Abstract
Background
Hyperventilation-induced hypocapnia (HV) reduces elevated intracranial pressure (ICP), a dangerous and potentially fatal complication of traumatic brain injury (TBI). HV decreases the arteriolar diameter of intracranial vessels, raising the risk of cerebral ischemia. The aim of this study was to characterize the effects of moderate short-term HV in patients with severe TBI by using concomitant monitoring of cerebral metabolism, brain tissue oxygen tension (PbrO2), and cerebral hemodynamics with transcranial color-coded duplex sonography (TCCD).
Methods
This prospective trial was conducted between May 2014 and May 2017 in the surgical intensive care unit (ICU) at the University Hospital of Zurich. Patients with nonpenetrating TBI older than 18 years of age with a Glasgow Coma Scale (GCS) score < 9 at presentation and with ICP monitoring, PbrO2, and/or microdialysis (MD) probes during ICU admission within 36 h after injury were included in our study. Data collection and TCCD measurements were performed at baseline (A), at the beginning of moderate HV (C), after 50 min of moderate HV (D), and after return to baseline (E). Moderate HV was defined as arterial partial pressure of carbon dioxide 4–4.7 kPa. Repeated measures analysis of variance was used to compare variables at the different time points, followed by post hoc analysis with Bonferroni adjustment as appropriate.
Results
Eleven patients (64% males, mean age 36 ± 14 years) with an initial median GCS score of 7 (IQR 3–8) were enrolled. During HV, ICP and mean flow velocity (CBFV) in the middle cerebral artery decreased significantly. Glucose, lactate, and pyruvate in the brain extracellular fluid did not change significantly, whereas PbrO2 showed a statistically significant reduction but remained within the normal range.
Conclusion
Moderate short-term hyperventilation has a potent effect on the cerebral blood flow, as shown by TCCD, with a concomitant ICP reduction. Under the specific conditions of this study, this degree of hyperventilation did not induce pathological alterations of brain metabolites and oxygenation.
Trial registration
NCT03822026. Registered on 30 January 2019.
Keywords: Traumatic brain injury, Hyperventilation, Intracranial pressure, Brain metabolites, Brain oxygenation, Transcranial color-coded duplex sonography
Introduction
Hyperventilation-induced arterial hypocapnia (HV) has been used for decades in neuroanesthesia and neurointensive care, especially in the management of patients with severe traumatic brain injury (TBI) and concomitant elevated intracranial pressure (ICP). Increased alveolar ventilation induces a dose-response decrease in arterial partial pressure of carbon dioxide (PaCO2), causing a parallel reduction of carbon dioxide partial pressure in the extracellular brain compartment with tissue alkalosis. This results in vasoconstriction of the cerebral arterioles [1, 2]. Consequently, cerebral blood flow (CBF), cerebral blood volume (CBV), and ultimately ICP decrease.
The most relevant criticism of the use of HV is the risk of cerebral ischemia and hypoxia, especially in the acute phase of TBI [3]. This risk has been documented in several studies [4, 5], but the net effects of HV on regional CBF and metabolism are still debated [6].
Despite those concerns, HV is still widely used, even in the absence of elevated ICP and without brain oxygenation monitoring [7]. However, there is an international consensus that HV could have deleterious effects, especially by reducing CBF and brain oxygenation. To minimize this risk, HV should be used only to counteract the raised ICP, rather than prophylactically, and only with appropriate monitoring of CBF and/or oxygenation [8–11]. While continuous global measurements of CBF are not feasible at the bedside, cerebral blood flow velocity in the middle cerebral artery (CBFV) may be measured by transcranial Doppler and has been used to monitor the effects of hypocapnia [12, 13]. The present study was conducted to quantify potential adverse effects of moderate short-term HV during the acute phase of severe TBI on cerebral hemodynamics, oxygenation, and metabolism.
Methods
The Institutional Ethics Committee of Zurich approved the research protocol of this prospective clinical trial (KEK-ZH 2012-0542). Informed consent was obtained from the patients’ next of kin prior to study enrollment and/or from the patients after ICU discharge.
