Extraskeletal (or ‘soft tissue’) calcifications are frequently seen in uraemic patients [1,2], especially in patients with end-stage renal failure [1–3]. Although periarticular calcifications, calcifications of medium-sized arteries, visceral calcifications mainly affecting the heart, lung and kidney, cutaneous/subcutaneous calcifications, and ocular calcifications [1] are described frequently in dialysis patients, their pathogenesis is still incompletely understood. Numerous underlying factors are thought to favour their formation, such as an increased calcium × phosphate (Ca × P) product, secondary hyperparathyroidism, advanced age, aluminium overload and low-turnover bone disease, administration of vitamin D analogues and impaired magnesium metabolism. In addition, a number of other systemic (changes in pH status, race, vitamin K, TGF-β) and local (focal tissue injury, local changes in pH or expression of growth factors) parameters have been implicated, the relative roles of which are, however, still uncertain.
Although calcifications of the endocranial vasculature have been reported in ∼9.6% of uraemic patients [4], the appearance of widespread intracranial calcifications is very rare. Herein, we present the case of a 72-year-old woman undergoing chronic haemodialysis, with brain calcinosis and neurological disorders strongly connected to hypoparathyroidism.
Case
A 72-year-old female patient with end-stage renal failure, who had been undergoing dialysis for the previous 9 months, presented with headaches, lightheadedness, dizziness, balance disorders, vision disturbance, fatigue, memory disturbances, depression and anxiety.
She had undergone a total thyroidectomy 54 years previously, due to goitre. Hypothyroidism requiring thyrormone supplementation and marginal hypocalcaemia were since reported. Twenty-three years prior to the current admission, she developed hypertension and incipient renal insufficiency. The primary cause was considered to be renovascular disease, but her case was not pursued further. Her recovered follow-up data revealed that 15 years previously, she had a serum creatinine of 1.4 mg/dl, normal urinary sediment, serum calcium of 8.1 mg/dl and serum phosphate of 6.8 mg/dl, while receiving alphacalcidol at 1 mg/day.
