Abstract
Background
High white blood cell (WBC) count and high blood glucose level are risk factors for mortality and pneumonia after acute ischemic stroke (AIS). We investigated the combined effect of high WBC count and high blood glucose level on hospital admission and in-hospital mortality and pneumonia in acute AIS patients.
Methods
A total of 3124 AIS patients enrolled from December 2013 to May 2014 across 22 hospitals in Suzhou city were included in the present study. We divided patients into four groups according to their level of WBC count and blood glucose: NWNG (normal WBC count and normal glucose), NWHG (normal WBC count and higher glucose), HWNG (higher WBC count and normal glucose), and HWHG (higher WBC count and higher glucose). Cox proportional hazard model and logistic regression model were used to estimate the combined effect of WBC count and blood glucose on all-cause in-hospital mortality and pneumonia in AIS patients.
Results
HWHG was associated with a 2.22-fold increase in the risk of in-hospital mortality in comparison to NWNG (adjusted hazard ratio [HR] 2.22; 95% confidence interval [CI], 1.21–4.07; P trend = 0.003). The risk of pneumonia was significantly higher in patients with HWHG compared to those with NWNG (adjusted odds ratio [OR] 2.61; 95% CI, 1.66–4.10; P trend < 0.001). The C-statistic for the combined WBC count and blood glucose was higher than WBC count or blood glucose alone for prediction of in-hospital mortality and pneumonia (all p < 0.01).
Conclusions
High WBC count combined with high blood glucose level at admission was independently associated with in-hospital mortality and pneumonia in AIS patients. Moreover, the combination of WBC count and blood glucose level appeared to be a better predictor than WBC count or blood glucose alone.
Electronic supplementary material
The online version of this article (10.1186/s12974-019-1422-7) contains supplementary material, which is available to authorized users.
Keywords: Acute ischemic stroke, White blood cell, Blood glucose, Combined effect, In-hospital outcomes
Introduction
Increased white blood cell (WBC) count and high glucose level are frequently found in patients with acute ischemic stroke (AIS) [1–4]. Several studies indicated that high WBC and high glucose level were not only associated with high risk of poor outcome and mortality but also pneumonia after stroke [2, 4–10].
Stress and inflammatory response are the two main causative factors leading to higher WBC and high glucose associated with mortality and pneumonia after acute stroke [2, 10–13]. There may exist a combined effect of WBC count and blood glucose level on stroke outcomes based on the similar mechanisms. Studies found that acute myocardial infarction (AMI) patients with co-existing higher WBC and high blood glucose had higher rates of in-hospital mortality and poor outcome compared to those with higher WBC or high blood glucose alone [14, 15]. A US study of 436 ischemic stroke patients indicated that those with both elevated total WBC count and high blood glucose had poor discharge outcome independent of elevations in either factor alone [16]. However, evidence on the association between the combined effect of WBC count and blood glucose at admission and in-hospital mortality and pneumonia in AIS patients are limited.
In the present study, we aimed to evaluate the possible association between combined effect of WBC count and blood glucose and in-hospital mortality and pneumonia in a large multicenter study of over 3000 AIS patients from Suzhou, China.
Methods
Study participants
From December 2013 to May 2014, we recruited patients with AIS or transient ischemic attack (TIA) from 22 hospitals in Suzhou, China (Additional file 1). Patients aged ≥ 18 years with a clinical diagnosis of AIS or TIA were considered eligible. Diagnosis of ischemic stroke was made according to the World Health Organization-defined criteria based on patient history, clinical data, and neuroimaging results (computed tomography [CT] or magnetic resonance imaging [MRI]). A team of investigators, including neurologists, reviewed the eligibility of study participants. Additional exclusion criteria were as follows: (1) diagnosis of TIA based on completely revised of symptoms and no acute infarct on the MRI or follow-up CT scans, (2) time from onset to admission over 7 days, and (3) lack of data on admission WBC or fasting glucose levels. Three thousand one hundred twenty-four patients were potentially eligible for this analysis (flowchart of participants selection; Fig. 1).
Fig. 1.
