Abstract
The multifunctional zinc-finger protein CCCTC-binding factor (CTCF) is a very strong candidate for the role of coordinating the expression level of coding sequences with their three-dimensional position in the nucleus, apparently responding to a ‘‘code’’ in the DNA itself. Dynamic interactions between chromatin fibers in the context of nuclear architecture have been implicated in various aspects of genome functions. However, the molecular basis of these interactions still remains elusive and is a subject of intense debate. Here we discuss the nature of CTCF-DNA interactions, the CTCF-binding specificity to its binding sites and the relationship between CTCF and chromatin, and we examine data linking CTCF with gene regulation in the three-dimensional nuclear space. We discuss why these features render CTCF a very strong candidate for the role and propose a unifying model, the ‘‘CTCF code,’’ explaining the mechanistic basis of how the information encrypted in DNA may be interpreted by CTCF into diverse nuclear functions.
Keywords: chromatin, epigenetics, gene expression, higher order structure
Introduction
Linear eukaryotic DNA is condensed ~100,000-fold by packaging with nucleosomes into chromatin.(1) The arrangement of chromatin within the nucleus is not random: at the interphase, de-condensed chromosomes occupy areas termed chromosome territories,(2,3) which are dynamic compartments able to intermingle with each other.(4) Chromosomes are generally organized in radial positions in the nucleus, with most gene-poor chromosomes located close to the periphery, and gene-dense chromosomes toward the center of the nucleus.(5) Recent findings have indicated that the positioning of genes within the nucleus – in relation to other genes or nuclear structures – is important and is able to modulate gene function.(6) These patterns illustrate a general principle of nuclear organization – multiple, specialized nuclear compartments associated with particular biological functions(7): transcription can take place at nuclear sites called ‘‘transcription factories,’’ DNA replication at ‘‘replication factories,’’ and DNA repair at specialized repair sites.(8)
Such organization in the nucleus is likely to result from more or less stochastic interactions of self-assembling nuclear proteins, as demonstrated for Cajal bodies.(9) Importantly, the existence of specialized compartments provides opportunities for interactions (‘‘crosstalk’’) between chromatin fibers. Recent technological advancements, in particular the chromosome conformation capture (3C) method and its derivatives (Box 1), allow researchers to study interactions between chromatin regions at high resolution. These approaches are helping to reveal that there are non-random interactions between different regions of chromatin and that these chromatin interactions can influence the function of the genome.(10–12) In particular, they have been implicated in affecting transcription(3) and replication(13) in mammals. However, important questions remain as to how these specific interactions are set up, maintained, and controlled.
Box 1: Chromosome conformation capture (3C) methods.
Awareness of the importance of the folding of chromosomes and how the chromatin fiber can establish both intra- and inter-chromosomal interactions prompted the development of methods to analyze, at high resolution, the frequency of interactions, and the sequences involved. The first method to be used for this purpose, DNA FISH, provides a measure of frequencies of physical juxtapositions(2) Although still useful, its low resolution, >0.2 mm, precludes detailed analysis of the interacting regions. Moreover, the DNA FISH approach requires a prior knowledge of candidate interacting regions, which may generate a bias in interpretation. One of these pitfalls, the low resolution, was overcome by the 3C method(108)
The 3C method has proven very useful in determining close physical proximity of sequences (with a resolution of a few kilobases) from remote locations within formaldehyde-cross-linked chromatin. Briefly, cross-linked chromatin is opened up by detergents, digested with chosen restriction enzymes, and followed by ligation under very dilute conditions, which favors intra-molecular ligation events. Subsequently, interacting chromatin fibers can be identified after reversal of the cross-links, using PCR primers representing either of the sequences of interest.
Another variation of 3C, termed ChIP-loop, combines the 3C analysis with chromatin immunopurification (ChIP) and allows determination of the interacting sequences that are dependent on a particular protein.(76,107)
However, the strategy of using defined PCR primers for either interacting sequence means that 3C is unsuitable where the sequences of interest are unknown. To deal with this shortcoming, alternative methods based on the 3C approach were developed. They are known as 4C techniques and were independently developed in two different laboratories (86,109,110) The name-giving versions of the 4C techniques differ from the 3C method by the inclusion of a circularization step that enables the identification of interacting sequences using primers positioned on one of the sequences (the bait), but close to the junction between the bait and interacting sequence. This trick allows high throughput screening of physical interactions between chromosomes without a preconceived idea of the interacting partners of the sequence of interest. Despite similar strategies, there are fundamental differences in the details of the 4C techniques, such as what restriction enzyme(s) were used, at what stage the circular DNA is generated, and how the resulting PCR-amplified fragments were analyzed. As a further caveat, the 4C methods allow at best a semi-quantitative estimate of genome-wide patterns of interactions from particular baits. Moreover, their inability to readily assess the frequency of patterns of interactions necessitates complementing the 4C screens with DNA FISH analysis. The currently low sensitivity of the 4C technology (usually requiring at least a million cells) offers only snapshots of accumulated interactions. Thus, it should be expected that the number of interacting sequences is quite limited at any given time point and that dynamic on-off patterns of interactions generates a wide range of interacting sequences in large cell populations. Complete patterns of chromosomal fiber interactions with both unbiased interactor and bait will remain unattainable until a sufficiently affordable high throughput sequencing method has been developed. Moreover, there will be a need to develop 3C/4C-like techniques that are able to examine chromatin fiber interactions in individual cells and compare these, e.g., with chromatin states and cell cycle parameters.
A growing body of evidence suggests that the same highly conserved multifunctional protein CCCTC-binding factor (CTCF) is a universal ‘‘master weaver’’ of diverged genomes in all multicellular organisms, which may be important for coordinating the organization and regulation of a whole range of distinct genomic functions in three dimensions (recently reviewed by Phillips and Corces(14)). The main aim of this essay is to discuss properties and functions of CTCF, which we believe are keys to the understanding why CTCF is such an attractive candidate for specifying functional three-dimensional interactions between chromatin fibers. Our principal focus will be on mammalian systems. Finally, we will introduce a model (the ‘‘CTCF code’’) explaining how the information written in the DNA can be interpreted by CTCF into diverse nuclear functions.
CTCF and brother of the regulator of imprinted sites (BORIS)
CTCF (Table 1 and Fig. 1A) was initially discovered as a transcriptional regulator that binds to a CT-rich region in the promoter of the chicken c-myc gene.(15) However, subsequent studies revealed that CTCF can recognize and bind to highly diverse sequences,(16) termed CTCF binding (or target) sites (CTSs). Numerous CTSs have now been identified in various genic and non-genic regions of the genome (including promoters, exons, introns, repeat elements, and others).(16–19)
Table 1.