Patient population and initial stabilization
Inclusion criteria involved patients (≥ 18 years of age) with nonpenetrating head injury, with initial GCS score < 9 prior to sedation and intubation, extended neuromonitoring with ICP, PbrO2, and/or microdialysis probes, undergoing invasive mechanical ventilation with fraction of inspired oxygen < 60% and positive end-expiratory pressure < 15 cmH2O. Exclusion criteria were decompressive craniectomy, pregnancy, preexisting neurologic disease, previous TBI, acute cardiovascular disease, severe respiratory failure, acute or chronic liver disease, sepsis, and failure to obtain satisfactory bilateral TCCD signals. Patients with persisting hypovolemia or hemodynamic instability despite previous fluid resuscitation (defined as global end-diastolic volume index < 680 ml/m2, central venous oxygen saturation [ScvO2] < 60%, or increase in mean arterial blood pressure [MAP] > 15% after passive leg raise test) were excluded. All patients were treated according to a cerebral perfusion orientated protocol aiming to achieve cerebral perfusion pressure (CPP) > 70 mmHg, ICP ≤ 20 mmHg, and PbrO2 > 15 mmHg. PaCO2 was targeted at 4.8–5.2 kPa.
Monitoring
MAP, ICP, CPP, arterial oxygen saturation (SaO2), end-tidal CO2 (etCO2), and PbrO2 were continuously monitored. ICP was measured with a fiberoptic device (Camino, San Diego, CA, USA) and PbrO2 with a Clark-type microcatheter (Licox GmbH, Kiel, Germany).
Cerebral microdialysis (MD) was performed using a catheter (CMA70; Microdialysis, Solna, Sweden) with a membrane length of 10 mm and a molecular mass cutoff of 20 kDa, which was perfused by a microinjection pump (CMA 106; Microdialysis) with artificial cerebrospinal fluid at a flow rate of 0.3 μl/min. Samples were collected every hour and immediately analyzed for glucose, lactate, and pyruvate with a bedside MD analyzer (CMA 600; CMA/Microdialysis). Sampling was performed at 1-h intervals in order to allow the detection of sufficient concentration of analytes in the microdialysate. ICP, PbrO2, and MD catheters were placed in the white frontal matter, usually on the side of the brain with more significant injury. The correct position of the catheters was confirmed by computed tomography.
TCCD examinations of the middle cerebral artery (MCA) were performed bilaterally through the transtemporal acoustic window by two experienced physicians (SK, GB) according to standard techniques [14, 15] using a 2-MHz Probe (Philips CX 50; Philips Healthcare, Andover, MA, USA). Three repeated measurements of the peak systolic velocity (PSV) and end-diastolic velocity (EDV) were performed for each side. The device automatically calculated CBFV and pulsatility index (PI). PI was chosen for its high predictive value in case of increased ICP and low CPP [16].
Study protocol
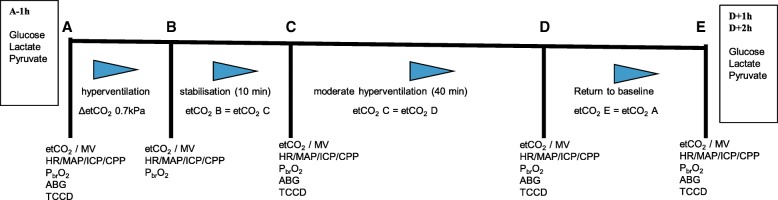
Patients were enrolled in the study within 36 h of sustaining trauma. Under baseline conditions, a TCCD examination was performed, and all parameters were recorded (Fig. 1, point A). The alveolar ventilation was then increased over a 10-min period to obtain moderate HV by a stepwise increase in tidal volume and respiratory rate until a reduction of etCO2 of 0.7 kPa (Fig. 1, point B) was achieved.
Fig. 1.
Study protocol. At different time points, several variables were collected and transcranial color-coded duplex sonography (TCCD) measurements were performed. A = Baseline. B = Increasing minute ventilation. C = Begin moderate hyperventilation with target PaCO2 4–4.7 kPa. D = After moderate hyperventilation for 50 min. E = Return to baseline. Glucose, lactate, and pyruvate concentrations in the extracellular fluid were sampled by microdialysis 1 h before A (A − 1 h) and 1 h (D + 1 h) and 2 h after D (D + 2). etCO2 End-tidal CO2 (kPa), MV Minute ventilation (L/min), HR Heart rate (beats/min), MAP Mean arterial pressure (mmHg), ICP Intracranial pressure (mmHg), CPP Cerebral perfusion pressure (mmHg), PbrO2 Brain tissue oxygen tension (mmHg), ABGA Arterial blood gas analysis
After 10 min of stable etCO2, a second TCCD measurement was made (beginning of HV; Fig. 1, point C). The etCO2 value was kept stable for 40 min, followed by a third TCCD examination (Fig. 1, point D). Finally, normoventilation was reestablished over 10 min, and all variables were allowed to return to baseline (Fig. 1, point E). A final TCCD examination was conducted at this time point. At each time point, MAP, ICP, CPP, PbrO2, SpO2, and etCO2 were recorded.