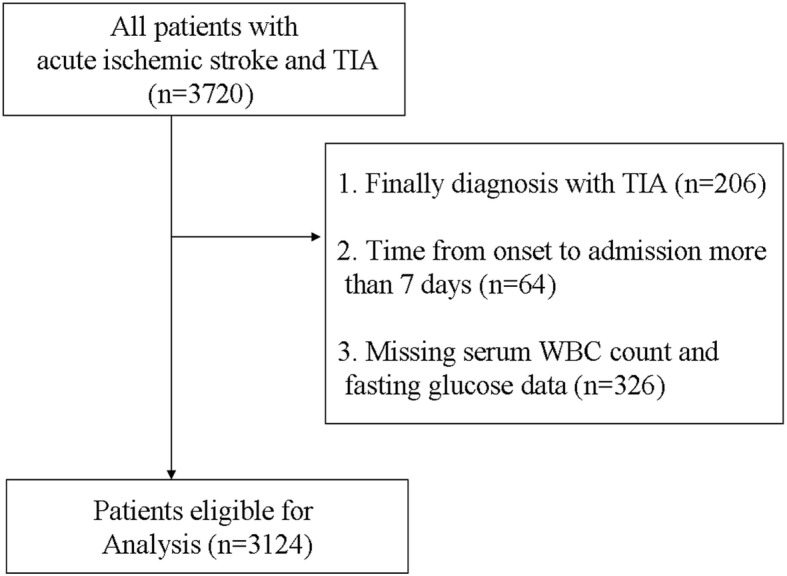
Patient flowchart. TIA indicates transient ischemic attack. WBC, white blood cell
Data collection and outcome assessment
We collected baseline information, including patient demographics, vascular risk factors, stroke severity (as measured by the National Institutes of Health Stroke Scale, NIHSS, and modified Rankin Scale, mRS), medication use, imaging data, and diagnosis-related information. Vascular risk factors included history of stroke, history of hypertension, history of diabetes mellitus, history of atrial fibrillation, history of coronary heart disease, current or previous smoking status, and alcohol consumption. Information on the aforementioned factors were obtained by interviews with patients or their family members (if patients were not able to communicate). Current smoking status was defined as having smoked at least one cigarette per day for the previous year or more. Data on the amount and type of alcohol consumed during the past year was collected. Alcohol consumption was defined as having consumed at least one alcoholic drink per day during the last year. Hypertension was defined as having a systolic blood pressure (BP) ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg or use of antihypertensive medications. Diabetes mellitus was defined as having fasting glucose ≥ 7.0 mmol/L (126 mg/dL), non-fasting glucose ≥ 11.1 mmol/L (200 mg/dL) with classic symptoms of hyperglycemia or hyperglycemic crisis, and use of glucose-lowering drugs. Atrial fibrillation was defined as having a history of atrial fibrillation, confirmed by ≥ 1 electrocardiogram or the presence of arrhythmia during hospitalization. Pneumonia after AIS was diagnosed by treating physicians according to the criteria of US Center for Disease Control and Prevention for hospital-acquired pneumonia, based on clinical and laboratory test [17]. Blood samples were collected within 24 h of hospital admission. The WBC count was determined at hospital admission by automated cell counters via standard techniques, and blood glucose and other biochemical parameters were analyzed enzymatically by automatic biochemical analyzer using fasting blood at local laboratories. The outcome of this study was all-cause in-hospital mortality and pneumonia.
Statistical analysis
Study participants were divided into four groups, based on serum WBC count and fasting glucose levels at admission: normal WBC count and normal glucose, NWNG (WBC count < 10 × 109/L and fasting glucose < 7.0 mmol/L), normal WBC count and higher glucose, NWHG (WBC count < 10 × 109/L and fasting glucose ≥ 7.0 mmol/L), higher WBC count and normal glucose, HWNG (WBC count ≥ 10 × 109/L and fasting glucose < 7.0 mmol/L), and higher WBC count and higher glucose, HWHG (WBC count ≥ 10 × 109/L and fasting glucose ≥ 7.0 mmol/L) [18, 19]. Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range [IQR]) and were compared using the analysis of variance or Wilcoxon rank-sum test. Categorical variables were expressed as frequency (%) and were compared using the chi-square test.
The crude cumulative risks of in-hospital mortality for each patient group based on admission serum WBC count and blood glucose were shown in a Kaplan-Meier plot and compared using the log-rank test. Crude and multivariable Cox proportional hazards regression model and logistic regression models were used to estimate the risk of in-hospital mortality and the risk of in-hospital pneumonia respectively. Hazard ratios (HRs), odds ratios (ORs), and 95% confidence interval (CIs) were calculated for each group with the NWNG as reference. Potential confounders that were adjusted in the multivariable models included age, sex, systolic BP, body temperature, time from onset to admission, cigarette smoking status, alcohol drinking, history of hypertension, history of diabetes mellitus, history of coronary heart disease, history of atrial fibrillation, history of stroke, thrombolysis treatment, baseline NIHSS score, Oxfordshire Community Stroke Project (OCSP) classification, and estimated glomerular filtration rate (eGFR) levels. To assess the robustness of the association between different serum WBC count and blood glucose levels and in-hospital mortality and pneumonia, we also performed sensitivity analyses (by restricting to patients with first-ever strokes and those with time from onset to admission ≤ 24 h) and conducted subgroup analysis (in patients with or without diabetes). In addition, we tested the discriminatory ability of WBC count and blood glucose (combined and separately) to predict in-hospital mortality and pneumonia by calculating C-statistics (areas under receiver operating characteristic [ROC] curves). All P values were two-tailed, and a significance level of 0.05 was used. All analyses were conducted using the SPSS Version 17.0 statistical software.
Results
Complete data on conventional risk factors and WBC count and blood glucose levels at admission were available for 3124 patients whose mean age was 68.6 years (± 12.9), with a median NIHSS score of 4.0 (IQR, 2.0–7.0). In comparison to NWNG participants, those with HWHG were more likely to be younger, male, and had more severe stroke (higher NIHSS) and other co-morbidities including hypertension, diabetes mellitus, coronary heart disease, and atrial fibrillation. HWHG patients also differed in metabolic profile (higher fasting glucose levels and serum total cholesterol, low-density lipoprotein cholesterol and WBC count level, and higher baseline diastolic BP and shorter time from onset to hospital) (Table 1).
Table 1.