Nine years prior to the present admission, while her serum creatinine was 1.8 mg/dl, she underwent a radionuclide study with 99mTc–Mag3 showing decreased renal perfusion and abnormal parenchymal function with reduced kidney size. Alphacalcidol was discontinued 5 years ago due to hypercalcaemia, when her serum creatinine had risen to 2 mg/dl. Her renal function gradually worsened and the patient finally reached end-stage renal failure. Renal replacement treatment with haemodialysis was initiated 9 months prior to admission. Intact parathormone (iPTH) measurements at that time were in the range 0–1 pg/ml.
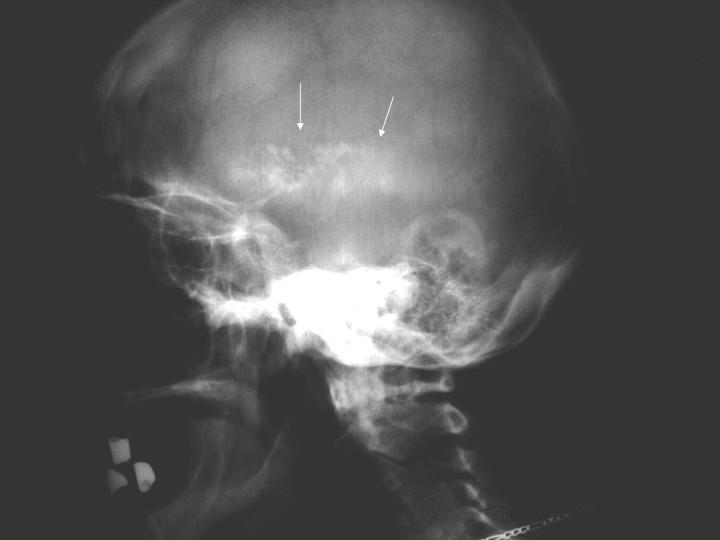
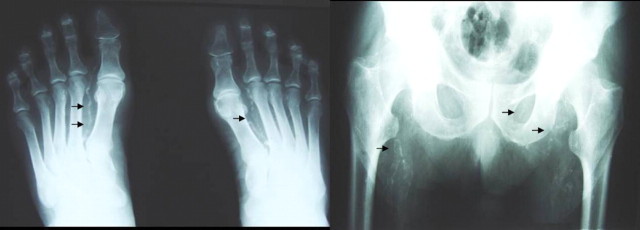
On admission, the patient was drowsy and possessed a BP of 130/80 mmHg and temperature of 36.4°C. Physical examination revealed a mild systolic murmur at the heart apex that radiated to the neck, and a systole–diastolic murmur at the umbilical area and groin region. Neurological examination was remarkable for fixed, symmetrical pupils, normal pyramidal signs and reflexes, reduced muscle tone, a Parkinsonian tremor, impaired posture and gait. The patient was alert, but slightly disoriented with memory defects. Fundoscopy showed mild papillary oedema. Blood gases were within normal limits. The skull x-ray revealed discrete intracranial calcifications (Figure 1). A CT scan of the head demonstrated the presence of numerous, symmetrically located calcifications in both frontal lobes, subcortical nuclei, the paraventricular region, brain fornix, as well as both cerebellar hemispheres (Figure 2). Further radiological evaluation revealed extended calcification of arteries of various sizes (Figure 3).
Fig. 1.
Skull x-ray showing discrete intracranial calcifications.
Fig. 2.
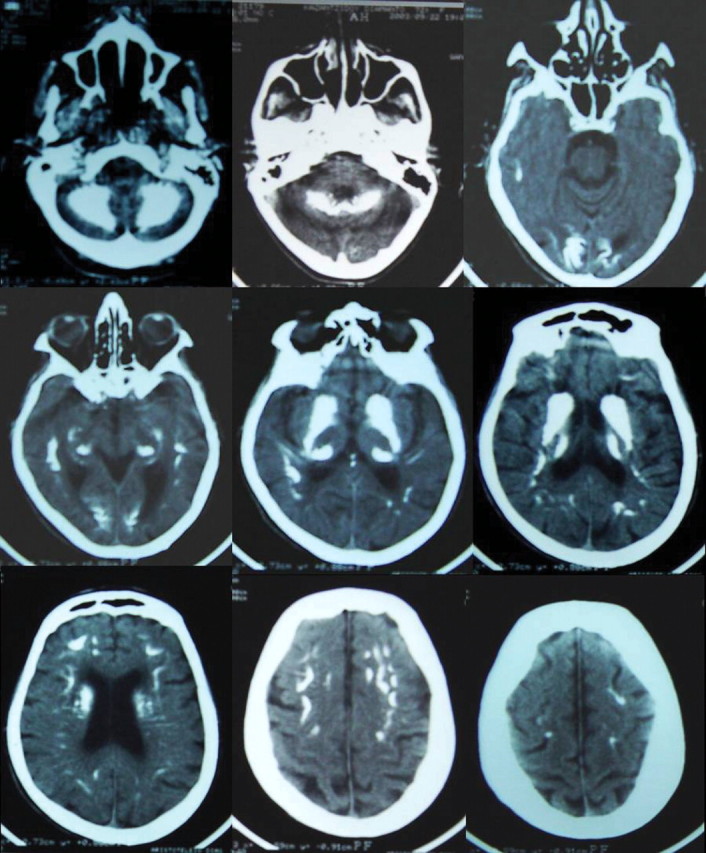
Series of brain CT scanning showing widespread, symmetrically located calcifications in both the frontal lobes, subcortical nuclei, paraventricular region, brain fornix, as well as both cerebellar hemispheres.
Fig. 3.
Plain X-ray film showing arterial calcification of dorsal, pelvic and femoral.
Discussion
Hypoparathyroidism is a clinical disorder that manifests when the parathyroid hormone (PTH) produced by the parathyroid gland is insufficient to maintain the extracellular fluid calcium within normal range, or when adequate circulating concentrations of PTH are unable to function optimally in target tissues to maintain normal calcium levels. The most common cause of hypoparathyroidism is surgical excision of, or damage to, the parathyroid glands as a result of total thyroidectomy, radical neck dissection for various cancer types or repeated operations for hyperparathyroidism.