Microdialysate samples for glucose, lactate, and pyruvate concentrations were collected 1 h before A (A − 1 h) as a baseline measurement and 1 h (D + 1 h) and 2 h (D + 2 h) after D to detect possible changes induced by moderate HV. Arterial blood gas tests (ABG) were obtained at points A, C, D, and E to monitor the changes of pH and PaCO2.
Statistical analysis
Data were analyzed to produce mean ± SD or median with IQR, unless otherwise indicated. Repeated-measures analysis of variance (ANOVA) was used to compare all variables. When the F-ratio of the ANOVA reached a critical level (corresponding to p < 0.05), post hoc analysis with Bonferroni adjustment was used. All tests were performed two-sided, and p < 0.05 was considered statistically significant. Statistical analysis was performed using Stata version 12.1 software (StataCorp LP, College Station, TX, USA).
Results
Demographic data
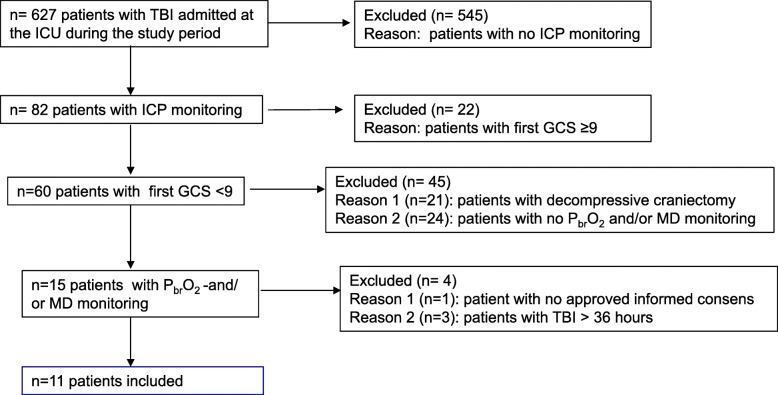
During the study period, 628 patients with TBI were admitted to the surgical ICU. A breakdown of reasons for exclusion is presented in Fig. 2. Eleven patients with severe TBI were included; 64% were males, with a mean age of 36 ± 14 years. The median first GCS score was 7 (IQR 3–8). The admission computed tomographic (CT) scan identified several brain lesions, very often combined. An epidural hematoma was diagnosed in three patients (27%), a subdural hematoma in seven (64%), contusions in eight (73%), and edema/swelling in three (27%).
Fig. 2.
Flowchart of the study. During the study period, 627 patients were admitted with a diagnosis of traumatic brain injury (TBI). Reasons for exclusion from the study are shown on the right side of the chart. ICU Intensive care unit, ICP intracranial pressure, GCS Glasgow Coma Scale, PbrO2 Brain tissue oxygen tension, MD Cerebral microdialysis
On average, the Injury Severity Score (ISS) was 31 ± 11, and the Simplified Acute Physiology Score (SAPS) II 24 h after TBI was, on average, 48 ± 9. The study was performed, on average, 23 h after TBI.
Ventilation parameters
Changes in PaCO2 during the test and other respiratory variables are shown in Table 1.
Table 1.
