Cardiovascular disease and uraemia
Uraemic syndrome can be defined as the combination of all clinical problems that occur when kidney function is progressively declining. Those are to a large extent attributed to the retention of compounds, in the body, that under normal conditions would be removed from the blood stream by the healthy kidneys; they are therefore named uraemic retention solutes. As long as these molecules exert biological/biochemical impact, they are called uraemic toxins, and in that case they contribute to the uraemic syndrome. Some uraemic retention products may be useful markers of solute removal and retention, even if they are not toxic.
A highly bothersome complication of chronic kidney disease (CKD) is the concomitant cardiovascular damage. Although first recognized more than three decades ago [1], the true dimensions of the problem became clear only some 10 years ago [2]. The general impact on public health has long been underestimated, but is now accepted to be at least as important as that of diabetic cardiovascular damage [3].
Whereas cardiovascular disease was originally considered relevant especially in the dialysed population [2], and hence seemed to affect only a minor fraction of the general population, later on it became clear that increased morbidity and mortality were present at much earlier stages of CKD, long before the development of the need for dialysis. There is now a general consensus that cardiovascular risk increases from a glomerular filtration rate (GFR) of 60 mL/ min/1.73 m2 on [3], which corresponds to a loss of kidney function of only 50%. According to some studies, risk becomes significant even earlier, from a GFR of 90 mL/min/1.73 m2 on [4]. Consequently, the group at risk extends over a large proportion of the general population, allegedly from 7 to 30% of all subjects. Since it is conceivable that uraemic retention starts from the moment kidney function decreases, the solutes that we will describe below start to play a role already at these early stages of kidney failure.
Of note, the quality of vessels is to a large extent different in the uraemic population, as compared to the population with normal kidney function, irrespective of stenotic or thrombotic lesions.
The arterial system undergoes a specific remodelling process, characterized by increased wall stiffness in parallel with the decline in renal function [5], resulting in a decreased dampening of pressure and flow oscillations and a progressive disturbance of the normal ventricular–vascular coupling. The ejection of the same blood volume in a stiffer central arterial system generates a high-pressure wave, which propagates faster towards the periphery (increased pulse wave velocity) and whose reflection comes back earlier during the cardiac cycle (wave reflection). The resulting mismatch between the higher systolic workload and decreased diastolic perfusion leads to a lower coronary reserve, irrespective of stenotic or thrombotic lesions.
To some extent, arterial stiffness can be attributed to medial wall calcification or ‘elastocalcinosis’, a specific feature of the overall increased propensity of patients with kidney disease to develop calcifications.
Although patients suffering from CKD are frequently affected by a number of other conditions and/or comorbidities that enhance cardiovascular risk, such as hypertension, insulin resistance, fluid overload, anaemia, diabetes mellitus and dyslipidaemia, the weight of these factors per se has been shown to be insufficient to explain the entire uraemic cardiovascular problem; therefore, it has been suggested that factors specific to CKD such as the uraemic milieu must play a central role [6].
One of these factors is (micro) albuminuria, although this might be considered as a marker of endothelial damage rather than a real cause. Another likely and more direct explanation is the role played by uraemic retention solutes [7]. Several of these compounds have been shown to have the capacity to cause cardiovascular damage [7], and this property is conceivably intensified by their increased concentration following the incapacity of the kidneys to excrete them.
In this article, we will first review current knowledge on uraemic toxins with a potential cardiovascular impact; we will then give an example of how this knowledge can be translated into therapeutic measures improving outcome in the population with CKD. Finally, we will consider options for the future and we will try to outline strategies showing how this knowledge can be extended and applied for a further improvement of the clinical condition of the affected patients.
Potential culprits: baseline in vitro and epidemiological data
Several uraemic retention solutes have been associated with cardiovascular damage [7]. Especially during the last decade, knowledge about compounds with the potential to affect the cardiovascular system has been continuously growing [7]. Due to space limitations, in what follows we will give a few examples, especially on compounds regarding which our current knowledge has recently been changed fundamentally. An extensive overview per uraemic solute of effects with the potential to damage heart or vessels and of epidemiological studies linking these compounds to outcome is given in Table 1.
Table 1.
Uraemic retention solutes with cardiovascular damaging potential
| Vascular damaging effect | Clinical correlate | |
|---|---|---|
| Small water-soluble compounds | ||
| ADMA | Vasoconstriction | Vascular outcome |
| Inhibition iNOS | ||
| Guanidino compounds | Inflammation | |
| Phosphate | Calcification | Outcome |
| SDMA | Inhibition iNOS | |
| Leukocyte activation | ||
| Protein-bound moleculesa | ||
| Homocysteine | Leukocyte activation | Outcomeb |
| Smooth muscle cell proliferation | ||
| Endothelial function | ||
| Indoles | Endothelial function | |
| P-cresylsulphate | Leukocyte activation | Outcomec |
| Hospitalizationc | ||
| Uraemic symptomsc | ||
| Phenylacetic acid | Inhibition iNOS | |
| Middle molecules | ||
| AGEs | Inflammation | |
| AOPPs | Inflammation | |
| Angiotensin A | Vasoconstriction | |
| β2-microglobulin | Monocyte migrationd | Outcome |
| Cytokine secretiond | ||
| BFGF | Smooth muscle cell proliferation | |
| Complement factor D | Activation alternative complement pathway | |
| Cytokines | Inflammatione | Outcome |
| Endothelial function | ||
| Thrombocyte activation | ||
| Smooth muscle cell proliferation | ||
| Dinucleotide polyphosphates | Vasoconstriction | |
| Smooth muscle cell proliferation | ||
| Leukocyte activation | ||
| Endothelin | Vasoconstriction | |
| Arterial stiffness | ||
| Oxidative stress | ||
| Inflammation | ||
| Insulin resistance | ||
| Hyaluronic acid | Expression VCAM-1 | Outcome |
| Expression MCP-1 | ||
| Leptin | Inflammation | CRP |
| Endothelial function | Nutritional status | |
| Thrombocyte activation | ||
| Neuropeptide-Y | Vasoconstriction | Cardiovascular complications |
| Parathyroid hormone | Inflammation | Outcome |
| Vessel-wall calcification | ||
| Resistin | Inflammation | |
| Insulin resistance |
ADMA: asymmetric dimethylarginine; iNOS: inducible nitric oxide synthase (endothelial protection); SDMA: symmetric dimethylarginine; AGEs: advanced glycation end products; AOPPs: advanced oxidation protein products; BFGF: basic fibroblast growth factor; VCAM-1: vascular cell adhesion molecule-1; MCP-1: monocyte chemoattractant protein-1; CRP: C-reactive protein.
