 cm H2O (QoE +++; strong recommendation).
Accompanying measures
Sedation, analgesia, management of delirium
The recommendations of the German S3 guideline on analgesia, sedation, and delirium management in intensive-care medicine (10) were adopted. The strong recommendations for the use of the Richmond Agitation-Sedation Scale (RASS) with a target value of 0 to -1 in intensive-care patients for whom mild sedation is not contraindicated, and for individually adapted pain management, are of particular importance when a patient is being weaned off mechanical ventilation.
Early mobilization
The recommendations of the S2e guideline on positioning therapy and early mobilization for the prevention or treatment of pulmonary dysfunction (21) were adopted, with additional consideration of more recent study findings: early mobilization (= 72 hours after admission to the intensive care unit) is considered to be a safe measure contributing to an improved outcome (shorter ICU and hospital stays) and is therefore strongly recommended, unless contraindicated.
Stress-ulcer prophylaxis
In 2013, the German Commission for Hospital Hygiene and Infection Prevention (Kommission für Krankenhaushygiene und Infektionsprävention, KRINKO) recommended that alkalizing drugs to prevent stress ulcers should not be given to patients receiving enteral nutrition, and that an individual decision should be made on this matter for critically ill patients receiving parenteral nutrition (22). In a recent meta-analysis, a subgroup analysis of appropriately randomized studies revealed no relevant effects on the rates of pneumonia and clinically relevant gastrointestinal hemorrhage. It is, therefore, recommended that mechanically ventilated patients should not routinely be given H2-blockers or proton-pump inhibitors for stress-ulcer prophylaxis (QoE ++; strong recommendation).
Tracheostomy
In the absence of high-quality clinical trials, the guideline group adopted the recommendation formulated by expert consensus in multiple international guidelines (23– 25) that the need for a tracheostomy should always be evaluated individually in each patient.
The timing of tracheostomy has been the subject of many randomized, controlled trials; two recent meta-analyses yielded contradictory findings (26, 27). The subgroup analysis on 1-year mortality revealed no significant effect of early tracheostomy (i.e., within one week of intubation; meta-analysis, 788 patients, early tracheostomy 49.7% versus late tracheostomy 53.4 %). Nor was there any demonstrable reduction of the rate of ventilator-associated pneumonia (27). On the other hand, the danger of unnecessary tracheostomy is well-documented (meta-analysis, 2689 patients, rate of tracheostomy actually performed: early tracheostomy group, 86.7%, versus late tracheostomy group, 53.4%) (26). These data provide the basis for a recommendation against early tracheostomy in mechanically ventilated patients (QoE +++; strong recommendation).
The treatment of severe or refractory impairment of gas exchange
Recruitment maneuvers
In a meta-analysis on the use of recruitment maneuvers (RM), i.e., ventilation maneuvers in which the peak inspiratory pressure is raised in order to reopen atelectatic areas of the lungs, in-hospital mortality was found to be lower in the treatment group than in the control group (36% versus 32%, relative risk [RR] 0.84, 95% confidence interval [0.74; 0.95]). However, in many of the trials underlying this meta-analysis, RM was but one component of a bundle of therapeutic measures that were provided, and there was therefore a high risk of bias (28). In the trials with low bias, no mortality-lowering effect was seen. Therefore, it was decided not to make any recommendation in the present guideline either for against the performance of recruitment maneuvers in patients with ARDS, despite the presence of such a recommendation in the international sepsis guideline (29).
Prone positioning
The recommendations of the S2e guideline on positioning therapy (21) were examined and compared with the current literature. Prone positioning, when initiated soon after the diagnosis of severe acute pulmonary failure (here: PaO2/FiO2<150 mmHg) and continued for at least 16 hours, is associated with reduced long-term mortality (meta-analysis, 977 patients in subgroup with PaO2/FiO2<150 mmHg: long-term mortality reduced from 54.7% to 41.5%, RR 0.77 [0.65; 0.92]) (30). The guideline group therefore adopted the strong recommendation of the S2e guideline that ARDS patients with a severely impaired oxygenation (here: PaO2/FiO2<150 mm Hg) should be intermittently positioned prone.