Respiratory variables
| n = 11 | A Baseline |
B Minute ventilation |
C Moderate hyperventilation |
D 50 min of moderate hyperventilation |
E Return to baseline |
|---|---|---|---|---|---|
| etCO2 (kPa) | 5.0 (0.7) | 4.3 (0.6)* | 4.2 (0.6)*§ | 4.0 (0.7)*§ | 4.7 (0.7)*§¶Ŧ |
| MV (L/min) | 6.9 (1.4) | 8.6 (1.6)* | 8.7 (1.7)* | 8.4 (1.5)* | 6.8 (1.4)§¶Ŧ |
| pH | 7.37 (0.09) | 7.45 (0.02)* | 7.46 (0.03)*¶ | 7.41 (0.03)¶Ŧ | |
| PaCO2 (kPa) | 5.0 (0.2) | 4.3 (0.2)* | 4.1 (0.4)*¶ | 4.7 (0.4)*¶Ŧ | |
| PaO2 (kPa) | 17.2 (1.5) | 17.9 (1.9) | 18.8 (2.3)*¶ | 17.4 (1.2)Ŧ |
Abbreviations: A Baseline, B Increasing minute ventilation, C Begin moderate hyperventilation with target PaCO2 4–4.7 kPa, D After moderate hyperventilation for 50 min, E Return to baseline, etCO2 End-tidal CO2, MV Minute ventilation (L/min), PaCO2 Partial pressure arterial carbon dioxide (kPa), PaO2 Partial pressure arterial oxygen (kPa)
Data are expressed as mean (SD)
* p < 0.05 compared with A
§ p < 0.05 compared with B
¶ p < 0.05 compared with C
Ŧ p < 0.05 compared with D
Effects of hyperventilation on systemic and cerebral hemodynamics
MAP and HR (Table 2) did not change significantly during the study. Mean ICP was 16 ± 6 mmHg (A), decreased significantly to 8 ± 6 mmHg (p < 0.0001) during C, and remained stable during D (10 ± 7 mmHg; p = 0.30). On return to normoventilation (E), ICP increased significantly to 14 ± 6 mmHg (p = 0.003 compared with D) and was similar to A (p = 0.06).
Table 2.
Systemic and cerebral hemodynamics
| n = 11 | A Baseline |
B Minute ventilation |
C Moderate hyperventilation |
D 50 min of moderate hyperventilation |
E Return to baseline |
|---|---|---|---|---|---|
| HR (beats/min) | 72 (16) | 72 (17) | 71 (17) | 71 (17) | 66 (16) |
| MAP (mmHg) | 92 (10) | 92 (10) | 93 (12) | 90 (10) | 94 (13) |
| ICP (mmHg) | 16 (6) | 8 (5)* | 8 (6)* | 10 (7)* | 14 (6)§¶Ŧ |
| CPP (mmHg) | 77 (9) | 84 (9)* | 85 (10)* | 81 (14) | 80 (11)§¶ |
| PbrO2 (mmHg) | 32 (10) | 33 (9) | 33 (10) | 30 (8)§¶ | 30 (8)§ |
Abbreviations: A Baseline, B Increasing minute ventilation, C Begin moderate hyperventilation with target PaCO2 4–4.7 kPa, D After moderate hyperventilation for 50 min, E Return to baseline, HR Heart rate (beats/min), MAP Mean arterial pressure (mmHg), ICP Intracranial pressure (mmHg), CPP Cerebral perfusion pressure (mmHg), PbrO2 Brain tissue oxygen tension (mmHg)
N = 11 patients; + = 10 patients. Data are expressed as mean (SD)
* p < 0.05 compared with A
§ p < 0.05 compared with B
¶ p < 0.05 compared with C
Ŧ p < 0.05 compared with D
Effects of hyperventilation on mean flow velocity and PI in the middle cerebral artery
TCCD measurements in the right and left MCAs were analyzed separately. CBFV was 81 ± 23 cm/s and 80 ± 22 in the right and left MCAs, respectively. During C, CBFV decreased to 69 ± 21 cm/s in the right MCA (p = 0.0002) and to 66 ± 17 cm/s in the left MCA (p = 0.006). CBFV did not change significantly during D (71 ± 20 cm/s, p = 0.38, and 63 ± 15 cm/s, p = 0.48, in the right and left MCA, respectively) compared with C. During E, CBFV was 77 ± 28 cm/s (p = 0.21, compared with D) and 75 ± 24 cm/s (p = 0.005, compared with D) at the right and left sides, respectively.
PI was 1.03 ± 0.17 and 0.96 ± 0.15 in the right and left MCAs, respectively. During the study, PI did not change significantly in the right MCA (Table 3). In the left MCA, PI remained stable during C (1.06 ± 0.10, p = 0.056, compared with A), increased significantly during D (1.07 ± 0.14, p < 0.03, compared with A), and remained stable during E (1.02 ± 0.14, p = 0.12, compared with D).