Although rare in the general population, central nervous system calcifications have long been noted in association with hypoparathyroidism of various origins and are present in as many as 80% of unselected cases [5–11]. Calcifications may be located in many areas of the brain, but are particularly common in the basal ganglia, where their presence has been associated with seizure disorders and extrapyramidal motor dysfunction [12,13]. Dementia, characterized by ‘poor concentration, impaired memory, disorientation and apathy’ has been noted in hypoparathyroid patients at the time of diagnosis [14], but it usually resolves with the correction of hypocalcaemia [14]. Furthermore, more permanent cognitive deficits have been noted in patients with chronic hypoparathyroidism [11,15,16].
Calcifications of the basal ganglia or more widespread intracranial structures may be detected on routine radiographs or by enhanced imaging (CT or MRI), and EEG changes may be present. Using conventional skull radiography, intracranial calcifications were noted in 20% of patients with chronic hypoparathyroidism, while CT and MRI imaging revealed similar lesions in patients with negative skull x-rays [17]. It is estimated that the caudate nucleus is always affected. Brain calcifications are symmetrical and besides, the basal ganglia may rarely affect the symmetrical cortex, occurring predominantly in the frontal lobe, subcortical white matter, thalamus and cerebellum [18].
The positive correlations between the extent of intracranial calcifications and both the degree of cognitive loss and presence of motor abnormalities suggest a pathophysiological relationship, the nature of which is obscure, but presumably associated with neuronal loss. Although Virchow [19] and Bamberger [20] independently described the histology of bilateral basal ganglia calcifications in 1855, it was not until 1939 that their association with hypoparathyroidism was recognized by Eaton et al. [21]. Bhimani et al. have identified the microscopic location of brain calcification in postsurgical hypoparathyroidism patients as primarily vascular and perivascular [22]. Thus, an ischaemic mechanism might contribute to the abnormalities.
The primary pathogenic mechanism by which hypoparathyroidism results in intracranial calcification is far from clear [3,23]. Hyaline degeneration and calcification of the media and adventitia of the small blood vessels have been described, and abnormal calcium and phosphorus concentrations in the extracellular environment have been suggested to play a role in the development of calcinosis [23]. However, the concentrations of calcium and phosphorus in the cerebrospinal fluid are unchanged in conditions associated with an excess or deficiency of PTH [23]. Several investigators suggest that PTH can penetrate the blood–brain barrier and can have a direct effect on brain function, while others have hypothesized an interaction between PTH and basal ganglia-specific binding sites [23,24]. Of importance is the fact that defects were observed in patients who were, for the most part, adequately treated [3].
Smits et al. reported that the extent of basal ganglia and dentate nuclei calcifications increased during normocalcaemia, which was caused by dihydrotachysterol therapy [25]. Whether conventional therapy with calcium and vitamin D supplementation may prevent or, conversely, contribute to the development of cognitive deficits, has not been addressed. A potential role for vitamin D in the pathogenesis of soft tissue calcifications, even in the absence of increased calcium or phosphorus levels, is supported by numerous clinical observations in patients with vitamin D intoxication [1]. It may occur through an enhancement of calcium and phosphorus absorption with subsequent direct precipitation in soft tissues, and/or via an increase in the expression of calcification-regulating proteins, such as osteopontin in the skin or in atheromatous blood vessels. Vitamin D derivatives also stimulate intestinal oxalate absorption, increase plasma oxalate saturation, and thereby enhance the risk of ectopic calcium crystal deposition [26].
The aetiology of extraskeletal calcification in patients with chronic renal failure has been linked to a variety of factors, mainly age, male gender, smoking, inflammation, hypertension, dyslipidaemia, and diabetes as in the general population. In addition, metabolic and endocrine disturbances, which are more frequently found in uraemic than non-uraemic patients, contribute significantly to abnormal calcium deposition, such as hyperparathyroidism, hypercalcaemia, hyperphospataemia, high Ca × P product, oxidative stress and overtreatment with calcium-containing drugs and vitamin D derivatives. Calcifications of the intracranial vessels have been reported in up to 10% of uraemic patients [4]. However, widespread intracranial calcification is extremely rare. An e-database search revealed only two documented cases, which were attributed to severe uncontrolled hyperparathyroidism with a high Ca × P product under large doses of vitamin D [27,28]. It seems that intracranial calcifications can occur in both high and low parathyroid concentration and that brain calcinosis is more related to vitamin D treatments.