Inhaled nitric oxide (iNO) therapy
A pertinent meta-analysis showed no reduction of mortality by iNO therapy in ARDS patients (31); at the same time, an elevated rate of acute renal failure was seen (meta-analysis, 919 patients in the ARDS subgroup, renal failure rates 19% in the iNO group vs. 12.4% in the control group, RR 1.55 [1.15; 2.09]) (32). The routine use of iNO therapy is, therefore, not recommended for patients with ARDS (QoE +++; strong recommendation).
Extracorporeal gas-exchange techniques
No prospective, randomized, controlled trials have yet clearly demonstrated the mortality benefit of extracorporeal gas-exchange techniques for patients with ARDS. On the basis of expert consensus, the use of veno-venous ECMO is recommended only as a rescue measure for patients with severe ARDS and refractory hypoxemia, after all other treatment options have been exhausted (expert consensus, strong recommendation).
ECMO carries a high risk of severe complications and requires a complex, multidisciplinary care structure. It is, therefore, strongly recommended that patients with severe ARDS and refractory hypoxemia should be cared for in a center where ECMO is available and is, as a rule, performed in at least 20 patients per year (expert consensus, strong recommendation).
Purely extracorporeal CO2 elimination with low-flow systems has not been shown to date to have any effect on survival or on the duration of ICU stays, but it does have a higher rate of clinically relevant complications (33, 34). In order to avoid additional harm in the absence of any demonstrated benefit, it is strongly recommended that low-flow systems for extracorporeal CO2 elimination should not be used to lessen the invasiveness of ventilation in patients with ARDS (expert consensus, strong recommendation).
Weaning off mechanical ventilation
Most patients can be rapidly weaned off mechanical ventilation, but weaning is difficult for some (24). Weaning protocols are used as an aid to the early evaluation of the patient’s ability to be weaned. The use of weaning protocols in adult patients who have been mechanically ventilated for more than 24 hours has been shown to significantly lessen the mean duration of ventilation (from 72 hours to 54 hours) and is therefore recommended for such patients (QoE +++; strong recommendation) (35).
Specific long-term sequelae
In the German S3 guideline on analgesia, sedation, and delirium management in intensive-care medicine (10), attention was drawn to post-traumatic stress disorder (PTSD) as a potential long-term sequela of treatment in an intensive care unit. This risk motivated the recommendation that patients’ families should be informed about PTSD and anxiety disorders in a documented conversation that includes structured information on how best to deal with patients requiring mechanical ventilation in an intensive care unit (expert consensus, weak recommendation).
Important clinical trials that are relevant to the topic of this guideline, but were published after the end of the guideline development process, are listed in Box 1.
Conclusion
The main objectives of this guideline are: (1) the improved clinical implementation of therapeutic measures that have been shown to be effective in mechanically ventilated patients, and (2) the reduction of excessive treatment with measures whose benefit has not been clearly demonstrated. In pursuit of these aims, the guideline group defined 119 recommendations, among which are 27 key recommendations, which, if consistently implemented, should improve the quality of clinical care in intensive care units.
The guideline is organized along the lines of the temporal course of an individual patient’s treatment in order to make it easier to use. To improve its rapid accessibility, multiple shortened versions (“Short Version,” “Key Recommendations and Quality Indicators,” eTables, eBox) and a “Pocket Edition” (box 2) were also developed. The Pocket Edition reproduces the central statements of the guideline. An accompanying implementation study is now being carried out to identify and analyze any major barriers that might impede the implementation of the recommendations contained in the guideline. Based on the findings of this study, an implementation manual will be created that will make it easier for users to apply the scientific evidence on mechanical ventilation therapy in their clinical work in the intensive care unit.
BOX 2. Evidence-based treatment algorithm for acute respiratory insufficiency.
Prerequisites: adult patient, systematic diagnostic assessment (x-ray, cardiac echocardiography and pulmonary ultrasonography, bronchoscopy, computerized tomography, microbiology), and treatment of treatable causes.
Try to avoid mechanical ventilation by first giving non-invasive ventilation or high-flow oxygen therapy. (Exceptions: severely impaired oxygenation [PaO2/FiO2 <150 mm Hg] or unsecured airway—initiate mechanical ventilation early in such cases.)
Enable spontaneous breathing. (Exceptions: severe ARDS—consider relaxation vs. spontaneous breathing; right-heart failure, intracranial hypertension—in such cases, assess the benefits and risks critically.)
Implement protective ventilator settings consistently. (SaO2 90–94%, appropriate PEEP*1, Vt 6–8 mL/kg [ARDS: Vt = 6 mL/kg], Pmax = 30 cm H2O, ?P = 15 cm H2O.)