Table 3.
Transcranial color-coded duplex sonography variables measured in middle cerebral arteries bilaterally
| n = 11 | A Baseline |
C Moderate hyperventilation |
D 50 min of moderate hyperventilation |
E Return to baseline |
|---|---|---|---|---|
| CBFV right (cm/s) | 81 (23) | 69 (21)* | 71 (20)* | 77 (28) |
| CBFV left (cm/s) | 80 (22) | 66 (17)* | 63 (15)* | 75 (24)¶Ŧ |
| PSV right (cm/s) | 136 (37) | 117 (34)* | 120 (33)* | 129 (42) |
| PSV left (cm/s) | 131 (35) | 110 (25)* | 107 (24)* | 126 (37)¶Ŧ |
| EDV right (cm/s) | 56 (20) | 45 (16)* | 46 (16)* | 51 (22)¶ |
| EDV left (cm/s) | 54 (16) | 43 (13)* | 41 (11)* | 52 (17)¶Ŧ |
| PI right | 1.03 (0.17) | 1.08 (0.16) | 1.05 (0.23) | 1.05 (0.17) |
| PI left | 0.96 (0.15) | 1.06 (0.10) | 1.07 (0.14)* | 1.02 (0.14) |
Abbreviations: A Baseline, B Increasing minute ventilation, C Begin moderate hyperventilation with target PaCO2 4–4.7 kPa, D After moderate hyperventilation for 50 min, E Return to baseline. CBFV Mean flow velocity of the middle cerebral artery (cm/s), PSV Peak systolic velocity (cm/s), EDV End-diastolic velocity (cm/s), PI Pulsatility index
* p < 0.05 compared with A
¶ p < 0.05 compared with C
Ŧ p < 0.05 compared with D
N = 11 patients, + = 10 patients. Data are expressed as mean (SD)
Changes of PSV and EDV on both sides are shown in Table 3.
Brain tissue oxygen tension and cerebral metabolism
PbrO2 and MD were measured in 10 and 9 patients, respectively. Eight patients got PbrO2 and MD simultaneously, and both probes were placed close to each other. The parenchyma surrounding the probes appeared normal on the CT scan in nine patients, seven of them with both PbrO2 and MD monitoring, one with only MD monitoring, and one with only PbrO2. In two cases, the parenchyma surrounding the probes appeared pathological; one of them had PbrO2 and MD probes inserted, and one only a PbrO2 probe.
Mean PbrO2 was 32 ± 10 mmHg; it remained stable during C (33 ± 10 p = 0.50), decreased during D (30 ± 8 p = 0.003, compared with C), and remained stable during E (30 ± 8 p = 0.90 compared with D) (Table 2).
The mean extracellular fluid concentrations of glucose, lactate, pyruvate, and the lactate/pyruvate ratio in the brain 1 h before and 1 and 2 h after moderate HV are reported in Table 4. No significant changes were observed.
Table 4.
Cerebral microdialysis
| n = 9 | Baseline (A − 1 h) | 1 h after moderate hyperventilation for 50 min (D + 1 h) | 2 h after moderate hyperventilation for 50 min (D + 2 h) |
|---|---|---|---|
| Brain glucose (mmol/L) | 1.5 (1.0)Φ | 1.4 (0.7) | 1.4 (0.7) |
| Brain lactate (mmol/L) | 3.3 (1.0)Φ | 3.5 (1.3) | 3.4 (1.4) |
| Brain pyruvate (μmol/L) | 101.4 (38.3)Φ | 99.6 (39.5) | 97.3 (44.7)Φ |
| LP ratio | 34.2 (8.2)Φ | 39.0 (17.7) | 37.5 (11.4)Φ |
LP Lactate/pyruvate ratio
Data are expressed as mean (SD). N = 9 patients
Φ Eight patients
Discussion
Main findings
The aim of this study was to detect possible adverse effects of moderate HV in patients with severe TBI during the acute phase postinjury. To our knowledge, this is the first study using a multimodal monitoring set (MD, PbrO2, and TCCD, together with ICP and CPP) to investigate this.
Our data suggest that moderate short-term HV was effective in lowering ICP with a remarkable effect on CBF velocities, as shown by the reduction of the CBFV in the MCAs bilaterally and by a slight increase in PI. The CBFV in the MCAs in the study population at baseline was normal, suggesting a physiological global CBF. In the course of moderate HV, CBFV and hence CBF decreased significantly, as in previous studies [17, 18]. These findings are consistent with vasoconstriction in the brain vasculature leading to a reduction in CBF and likely in CBV.