aProtein-bound middle molecules will be mentioned in the section devoted to middle molecules and will be marked in italics.
bIf corrected for nutritional status.
cP-cresylsulphate measured as p-cresol after acidic hydrolysis.
dOnly AGE-modified β2-microglobulin AGE-modified.
eOnly for pro-inflammatory cytokines.
Small water-soluble compounds
• Guanidines.
Guanidines are, in a comparable way to urea, small and water-soluble products of protein breakdown. Historically, since long they have been considered as neurotoxins, but more recently, they were also linked to vascular damage, as it was demonstrated that several of the solutes of this group activated leukocyte function [8]. In addition, guanidines were also shown to modify albumin structure, in such a way as to decrease the protein binding of homocysteine (Hcy) [9], hence stimulating the release of free, active Hcy and enhancing cardiovascular damaging potential.
The concentration of another guanidine, asymmetric dimethylarginine (ADMA), has been related to several parameters of vascular outcome [10,11]. Symmetric dimethylarginine (SDMA), a structural variant of ADMA, was considered inert until recently, but has now equally been suggested to be related to vascular damage by inhibition of inducible nitric oxide synthase [12].
In spite of being small and water soluble, guanidines nevertheless show a kinetic behaviour that diverges from that of urea [13,14].
• Phosphate.
Phosphate is considered as one of the major uraemic toxins involved in the early phases of vascular calcification. More importantly, in contrast to its previously believed passive role in chemical complexation with calcium due to supersaturation of plasma, recent evidence indicates that phosphate may induce direct cellular effects. This involves the activation of genes (Cbfa-1) that cause vascular smooth cell transdifferentiation into osteoblast-like cells [15] capable of producing hydroxyappatite crystals. Therefore, arterial calcification is currently considered an active regulated process, similar to bone formation. Although large epidemiological studies have shown an independent relationship between phosphate concentrations and the outcome [16], the net effect also depends on levels of calcium and the integrity of counterregulatory mechanisms. Of potential clinical importance are systemic calcification inhibitors, such as fetuin-A, as well as local inhibitors such as matrix-GLA protein (vitamin K dependent, inhibited by warfarin), pyrophosphate (water soluble and eliminated by dialysis) and osteoprotegerin. Finally, the calcification process is also influenced by the complex interaction of disturbances in phosphorus handling and calcium balance with bone buffering capacity, level of parathyroid activity and vitamin D status.
Protein-bound molecules
• Homocysteine.
Homocysteine (Hcy), a sulphur-containing amino acid, is produced by the demethylation of dietary methionine. Moderate hyperhomocysteinaemia is an independent risk factor for cardiovascular disease in the general population [17]. Patients with chronic kidney failure have serum Hcy levels two to fourfold above normal. Hcy increases the proliferation of vascular smooth muscle cells, one of the most prominent hallmarks of atherosclerosis [18]. Moderate hyperhomocysteinaemia may induce endothelial dysfunction and generate oxidative oxygen species [19]. Hcy-induced superoxide anion generation is responsible for NF-κB activation and subsequent monocyte chemoattractant protein-1 (MCP-1) expression in macrophages [20] inducing inflammatory responses. The administration of excessive quantities of the Hcy precursor methionine to rats induces atherosclerosis-like alterations in the aorta [21]. Hcy also disrupts several anticoagulant functions in the vessel wall, which results in enhanced thrombogenicity [22]. Studies of the potential of folic acid or 5-methyltetrahydrophosphate (MTHF) to decrease Hcy levels emanated in contradictory results. Touam et al. were able to reduce total Hcy to normal in ∼50% of the studied population, by administration of folinic acid, a precursor of MTHF [23]. The study of van Guldener et al., on the other hand, showed that even when it was possible to decrease Hcy levels therapeutically, carotid artery stiffness was not altered [24].
• Indoles.
Indoles are a group of protein-bound compounds, which are generated by chemical transformation processes such as conjugation. Indoxyl sulphate, the most abundant indolic compound in uraemia, has been linked to endothelial damage, inhibition of endothelial regeneration and repair and endothelial free-radical production [25,26].
• P-cresylsulphate.
Although most of the pioneering research on phenolic compounds has been focused on the concentration and the toxicity of the mother compound p-cresol, later work revealed that genuine p-cresol was present at very low concentrations in patients with renal failure and most of the p-cresol, generated by the intestinal flora, was conjugated to p-cresylsulphate in the intestinal wall and p-cresylglucuronide in the liver [27,28]. Both conjugates are characterized by a strong protein binding. The reason for the incorrect previous emphasis on p-cresol was due to the fact that most determination methods were based on deproteinization by acidification, causing disintegration of the conjugates by hydrolysis. Application of deproteinization methods without acidification revealed the presence of the conjugate p-cresylsulphate [27]. Further studies indicated that the biochemical impact of the mother compound p-cresol is not necessarily the same as that of the conjugate. Whereas p-cresol suppresses activity of leukocytes, especially after their activation, p-cresylsulphate essentially appeared to be linked to baseline leukocyte activation [29]. Nevertheless, since there is probably a correlation between former p-cresol estimations and current p-cresylsulphate measurements, previously held conclusions regarding p-cresol about protein binding and relationship with clinical outcome parameters [30–32] are very likely still valid for the conjugates as well.