Comparison of the features of CTCF and BORIS
| Features | CTCF | BORIS (CTCFL) |
|---|---|---|
| Expression pattern | Ubiquitously expressed in various tissues and cells, in different organisms,(111) except primary spermatocytes(28) | Primarily in spermatocytes in the testes of therian mammals in a mutually exclusive fashion with CTCF.(22,28) Activated in a wide range of human cancers.(116–118) |
| Localization | Nuclear(111) | Nuclear and cytoplasmic.(28) |
| Functions | Growth, proliferation, apoptosis, differentiation, imprinting, X-chromosome inactivation, genome integrity, candidate tumor suppressor(14,16,20,22,51) | Linked with epigenetic reprogramming during germ-line development.(22,28) Can function as a dominant-negative version of CTCF. |
| Identified orthologs | Chicken,(111) human,(47) mouse,(47) Xenopus,(112) Drosophila,(113) zebrafish,(114) cattle,(115) tammar wallaby,(115) platypus,(115) central bearded dragon(115) | Human,(28) mouse,(28) cattle,(115) tammar wallaby,(115) platypus,(115) central bearded dragon(115) |
| Molecular mechanisms | Gene activation, repression, insulation and silencing(14,16,20,22,51) | Initiation of methylation(119) or de-methylation events(117,120) in context-dependent manners |
| CTCF target sequences | Recognizes and binds to highly diverse sequences usually in methylation-sensitive manners(16,20–22) | Likely to recognize the same set of DNA targets as CTCF(22,28) |
Figure 1.
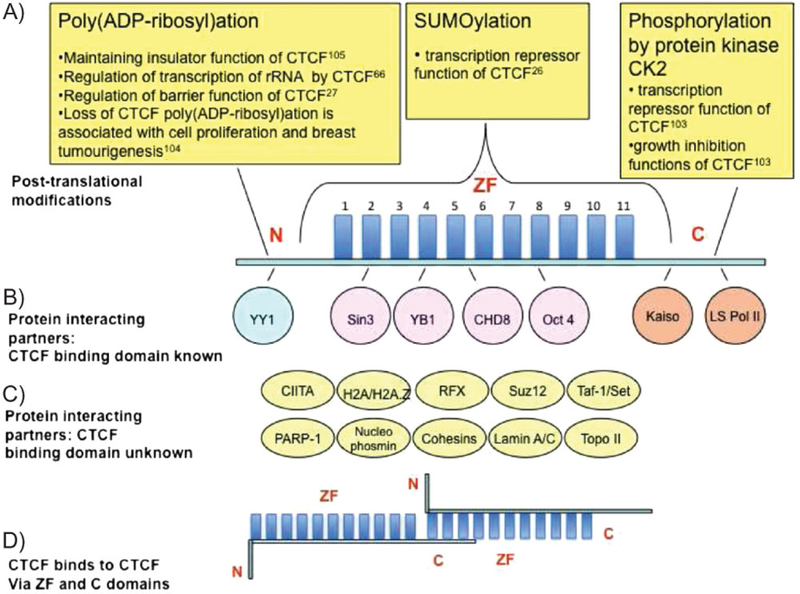
Structure of CTCF and its known modifications and interacting partners. A: Schematic representation of CTCF. It is composed of three structurally distinct domains: the N-terminal domain (N), 11 ZFs and a C-terminal domain (C). Post-translational modifications of CTCF are shown in the upper part of the figure, with the domain that is modified indicated. B: Proteins that interact with CTCF through the known domain, with domain of interaction indicated. C: Other proteins interacting with CTCF. D: CTCF interaction with another CTCF molecule via ZF and C-terminal domains. (For more details in panels B, C, see Table 2).
The DNA binding domain of CTCF, which is highly conserved between species, is composed of 11 zinc fingers (ZFs).(16) The conservation of the CTCF protein from Drosophila to human and its widespread expression in cells and tissues signify the importance of CTCF. Indeed, many cellular functions such as growth, proliferation, differentiation, apoptosis, imprinting, and X-chromosome inactivation are regulated by CTCF.(16,20–22) Conditional CTCF knockout mouse models have demonstrated that CTCF is essential for cell cycle progression in ab T cells(23) and cytokine expression in T helper 2 cells.(24) Together with data obtained recently in Drosophila(25) and mammalian cell models,(26,27) these results converge on regulation of cell cycle as one of the main functions of CTCF. Various post-translational modifications and interacting partners of CTCF have been identified (Fig. 1 and Table 2), which are likely to play an important role in CTCF function (see below).
Table 2.
CTCF protein partners
| Protein partner | General function of the partner | Functional role of CTCF – partner interaction | References |
|---|---|---|---|
| Transcription enzymes | |||
| RNA polymerase II (large subunit), LS Pol II | The largest subunit of eukaryotic RNA polymerase II, enzyme transcribing all protein-coding genes and also non-coding regulatory RNAs | The largest subunit of RNA polymerase II and CTCF probably cooperate in the regulation of transcription and insulator functions | (102) |
| Transcription regulatory factors | |||
| CIITA (class II transactivator) | Transcriptional coactivator, master regulator of MHCII | CTCF, CIITA, and RFX are important for activation of transcription from HLA-DRB1 and HLA-DQA1 genes | (78) |
| CTCF | Multifunctional transcription factor | Dimerization with possible implications in the interaction between distant DNA regions | (49) |
| Kaiso | Member of the POZ (pox virus and ZF) family of ZF transcription factors | Kaiso regulates insulator function of CTCF. May contribute to epigenetic silencing of pRB following CTS methylation. | (121, 122) |
| Oct4 | Member of the family of POU transcription factors. Important for maintaining pluripotency of embryonic stem cells. | Oct4 interaction with CTCF is important for X-chromosome pairing and counting | (123) |
| RFX (regulatory factor X) | Promotes formation of MHCII enhanceosome, which can recruit CIITA as the co-activator | CTCF, CIITA, and RFX are important for the activation of transcription from HLA-DRB1 and HLA-DQA1 genes | (78) |
| YB1 | Multifunctional Y-box DNA/RNA-binding factor | CTCF and YB1 cooperate in transcriptional repression of c-myc. CTCF interferes with activation of the serotonin transporter (5-HTT) gene expression by YB-1. | (19, 124) |
| YY1 | Multifunctional ZF DNA-binding protein | Cooperation in transactivation of Tsix gene | (92) |
| Chromatin constituents | |||
| CHD8 | SNF2-like chromodomain helicase protein | CHD8 regulates insulator function of CTCF | (94) |
| Suz12 | Polycomb group protein that forms Polycomb repressive complexes | Suz12 is important for regulation of insulator function of CTCF at the H19 ICR. | (68) |
| aSin3A | Corepressor of histone deacetylases (HDAC) | Sin3 contributes to transcription repressor function of CTCF possibly via recruitment of histone deacetylases | (125) |
| bTaf-1/Set | Components of the INHAT (inhibitor of acetyltransferases) complex. Inhibit histone acetylation. | Not known | (88) |
| bH2A/H2A.Z | Component of nucleosome, non-allelic variant of histone H2A. Associates with functional regulatory elements. | H2A/H2A.Z and CTCF co-localize genome-wide | (33, 88) |
| Genome integrity | |||
| PARP-1 | Nuclear multifunctional enzyme involved in DNA repair, modulating chromatin structure, regulation of gene expression. | CTCF regulates crosstalk between poly(ADP-ribosyl)ation and DNA methylation | (66, 88) |
| Nuclear architectural proteins | |||
| Nucleophosmin/B23 | Nuclear chaperone, member of the nucleophosmin/nucleoplasmin (NPM) family | Nucleophosmin/B23 regulates insulator function of CTCF | (88) |
| bCohesins | Protein complex mediating cohesion of sister chromatid | Cohesins and CTCF co-localize genome-wide. Cohesins regulate insulator function of CTCF. | (37, 95, 96) |
| bLamin A/C | Components of nuclear lamina | Not known | (88) |
| bTopoisomerase II | DNA topology enzyme | Not known | (88) |
Limited to in vitro observations.