MAP and HR remained stable during moderate HV, as demonstrated by Minhas et al. [13] in volunteers. However, in patients receiving positive pressure ventilation, sedation, and hypovolemia, the interactions between hypocapnia and hemodynamics are more complex [2]. Due to the possible systemic effects of hypocapnia, patients with persisting hypovolemia or hemodynamic instability despite previous fluid resuscitation were excluded.
Our work included two additional variables, PbrO2 and MD, with the specific aim of demonstrating possible tissue hypoxia and/or disturbed metabolic patterns.
On the basis of our results, there was a PbrO2 reduction, but within a physiological range. Additionally, MD with a stable Lactate/pyruvate (L/P) ratio was not indicative of an energy crisis.
What is already known
In the last 30 years, HV has been fiercely debated. After initial widespread use [19], the effects of HV on outcome were questioned in a randomized study [20]. That study included a total of 113 patients in 3 groups and used different levels of HV in all arms. ICP was kept stable in all groups. At 3 and 6 months after injury, patients with a motor score of 4–5 in the hyperventilation group had less favorable outcomes than the other groups. However, the control group was hyperventilated and had a PaCO2 of 31–32 mmHg for the first 5 study days. Additionally, HV was induced prophylactically, regardless of ICP.
More accurate studies of pathophysiological changes induced by HV have been performed by two authoritative groups. Coles et al. measured a significant reduction of CBF with PET scans and an increase in the volume of severely hypoperfused tissue following HV [4] with a PaCO2 reduction from 4.8 to 3.9 kPa. Diringer et al., on the contrary, using an analogous PET technique, did not disclose any alteration of oxygen metabolism in two groups of patients with TBI managed with different degrees of HV [17]. They concluded that oxygen metabolism was maintained due to the low baseline metabolic rate and compensatory increase in oxygen extraction fraction.
How our data compare with the literature: limitations
Our data questioned if HV, even when effective in reducing CBF and ICP, induced measurable changes compatible with true ischemia (defined as a CBF insufficient for preserving adequate tissue oxygenation and metabolism). For a fair comparison with the existing evidence, however, the study limitations should be acknowledged. First, the small sample size limits the generalizability of the findings. More accurate analyses, for instance looking at sex differences in vasoreactivity or association between PI and outcome, were clearly not feasible [21, 22]. Second, we decided to perform only a moderate degree of short-term HV. This choice was dictated by two concerns: the risk of ischemia and the time frame of the test. We were aware that the brain is especially vulnerable to ischemia during the acute phase after trauma [23]. It may well be that we did not detect pathological changes because of the mild level of hypocapnia used for a short time interval. Third, the MD and PbrO2 probes explore only small areas of the brain; therefore, we cannot exclude concomitant changes in other brain regions [24, 25].
In our study population, however, the MD and the PbrO2 probes were placed, in the majority of the cases, in the most injured hemisphere of the brain. Interestingly, the cerebral glucose at baseline was normal, whereas the L/P ratio at baseline was markedly increased and pathological [26], reflecting tissue injury despite “normal” parenchyma on the CT scan. From this point of view, we were exploring a vulnerable portion of the brain, where potential side effects of HV would have been more likely to be detected. Furthermore, because none of the probes were placed directly in the site of contusion and only two were in pericontusional tissue, the effect of HV on PbrO2 should not have been compromised [24].
Conclusions
Our findings add evidence to the belief that a moderate degree of short-term HV is effective in reducing ICP through CBFV modifications as an estimation of CBF. Furthermore, a moderate degree of HV reduces PbrO2, but within a physiological range, and does not significantly change the cerebral energy metabolism. Further investigations of prolonged and more severe HV, in the context of intracranial hypertension and in a larger study population, are needed to characterize the risks and benefits of HV in severe TBI.
Acknowledgements
Not applicable.