• Phenylacetic acid.
Phenylacetic acid (PAA) is a degradation product of the amino acid phenylalanine. Plasma concentrations of PAA in stage 5 CKD patients strongly exceeded those in healthy controls. PAA was identified as an inhibitor of Ca2+-ATPase activity [33] and was shown to inhibit expression of inducible nitric oxide synthase (iNOS), a protective enzyme of the endothelium [34]. Consequently, inhibition of NO production might reinforce vascular damage. Recently, PAA concentrations in CKD stage 5 patients were shown to correlate with arterial properties corresponding to vascular damage [35].
Middle molecules
• Advanced glycation end products (AGEs) and advanced oxidation protein products (AOPPs).
AGEs and AOPPs are generated by oxidative processes and are often incorporated into larger molecules and peptide/protein structures. Although the basic elements composing AGEs and AOPPs do not necessarily have a high molecular weight, once they are linked to peptidic or protein structures, their molecular weight becomes important enough to hamper their removal by standard dialysis strategies [36].
AGEs and AOPPs activate inflammatory processes, which are involved in vascular damage [37,38]. Recent data suggest that AOPP concentrations measured with classical methods [39] are biased by a background noise created by triglycerides, coagulation factors and very likely other as yet unidentified factors [40,41].
• Angiotensin A.
Next to genuine angiotensin, structural variants, such as angiotensin A, characterized by the decarbolization of the asparagine molecule in the angiotensin peptide, have been described [42]. These structural variants also have vasoactive properties, albeit not necessarily to the same extent as genuine angiotensin II; the concentration of angiotensin A is relatively increased in CKD as compared to subjects with normal kidney function. It is conceivable that other variants of angiotensin might exist, be retained in kidney disease and play a pathophysiological role in vascular dysfunction as well.
• Cytokines.
Related to the strong association among atherosclerosis, malnutrition and inflammation [43], pro-inflammatory cytokine system activity is elevated in CKD [44]. Concentration of pro-inflammatory cytokines is correlated to adjusted mortality of haemodialysis patients [44].
• Dinucleotide polyphosphates.
Dinucleoside polyphosphates are a group of substances involved in the regulation of vascular tone, as well as in the proliferation of vascular smooth muscle cells [45] and mesangial cells [46]. Specific members of this group, diadenosine polyphosphates, were detected in hepatocytes, human plasma [47] and platelets. In addition, concentrations of diadenosine polyphosphates were increased in platelets from haemodialysis patients [48]. Recently, uridine adenosine tetraphosphate (Up4A) was isolated and identified as a novel endothelium-derived vasoconstrictive factor. Its vasoconstrictive effects, plasma concentration and release upon endothelial stimulation strongly suggest that Up4A has a functional vasoregulatory role [49].
• Resistin.
Resistin levels are higher in dialyzed than in non-dialyzed CKD patients [50], and in the non-dialyzed, concentrations correlate inversely with GFR [51]. In CKD, circulating levels are also strongly associated with inflammatory biomarkers [52], but not with insulin resistance [52,53], although resistin administration to laboratory animals impairs glucose tolerance and the action of insulin [54].
Summary
Many of the compounds accumulated in CKD have the capacity to interfere with the metabolic systems in the body so as to create cardiovascular damage (Table 1). If we translate this information into physicochemical characteristics impacting on removal by dialysis, as of today still the most important method to eliminate these molecules and their effects, it is clear that the majority of these compounds are so-called molecules that are difficult to remove by dialysis, such as middle molecules or protein-bound molecules (Table 1). Such compounds are not or barely removed by standard dialysis strategies and, to eliminate them more efficiently, one has to utilize more complex methods such as high-flux dialysis or convective strategies, and/or one has to modify standard conditions, e.g. by prolonging dialysis time. Even guanidines, in spite of being small and water soluble, have markedly different kinetic characteristics from our current marker urea.
Hence, it should be realized that in uraemic retention in general, as well as in uraemic retention related to cardiovascular risk, there is more than urea alone. In addition, in uraemic toxin removal, it may also be useful to pursue more than urea removal alone.
Translation of knowledge into clinical outcome studies
In what follows, we will first give an example of how knowledge collected from in vitro studies and epidemiological assessments, such as the data summarized in the section above and in Table 1, has ultimately led to information permitting an improvement of the therapeutic approach and condition of patients. We will then consider which further strategies should be considered for the future, in an attempt to influence various aspects of uraemic retention.
Current status—updated example
As mentioned above, a host of uraemic solutes with the potential to damage heart and vessels, are larger so-called middle molecules that are not removed by standard dialysis using dialyzers with small pores (low flux), but only via open high-flux membranes with larger pores [55,56].
In the past, several observational or epidemiological studies demonstrated that high-flux membranes were related to a better patient outcome [55,57,58].
The HEMO study, a four-armed study not only comparing low-flux to high-flux haemodialysis, but also standard dialysis adequacy to high adequacy as defined by different levels of Kt/Vurea, was the first controlled study evaluating the impact of dialyzer pore size on patient outcome [59]. At primary analysis, no difference in overall survival could be found between low-flux and high-flux haemodialyses. Such a difference in favour of high-flux haemodialysis was present, however, at several secondary analyses while considering (1) cardiac mortality or cardiac mortality plus hospitalization for cardiac reasons for the entire population [59]; (2) overall outcome in those patients who had been enrolled into the study after a time on dialysis that was longer than the median for the entire group (>3.7 years) [60] and (3) cerebrovascular mortality [61]. In addition, irrespective of the membrane type, outcome was inversely correlated to mean cumulative pre-dialysis β2-microglobulin concentration [62]. In the second wave of European Best Practice Guidelines for haemodialysis, which were published in May 2007, all these data together led to the recommendation to preferably use high-flux dialyzer membranes for haemodialysis treatment [63].