Direct interaction has not been demonstrated.
BORIS (Table 1), also known as CTCFL (CTCF-like), is a paralog of CTCF. The 11ZF domain of BORIS is identical to that of CTCF at every amino acid relevant to DNA binding, suggesting that BORIS does not differ from CTCF with respect to recognition of the same set of DNA targets.(22,28) The flanking N- and C-terminal domains of CTCF and BORIS are different, indicating distinct functions for these domains in the two proteins. In contrast to the ubiquitous CTCF, BORIS is primarily expressed in the testes of therian (placental and marsupial) mammals, in a mutually exclusive fashion with CTCF in mice and humans.(22,28)
CTCF-DNA interactions
What do consensus motifs for CTSs mean?
Identification of the consensus DNA sequence for the binding of a transcription factor is important for prediction of potential target sites and, subsequently, genes regulated by this factor. As prediction of the recognition motifs on the basis of the structure of DNA-binding proteins (including ZF factors) appears to be extremely difficult,(29) these motifs are usually determined experimentally, often in combination with bioinformatics tools.
Given the biological importance of CTCF, significant efforts have been made to elucidate the consensus motif for CTSs. Interestingly, analysis of the CTSs discovered prior to genome-wide screening highlighted the diverse nature of the CTSs, with individual CTSs having strikingly dissimilar primary DNA sequences.(16) This diversity made it very difficult to elucidate a single consensus motif to fit the CTSs then known.(30–32)
More recently, a combination of computational methods and genome-wide screens has generated a number of CTCF ‘‘consensus’’ motifs for different organisms by independent approaches (Fig. 2A).(17,33–38) The three most consistent types of the consensus motif have been generated from the ChIP-seq datasets, which provide a better resolution compared to the ChIP-on-chip data.(39)
Figure 2.

CTCF binding to CTCF target sites. A: Characteristic examples of DNA logos of CTCF ‘‘consensus’’ motifs identified via genome-wide screens. DNA logos of the consensus motifs identified in screening for binding sequences of cohesin subunits, Rad21 and Scc1, are shown at the bottom. The size of each base is indicative of the probability of its presence at a particular position within a binding site. Nucleotides conserved between species are boxed; the invariant core sequence is indicated (‘‘n’’ signifies any nucleotide). B: Different combinations of ZFs are employed depending on the primary sequence of the CTS. Several characteristic examples are shown. The panel on the left summarizes which ZFs of CTCF are involved in binding to the CTS shown on the right. Filled boxes indicate fingers that are essential for binding, lighter boxes indicate fingers less important for binding and empty boxes are fingers dispensable for binding. The panel on the right represents the sequences of the CTSs; contact guanine nucleotides are shown in red. The mutated guanine nucleotide in Xist C(—43)G mutation is shown in lower case in the third example. The CTSs are aligned with the consensus motif,(17) on plus and minus DNA strands; the invariant nucleotides are highlighted in gray. A match with the invariant core is indicated in the column in the middle. In human c-myc-A CTS, the mutation of ‘‘G’’ outside the consensus (indicated by the asterisk) leads to complete loss of CTCF binding. C: The effect of mutations in the CTCF ZF3 and ZF7 on CTS recognition. The range of CTSs recognized by CTCF carrying tumor-specific mutations of individual ZFs(48) in gel retardation experiments is shown. Filled boxes indicate strong binding, lighter boxes indicate partial loss of binding, and empty boxes indicate no binding. The mutations are: K344E, R339W, and H345R in ZF3 and R448Q in ZF7.
The CTCF consensus motifs revealed both commonalities and discrepancies: some nucleotides were conserved at certain positions between species (‘‘the invariant core,’’ boxed in Fig. 2A), whereas motifs identified even within the same species by independent means were not identical. The causes of these discrepancies might include the use of different antibodies that detect CTCF epitopes with different availability after formaldehyde cross-linking (a step in chromatin immunoprecipitation used in most genome-wide studies of CTCF binding), as well as the type of screening procedure adopted. A common problem of such genome-wide screening is that although high-affinity CTCF sites are well represented, there is poor coverage of CTSs weakly bound by CTCF. In addition, it recently emerged that ChIP assays generally have a bias for active chromatin, with heterochromatic regions being under-represented;(40) this may also be relevant to genome-wide CTS detection assays. As discussed below, it is also possible that CTCF could mediate a loop formation between a CTS and a non-CTS. Presence of such non-CTSs, detected by ChIP, in the CTSs population may be an additional factor contributing to the controversial nature of the consensus.
Owing in part to these experimental differences, the number of CTSs determined by genome-wide screening varies. The highest estimate is ~40,000 CTSs in mouse ES cells.(35) A comprehensive ‘‘CTCF binding sites database’’ (http://insulatordb.utmem.edu) has been built(41) from CTSs obtained through genome-wide screens(17,33,42,43) and a further 55 dissimilar CTSs that have been manually characterized (with respect to sequences occupied by CTCF and contact guanines responsible for contacts with CTCF ZFs). Of the CTSs collated from one of the genome-wide studies, the majority (46%) map to intergenic regions of the genome; the remaining CTSs are located in promoters (20%), exons (12%), and introns (22%).(17)
Due to an only partial overlap between the sites identified by the different genome-wide screens, there is a need to experimentally assess the CTCF binding properties of subpopulations of CTSs that were identified in one report but not in others. The importance of such experimental validation is reinforced by the recent discovery of several functional CTSs that do not match any in-silico-generated CTSs consensus motif. Examples include CTSs in the hTERT(44) and ALF(45) gene promoters, and numerous methylation-sensitive and methylation-resistant CTSs in the promoter regions of the human BORIS gene(46) and in pericentromeric gamma-satellite DNA arrays.(18) Finally, the data on CTSs provided by genome-wide screening have limitations as although these studies present indications of the binding position, they only give approximations of the length of the region of DNA bound by CTCF.