Baseline characteristics of 3124 acute ischemic stroke patients according to white blood cell and blood glucose level
| Characteristicsa | NWNG | NWHG | HWNG | HWHG | P value |
|---|---|---|---|---|---|
| Number of subjects | 2025 | 681 | 266 | 152 | |
| Demographics | |||||
| Age, years | 68.8 ± 12.8 | 68.8 ± 11.4 | 66.5 ± 16.3 | 67.6 ± 14.3 | 0.031 |
| Male sex | 1181 (58.3) | 356 (52.3) | 170 (63.9) | 93 (61.2) | 0.004 |
| Cigarette smoking status | 420 (20.7) | 119 (17.5) | 54 (20.3) | 25 (16.4) | 0.206 |
| Alcohol consumption | 205 (10.1) | 62 (9.1) | 21 (7.9) | 16 (10.5) | 0.617 |
| Clinical features | |||||
| Time from onset to hospital, hours | 24.0 (6.0–72.0) | 24.0 (5.0–72.0) | 24.0 (4.0–48.0) | 12.0 (3.0–48.0) | < 0.001 |
| Hospital stay, days | 10.0 (8.0–13.0) | 11.0 (8.0–15.0) | 11.0 (7.0–15.0) | 12.0 (7.5–19.0) | < 0.001 |
| Baseline systolic BP, mm Hg | 151.0 ± 22.6 | 154.6 ± 22.0 | 153.4 ± 25.0 | 155.5 ± 22.6 | 0.001 |
| Baseline diastolic BP, mm Hg | 85.1 ± 13.0 | 85.7 ± 12.5 | 87.1 ± 14.7 | 86.1 ± 14.6 | 0.087 |
| TG, mmol/L | 1.2 (0.9–1.6) | 1.4 (1.0–2.2) | 1.1 (0.9–1.6) | 1.2 (0.9–1.8) | < 0.001 |
| TC, mmol/L | 4.5 (3.8–5.1) | 4.7 (4.0–5.5) | 4.6 (3.9–5.3) | 4.9 (4.2–5.6) | < 0.001 |
| LDL-C, mmol/L | 2.6 (2.1–3.2) | 2.7 (2.1–3.4) | 2.7 (2.2–3.2) | 3.1 (2.3–3.7) | < 0.001 |
| HDL-C, mmol/L | 1.2 (1.0–1.4) | 1.2 (1.0–1.4) | 1.2 (1.0–1.4) | 1.2 (1.0–1.5) | 0.079 |
| FG, mmol/L | 5.3 (4.9–5.9) | 8.9 (7.7–11.3) | 5.6 (5.0–6.1) | 9.2 (8.1–11.6) | < 0.001 |
| WBC, 103/uL | 6.3 (5.2–7.5) | 6.8 (5.6–8.0) | 11.5 (10.6–13.0) | 11.6 (10.6–13.7) | < 0.001 |
| eGFR, ml/min/1.73 m2 | 94.3 (76.2–114.8) | 99.3 (78.2–124.6) | 93.2 (71.2–117.2) | 94.9 (64.3–115.8) | 0.004 |
| Baseline NIHSS score | 3.0 (2.0–6.0) | 4.0 (2.0–7.0) | 6.0 (3.0–12.0) | 7.5 (3.5–15.0) | < 0.001 |
| Medical history | |||||
| History of hypertension | 1542 (76.1) | 569 (83.6) | 211 (79.3) | 129 (84.9) | < 0.001 |
| History of diabetes mellitus | 236 (11.7) | 458 (67.3) | 26 (9.8) | 84 (55.3) | < 0.001 |
| History of coronary heart disease | 97 (4.8) | 48 (7.0) | 15 (5.6) | 14 (9.2) | 0.029 |
| History of atrial fibrillation | 283 (14.0) | 108 (15.9) | 56 (21.1) | 30 (19.7) | 0.007 |
| History of stroke | 464 (22.9) | 144 (21.1) | 46 (17.3) | 39 (25.7) | 0.122 |
| Medication history | |||||
| Antihypertensive therapy | 1133 (56.0) | 449 (65.9) | 148 (55.6) | 102 (67.1) | < 0.001 |
| Antiplatelet therapy | 161 (8.0) | 52 (7.6) | 11 (4.1) | 10 (6.6) | 0.162 |
| Anticoagulation therapy | 21 (1.0) | 8 (1.2) | 3 (1.1) | 3 (2.0) | 0.810 |
| Antiglycemic therapy | 179 (8.8) | 338 (49.6) | 19 (7.1) | 49 (32.2) | < 0.001 |
| Statin therapy | 76 (3.8) | 13 (1.9) | 3 (1.1) | 3 (2.0) | 0.016 |
| Thrombolysis treatment | 43 (2.1) | 11 (1.6) | 12 (4.5) | 11 (7.2) | < 0.001 |
| Stroke syndrome | < 0.001 | ||||
| TACS | 143 (7.1) | 62 (9.1) | 46 (17.3) | 44 (28.9) | |
| PACS | 1076 (53.1) | 311 (45.7) | 129 (48.5) | 48 (31.6) | |
| POCS | 416 (20.5) | 209 (30.7) | 70 (26.3) | 48 (31.6) | |
| LACS | 390 (19.3) | 99 (14.5) | 21 (7.9) | 12 (7.9) | |
aContinuous variables are expressed as mean ± standard deviation or as median (interquartile range). Categorical variables are expressed as frequency (percent)
Abbreviations: BP blood pressure, TG triglycerides, TC total cholesterol, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, FG fasting glucose, eGFR estimated glomerular filtration rate, mRS modified Rankin Scale, NIHSS National Institutes of Health Stroke Scale, TACS total anterior circulation syndrome, PACS partial anterior circulation syndrome, POCS posterior circulation syndrome, LACS lacunar syndrome, Q quartile
During hospitalization, 104 patients (3.3%) died from all causes. HWHG patients had the highest cumulative incidence of in-hospital mortality (log-rank P < 0.001; Fig. 2). In the unadjusted model, the HR of in-hospital mortality was significantly higher among study participants with admission HWHG, NWHG, and HWNG compared with NWNG (P trend < 0.001). After adjusting for age, sex, time from onset to admission, baseline NIHSS score, and other covariates, the HR (95% CI) of admission HWHG was 2.22 (1.21–4.07) and HWNG was 2.08 (1.15–3.78) for mortality, as compared with NWNG (P trend = 0.003) (Table 2). HWHG was also shown to be associated with a higher risk of in-hospital mortality in all sensitivity analyses (Table 2).