The Membrane Permeability Outcome (MPO) study is a European study with some striking points of difference with the HEMO study (Table 2) [64]. The MPO study was relatively well powered, with a targeted sample of 660 patients (exceeded at the end of enrolment by 78 additional patients) for only two study arms. Randomization was performed separately for the two albumin strata. In the MPO study, the threshold pore size defining high-flux membranes was larger than in the HEMO study, as exemplified by a markedly higher ultrafiltration coefficient and β2-microglobulin clearance. The length of the follow-up was considerably longer than that in the HEMO study, with focus on incident patients starting with dialysis under a poorer clinical condition upon enrolment (serum albumin ≤4.0 g/dL for the large majority of patients). Although the study was originally designed only for such hypoalbuminaemic patients, the protocol was amended after 11 months due to too slow patient inclusion into the study, and from then on normoalbuminaemic patients were also enrolled. At the end of the study, however, 76.2% of patients under evaluation appeared to have been still hypoalbuminaemic, according to the study definitions.
Table 2.
Comparison of the HEMO and MPO studies
| HEMO | MPO | |
|---|---|---|
| Study arms | 4 | 2 |
| Randomization | Stratified by centre, | Stratified by centre and |
| diabetes status and age | serum albumin | |
| Follow-up | 1–5 years | 3–7.5 years |
| Study sites | USA | nine European countries |
| Type of patients | Prevalent | Incident |
| Albumin status | ≥2.6 g/dL | Large majority (75%) |
| ≤4.0 g/dL | ||
| Treatment time | ≤4.5 h | ≥3 h |
| Dialyzer reuse | Yes | No |
| Definition of | Including ‘medium flux’ | Strictly ‘high flux’ |
| high flux |
As compared to low flux, outcome was significantly better in the 492 hypoalbumaemic patients treated with high-flux membranes (P = 0.032) (presentation by F. Locatelli at the ERA–EDTA meeting in Barcelona, 2007). There was also a survival advantage for high flux in the patients with diabetes mellitus, either when analysing the overall group (P = 0.0385) or the group with hypoalbuminaemia (P = 0.006). The latter data are strikingly parallel with the findings of a secondary analysis of the 4D study, originally designed to evaluate the impact of a statin on survival of diabetic dialysis patients, since here also a survival advantage for high-flux membranes was demonstrated [65].
All in all, the MPO study shows a survival advantage for high flux in hypoalbuminaemic and diabetic subjects, i.e. the subgroups on dialysis with poor general condition and outcome prognosis. Conceivably, it is easier to reach sufficient statistical power to demonstrate an outcome difference in such populations with less brilliant prospects. Of note, both hypoalbuminaemia and diabetes are frequent conditions among dialysis patients, and with a continuously growing incidence.
In a broader perspective, these data show how increasing knowledge of uraemic toxins with middle molecular weight and their potential to induce vascular damage has led to an increasing use of membranes with larger pore size and ultimately to controlled studies showing a survival advantage for such membranes.
Is this the ultimate endpoint or are more questions arising ahead of the knowledge that we have already acquired? Large molecules and even protein-bound molecules might be more efficiently removed by applying convective strategies. In that case, plasma water is ultrafiltered from the body and replaced by equivoluminous amounts of sterile saline. Although this substitution was originally administered as pharmacologically prepared solutions in bags, hence limiting the administered amount essentially due to cost limitations, more recently it has become possible to administer substitution fluid as ultrapure dialysate in much more substantial amounts, due to improvements in dialysis water preparation strategies.
Since in the HEMO study, survival was inversely linked to β2-microglobulin concentrations [62], it is conceivable that further increasing middle molecule removal by adding convection to diffusive dialysis will lead to a further outcome benefit. A number of observational studies point in this direction [66–68]. At the moment, several large controlled multicentre studies are being conducted to test this hypothesis [69–71].
Strategies for the future
It is clear from the previous sections that on one hand, our knowledge about the factors involved in uraemic syndrome and their responsibility in provoking cardiovascular damage is incomplete, but on the other hand, it is also clear that gaining knowledge in this area may be of help for developing and testing new preventive or therapeutic methods with clinical benefit.
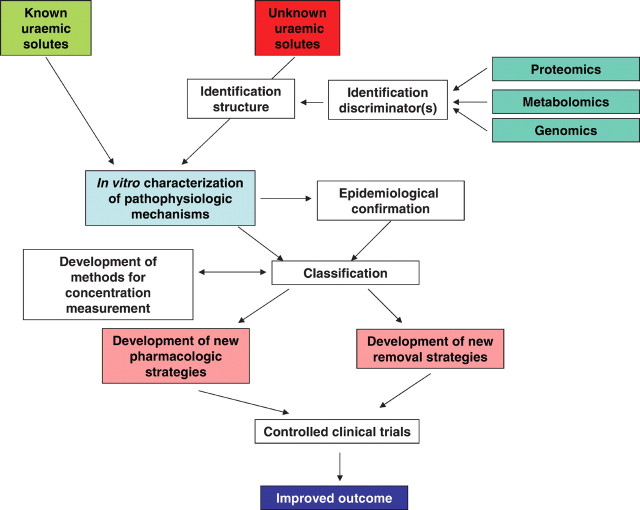
In what follows we describe an approach for the future that could be of help in finding new therapeutic strategies to improve quality of life and survival of uraemic patients. This strategy is summarized as a flowchart in Figure 1.