What does determine the binding specificity of CTCF?
Early in CTCF research it was demonstrated that the sensitivity of CTCF-CTS complexes to proteases reflected the nature of the CTSs.(47) The realization that the utilization of the ZFs of CTCF for DNA binding might be CTS-specific, led to the development of the missing fingers approach.(47) By employing truncated versions of the ZF domain it was confirmed that, at least in vitro, different combinations of ZFs are employed depending on the sequence of the CTS(16) (Fig. 2B; for more examples see(16)). Inline with these observations, naturally occurring tumor-specific point mutations in the CTCF ZFs completely altered the range of CTSs recognized by full-length CTCF (Fig. 2C).(48)
An interesting aspect of different utilization of binding surfaces by CTCF at different CTSs was shown by in vitro assays using binding sites 3 and 4 at the H19 imprinting control region (ICR).(49) CTCF-DNA complexes formed at site 3 and at site 4 were able to interact with each other through CTCF dimerization. However, interaction did not occur between two complexes bound at separate copies of site 3 or at separate copies of site 4 (illustrated in Fig. 1D). This showed that the surfaces available for dimerization varied depending on the underlying CTS; this might have important functional implications (see below).
Importantly, the pattern of guanine nucleotides that contact CTCF differs between individual CTSs, irrespective of whether or not they conform to the consensus motif(16) (Fig. 2B). Moreover, as demonstrated in vitro(47) and in vivo(26) for the myc-A CTS, which is located immediately downstream of the c-myc P2 promoter, a single-base mutation of a ZF contact outside of the consensus region resulted in the loss of CTCF binding and abrogated activation of the c-myc P2 promoter in a stably chromatin-integrated reporter.(47) Also, naturally occurring mutations in CTSs can dramatically change the binding specificity of CTCF. This is exemplified by C-to-A or C-to-G mutations in a CTS at the 43 position relative to transcriptional start site of the XIST gene. Whereas the C(—43)A mutation abrogated CTCF binding (leading to inactive XIST), the C(—43)G mutation dramatically increased CTCF-binding efficiency (leading to active XIST). In addition, the C(—43)G mutation also altered the ZFs used by CTCF to contact the DNA; so CTCF used different contact nucleotides at the mutant site (Fig. 2B).(50)
Taken together, these observations suggest that a complex combination of the determinants (ZF utilization, the CTS primary sequence, and the CTS context) specify interactions between CTCF and CTSs. It is likely that, in vivo, these determinants have combinatorial effects that diversify CTCF function. In addition to these factors, chromatin context also affects CTCF binding; this is discussed in more detail below.
What do CTSs do?
The enhanced interest in CTCF during the last decade stems from the finding that mammalian chromatin insulators – with apparently no known exceptions – depend on CTSs(25,51) (insulators are discussed further below). As a direct result of this interest, it is currently common in the literature to state that the presence of a CTS equals a chromatin insulator. However, experimental evidence shows that different CTSs are not equal and cannot readily functionally substitute for one another. For example, replacement of the H19 ICR by the β-globin insulator FII leads to the loss of imprinting.(52) Another study shows that mutation of just a single CTS in the cluster of four sites of the mouse H19 ICR results in deregulation of the whole locus and partial loss of imprinting.(49)
In addition to insulation, CTSs have been linked with transcriptional repression (at the chicken and human MYC gene),(47) activation (at the APP gene),(53) silencing (at the chicken lysozyme gene),(54) control of transcriptional elongation,(55) chromatin domain boundaries that control the spread of non-coding RNA,(56) heterochromatinization,(56) and micro-satellite repeat expansion.(57) While it is possible that CTSs will often display an insulator function in a plasmid-based insulator assay, these sites might, however, have other properties in other assays. This issue comprises the task to assign precise functional in vivo properties to CTSs from in vitro assays. Deciphering the function of CTSs is further complicated by the fact that neighboring transcription factor sites can also modulate CTCF function. For example, CTSs flanked by thyroid hormone response elements (TREs) cooperate in enhancer blocking regulation.(58) These data demonstrate that CTCF, via CTSs, is involved in the regulation of multiple cellular functions. Some aspects of this regulation, related to the organization of three-dimensional nuclear space by CTCF, will be discussed below.
The complex relationship between CTCF and chromatin
Nucleosome positioning and CTCF binding
Many transcription factors act by accessing DNA binding sites in a chromatin structure-dependent manner, and CTCF is no exception.(20,21) CTCF cannot interact with CTSs covered by nucleosomes;(59) therefore it is not surprising that CTSs are located in the linker regions between nucleosomes, as originally demonstrated for the H19 ICR(59) and, subsequently, for the entire genome.(60) Interestingly, linker regions are similar in length to the 50–60 bp length of CTCF binding sites, thus suggesting that CTCF fits tightly into the linker regions between positioned nucleosomes. A case of the influence of nucleosome reorganization on CTCF binding was recently reported for the lysozyme gene: transcription of a non-coding RNA, induced by pro-inflammatory stimulus, led to nucleosome repositioning and release of CTCF from the CTS in the negative regulatory element of the lysozyme gene.(61) Whether CTCF itself plays a role in nucleosome repositioning is not well understood; at least within the H19 ICR(59) it is the underlying DNA sequence, not CTCF, that regulates nucleosome position.(62) While such features might have co-evolved with the need to keep some CTSs constitutively available in some regions, such as at the H19 ICR, it is possible that in other instances CTCF might play a more active role in nucleosome repositioning, potentially acting in concert with chromatin remodeling factors to evict or slide nucleosomes covering CTSs.(60)
CTCF and epigenetic states
In addition to the impact of nucleosomes on CTCF binding, CTCF binding to many (although not all) CTSs that contain CpG dinucleotides is dependent on these sequences being unmethylated.(49,63–65) Conversely, mutations in CTSs and loss of CTCF binding in the maternal copy of the H19 ICR lead to hypermethylation (‘‘paternalization’’) of the maternal ICR(49,63,64) and acquisition of histone modifications that are characteristic of inactive chromatin across the H19 locus.(65) Therefore, binding of CTCF to the CTSs at the maternal H19 ICR allele prevents their de novo methylation. This pattern is consistent with observations that CTCF maps to predominantly methylation-free CTSs genome-wide.(42) Furthermore, CTCF-protein complexes might have an indirect inhibitory effect on the DNA methyltransferase Dnmt1 via PARP-1, resulting in DNA hypomethylation(66) (also see Table 2). The importance of controlling DNA methylation at CTSs is further illustrated by the example of the spinocerebellar ataxia type 7 (SCA7) loci. Here, a methylation-free status at the CTSs is necessary to control the CTG repeat extension;(57) such a mechanism may be common for many disorders resulting from microsatellite repeat expansion. In addition, CTCF binding to the CTS in the DM1 locus(56) can control the spread of the non-coding RNA and heterochromatin, thus restricting the CTG repeat expansion.