Funding
None.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
GB helped in the conception and design of the study; the acquisition, analysis, and interpretation of data; and drafting the work and revising it critically for key content. NS helped in the interpretation of data, as well as in drafting the work and revising it for key content. AP helped in the analysis and interpretation of data, as well as in revising the work for key content. FS helped in the acquisition of data and critical revision of key content. PS helped in the conception and design of the study. SK helped in the conception and design of the study, the acquisition of data, and revising the work critically for key content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Institutional Ethics Committee of Zurich approved the research protocol of this prospective clinical trial (KEK-ZH 2012-0542). Informed consent was obtained from the patients’ next of kin prior to study enrollment and/or from the patients after ICU discharge.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Giovanna Brandi, Phone: +41 44 255 23 76, Email: giovanna.brandi@usz.ch.
Nino Stocchetti, Email: nino.stocchetti@policlinico.mi.it.
Alberto Pagnamenta, Email: Alberto.Pagnamenta@eoc.ch.
Federica Stretti, Email: federicastretti@gmail.com.
Peter Steiger, Email: peter.steiger@usz.ch.
Stephanie Klinzing, Email: stephanie.klinzing@usz.ch.
References
- 1.Muizelaar JP, van der Poel HG, Li ZC, Kontos HA, Levasseur JE. Pial arteriolar vessel diameter and CO2 reactivity during prolonged hyperventilation in the rabbit. J Neurosurg. 1988;69(6):923–927. doi: 10.3171/jns.1988.69.6.0923. [DOI] [PubMed] [Google Scholar]
- 2.Stocchetti N, Maas AI, Chieregato A, van der Plas AA. Hyperventilation in head injury: a review. Chest. 2005;127(5):1812–1827. doi: 10.1378/chest.127.5.1812. [DOI] [PubMed] [Google Scholar]
- 3.Godoy DA, Seifi A, Garza D, Lubillo-Montenegro S, Murillo-Cabezas F. Hyperventilation therapy for control of posttraumatic intracranial hypertension. Front Neurol. 2017;8:250. doi: 10.3389/fneur.2017.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coles JP, Minhas PS, Fryer TD, Smielewski P, Aigbirihio F, Donovan T, et al. Effect of hyperventilation on cerebral blood flow in traumatic head injury: clinical relevance and monitoring correlates. Crit Care Med. 2002;30(9):1950–1959. doi: 10.1097/00003246-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Coles JP, Fryer TD, Coleman MR, Smielewski P, Gupta AK, Minhas PS, et al. Hyperventilation following head injury: effect on ischemic burden and cerebral oxidative metabolism. Crit Care Med. 2007;35(2):568–578. doi: 10.1097/01.CCM.0000254066.37187.88. [DOI] [PubMed] [Google Scholar]
- 6.Diringer MN, Yundt K, Yideen TO, Adams RE, Zazulia AR, Deibert E, et al. No reduction in cerebral metabolism as a result of early moderate hyperventilation following severe traumatic brain injury. J Neurosurg. 2000;92(1):7–13. doi: 10.3171/jns.2000.92.1.0007. [DOI] [PubMed] [Google Scholar]
- 7.Chesnut RM, Temkin N, Dikmen S, Rondina C, Videtta W, Petroni G, et al. A method of managing severe traumatic brain injury in the absence of intracranial pressure monitoring: the imaging and clinical examination protocol. J Neurotrauma. 2018;35(1):54–63. doi: 10.1089/neu.2016.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(9):1189–1209. doi: 10.1007/s00134-014-3369-6. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal G, Hemphill JC, 3rd, Sorani M, Martin C, Morabito D, Obrist WD, et al. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit Care Med. 2008;36(6):1917–1924. doi: 10.1097/CCM.0b013e3181743d77. [DOI] [PubMed] [Google Scholar]