For severe ARDS: perform prone positioning (as soon as PaO2/FiO2 <150 mm Hg) and restrict fluids. Critically assess recruiting maneuvers and relaxation. Make early contact with the regional ARDS/ECMO center.*2
Mobilize the patient early (within 72 hours of ICU admission) and wean according to a protocol, with daily trials of spontaneous breathing and evaluation of stopping mechanical ventilation.
Inform patients and their relatives of the long-term sequelae of mechanical ventilation.
*1 PEEP settings:
PEEP settings are easily chosen with the aid of ventilatory protocal cards of the ARDS Network, but this method fails to take individual respiratory mechanics into account and can therefore be recommended only as a rough guide.
Ventilatory protocol card “lower PEEP/higher FiO2”
| FiO2 | 0.3 | 0.4 | 0.4 | 0.5 | 0.5 | 0.6 | 0.7 | 0.7 | 0.7 | 0.8 | 0.9 | 0.9 | 0.9 | 1.0 |
| PEEP | 5 | 5 | 8 | 8 | 10 | 10 | 10 | 12 | 14 | 14 | 14 | 16 | 18 | 18-24 |
| FiO2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 | 0.4 | 0.5 | 0.5 | 0.5-0.8 | 0.8 | 0.9 | 1.0 | 1.0 |
| PEEP | 5 | 8 | 10 | 12 | 14 | 14 | 16 | 16 | 18 | 20 | 22 | 22 | 22 | 24 |
Alternative ways to determine PEEP settings for individualized PEEP titration:
use of the static or quasi-static inspiratory compliance curve
determination of maximal compliance with minimally impaired oxygen-carrying capacity of the circulation
highest possible PEEP for Vt = 6 mL/kg body weight and Pinsp in the range of 28–30 cm H2O
determination of the stress index
Acute hypercapnic respiratory failure:
Setting of extrinsic peep at a level up to 85% of the intrinsic PEEP
*2 Contact data of the regional ARDS/ECMO center:
Abbreviations:
ARDS: acute respiratory distress syndrome, ECMO: extracorporeal membrane oxygenation, FiO2: fraction of inspired oxygen; PaO2: partial pressure of oxygen in arterial blood; PEEP: positive end-expiratory pressure; Pmax: highest inspiratory pressure; ?P: driving pressure; SaO2: arterial oxygen saturation; Vt: tidal volume.
BOX 1. Important clinical trials that were published after the end of the literature search period.
After the conclusion of the systematic literature search in June 2016, no further clinical trials were published that, to the authors’ knowledge, would have necessitated a change in any recommendation in the guideline. The guideline group is of the opinion, however, that three trials published after this date do indeed further support and refine some of the individual recommendations made in the guideline:
Girardis et al. (36) showed that restrictive oxygen administration to a target oxygen saturation of 94-97% lowers mortality and organ failure rates. This was the first randomized, controlled clinical trial to support the recommendation made in the guideline, on the basis of an expert consensus, that the inspiratory oxygen concentration (FiO2) in mechanically ventilated patients should be kept as low as possible.
In the ART trial (37), the use of recruiting maneuvers followed by PEEP (positive end-expiratory pressure) titration was compared with PEEP application according to the ARMA trial for patients with moderate to severe acute respiratory distress syndrome (ARDS) (38). It was found that recruiting maneuvers followed by PEEP titration led to an increased 28-day mortality. This finding underscores the recommendation already made in the guideline that patients with ARDS should be treated with higher PEEP settings based on the PEEP specifications of the ARDSnet studies. It also implies that—in contrast to the neutral position taken in the guideline—recruiting maneuvers are associated with elevated mortality in ARDS patients and should therefore be used with utmost caution.
In the EOLIA trial (39), veno-venous extracorporeal membrane oxygenation (ECMO) as a standard measure was compared with conventional treatment for patients with very severe ARDS; in the control group, ECMO was permissible as a rescue measure for refractory hypoxemia. No increased survival was found for standard veno-venous ECMO in this group of patients. This confirms the assessment of the guideline group that veno-venous ECMO should be used only as a rescue measure for ARDS patients with refractory hypoxemia.
Key Messages.