Fig. 2.
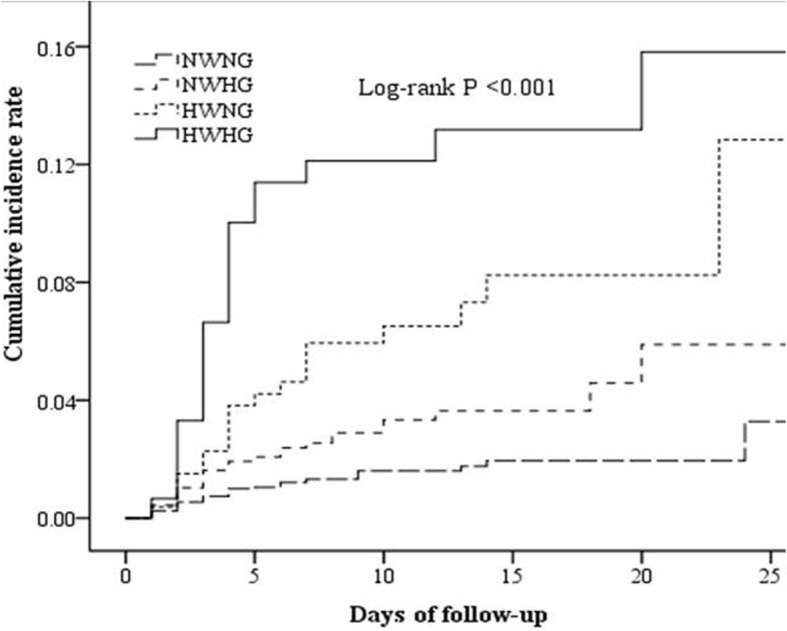
Cumulative incidence curves of in-hospital mortality by WBC count and blood glucose level. WBC indicates white blood cell; NWNG, normal WBC count and normal glucose; NWHG, normal WBC count and higher glucose; HWNG, higher WBC count and normal glucose; HWHG, higher WBC count and higher glucose
Table 2.
Hazard ratios and 95% confidence intervals of in-hospital mortality according to level of white blood cell and blood glucose
| NWNG | NWHG | HWNG | HWHG | P trend | |
|---|---|---|---|---|---|
| No. | 2025 | 681 | 266 | 152 | |
| No. of deaths | 35 (1.7) | 26 (3.8) | 19 (7.1) | 24 (15.8) | |
| Crude | 1.00 | 2.02 (1.21–3.56) | 3.89 (2.21–6.77) | 7.59 (4.47–12.88) | < 0.001 |
| Model 1 | 1.00 | 2.12 (1.27–3.54) | 3.95 (2.26–6.91) | 7.68 (4.53–13.01) | < 0.001 |
| Model 2 | 1.00 | 1.46 (0.84–2.56) | 2.08 (1.15–3.78) | 2.22 (1.21–4.07) | 0.003 |
| Sensitivity analysis | |||||
| Model 3 | 1.00 | 1.28 (0.68–2.40) | 1.69 (0.88–3.25) | 2.11 (1.04–4.31) | 0.022 |
| Model 4 | 1.00 | 1.37 (0.75–2.50) | 2.16 (1.18–3.98) | 1.72 (0.88–3.37) | 0.025 |
Model 1, adjusted for age and sex;
Model 2, adjusted for age, sex, systolic BP, body temperature, time from onset to admission, cigarette smoking status, alcohol drinking, history of hypertension, history of diabetes mellitus, history of coronary heart disease, history of atrial fibrillation, history of stroke, thrombolysis treatment, baseline National Institutes of Health Stroke Scale score, eGFR levels, Oxfordshire Community Stroke Project classification, and pneumonia
Model 3, adjusted for model 2 and further restricted to patients with first-ever stroke
Model 4, adjusted for model 2 and further restricted to patients with time from onset to admission ≤ 24 h
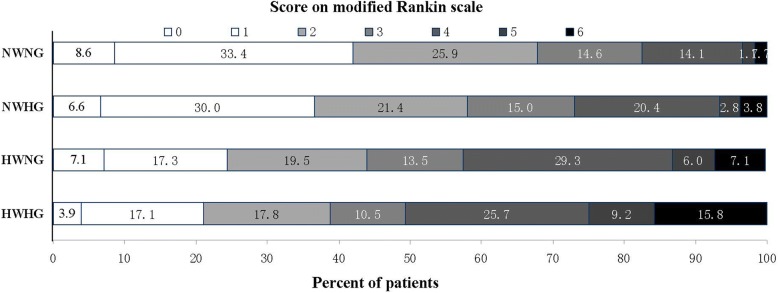
NWNG patients had a median mRS score of 2 (IQR 1–3), in comparison to HWHG patients with a median mRS score of 4 (IQR 2–4.5) during hospital discharge. Figure 3 shows the distribution of disability scores on the mRS during hospital discharge for patients classified according to combined WBC count and blood glucose levels (p < 0.001).