Despite the diversity in the relationships between CTCF and epigenetic regulation reported so far, a common feature has recently emerged from the genome-wide studies. Although mainly correlative, these studies have found that the border between the repressive chromatin mark histone H3 trimethylated at lysine 27 (H3K27me3) and the active mark histone H2A acetylated at lysine 5 (H2AK5ac) are marked by CTCF.(33,34) Another study showed that the borders of heterochromatic lamina-associated domains are also frequently separated by CTCF.(67) These observations raise the possibility that CTCF could maintain the local balance between active and repressive chromatin marks; this implies a need for cooperation between CTCF and chromatin modifiers. Indeed, association between CTCF and repressive complexes has been reported.(68)
The conclusion from these observations is that the relationship between CTCF and chromatin is regulated in a complex manner through a variety of molecular mechanisms, including methylation-sensitive binding of CTCF to CTSs, nucleosome positioning, transcription of non-coding RNA, and regulation of local balance between active and repressive chromatin marks. As we discuss below, the link between CTCF and chromatin is likely to be crucial to explaining the mechanistic and functional aspects of interactions between different regions of DNA.
CTCF and three-dimensional aspects of genome function
It is now widely accepted that sub-chromosomal regions have a dynamic localization relative to nuclear compartments, where they encounter other parts of the genome. The trivial explanation for the existence of inter- and intra-chromosomal interactions is that they result from random collisions in the nucleus. However, a growing body of evidence demonstrates that close spatial proximity of genes might result from a sharing of common resources(69) (such as transcriptional machinery) and might also have regulatory functions in the selective activation or silencing of alleles.(70,71) Although the mechanisms underlying spatial arrangement are currently poorly understood, observations relating to three-dimensional organization using the 3C technique and its derivatives (Box 1) are already significantly influencing our perspective of how gene functions are established and maintained by both cis- and trans-acting influences. In this section, we review current data that link CTCF with spatial regulation of genome functions.
Chromatin insulator and barrier function
Chromatin insulators are commonly defined as DNA elements that prevent inappropriate interactions between neighboring genes. Insulators are classified into enhancer blockers and barrier elements. Enhancer blockers prevent enhancer-promoter interactions if placed between these elements, thus repressing transcription. In contrast, chromatin barriers prevent spreading of heterochromatin (a repressive chromatin environment) into neighboring active domains.(72)
The CTCF-insulator link was first demonstrated for the chicken HS4 element in the 5ʹ boundary of the β-globin locus.(30) Subsequently, CTCF binding sites were identified at the H19 ICR insulator.(31,73,74) The number of characterized insulator sites increased with the development of a high throughput microarray analysis scoring for insulator functions.(42) However, common to all of these, and numerous other, reports is the use of plasmid systems with CTSs placed between enhancers and promoters of reporter genes;(75) i.e., testing for enhancer blocker activity. Such artificial systems suffer from a number of limitations: apart from poorly mimicking the chromatin context of endogenous CTSs, the plasmid systems do not allow an assessment of very long-range chromatin effects, which have emerged as a crucial element in the regulation of insulator function.(76) There is a strong possibility, therefore, that the insulator plasmid systems, irrespective of whether they are maintained episomally or incorporated into the genome, do not reflect an in vivo situation. To rigorously determine enhancer blocker function in vivo, there is a need not only to delete the insulator region, but also to insert the endogenous enhancer between the insulator and the transcriptional unit in question by genetic engineering approaches. Currently, the H19 ICR is the only mammalian insulator that has undergone such a robust in vivo analysis.(63,64)
It has for some time been unclear whether or not CTCF is involved also in chromatin barrier function. Although it was reported that the chromatin barrier at the 5ʹ boundary of the chicken β-globin locus region contains no CTSs(77) and is CTCF-independent, three different recent reports have demonstrated that chromatin barrier functions can be CTCF-dependent: it was observed that CTSs in several instances demarcate repressive (H3K27me3) from active (H2AK5ac) domains in cell type-specific patterns;(34) gamma-satellite sequences bind CTCF to protect against epigenetic silencing;(18) and the tumor suppressor gene p16INK4 can be aberrantly silenced by the eviction of CTCF from CTSs at or near a chromatin boundary.(27) Therefore it appears that at a subset of CTSs, CTCF binding has the ability to block either enhancing or silencing effects on transcriptional units.
Mechanisms of CTCF action in cis
The observations that long-range chromosome interactions are involved in the regulation of chromatin insulators hinted at two – not mutually exclusive – ways in which insulators might function. First, as exemplified by the Igf2-H19(76)and MHC-II(78) loci, CTCF-dependent insulators can organize long-range interactions, which in turn may influence epigenetic states at key cis regulatory elements and affect transcription.
Second, CTCF might drive the organization of insulated domains, probably via formation of loops and hubs, which restrict access to the transcriptional machinery. The active hub (ACH) concept(79) was first developed to suggest physical interaction in the nucleus between active β-globin genes and multiple cis-regulatory elements, whereas inactive genes were looped out. The role of CTCF in this system appears to be to regulate epigenetic states and long-range chromatin interactions, at the early stages of erythroid differentiation, with no effect on transcription.(80,81) The latter finding is puzzling and may be explained by the presence of additional CTCF binding sites and/or different function of these CTSs in this locus.(81)
The ACH concept provided a framework to explain CTCF-mediated interchromosomal interactions in the Igf2 locus, which include several models. In the ‘‘inactive loop’’ model, the insulator is proposed to act as a topological barrier, creating a tight and transcriptionally inactive loop around the maternal Igf2 locus.(76) In the ‘‘unproductive loop’’ model, the insulator competes with the enhancer for promoters, acting as a promoter decoy for enhancers.(82) In the ‘‘knotted loop’’ model, the insulator forms a composite physical barrier (which might even involve interactions between different chromosomes) that blocks the access of all enhancers to Igf2, thereby silencing the maternal Igf2.(83) Irrespective of the specific model, the function of a loop would be to maintain, directly or indirectly, the silent state of the Igf2 at the maternal chromosome. Indeed, CTCF interacts with Suz12, a component of the PRC2 complex,(68) which establishes repressive H3K27me3 marks. The juxtaposition of PRC2 at the H19 ICR with the Igf2 promoter/DMR1 region might, via long-range chromatin fiber interaction and association to Pc bodies, contribute to the repressed status of the maternal Igf2 locus.