In conclusion, we report a dialysis patient who manifested a neurological disorder due to intracranial calcifications related to chronic hypoparathyroidism. The course of the patient was serious, with gradual deterioration of the neurological deficits leading to coma and ultimately to death.
Teaching points
Intracranial calcifications (brain calcinosis) are a frequent complication in long-standing hypoparathyroidism.
Although brain calcinosis has been linked to low PTH concentrations, it has also been reported to occur in uraemic patients with severe uncontrolled hyperparathyroidism receiving large doses of vitamin D treatment.
The potential role of vitamin D in the pathogenesis of intracranial calcifications should caution nephrologists when prescribing prolonged treatments with vitamin D analogues.
Conflict of interest statement. None declared.
References
- 1.Drüeke TB. A clinical approach to the uraemic patient with extraskeletal calcifications. Nephrol Dial Transplant. 1996;11(Suppl 3):37–42. doi: 10.1093/ndt/11.supp3.37. [DOI] [PubMed] [Google Scholar]
- 2.Parfitt AM. Soft-tissue calcification in uremia. Arch Intern Med. 1969;124:544–556. [PubMed] [Google Scholar]
- 3.Kuzela DC, Huffer WE, Conger JD, et al. Soft tissue calcification in chronic dialysis patients. Am J Pathol. 1977;86:403–424. [PMC free article] [PubMed] [Google Scholar]
- 4.Savazzi GM, Cusmano F, Musini S. Cerebral imaging changes in patients with chronic renal failure treated conservatively or in haemodialysis. Nephron. 2001;89:31–6. doi: 10.1159/000046040. [DOI] [PubMed] [Google Scholar]
- 5.Kowdley KV, Coull BM, Orwoll ES. Cognitive impairment and intracranial calcification in chronic hypoparathyroidism. Am J Med Sci. 1999;317:273–281. doi: 10.1097/00000441-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Koller WC, Cochran JW, Klawans HL. Calcification of the basal ganglia: computerized tomography and clinical correlation. Neurology. 1979;29:328–333. doi: 10.1212/wnl.29.3.328. [DOI] [PubMed] [Google Scholar]
- 7.Murphy MJ. Clinical correlations of CT scan-detected calcifications of the basal ganglia. Ann Neurol. 1979;6:507–511. doi: 10.1002/ana.410060608. [DOI] [PubMed] [Google Scholar]
- 8.Sachs C, Sjoberg HE, Erickson K. Basal ganglia calcifications on CT: relation to hypoparathyroidism. Neurology. 1982;21:779–782. doi: 10.1212/wnl.32.7.779. [DOI] [PubMed] [Google Scholar]
- 9.Posen S, Clifton-Bligh P, Cromer T. Computerized tomography of the brain in surgical hypoparathyroidism. Ann Intern Med. 1979;91:515–517. doi: 10.7326/0003-4819-91-3-415. [DOI] [PubMed] [Google Scholar]
- 10.Illum F, Dupont E. Prevalence of CT-detected calcification in the basal ganglia in idiopathic hypoparathyroidism and pseudo-hypoparathyroidism. Neuroradiology. 1985;27:32–37. doi: 10.1007/BF00342514. [DOI] [PubMed] [Google Scholar]
- 11.Denko JD. The psychiatric aspects of hypoparathyroidism. Acta Psych Scand. 1962;38:1–70. [PubMed] [Google Scholar]
- 12.Uncini A, Tartaro A, Stefano D, et al. Parkinsonism, basal ganglia calcification and epilepsy as late stage complications of postoperative hypoparathyroidism. J Neurol. 1985;232:109–111. doi: 10.1007/BF00313910. [DOI] [PubMed] [Google Scholar]