Fig. 3.
Relationship between mRS and combined WBC count and blood glucose type during hospital discharge. mRS indicates modified Rankin Scale; WBC, white blood cell; NWNG, normal WBC count and normal glucose; NWHG, normal WBC count and higher glucose; HWNG, higher WBC count and normal glucose; HWHG, higher WBC count and higher glucose
During hospitalization, 535 patients (17.1%) had pneumonia. In the unadjusted regression model, the odds of pneumonia were significantly higher among HWHG, NWHG, and HWNG participants compared with NWNG patients (P trend < 0.001). After adjusting for age, sex, time from onset to admission, baseline NIHSS score, eGFR, and other traditional risk factors, the OR (95% CI) for the HWHG group was 2.61 (95% CI 1.66–4.10) and HWNG group was 2.05 (95% CI 1.45–2.91) as compared with the NWNG for pneumonia (P trend < 0.001) (Table 3). Similar associations between HWHG, HWNG, and pneumonia were shown in all sensitivity analyses (Table 3).
Table 3.
Odds ratios and 95% confidence intervals of pneumonia according to level of white blood cell and blood glucose
| NWNG | NWHG | HWNG | HWHG | P trend | |
|---|---|---|---|---|---|
| No. | 2025 | 681 | 266 | 152 | |
| No. of Pneumonia | 265 (13.1) | 125 (18.4) | 85 (32.0) | 60 (39.5) | |
| Crude | 1.00 | 1.49 (1.18–1.89) | 3.12 (2.34–4.16) | 4.33 (3.05–6.15) | < 0.001 |
| Model 1 | 1.00 | 1.59 (1.25–2.02) | 3.67 (2.70–5.00) | 5.26 (3.61–7.66) | < 0.001 |
| Model 2 | 1.00 | 1.34 (0.99–1.83) | 2.05 (1.45–2.91) | 2.61 (1.66–4.10) | < 0.001 |
| Sensitivity analysis | |||||
| Model 3 | 1.00 | 1.13 (0.79–1.63) | 2.15 (1.45–3.17) | 2.43 (1.44–4.10) | < 0.001 |
| Model 4 | 1.00 | 1.27 (0.88–1.82) | 1.61 (1.06–2.45) | 2.48 (1.46–4.20) | < 0.001 |
Model 1, adjusted for age and sex
Model 2, adjusted for age, sex, systolic BP, body temperature, time from onset to admission, cigarette smoking status, alcohol drinking, history of hypertension, history of diabetes mellitus, history of coronary heart disease, history of atrial fibrillation, history of stroke, thrombolysis treatment, baseline National Institutes of Health Stroke Scale score, Oxfordshire Community Stroke Project classification, and eGFR levels
Model 3, adjusted for model 2 and further restricted to patients with first-ever stroke
Model 4, adjusted for model 2 and further restricted to patients with time from onset to admission ≤24 h
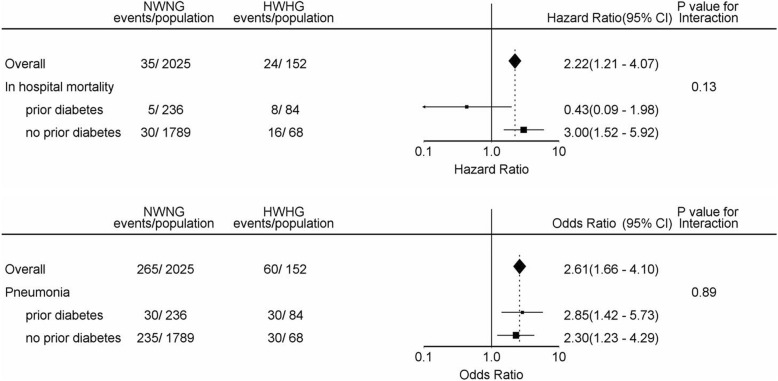
Subgroup analysis showed that HWHG was associated with in-hospital mortality and pneumonia in patients with and without diabetes. No significant interaction was observed (P-interaction > 0.05 for all, Fig. 4).
Fig. 4.