Mechanical ventilation is recommended as primary treatment only for patients with severe ARDS (expert consensus, weak recommendation). For all other groups of patients with acute respiratory insufficiency, it is recommended that a trial of non-invasive ventilation should be carried out first (quality of evidence: very low to high; weak/strong recommendations).
For mechanically ventilated patients who do not have severe ARDS, it is recommended that spontaneous breathing should be enabled early (quality of evidence: very low; weak recommendation).
To avoid harm, high-frequency ventilation is not recommended for adult patients with ARDS (quality of evidence: high; strong recommendation).
Early, consistent prone positioning of patients with severe ARDS is recommended, as it lessens mortality quality of evidence: high; strong recommendation).
Veno-venous ECMO carries a high complication rate and is recommended for patients with severe ARDS and refractory hypoxemia only as a rescue measure after all other treatment options have been exhausted, and only in specialized centers (expert consensus, strong recommendation).
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement Prof. Moerer has received lecture honoraria from Pulsion, CareFusion, and Maquet. His department has received research funding from Maquet, CareFusion, B. Braun, CSL Behring, LMA, Pulsion, and Med. Systems.
Prof. Weber-Carstens has received funding for a research project that he initiated, as well as for the performance of clinical trials, from the Dräger company (and he therefore abstained from voting on related subjects during the development of this S3 guideline).
PD Dr. Laudi, Dr. Fichtner, Dr. Nothacker, and Prof. Kaisers state that they have no conflict of interest.
References
- 1.Raymondos K, Dirks T, Quintel M, et al. Outcome of acute respiratory distress syndrome in university and non-university hospitals in Germany. Crit Care. 2017;21 doi: 10.1186/s13054-017-1687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 3.Karagiannidis C, Brodie D, Strassmann S, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med. 2016;42:889–896. doi: 10.1007/s00134-016-4273-z. [DOI] [PubMed] [Google Scholar]
- 4.Langer G, Meerpohl JJ, Perleth M, Gartlehner G, Kaminski-Hartenthaler A, Schünemann H. GRADE-Leitlinien: 1 Einführung - GRADE-Evidenzprofile und Summary-of-Findings-Tabellen. Z Evid Fortbild Qual Gesundhwes. 2012;106:357–368. doi: 10.1016/j.zefq.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Schünemann H. Integrative assessment of evidence in healthcare: the GRADE system. Z Evid Fortbild Qual Gesundhwes. 2009;103:261–268. doi: 10.1016/j.zefq.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Kaminski-Hartenthaler A, Meerpohl JJ, Gartlehner G, et al. GRADE Leitlinien: 14 Von der Evidenz zur Empfehlung: Die Bedeutung und Darstellung von Empfehlungen. Z Evid Fortbild Qual Gesundhwes. 2014;108:413–420. doi: 10.1016/j.zefq.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 8.Deutsche Gesellschaft für Palliativmedizin. S3-Leitlinie Palliativmedizin. www.awmf.org/uploads/tx_szleitlinien/128-001OLl_S3_Palliativmedizin_2015-07.pdf (last accessed on 6 January 2018) [Google Scholar]
- 9.Shehabi Y, Bellomo R, Reade MC, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 10.Baron R, Binder A, Biniek R, et al. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine Revision 2015 DAS-Guideline 2015 short version. Ger Med Sci. 2015 doi: 10.3205/000223. 13:Doc19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 12.Sud S, Sud M, Friedrich JO, et al. High-frequency oscillatory ventilation versus conventional ventilation for acute respiratory distress syndrome. Cochrane Database Syst Rev. 2016;4 doi: 10.1002/14651858.CD004085.pub4. CD004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson ND, Cook DJ, Guyatt GH, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 14.Santa Cruz R, Rojas JI, Nervi R, Heredia R, Ciapponi A. High versus low positive end-expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013;6 doi: 10.1002/14651858.CD009098.pub2. CD009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 16.Damiani E, Adrario E, Girardis M, et al. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2014;18 doi: 10.1186/s13054-014-0711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panwar R, Hardie M, Bellomo R, et al. Conservative versus liberal oxygenation targets for mechanically ventilated patients A pilot multicenter randomized controlled trial. Am J Respir Crit Care Med. 2016;193:43–51. doi: 10.1164/rccm.201505-1019OC. [DOI] [PubMed] [Google Scholar]