However, it is noteworthy that the ICR insulator needs to be continuously present to suppress maternal Igf2 expression in somatic cells, suggesting that any such epigenetic mark is not stably maintained.(84) Exactly how this relates to the mechanism of enhancer function is not clear, although it has been argued that the H19 enhancer tracks along the chromatin fiber.(85) In this model, the H19 ICR insulator both provides a topological barrier and acts as a long-distance facilitator of repressed states.
CTCF action in trans
As the distribution of CTSs closely parallels gene density profiles,(17) the relationship between the gene density of chromosomes and their position within the nucleus is likely to influence the nuclear distribution of CTSs and thereby influence opportunities for CTCF-dependent chromatin fiber interactions. Although the data available are currently limited, the application of 3C and related techniques has provided data that support the existence of many CTCF-dependent trans-chromatin fibers. In these experiments, CTSs at the H19 ICR were shown to interact with a wide range of different loci both from the same and different chromosomes.(76,86,87) Furthermore, physical associations between chromatin fibers from different chromosomes were shown to affect transcription; e.g., deletion of the maternal H19 ICR allele on chromosome 7 led to alteration in the expression level of the Wsb1/Nf1 locus on chromosome 11.(87) Similarly, the expression levels of an imprinted domain on chromosome 18, the Osbpl1a/Impact locus, depended on CTSs of the maternal H19 ICR allele.(86) It is not yet known, however, whether it is CTCF itself, its interaction partners, or the physical interaction of the chromatin that influences transcription at these loci.
The functions of CTCF-dependent complexes might also be linked to their positions relative to sub-nuclear compartments. For example, interaction of CTCF with nucleophosmin could possibly direct some CTSs to nucleoli,(88) and, as was recently demonstrated, to the nuclear membrane, in cooperation with lamin A.(89) The subset of CTSs that flank heterochromatic blocks, and possibly function as chromatin barrier, are likely to be found at the nuclear membrane where these blocks are often positioned.(67,89) In combination with random or directed chromatin movements within and beyond the boundaries of chromosomal territories, localization to nuclear compartments can further diversify patterns of intra-and inter-chromosomal interactions by providing different chromatin environments. For example, a transvection-like phenomenon in human cancer cells may rely on CTCF and its ability to relocate a subset of CTSs to the nucleolar compartment: at the nucleolus of mantle cell lymphoma cells, interaction between a CCND1 allele in its normal chromosomal location and a translocated allele might be dependent on CTCF-nucleophosmin complexes associated with key regulatory elements at the translocated allele.(90) Interestingly, the epigenetic status of the translocated allele was able to influence the methylation status of the non-rearranged CCND1 allele and hence influence its transcription potential. Similarly, it has been proposed that interaction between X-chromosomes in female mammals, during the counting phase of X-chromosome inactivation, might involve CTSs on X-chromosomes.(91,92)
The role of cohesin
For loops to form in a primary chromatin fiber, it has been suggested that the interacting sites must, by default, be at least 10 kb apart.(93) Higher-order chromatin conformations might impose further hindrances on chromatin folding. Of course, chromatin flexibility can be influenced by several factors, such as helicase activity. Interestingly, CTCF recruits CHD8, a chromatin-remodeling factor with helicase activity, to CTSs.(94) Thus, CHD8 could potentially release torsional stress that is hampering CTCF-dependent loop formation.
It is not known how an encounter between chromatin fibers is initiated and then stabilized, although it has been proposed to involve distinct steps that reflect stochastic or directed chromatin movements. Once the physical distance between two chromosomal regions is sufficiently small, protein-DNA complexes might – perhaps transiently – stabilize the direct interaction between chromatin fibers. Members of the cohesin complex could play such a role. Cohesin complex binding extensively overlaps with CTSs in mammalian cells(37,95,96) and the majority of chromatin-cohesin associations are dependent on CTSs (Fig. 2A). As CTCF(49) (Fig. 1D) and cohesins(97) can homodimerize, it has been proposed that contact between two distal CTSs might be initiated via interaction between two CTCF and/or two cohesin molecules. Indeed, analysis of the molecular features of short-range (locus-specific) chromatin fiber interactions at the Ifng locus showed that the CTCF-cohesin complex regulates chromatin interactions within the Ifng locus in human T helper 1 cells.(98) Although the parental Ifng alleles have been shown to interact in trans to facilitate the kinetics of IFN-γ expression upon pathogen insults,(99) in this case the CTCF-cohesin complex appears to be operating only in cis, consistent with the function of the cohesin complex in constraining chromosome topology.
The Ifng example described above suggests that the CTCF-cohesin complex might be specific to particular developmental or cellular stages. Similarly, contraction of the immunoglobulin heavy and light chain loci appears to involve stage-specific recruitment of the cohesin complex to CTSs during B cell development.(100) The concept that CTCF might interact stably with CTSs whereas the cohesin complex forms transiently at CTSs was further supported by the recent observation that the CTCF-cohesin association at chromatin peaks at mid-S phase and is dissolved during G2/M.(101) Such a dynamic behavior of the CTCF-cohesin interaction is consistent with the possibility that complexes of CTCF with other proteins (Table 2) vary between different cellular and chromosomal contexts.
A model for the diverse functions of CTCF
This review has summarized current evidence that has shown CTCF to be a versatile factor implicated in a large variety of nuclear functions. How can one factor be involved in such a range of biological processes? We believe that CTCF possesses features that might help to explain the molecular basis for organization of chromatin domains and epigenetic states both in cis and in trans. These features include: the presence of numerous binding sites (CTSs) – alone or in tandem – in the genome; the diversity of CTSs, resulting in the utilization of different ZFs; the ability of CTCF to interact with itself and/or with a wide range of protein partners; the regulation of CTCF by post-translational modifications (Fig. 1); and links between CTCF and epigenetic regulation. This combination of properties might be able to provide sufficient flexibility for the organization of dynamic nuclear chromatin domains and at the same time be able to provide specificity for selection of non-random interactions.
To draw together the diverse observations relating to CTCF function, we propose a unifying model, which we term the CTCF code (Fig. 3). The central concept of this model is that CTCF can ‘‘read’’ the diverse functional information written in the DNA sequence of CTSs, such that differences in the nucleotide sequences, within or outside the consensus CTS motifs, can be sensed by various combinations of CTCF ZFs. As a result of using different ZFs to bind different CTSs, the exposed surface of CTCF that is available for interaction with another CTCF molecule(49) or with CTCF protein partners varies. Evidence for different CTSs having different intrinsic properties, for the differential utilization of ZF by different CTSs, and for the interaction between two different CTCF-CTSs complexes(16,49) has been discussed above. A direct association of certain CTSs with CTCF bound to specific protein partners has been demonstrated on a genome scale by serial ChIP analysis for CTCF-RNA polymerase II.(102) This type of analysis needs to be extended to other CTCF partners in order to test whether specific CTSs are consistently associated with specific CTCF-protein complexes.