Association between co-existing higher WBC count and higher blood glucose on in-hospital mortality and pneumonia in AIS patients with or without diabetes. WBC indicates white blood cell; AIS, acute ischemic stroke; NWNG, normal WBC count and normal glucose; HWHG, higher WBC count and higher glucose
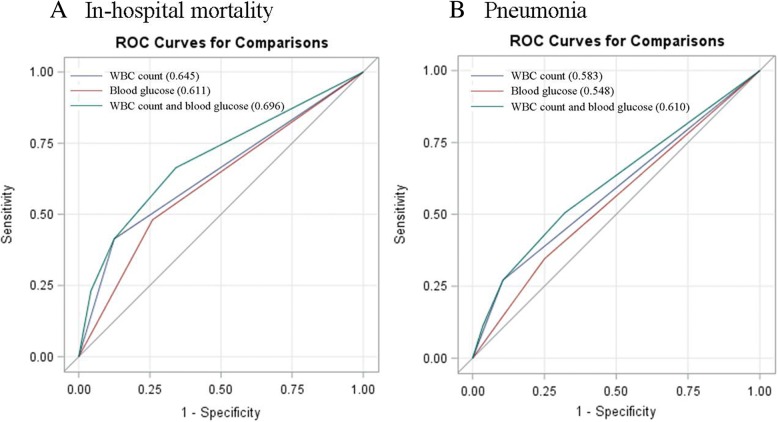
ROC curves comparing the predictive ability of combined WBC count and blood glucose, WBC count or blood glucose alone on in-hospital mortality and pneumonia are shown in Fig. 5a and b. The C-statistic was significantly greater for the combined effect of WBC count and blood glucose than WBC count or blood glucose alone for both outcomes (all p < 0.01).
Fig. 5.
a ROC curve of combined WBC count and blood glucose level on in-hospital mortality. ROC indicates receiver operating characteristic; WBC, white blood cell. b ROC curves of combined WBC count and blood glucose level on in-hospital pneumonia. ROC indicates receiver operating characteristic; WBC, white blood cell
Discussion
The present study of 3124 patients demonstrated the prognostic effect of combined high WBC count and high glucose on in-hospital mortality and pneumonia in AIS. Patients with high WBC count and high glucose level appeared to be associated with a 2.22-fold and 2.61-fold increase in the risk of in-hospital mortality and pneumonia respectively, as well as poor functional outcome at discharge. The combined effect of high WBC count and high glucose on in-hospital mortality and pneumonia were comparable in patients with or without diabetes. Furthermore, the predictive value of combined WBC and glucose levels on in-hospital mortality and pneumonia appears to be better than WBC or glucose alone.
A growing body of studies has identified an association between high WBC or high glucose at admission and mortality or poor outcome after AIS [2, 4, 5, 20, 21]. However, few studies have investigated the combined effect of high WBC count and high blood glucose on poor outcome and mortality in patients with MI and AIS [14–16, 22]. It is plausible that inflammatory and stress response are the potential causative factors leading to raised WBC and blood glucose levels after AIS [2, 10–13, 23]. The Japanese Acute Coronary Syndrome Study (JACSS) reported that combined WBC count and blood glucose level were independently associated with in-hospital mortality in patients with AMI [14]. A study of 436 AIS patients also indicated that HWHG significantly increased the risk of poor outcome at hospital discharge compared to patients with NWNG [16]. Zhou et al. study also found a combined effect of hyperglycemia and inflammation markers, including elevated WBC count on the neurologic deficiency or death at discharge in AIS patients [23]. Consistent with prior literatures, the current study of 3124 AIS patients showed coexistence of high WBC count and blood glucose was associated with 2.22-fold of in-hospital mortality compared to those with normal WBC count and blood glucose. We also found the predictive value of combined WBC count and blood glucose for in-hospital mortality was better than WBC count or blood glucose alone.
Prior studies had demonstrated high WBC and high blood glucose separately as independent predictors of pneumonia after AIS [2, 6, 7, 10]. The latest study from the Netherlands also revealed that AIS patients with admission blood glucose ≥ 7.8 mmol/L had 2.31-fold increased risk of pneumonia [10]. However, little evidence exists to support an association between a combination effect of WBC count and blood glucose and the pneumonia after AIS. Our study is the first to show a combined predictive effect of WBC count and blood glucose on pneumonia after AIS and that the predictor value of combined WBC count and blood glucose is better than WBC count or blood glucose alone. These results indirectly suggest that elevated blood glucose is an inflammation marker after AIS, consistent with previous studies [10, 11, 24, 25].
Several studies including a systematic review indicated an association between high blood glucose and short-term mortality after AIS in patients without diabetes but not in diabetic patients [4, 26–28]. The relationship between admission hyperglycemia and the post-stroke pneumonia was also shown in nondiabetic patients but not in diabetic patients [10]. In the present study, we found the combined effect of WBC count and blood glucose on in-hospital mortality and pneumonia was not significantly different in patients with or without diabetes. Therefore, combined WBC count and blood glucose maybe was a more useful predictor for mortality and pneumonia for all patients (diabetic and non-diabetic).
Strengths of our study include having a large dataset of patients from multiple centers and being the first study to evaluate the combination effect of WBC count and blood glucose on in-hospital mortality and pneumonia. However, there are still some potential limitations that merit consideration. First, this cohort included some patients whose time from onset to admission exceeded 24 h; therefore, the levels of WBC and blood glucose might not accurately reflect the levels at stroke onset. However, our sensitivity analysis showed that the significance of the association remained when we restricted to patients with time from onset to admission ≤ 24 h. Secondly, a proportion of patients were excluded due to a lack of WBC and blood glucose data, which may cause selection bias. Thirdly, we were precluded investigating the possible mechanism between WBC count and blood glucose and in-hospital mortality as we lacked information on the exact cause of death. Also, data on pre-stroke disability and Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification, which may also influence in-hospital mortality, were not collected. Finally, the follow-up period of our study is relatively short; thus, we were unable to evaluate the combined long-term effect of WBC count and blood glucose on AIS outcomes and the values of area under ROC curve in both models in outcome prediction were relatively low.