- 18.Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med. 2009;151:566–576. doi: 10.7326/0003-4819-151-8-200910200-00011. [DOI] [PubMed] [Google Scholar]
- 19.Petrucci N, de Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane database of systematic reviews. Syst Rev. 2013;2 doi: 10.1002/14651858.CD003844.pub4. CD003844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serpa Neto A, Hemmes SNT, Barbas CS, et al. Protective versus conventional ventilation for surgery: a systematic review and individual patient data meta-analysis. Anesthesiol. 2015;23:66–78. doi: 10.1097/ALN.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 21.Bein T, Bischoff M, Bruckner U, et al. Kurzversion S2e-Leitlinie - „Lagerungstherapie und Frühmobilisation zur Prophylaxe oder Therapie von pulmonalen Funktionsstorungen“. Der Anaesthesist. 2015;64:596–611. doi: 10.1007/s00101-015-0060-4. [DOI] [PubMed] [Google Scholar]
- 22.Prävention der nosokomialen beatmungsassoziierten Pneumonie. Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:1578–1590. [PubMed] [Google Scholar]
- 23.Barbas CSV, Ísola AM, Farias, AM, et al. Brazilian recommendations of mechanical ventilation 2013. Part I. Rev Bras Ter Intensiva. 2014 doi: 10.5935/0103-507X.20140017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schönhofer B, Geiseler J, Dellweg D, et al. Prolongiertes Weaning: S2k-Leitlinie herausgegeben von der Deutschen Gesellschaft für Pneumologie und Beatmungsmedizin e. V. Pneumologie. 2014;68:19–75. doi: 10.1055/s-0033-1359038. [DOI] [PubMed] [Google Scholar]
- 25.Davidson AC, Banham S, Elliott M, et al. BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults. Thorax. 2016;71(Suppl 2):ii1–ii35. doi: 10.1136/thoraxjnl-2015-208209. [DOI] [PubMed] [Google Scholar]
- 26.Hosokawa K, Nishimura M, Egi M, Vincent JL. Timing of tracheotomy in ICU patients: a systematic review of randomized controlled trials. Crit Care. 2015;19 doi: 10.1186/s13054-015-1138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siempos II, Ntaidou TK, Filippidis FT, Choi AMK. Effect of early versus late or no tracheostomy on mortality and pneumonia of critically ill patients receiving mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:150–158. doi: 10.1016/S2213-2600(15)00007-7. [DOI] [PubMed] [Google Scholar]
- 28.Suzumura EA, Figueiro M, Normilio-Silva K, et al. Effects of alveolar recruitment maneuvers on clinical outcomes in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Intensive Care Med. 2014;40:1227–1240. doi: 10.1007/s00134-014-3413-6. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 30.Bloomfield R, Noble DW, Sudlow A. Prone position for acute respiratory failure in adults. Cochrane Database Syst Rev. 2015;11 doi: 10.1002/14651858.CD008095.pub2. CD008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adhikari NK, Dellinger RP, Lundin S, et al. Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis. Crit Care. 2014;42:404–412. doi: 10.1097/CCM.0b013e3182a27909. [DOI] [PubMed] [Google Scholar]
- 32.Ruan SY, Huang TM, Wu HY, Wu HD, Yu CJ, Lai MS. Inhaled nitric oxide therapy and risk of renal dysfunction: a systematic review and meta-analysis of randomized trials. Crit Care. 2015;19 doi: 10.1186/s13054-015-0880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzgerald M, Millar J, Blackwood B, et al. Extracorporeal carbon dioxide removal for patients with acute respiratory failure secondary to the acute respiratory distress syndrome: a systematic review. Crit Care Med. 2014;18 doi: 10.1186/cc13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sklar MC, Beloncle F, Katsios CM, Brochard L, Friedrich JO. Extracorporeal carbon dioxide removal in patients with chronic obstructive pulmonary disease: a systematic review. Intensive Care Med. 2015;41:1752–1762. doi: 10.1007/s00134-015-3921-z. [DOI] [PubMed] [Google Scholar]
- 35.Blackwood B, Burns KE, Cardwell CR, O‘Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev. 2014;11 doi: 10.1002/14651858.CD006904.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the Oxygen-ICU Randomized Clinical Trial. JAMA. 2016;316:1583–1589. doi: 10.1001/jama.2016.11993. [DOI] [PubMed] [Google Scholar]
- 37.Cavalcanti AB, Suzumura ÉA, Laranjeira LN, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 39.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]