Figure 3.
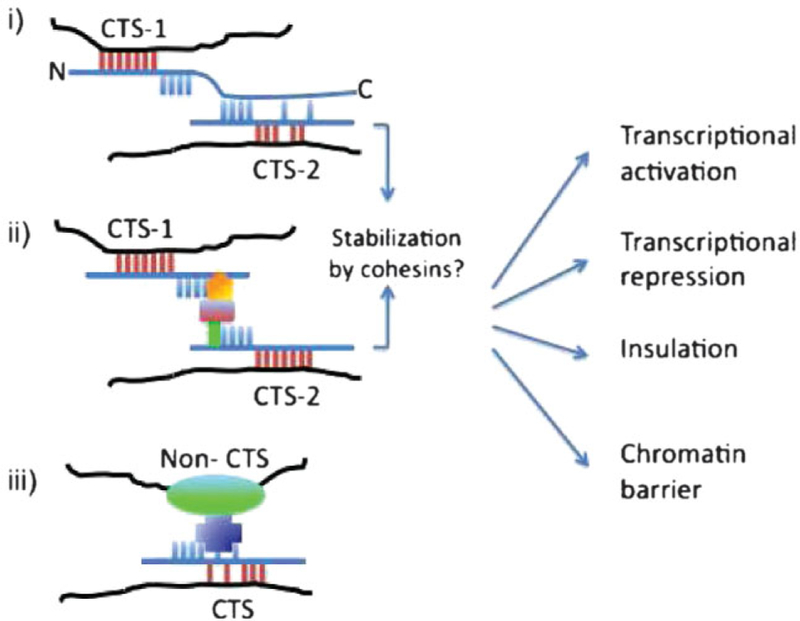
The CTCF code. Formation of diverse CTCF-protein complexes at different CTCF target sites (CTSs). The CTSs (CTS-1 and CTS-2) are recognized (‘‘read’’) by CTCF (blue line with ZFs shown as bars, and N- and C-terminal domains as an extension of the line). The red bars indicate the ZFs contacting the CTSs, and blue bars the ZFs that do not contact the CTSs. DNA is shown as a black line. Out of numerous possibilities we depict three general cases. In the first (i), CTCF uses different ZFs to contact two different CTSs. CTCF molecules bound at the two sites make direct contact with each other via, e.g., C-terminal ZF domain interactions (see Ref.(49)). This brings together two CTSs. In the second example (ii), CTS-1 and CTS-2 are brought together by protein partners, which contact CTCF directly or indirectly via protein-protein bridges (green, red, and orange symbols in the image). Cohesin may act to stabilize the interactions in cases (i) and (ii), as demonstrated in Ref.(98) In the third example (iii), CTCF may directly (see, e.g., Ref.(92)) or indirectly make contact with another DNA-binding trans-acting factor to bridge a CTS with a non-CTS. In this model, use of different ZFs at different CTSs results in different surfaces on CTCF being available for inter-action with protein partners. The resulting combination of the CTCF-CTSs-protein complexes specifies a particular nuclear function. According to the model, the underlying CTS determines the pattern of ZF utilization and hence the choice of protein partner in combina-tion with post-translational modifications to dictate the functional outcome, as indicated in the figure.
The differences between the CTCF-DNA complexes that form at different CTSs across the genome might create a network of regulatory complexes that are capable of receiving, translating, and integrating intrinsic and extrinsic cues into functional outcomes. This model does not rule out the possibility of interactions between a CTCF complex and another type of protein complex, e.g., formed by another transcription factor at its cognate site (Fig. 3iii). Post- translational modifications of CTCF (Fig. 1)(26,27,66,103–105) might also contribute to the diversity of outcomes of CTCF binding and complex formation.
Currently the data available to support this model are limited and are largely based on in vitro experiments. However, the model outlines predictions that can be tested in vivo, such as analysis of the CTSs bound by particular CTCF-protein complexes and identification of which proteins are associated with specific CTSs.
Conclusions
The large number of different CTSs across the genome and the apparent range of possible CTCF-protein interactions (‘‘CTCF-interactome’’) might be involved in the translation of a myriad of regulatory signals into specific nuclear functions. This could create a very extensive web of molecular interactions in the nucleus involving CTCF, its partners and CTSs. In order to elucidate the molecular details of CTCF action – and to test the concept of the CTCF code and the CTCF-interactome – further experimentations are required. These may include, e.g., elucidation of the biochemical properties of CTCF when it interacts with different CTSs, further characterization of the effect of missing ZFs, structural analysis of CTCF-CTSs complexes, as well as a more complete identification of proteins associated with specific CTSs in vivo.(106) Another aspect of CTCF function that warrants further investigation is whether and how it can act in an indirect manner, e.g., by maintaining methylation-free regions that would allow the binding of other protein factors.
Despite a large body of experimental data, the modus operandi of CTCF in the establishment of the long-distance interactions between chromatin fibers remains poorly understood. How can particular binding sites on separate chromosomes recognize each other amongst several tens of thousands of binding sites scattered all over the genome? This is a central issue in any approach that attempts to understand the mechanistic basis of long-range interactions between chromatin fibers. A plausible explanation is that the higher-order chromatin conformations of interacting chromosomes provide three-dimensional epigenetic information: an initial encounter that transiently generates an inter-chromosomal interaction facilitates protein-protein interactions that can stabilize specific interactions.
The fact that CTCF is expressed in most, if not all, somatic cells is one feature that makes it stands out amongst other key architectural nuclear factors, such as SatB1.(107) This expression pattern and the profound consequences of CTCF mutation and knock-down suggest that CTCF is an indispensable factor associated with essential functions in somatic cells. Given the relationship between CTCF and epigenetic modifications, it seems likely that CTCF complexes need to be dissolved during reprogramming events. In contrast to CTCF, BORIS expression is normally restricted to cells that are being epigenetically reprogrammed. Therefore it is possible that BORIS antagonizes CTCF interactions. Whether BORIS operates as a dominant-negative version of CTCF and/or as a CTCF-like factor able to establish other types of chromatin fiber interactions with its own set of protein partners remains to be determined.
Our understanding of human diseases might also benefit from a research into how loss of CTCF functions can perturb nuclear architecture. One can envisage that disease-associated changes in CTCF-dependent features of the nuclear architecture might be owing to epigenetic changes at CTCF binding sites or changes at other levels including CTCF ZF mutations, lesions that affect post-translational modifications, altered responses to signaling pathways, loss of function of CTCF protein partners, or the unscheduled activation of BORIS. Such effects could act locally or more globally, thus affecting the three-dimensional chromatin network in the whole nucleus in a pleiotropic manner.(69) Clearly, we are only seeing the beginning of a new and exciting research area that will improve our understanding of what functionally connects chromatin fibers within the nucleus.