Conclusion
Co-existing high WBC count and high glucose at admission was independently associated with in-hospital mortality and pneumonia in acute stroke patients and its predictive value is better than WBC count and blood glucose alone. The association between combined WBC count and glucose and in-hospital mortality and pneumonia was not different by diabetes status.
Additional file
Investigation group. (DOC 31 kb)
Acknowledgements
We thank the study participants and their relatives and the clinical staff for their support and contribution to this study.
Funding
This work was supported in part by grants from the National Natural Science Foundation of China (81471195), Basic research of Suzhou Medical and health care (SYS201724), Suzhou Clinical Research Center of Neurological Disease (Szzx201503), the Second Affiliated Hospital of Soochow University Preponderant Clinic Discipline Group Project Funding (XKQ2015002). Suzhou health science and technology project (Gwzx201503). This was also partly supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Availability of data and materials
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AIS
Acute ischemic stroke
- AMI
Acute myocardial infarction
- BP
Blood pressure
- CI
Confidence interval
- CT
Computed tomography
- eGFR
Estimated glomerular filtration rate
- FG
Fasting glucose
- HR
Hazard ratio
- HWHG
Higher WBC count and higher glucose
- HWNG
Higher WBC count and normal glucose
- IQR
Interquartile range
- JACSS
Japanese Acute Coronary Syndrome Study
- MRI
Magnetic resonance imaging
- mRS
Modified Rankin Scale score
- NIHSS
National Institutes of Health Stroke Scale
- NWHG
Normal WBC count and higher glucose
- NWNG
Normal WBC count and normal glucose
- OCSP
Oxfordshire Community Stroke Project
- OR
Odds ratio
- ROC
Receiver operating characteristic
- SD
Standard deviation
- TIA
Transient ischemic attack
- TOAST
Trial of Org 10172 in Acute Stroke Treatment
- WBC
White blood cell
Authors’ contributions
SJY, YJC, and CFL contributed to the concept and rationale for the study. SJY and WZ were responsible for the first draft. DZ, SJY, and CKZ contributed statistical analyses. ZJO, XFD, CHQ, and TSL performed the data collection. YJC and CFL contributed to the first revision. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Soochow University, as well as ethical committees at the participating hospitals. Written consent was obtained from all study participants or their immediate family members.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shoujiang You, Email: 0319503013@163.com.
Zhijie Ou, Email: m18351637964@163.com.
Wei Zhang, Email: dadazhangwei@126.com.
Danni Zheng, Email: dzheng@georgeinstitute.org.au.
Chongke Zhong, Email: zckzxysuda@163.com.
Xiaofeng Dong, Email: dxf561735@126.com.
Chenhong Qiu, Email: 13962528227@163.com.
Taosheng Lu, Email: lutaosheng@163.com.
Yongjun Cao, Phone: (86) 512-6778-3663, Email: yongjuncao@126.com.
Chun-Feng Liu, Phone: (86) 512-6778-3307, Email: liuchunfeng@suda.edu.cn.
References
- 1.Christensen H, Boysen G. C-reactive protein and white blood cell count increases in the first 24 hours after acute stroke. Cerebrovasc Dis. 2004;18:214–219. doi: 10.1159/000079944. [DOI] [PubMed] [Google Scholar]
- 2.Furlan JC, Vergouwen MD, Fang J, Silver FL. White blood cell count is an independent predictor of outcomes after acute ischaemic stroke. Eur J Neurol. 2014;21:215–222. doi: 10.1111/ene.12233. [DOI] [PubMed] [Google Scholar]
- 3.Allport L, Baird T, Butcher K, Macgregor L, Prosser J, Colman P, et al. Frequency and temporal profile of poststroke hyperglycemia using continuous glucose monitoring. Diabetes Care. 2006;29:1839–1844. doi: 10.2337/dc06-0204. [DOI] [PubMed] [Google Scholar]
- 4.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 5.Kazmierski R, Guzik P, Ambrosius W, Ciesielska A, Moskal J, Kozubski W. Predictive value of white blood cell count on admission for in-hospital mortality in acute stroke patients. Clin Neurol Neurosurg. 2004;107:38–43. doi: 10.1016/j.clineuro.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Harms H, Grittner U, Droge H, Meisel A. Predicting post-stroke pneumonia: the PANTHERIS score. Acta Neurol Scand. 2013;128:178–184. doi: 10.1111/ane.12095. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Zhang LH, Xu WP, Hu JM. Risk assessment of ischemic stroke associated pneumonia. World J Emerg Med. 2014;5:209–213. doi: 10.5847/wjem.j.issn.1920-8642.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baird TA, Parsons MW, Phan T, Butcher KS, Desmond PM, Tress BM, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208–2214. doi: 10.1161/01.STR.0000085087.41330.FF. [DOI] [PubMed] [Google Scholar]
- 9.Ji R, Shen H, Pan Y, Wang P, Liu G, Wang Y, et al. Novel risk score to predict pneumonia after acute ischemic stroke. Stroke. 2013;44:1303–1309. doi: 10.1161/STROKEAHA.111.000598. [DOI] [PubMed] [Google Scholar]