- 13.Kartin P, Zupevc M, Pogacnik T, et al. Calcification of basal ganglia, postoperative hypoparathyroidism and extrapyramidal, cerebellar, pyramidal motor dysfunction. J Neurol. 1982;227:171–176. doi: 10.1007/BF00313572. [DOI] [PubMed] [Google Scholar]
- 14.Hossain M. Neurological and psychiatric manifestations in idiopathic hypoparathyroidism: response to treatment. J Neurol Neurosurg Psychiatry. 1970;33:153–156. doi: 10.1136/jnnp.33.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shu-Lian Y, Chang-Hua W, Ying-Kun F. Neurologic and psychiatric manifestations presenting as hypoparathyroidism. Chin Med J. 1984;97:267–272. [PubMed] [Google Scholar]
- 16.Nikolai A, Lazzarino LG. Dementia syndrome in patients with postsurgical hypoparathyroidism and extensive brain calcifications. Eur Neurol. 1994;34:230–235. doi: 10.1159/000117045. [DOI] [PubMed] [Google Scholar]
- 17.Tsuruda JS, Bradley WG. MR detection of intracranial calcification: a phantom study. Am J Neuroradiol. 1986;8:1049–1055. [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman JH, Chiucchini I, Tucci JR. Idiopathic hypoparathyroidism with extensive brain calcification and persistent neurologic dysfunction. Neurology. 1987;37:307–309. doi: 10.1212/wnl.37.2.307. [DOI] [PubMed] [Google Scholar]
- 19.Virchow R. Kalk-Metastasen. Virchows Arch Pathol Anat. 1855;8:103–113. [Google Scholar]
- 20.Bamberger K. Lehrbuch der pathologischen Anatomie. Vol. 2. Vienna: Wilhelm Braumuller; 1856. [Google Scholar]
- 21.Eaton LM, Camp JD, Love JG. Symmetric cerebral calcification, particularly of the basal ganglia, demonstrable roentgenographically; calcification of the finer cerebral blood vessels. Arch Neurol Psychiatry. 1939;41:921–942. [Google Scholar]
- 22.Bhimani S, Sarwar M, Virapongse C, et al. Computed tomography of cerebrovascular calcifications in postsurgical hypoparathyroidism. J Comput Assist Tomogr. 1985;232:109–111. doi: 10.1097/00004728-198501000-00022. [DOI] [PubMed] [Google Scholar]
- 23.Sztriha L, Punnose J, Prais V, et al. Idiopathic hypoparathyroidism with basal ganglia calcification, epilepsy and interictal focal hypoperfusion. J Child Neurol. 1998;13:189–192. doi: 10.1177/088307389801300410. [DOI] [PubMed] [Google Scholar]
- 24.Harvey S, Fraser RA. Parathyroid hormone: neural and neuroendocrine perspectives. J Endocrinol. 1993;139:353–361. doi: 10.1677/joe.0.1390353. [DOI] [PubMed] [Google Scholar]
- 25.Smits M, Gabreels F, Froeling P, et al. Autosomal dominant idiopathic hypoparathyroidism and nervous system dysfunction: report of three cases and review of the literature. J Neurol. 1982;228:113–122. doi: 10.1007/BF00313756. [DOI] [PubMed] [Google Scholar]
- 26.Marangela M, Vitale C, Cosseder D, et al. Effect of oral and intravenous calcitriol on serum calcium oxalate saturation in dialysis patients. Clin Sci. 1993;85:309–314. doi: 10.1042/cs0850309. [DOI] [PubMed] [Google Scholar]
- 27.Seyahi N, Apaydin S, Sariyar M, et al. Intracranial calcification and tumoural calcinosis during vitamin D therapy. Nephrology. 2004;9:89–93. doi: 10.1111/j.1440-1797.2003.00237.x. Carlton. [DOI] [PubMed] [Google Scholar]
- 28.Bilge I, Sadikoglu B, Emre S, et al. Brain calcification due to secondary hyperparathyroidism in a child with chronic renal failure. Turk J Pediatr. 2005;47:287–90. [PubMed] [Google Scholar]