Fig. 1.
Suggested approach for the future, helping to find new therapeutic strategies to improve quality of life and survival of uraemic patients.
In view of our incomplete knowledge of the toxic effects of uraemic solutes, in vitro efforts should be continued to identify as yet unknown toxic effects, on one hand by continuing the study of already known solutes and on the other by the evaluation of newly detected toxins. Approaches that might be helpful for the detection of new toxins are recent acquisitions in the fields of proteomics, metabonomics and/or genomics. All these strategies make use of comparison of well-defined patient groups (e.g. those with previous cardiovascular events and those without; those with and without malnutrition), which are assessed by highly efficient analytical and biostatistical tools allowing to detect discriminators between patient groups, which are present in one group and absent in another. After identification of the structure of those compounds, they then can be submitted to further in vitro analysis.
Our research and understanding can also benefit from the application or integration of advances in other medical areas, such as cardiovascular medicine and endocrinology (hormonal and bone diseases), as well as from the understanding of rare monogenic disorders that show similarities with metabolic or pathophysiological disturbances observed in kidney disease. Examples are the relationship between osteoporosis and vascular calcification in non-CKD patients, and the Keutel syndrome, an autosomal recessive disorder resulting in a non-functional matrix-GLA protein and characterized by severe vascular media calcification [72].
In vitro analysis, be it on well-known or newly detected compounds, is best done in a standardized manner so that results from different laboratories can be compared and allow classification if several potential culprits emanate from a series of analyses. Recommendations for such a standardized approach have been formulated recently [73].
Once potentially responsible compounds have been recognized in vitro, their in vivo impact can be studied clinically in epidemiological studies, evaluating their relationship with outcome and/or relevant complications/ comorbidities.
In vitro analysis in combination with epidemiological studies should enable classification of potential culprits, in order to develop a sequence of importance for further analysis of involved pathways, which in turn can then lead towards new therapeutic strategies.
In particular, the compounds with the strongest impact should be submitted to evaluation of the involved metabolic pathways, which should allow us to develop pharmacological therapies to counteract these pathways. These approaches can then be applied not only at the latest stages of CKD, but also during earlier non-dialytic stages. This extends the group of interest up to 5 to 10% of the general population, which is much more than the ± 0.1% of people who are candidates for renal replacement therapy. The group of interest also comprises renal transplant recipients who almost always suffer from a certain degree of renal failure. It could be argued that such preventive pharmacologic measures already exist, e.g. the administration of ACE-inhibitors or aspirin. The possibility should be considered, however, that many other pathways remain unknown and counteracting them might have a more specific and intensive effect than what we have available right now, largely on empirical grounds. Of note, most of the currently applied preventive and therapeutic approaches have not been developed for the renal population and tested in this patient group either.
The other applications that can be developed once the most important culprits are known are removal strategies. Hereby one might think about dialysis strategies, but more specific approaches might include adsorption, which should not necessarily be applied in contact with blood, other options being plasma, ultrafiltrate or gastrointestinal contents.
Once such therapies are developed, they should be tested in controlled clinical trials, and in the case of a positive result, application will conceivably lead to further improvements of patient condition and survival.
Conclusions
Uraemic retention results in a complex picture of various different compounds being accumulated in the body of patients affected by kidney failure. Those compounds in turn modify several biochemical/biological systems normally involved in the homeostasis and normal functioning of the body. If these functions deteriorate, uraemic syndrome ensues. Many organic disturbances are part of uraemic syndrome, but one of the most vexing problems is the severe cardiovascular disease that weighs heavily on the survival and quality of life.
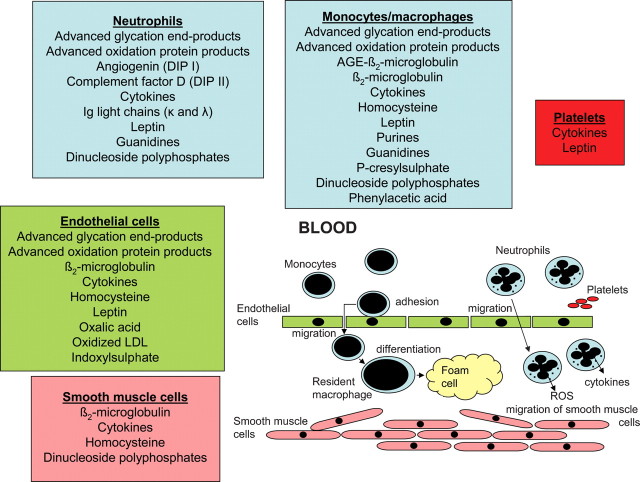
During the last decade, our knowledge of uraemic toxins and their functional effects has been largely extended (Figure 2). Obviously, compounds that are ‘difficult to remove by dialysis’, especially larger ‘middle’ molecules and protein-bound solutes, have been related to many toxic effects, especially cardiovascular disease.
Fig. 2.
The presently known uraemic retention solutes with a negative impact on the major cell types involved in the development of cardiovascular disease (adapted and extended from [7]).
Enhancing the removal of these molecules by increasing dialyzer pore size and possibly also by adding convection, has a beneficial impact on outcome. Based on this experience, our research for the future should focus on the detection of new responsible compounds and/or mechanisms, which should lead to the development of new removal methods and pharmacological strategies blocking responsible pathways, ultimately improving patient condition.
Conflict of interest statement. None declared.