- 10.Zonneveld TP, Nederkoorn PJ, Westendorp WF, Brouwer MC, van de Beek D, Kruyt ND. Hyperglycemia predicts poststroke infections in acute ischemic stroke. Neurology. 2017;88:1415–1421. doi: 10.1212/WNL.0000000000003811. [DOI] [PubMed] [Google Scholar]
- 11.Slowik A, Turaj W, Pankiewicz J, Dziedzic T, Szermer P, Szczudlik A. Hypercortisolemia in acute stroke is related to the inflammatory response. J Neurol Sci. 2002;196:27–32. doi: 10.1016/S0022-510X(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 12.O'Neill PA, Davies I, Fullerton KJ, Bennett D. Stress hormone and blood glucose response following acute stroke in the elderly. Stroke. 1991;22:842–847. doi: 10.1161/01.STR.22.7.842. [DOI] [PubMed] [Google Scholar]
- 13.Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol. 2010;6:145–155. doi: 10.1038/nrneurol.2009.231. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara M, Kojima S, Sakamoto T, Asada Y, Kimura K, Miyazaki S, et al. Usefulness of combined white blood cell count and plasma glucose for predicting in-hospital outcomes after acute myocardial infarction. Am J Cardiol. 2006;97:1558–1563. doi: 10.1016/j.amjcard.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 15.Kosuge M, Kimura K, Morita S, Kojima S, Sakamoto T, Ishihara M, et al. Combined prognostic utility of white blood cell count, plasma glucose, and glomerular filtration rate in patients undergoing primary stent placement for acute myocardial infarction. Am J Cardiol. 2009;103:322–327. doi: 10.1016/j.amjcard.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 16.Yoon SS, Zheng ZJ. Elevated total white blood cell count with high blood glucose is associated with poor outcome after ischemic stroke. J Stroke Cerebrovasc Dis. 2005;14:88–93. doi: 10.1016/j.jstrokecerebrovasdis.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 18.Grond-Ginsbach C, Giossi A, Aksay SS, Engelter ST, Lyrer PA, Metso TM, et al. Elevated peripheral leukocyte counts in acute cervical artery dissection. Eur J Neurol. 2013;20:1405-10. [DOI] [PubMed]
- 19.Xu RB, Kong X, Xu BP, Song Y, Ji M, Zhao M, et al. Longitudinal association between fasting blood glucose concentrations and first stroke in hypertensive adults in China: effect of folic acid intervention. Am J Clin Nutr. 2017;105:564–570. doi: 10.3945/ajcn.116.145656. [DOI] [PubMed] [Google Scholar]
- 20.Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A, et al. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology. 2002;59:67–71. doi: 10.1212/WNL.59.1.67. [DOI] [PubMed] [Google Scholar]
- 21.Hu GC, Hsieh SF, Chen YM, Hu YN, Kang CL, Chien KL. The prognostic roles of initial glucose level and functional outcomes in patients with ischemic stroke: difference between diabetic and nondiabetic patients. Disabil Rehabil. 2012;34:34–39. doi: 10.3109/09638288.2011.585213. [DOI] [PubMed] [Google Scholar]
- 22.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373:1798–1807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Wu J, Zhang J, Xu T, Zhang H, Zhang Y, et al. Association of stroke clinical outcomes with coexistence of hyperglycemia and biomarkers of inflammation. J Stroke Cerebrovasc Dis. 2015;24:1250–1255. doi: 10.1016/j.jstrokecerebrovasdis.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Ata A, Lee J, Bestle SL, Desemone J, Stain SC. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg. 2010;145:858–864. doi: 10.1001/archsurg.2010.179. [DOI] [PubMed] [Google Scholar]
- 25.Richards JE, Hutchinson J, Mukherjee K, Jahangir AA, Mir HR, Evans JM, et al. Stress hyperglycemia and surgical site infection in stable nondiabetic adults with orthopedic injuries. J Trauma Acute Care Surg. 2014;76:1070–1075. doi: 10.1097/TA.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 26.Hu GC, Hsieh SF, Chen YM, Hsu HH, Hu YN, Chien KL. Relationship of initial glucose level and all-cause death in patients with ischaemic stroke: the roles of diabetes mellitus and glycated hemoglobin level. Eur J Neurol. 2012;19:884–891. doi: 10.1111/j.1468-1331.2011.03647.x. [DOI] [PubMed] [Google Scholar]
- 27.Stollberger C, Exner I, Finsterer J, Slany J, Steger C. Stroke in diabetic and non-diabetic patients: course and prognostic value of admission serum glucose. Ann Med. 2005;37:357–364. doi: 10.1080/07853890510037356. [DOI] [PubMed] [Google Scholar]
- 28.Stead LG, Gilmore RM, Bellolio MF, Mishra S, Bhagra A, Vaidyanathan L, et al. Hyperglycemia as an independent predictor of worse outcome in non-diabetic patients presenting with acute ischemic stroke. Neurocrit Care. 2009;10:181–186. doi: 10.1007/s12028-008-9080-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Investigation group. (DOC 31 kb)
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.