- 10.Rohlwink UK, Zwane E, Fieggen AG, Argent AC, le Roux PD, Figaji AA. The relationship between intracranial pressure and brain oxygenation in children with severe traumatic brain injury. Neurosurgery. 2012;70(5):1220–1230. doi: 10.1227/NEU.0b013e318243fc59. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson EA, Barletta JF, Figueroa BE, Bonnell BW, Vanderkolk WE, McAllen KJ, et al. Cerebral perfusion pressure and intracranial pressure are not surrogates for brain tissue oxygenation in traumatic brain injury. Clin Neurophysiol. 2012;123(6):1255–1260. doi: 10.1016/j.clinph.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Ainslie PN, Celi L, McGrattan K, Peebles K, Ogoh S. Dynamic cerebral autoregulation and baroreflex sensitivity during modest and severe step changes in arterial PCO2. Brain Res. 2008;1230:115–124. doi: 10.1016/j.brainres.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 13.Minhas JS, Panerai RB, Robinson TG. Modelling the cerebral haemodynamic response in the physiological range of PaCO2. Physiol Meas. 2018;39(6):065001. doi: 10.1088/1361-6579/aac76b. [DOI] [PubMed] [Google Scholar]
- 14.McCarville MB, Goodin GS, Fortner G, Li CS, Smeltzer MP, Adams R, et al. Evaluation of a comprehensive transcranial Doppler screening program for children with sickle cell anemia. Pediatr Blood Cancer. 2008;50(4):818–821. doi: 10.1002/pbc.21430. [DOI] [PubMed] [Google Scholar]
- 15.Klinzing S, Steiger P, Schupbach RA, Bechir M, Brandi G. Competence for transcranial color-coded duplex sonography is rapidly acquired. Minerva Anestesiol. 2015;81(3):298–304. [PubMed] [Google Scholar]
- 16.Zweifel C, Czosnyka M, Carrera E, de Riva N, Pickard JD, Smielewski P. Reliability of the blood flow velocity pulsatility index for assessment of intracranial and cerebral perfusion pressures in head-injured patients. Neurosurgery. 2012;71(4):853–861. doi: 10.1227/NEU.0b013e3182675b42. [DOI] [PubMed] [Google Scholar]
- 17.Diringer MN, Videen TO. Yundt K, Zazulia AR, Aiyagari V, Dacey RG, Jr, et al. Regional cerebrovascular and metabolic effects of hyperventilation after severe traumatic brain injury. J Neurosurg. 2002;96(1):103–108. doi: 10.3171/jns.2002.96.1.0103. [DOI] [PubMed] [Google Scholar]
- 18.Cold GE. Does acute hyperventilation provoke cerebral oligaemia in comatose patients after acute head injury? Acta Neurochir (Wien). 1989;96(3-4):100–106. doi: 10.1007/BF01456166. [DOI] [PubMed] [Google Scholar]
- 19.Obrist WD, Langfitt TW, Jaggi JL, Cruz J, Gennarelli TA. Cerebral blood flow and metabolism in comatose patients with acute head injury: relationship to intracranial hypertension. J Neurosurg. 1984;61(2):241–253. doi: 10.3171/jns.1984.61.2.0241. [DOI] [PubMed] [Google Scholar]
- 20.Muizelaar JP, Marmarou A, Ward JD, Kontos HA, Choi SC, Becker DP, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75(5):731–739. doi: 10.3171/jns.1991.75.5.0731. [DOI] [PubMed] [Google Scholar]
- 21.Minhas JS, Panerai RB, Robinson TG. Sex differences in cerebral haemodynamics across the physiological range of PaCO2. Physiol Meas. 2018;39(10):105009. doi: 10.1088/1361-6579/aae469. [DOI] [PubMed] [Google Scholar]
- 22.Minhas JS, Panerai RB, Ghaly G, Divall P, Robinson TG. Cerebral autoregulation in hemorrhagic stroke: a systematic review and meta-analysis of transcranial Doppler ultrasonography studies. J Clin Ultrasound. 2019;47(1):14–21. doi: 10.1002/jcu.22645. [DOI] [PubMed] [Google Scholar]
- 23.Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF. Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J Neurosurg. 1991;75(5):685–693. doi: 10.3171/jns.1991.75.5.0685. [DOI] [PubMed] [Google Scholar]
- 24.Hawryluk GW, Phan N, Ferguson AR, Morabito D, Derugin N, Stewart CL, et al. Brain tissue oxygen tension and its response to physiological manipulations: influence of distance from injury site in a swine model of traumatic brain injury. J Neurosurg. 2016;125(5):1217–1228. doi: 10.3171/2015.7.JNS15809. [DOI] [PubMed] [Google Scholar]
- 25.Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, et al. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol. 2012;590(14):3261–3275. doi: 10.1113/jphysiol.2012.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinstrup P, Stahl N, Mellergard P, Uski T, Ungerstedt U, Nordstrom CH. Intracerebral microdialysis in clinical practice: baseline values for chemical markers during wakefulness, anesthesia, and neurosurgery. Neurosurgery. 2000;47(3):701–709. doi: 10.1097/00006123-200009000-00035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.