References
- 1.Lindner A, Charra B, Sherrard DJ, et al. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974;290:697–701. doi: 10.1056/NEJM197403282901301. [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16–S23. [PubMed] [Google Scholar]
- 3.Vanholder R, Massy Z, Argiles A, et al. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant. 2005;20:1048–1056. doi: 10.1093/ndt/gfh813. [DOI] [PubMed] [Google Scholar]
- 4.Van Biesen W, De Bacquer D, Verbeke F, et al. The glomerular filtration rate in an apparently healthy population and its relation with cardiovascular mortality during 10 years. Eur Heart J. 2007;28:478–483. doi: 10.1093/eurheartj/ehl455. [DOI] [PubMed] [Google Scholar]
- 5.Briet M, Bozec E, Laurent S, et al. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int. 2006;69:350–357. doi: 10.1038/sj.ki.5000047. [DOI] [PubMed] [Google Scholar]
- 6.Cheung AK, Sarnak MJ, Yan G, et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58:353–362. doi: 10.1046/j.1523-1755.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 7.Vanholder R, Argiles A, Baurmeister U, et al. Uremic toxicity: present state of the art. Int J Artif Organs. 2001;24:695–725. [PubMed] [Google Scholar]
- 8.Glorieux GL, Dhondt AW, Jacobs P, et al. In vitro study of the potential role of guanidines in leukocyte functions related to atherogenesis and infection. Kidney Int. 2004;65:2184–2192. doi: 10.1111/j.1523-1755.2004.00631.x. [DOI] [PubMed] [Google Scholar]
- 9.Perna AF, Ingrosso D, Satta E, et al. Plasma protein aspartyl damage is increased in hemodialysis patients: studies on causes and consequences. J Am Soc Nephrol. 2004;15:2747–2754. doi: 10.1097/01.ASN.0000141041.71717.11. [DOI] [PubMed] [Google Scholar]
- 10.Zoccali C, Bode-Boger S, Mallamaci F, et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–2117. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 11.Kielstein JT, Impraim B, Simmel S, et al. Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation. 2004;109:172–177. doi: 10.1161/01.CIR.0000105764.22626.B1. [DOI] [PubMed] [Google Scholar]
- 12.Bode-Boger SM, Scalera F, Kielstein JT, et al. Symmetrical dimethylarginine: a new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol. 2006;17:1128–1134. doi: 10.1681/ASN.2005101119. [DOI] [PubMed] [Google Scholar]
- 13.Eloot S, Torremans A, De Smet R, et al. Kinetic behavior of urea is different from that of other water-soluble compounds: the case of the guanidino compounds. Kidney Int. 2005;67:1566–1575. doi: 10.1111/j.1523-1755.2005.00238.x. [DOI] [PubMed] [Google Scholar]
- 14.Eloot S, Torremans A, De Smet R, et al. Complex compartmental behavior of small water-soluble uremic retention solutes: evaluation by direct measurements in plasma and erythrocytes. Am J Kidney Dis. 2007;50:279–288. doi: 10.1053/j.ajkd.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 16.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 17.Boushey CJ, Beresford SA, Omenn GS, et al. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 18.Tsai JC, Perrella MA, Yoshizumi M, et al. Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis. Proc Natl Acad Sci USA. 1994;91:6369–6373. doi: 10.1073/pnas.91.14.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massy ZA, Ceballos I, Chadefaux-Vekemens B, et al. Homocyst(e)ine, oxidative stress, and endothelium function in uremic patients. Kidney Int. 2001;59(Suppl 78):S243–S245. doi: 10.1046/j.1523-1755.2001.59780243.x. [DOI] [PubMed] [Google Scholar]
- 20.Au-Yeung KK, Yip JC, Siow YL. O K. Folic acid inhibits homocysteine-induced superoxide anion production and nuclear factor kappa B activation in macrophages. Can J Physiol Pharmacol. 2006;84:141–147. doi: 10.1139/Y05-136. [DOI] [PubMed] [Google Scholar]
- 21.Matthias D, Becker CH, Riezler R, et al. Homocysteine induced arteriosclerosis-like alterations of the aorta in normotensive and hypertensive rats following application of high doses of methionine. Atherosclerosis. 1996;122:201–216. doi: 10.1016/0021-9150(95)05740-4. [DOI] [PubMed] [Google Scholar]
- 22.Harpel PC, Zhang X, Borth W. Homocysteine and hemostasis: pathogenic mechanisms predisposing to thrombosis. J Nutr. 1996;126:1285S–1289S. doi: 10.1093/jn/126.suppl_4.1285S. [DOI] [PubMed] [Google Scholar]
- 23.Touam M, Zingraff J, Jungers P, et al. Effective correction of hyperhomocysteinemia in hemodialysis patients by intravenous folinic acid and pyridoxine therapy. Kidney Int. 1999;56:2292–2296. doi: 10.1046/j.1523-1755.1999.00792.x. [DOI] [PubMed] [Google Scholar]
- 24.van Guldener C, Lambert J, ter Wee PM, et al. Carotid artery stiffness in patients with end-stage renal disease: no effect of long-term homocysteine-lowering therapy. Clin Nephrol. 2000;53:33–41. [PubMed] [Google Scholar]
- 25.Dou L, Jourde-Chiche N, Faure V, et al. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost. 2007 doi: 10.1111/j.1538-7836.2007.02540.x. [DOI] [PubMed] [Google Scholar]
- 26.Dou L, Bertrand E, Cerini C, et al. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65:442–451. doi: 10.1111/j.1523-1755.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 27.Martinez AW, Recht NS, Hostetter TH, et al. Removal of p-cresol sulfate by hemodialysis. J Am Soc Nephrol. 2005;16:3430–3436. doi: 10.1681/ASN.2005030310. [DOI] [PubMed] [Google Scholar]
- 28.de Loor H, Bammens B, Evenepoel P, et al. Gas chromatographic-mass spectrometric analysis for measurement of p-cresol and its conjugated metabolites in uremic and normal serum. Clin Chem. 2005;51:1535–1538. doi: 10.1373/clinchem.2005.050781. [DOI] [PubMed] [Google Scholar]
- 29.Schepers E, Meert N, Glorieux G, et al. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant. 2007;22:592–596. doi: 10.1093/ndt/gfl584. [DOI] [PubMed] [Google Scholar]
- 30.De Smet R, Van Kaer J, Van Vlem B, et al. Toxicity of free p-cresol: a prospective and cross-sectional analysis. Clin Chem. 2003;49:470–478. doi: 10.1373/49.3.470. [DOI] [PubMed] [Google Scholar]
- 31.Bammens B, Evenepoel P, Keuleers H, et al. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006;69:1081–1087. doi: 10.1038/sj.ki.5000115. [DOI] [PubMed] [Google Scholar]
- 32.Bammens B, Evenepoel P, Verbeke K, et al. Removal of middle molecules and protein-bound solutes by peritoneal dialysis and relation with uremic symptoms. Kidney Int. 2003;64:2238–2243. doi: 10.1046/j.1523-1755.2003.00310.x. [DOI] [PubMed] [Google Scholar]
- 33.Jankowski J, Luftmann H, Tepel M, et al. Characterization of dimethylguanosine, phenylethylamine, and phenylacetic acid as inhibitors of Ca2+ ATPase in end-stage renal failure. J Am Soc Nephrol. 1998;9:1249–1257. doi: 10.1681/ASN.V971249. [DOI] [PubMed] [Google Scholar]
- 34.Jankowski J, Van Der GM, Jankowski V, et al. Increased plasma phenylacetic acid in patients with end-stage renal failure inhibits iNOS expression. J Clin Invest. 2003;112:256–264. doi: 10.1172/JCI15524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scholze A, Jankowski V, Henning L, et al. Phenylacetic acid and arterial vascular properties in patients with chronic kidney disease stage 5 on hemodialysis therapy. Nephron Clin Pract. 2007;107:c1–c6. doi: 10.1159/000105137. [DOI] [PubMed] [Google Scholar]
- 36.Fishbane S, Bucala R, Pereira BJ, et al. Reduction of plasma apolipoprotein-B by effective removal of circulating glycation derivatives in uremia. Kidney Int. 1997;52:1645–1650. doi: 10.1038/ki.1997.497. [DOI] [PubMed] [Google Scholar]
- 37.Witko-Sarsat V, Friedlander M, Nguyen KT, et al. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161:2524–2532. [PubMed] [Google Scholar]
- 38.Glorieux G, Helling R, Henle T, et al. In vitro evidence for immune activating effect of specific AGE structures retained in uremia. Kidney Int. 2004;66:1873–1880. doi: 10.1111/j.1523-1755.2004.00961.x. [DOI] [PubMed] [Google Scholar]
- 39.Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 40.Valli A, Suliman ME, Meert N, et al. Overestimation of advanced oxidation protein products in uremic plasma due to presence of triglycerides and other endogenous factors. Clin Chim Acta. 2007;379:87–94. doi: 10.1016/j.cca.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 41.Selmeci L, Szekely M, Soos P, et al. Human blood plasma advanced oxidation protein products (AOPP) correlates with fibrinogen levels. Free Radic Res. 2006;40:952–958. doi: 10.1080/10715760600818789. [DOI] [PubMed] [Google Scholar]
- 42.Jankowski V, Vanholder R, Van Der Giet M, et al. Mass-spectrometric identification of a novel angiotensin peptide in human plasma. Arterioscler Thromb Vasc Biol. 2007;27:297–302. doi: 10.1161/01.ATV.0000253889.09765.5f. [DOI] [PubMed] [Google Scholar]
- 43.Stenvinkel P, Heimburger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 44.Kimmel PL, Phillips TM, Simmens SJ, et al. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998;54:236–244. doi: 10.1046/j.1523-1755.1998.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogilvie A, Blasius R, Schulze-Lohoff E, et al. Adenine dinucleotides: a novel class of signalling molecules. J Auton Pharmacol. 1996;16:325–328. doi: 10.1111/j.1474-8673.1996.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 46.Heidenreich S, Tepel M, Schluter H, et al. Regulation of rat mesangial cell growth by diadenosine phosphates. J Clin Invest. 1995;95:2862–2867. doi: 10.1172/JCI117992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jankowski J, Jankowski V, Laufer U, et al. Identification and quantification of diadenosine polyphosphate concentrations in human plasma. Arterioscler Thromb Vasc Biol. 2003;23:1231–1238. doi: 10.1161/01.ATV.0000075913.00428.FD. [DOI] [PubMed] [Google Scholar]
- 48.Jankowski J, Hagemann J, Yoon MS, et al. Increased vascular growth in hemodialysis patients induced by platelet-derived diadenosine polyphosphates. Kidney Int. 2001;59:1134–1141. doi: 10.1046/j.1523-1755.2001.0590031134.x. [DOI] [PubMed] [Google Scholar]
- 49.Jankowski V, Tolle M, Vanholder R, et al. Uridine adenosine tetraphosphate: a novel endothelium- derived vasoconstrictive factor. Nat Med. 2005;11:223–227. doi: 10.1038/nm1188. [DOI] [PubMed] [Google Scholar]
- 50.Diez JJ, Iglesias P, Fernandez-Reyes MJ, et al. Serum concentrations of leptin, adiponectin and resistin, and their relationship with cardiovascular disease in patients with end-stage renal disease. Clin Endocrinol (Oxf) 2005;62:242–249. doi: 10.1111/j.1365-2265.2005.02207.x. [DOI] [PubMed] [Google Scholar]
- 51.Risch L, Saely C, Hoefle G, et al. Relationship between glomerular filtration rate and the adipokines adiponectin, resistin and leptin in coronary patients with predominantly normal or mildly impaired renal function. Clin Chim Acta. 2007;376:108–113. doi: 10.1016/j.cca.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 52.Axelsson J, Bergsten A, Qureshi AR, et al. Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance. Kidney Int. 2006;69:596–604. doi: 10.1038/sj.ki.5000089. [DOI] [PubMed] [Google Scholar]
- 53.Kielstein JT, Becker B, Graf S, et al. Increased resistin blood levels are not associated with insulin resistance in patients with renal disease. Am J Kidney Dis. 2003;42:62–66. doi: 10.1016/s0272-6386(03)00409-8. [DOI] [PubMed] [Google Scholar]
- 54.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 55.Koda Y, Nishi S, Miyazaki S, et al. Switch from conventional to high-flux membrane reduces the risk of carpal tunnel syndrome and mortality of hemodialysis patients. Kidney Int. 1997;52:1096–1101. doi: 10.1038/ki.1997.434. [DOI] [PubMed] [Google Scholar]
- 56.Locatelli F, Mastrangelo F, Redaelli B, et al. (the Italian Cooperative Dialysis Study Group). Effects of different membranes and dialysis technologies on patient treatment tolerance and nutritional parameters. Kidney Int. 1996;50:1293–1302. doi: 10.1038/ki.1996.441. [DOI] [PubMed] [Google Scholar]
- 57.Hornberger JC, Chernew M, Petersen J, et al. A multivariate analysis of mortality and hospital admissions with high-flux dialysis. J Am Soc Nephrol. 1992;3:1227–1237. doi: 10.1681/ASN.V361227. [DOI] [PubMed] [Google Scholar]
- 58.Woods HF, Nandakumar M. Improved outcome for haemodialysis patients treated with high-flux membranes. Nephrol Dial Transplant. 2000;15(Suppl 1):36–42. doi: 10.1093/oxfordjournals.ndt.a027962. [DOI] [PubMed] [Google Scholar]
- 59.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 60.Cheung AK, Levin NW, Greene T, et al. Effects of high-flux hemodialysis on clinical outcomes: results of the HEMO study. J Am Soc Nephrol. 2003;14:3251–3263. doi: 10.1097/01.asn.0000096373.13406.94. [DOI] [PubMed] [Google Scholar]
- 61.Delmez JA, Yan G, Bailey J, et al. Cerebrovascular disease in maintenance hemodialysis patients: results of the HEMO Study. Am J Kidney Dis. 2006;47:131–138. doi: 10.1053/j.ajkd.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 62.Cheung AK, Rocco MV, Yan G, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. 2006;17:546–555. doi: 10.1681/ASN.2005020132. [DOI] [PubMed] [Google Scholar]
- 63.Tattersall J, Martin-Malo A, Pedrini L, et al. EBPG guideline on dialysis strategies. Nephrol Dial Transplant. 2007;22(Suppl 2):ii5–ii21. doi: 10.1093/ndt/gfm022. [DOI] [PubMed] [Google Scholar]
- 64.Locatelli F, Hannedouche T, Jacobson S, et al. The effect of membrane permeability on ESRD: design of a prospective randomised multicentre trial. J Nephrol. 1999;12:85–88. [PubMed] [Google Scholar]
- 65.Krane V, Krieter DH, Olschewski M, et al. Dialyzer membrane characteristics and outcome of patients with type 2 diabetes on maintenance hemodialysis. Am J Kidney Dis. 2007;49:267–275. doi: 10.1053/j.ajkd.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 66.Canaud B, Bragg-Gresham JL, Marshall MR, et al. Mortality risk for patients receiving hemodiafiltration versus hemodialysis: European results from the DOPPS. Kidney Int. 2006;69:2087–2093. doi: 10.1038/sj.ki.5000447. [DOI] [PubMed] [Google Scholar]
- 67.Jirka T, Cesare S, Di Benedetto A, et al. Mortality risk for patients receiving hemodiafiltration versus hemodialysis. Kidney Int. 2006;70:1524–1525. doi: 10.1038/sj.ki.5001759. [DOI] [PubMed] [Google Scholar]
- 68.Bosch JP, Lew SQ, Barlee V, et al. Clinical use of high-efficiency hemodialysis treatments: long-term assessment. Hemodial Int. 2006;10:73–81. doi: 10.1111/j.1542-4758.2006.01178.x. [DOI] [PubMed] [Google Scholar]
- 69.Penne EL, Blankestijn PJ, Bots ML, et al. Effect of increased convective clearance by on-line hemodiafiltration on all cause and cardiovascular mortality in chronic hemodialysis patients—the Dutch CONvective TRAnsport STudy (CONTRAST): rationale and design of a randomised controlled trial [ISRCTN38365125] Curr Control Trials Cardiovasc Med. 2005;6:8. doi: 10.1186/1468-6708-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Canaud B, Morena M, Leray-Moragues H, et al. Overview of clinical studies in hemodiafiltration: what do we need now? Hemodial Int. 2006;10(Suppl 1):S5–S12. doi: 10.1111/j.1542-4758.2006.01183.x. [DOI] [PubMed] [Google Scholar]
- 71.Bolasco P, Altieri P, Andrulli S, et al. Convection versus diffusion in dialysis: an Italian prospective multicentre study. Nephrol Dial Transplant. 2003;18(Suppl 7):vii50–vii54. doi: 10.1093/ndt/gfg1080. [DOI] [PubMed] [Google Scholar]
- 72.Munroe PB, Olgunturk RO, Fryns JP, et al. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat Genet. 1999;21:142–144. doi: 10.1038/5102. [DOI] [PubMed] [Google Scholar]
- 73.Cohen G, Glorieux G, Thornalley P, et al. Review on uraemic toxins III—recommendations for handling uraemic retention solutes in vitro: towards a standardized approach for research on uraemia. Nephrol Dial Transplant. 2007;22(12):3381–3390. doi: 10.1093/ndt/gfm210. [DOI] [PubMed] [Google Scholar]