Acknowledgments:
We sincerely apologize to our colleagues in the field for not being able to cite many relevant original papers due to space limitation. Valuable advice from Dr. A. Gӧndӧr and Mr. P. Vince is gratefully acknowledged. This work was supported by the Swedish Science Research Council (RO), the Swedish Cancer Research Foundation (RO), the Swedish Pediatric Cancer Foundation (RO), the Lundberg Foundation (RO) as well as HEROIC and ChILL (EU integrated projects, RO), and in part funded by intramural research program of NIAID/NIH (VL), Medical Research Council, UK (EK), Breast Cancer Campaign, UK (EK), Breast Cancer Research Trust, UK (EK), University of Essex, UK (EK), and Colchester Hospital University NHS Foundation Trust, UK (EK).
Footnotes
Note added in proof. Shortly after this manuscript was accepted for publication, a report appeared in Genome Biology online (Essien K, Vigneau S, Apreleva S, Singh LN, Bartolomei MS, Hannenhalli S. ‘‘CTCF binding site classes exhibit distinct evolutionary, genomic, epigenomic and transcriptomic features’’ 2009. Genome Biol. Nov 18;10(11): R131. [Epub ahead of print]), which describes the existence of several different classes of CTCF binding sites with potentially different functions. These data provide evidence in support of the ‘‘CTCF code’’ model proposed in our essay.
References
- 1.Goetze S, Mateos-Langerak J, van Driel R. 2007. Three-dimensional genome organization in interphase and its relation to genome function. Semin Cell Dev Biol 18: 707–14. [DOI] [PubMed] [Google Scholar]
- 2.Cremer T, Cremer C. 2001. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet 2: 292–301. [DOI] [PubMed] [Google Scholar]
- 3.Sexton T, Schober H, Fraser P, et al. 2007. Gene regulation through nuclear organization. Nat Struct Mol Biol 14: 1049–1055. [DOI] [PubMed] [Google Scholar]
- 4.Branco MR, Pombo A. 2006. Intermingling of chromosome territories in interphase suggests role in translocations and transcription-dependent associations. PLoS Biol 4: e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert N, Gilchrist S, Bickmore WA. 2005. Chromatin organization in the mammalian nucleus. Int Rev Cytol 242: 283–336. [DOI] [PubMed] [Google Scholar]
- 6.Takizawa T, Meaburn KJ, Misteli T. 2008. The meaning of gene positioning. Cell 135: 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanctot C, Cheutin T, Cremer M, et al. 2007. Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat Rev Genet 8: 104–15. [DOI] [PubMed] [Google Scholar]
- 8.Misteli T 2007. Beyond the sequence: cellular organization of genome function. Cell 128: 787–800. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser TE, Intine RV, Dundr M. 2008. De novo formation of a subnuclear body. Science 322: 1713–7. [DOI] [PubMed] [Google Scholar]
- 10.Fraser P, Bickmore W. 2007. Nuclear organization of the genome and the potential for gene regulation. Nature 447: 413–7. [DOI] [PubMed] [Google Scholar]
- 11.Kumaran RI, Thakar R, Spector DL. 2008. Chromatin dynamics and gene positioning. Cell 132: 929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dekker J 2008. Gene regulation in the third dimension. Science 319: 1793–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gondor A, Ohlsson R. 2009. Replication timing and epigenetic reprogramming of gene expression: a two-way relationship? Nat Rev Genet 10: 269–76. [DOI] [PubMed] [Google Scholar]
- 14.Phillips JE, Corces VG. 2009. CTCF: master weaver of the genome. Cell 137: 1194–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobanenkov VV, Nicolas RH, Adler VV, et al. 1990. A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5’-flanking sequence of the chicken c-myc gene. Oncogene 5: 1743–53. [PubMed] [Google Scholar]
- 16.Ohlsson R, Renkawitz R, Lobanenkov V. 2001. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet 17: 520–7. [DOI] [PubMed] [Google Scholar]
- 17.Kim TH, Abdullaev ZK, Smith AD, et al. 2007. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell 128: 1231–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JH, Ebersole T, Kouprina N, et al. 2009. Human gamma-satellite DNA maintains open chromatin structure and protects a transgene from epigenetic silencing. Genome Res 19: 533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klenova E, Scott AC, Roberts J, et al. 2004. YB-1 and CTCF differentially regulate the 5-HTT polymorphic intron 2 enhancer which predisposes to a variety of neurological disorders. J Neurosci 24: 5966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filippova GN. 2008. Genetics and epigenetics of the multifunctional protein CTCF. Curr Top Dev Biol 80: 337–60. [DOI] [PubMed] [Google Scholar]
- 21.Recillas-Targa F, De La Rosa-Velazquez IA, Soto-Reyes E, et al. 2006. Epigenetic boundaries of tumour suppressor gene promoters: the CTCF connection and its role in carcinogenesis. J Cell Mol Med 10: 554–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klenova EM, Morse HC III, Ohlsson R, et al. 2002. The novel BORIS þ CTCF gene family is uniquely involved in the epigenetics of normal biology and cancer. Semin Cancer Biol 12: 399–414. [DOI] [PubMed] [Google Scholar]
- 23.Heath H, de Almeida CR, Sleutels F, et al. 2008. CTCF regulates cell cycle progression of alphabeta T cells in the thymus. EMBO J 27: 2839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro de Almeida C, Heath H, Krpic S, et al. 2009. Critical role for the transcription regulator CCCTC-binding factor in the control of Th2 cytokine expression. J Immunol 182: 999–1010. [DOI] [PubMed] [Google Scholar]
- 25.Bushey AM, Ramos E, Corces VG. 2009. Three subclasses of a Drosophila insulator show distinct and cell type-specific genomic distributions. Genes Dev 23: 1338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacPherson MJ, Beatty LG, Zhou W, et al. 2009. The CTCF insulator protein is posttranslationally modified by SUMO. Mol Cell Biol 29: 714–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witcher M, Emerson BM. 2009. Epigenetic silencing of the p16(INK4a) tumor suppressor is associated with loss of CTCF binding and a chromatin boundary. Mol Cell 34: 271–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loukinov DI, Pugacheva E, Vatolin S, et al. 2002. BORIS, a novel male germ-line-specific protein associated with epigenetic reprogramming events, shares the same 11-zinc-finger domain with CTCF, the insulator protein involved in reading imprinting marks in the soma. Proc Natl Acad Sci U S A 99: 6806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pabo CO, Nekludova L. 2000. Geometric analysis and comparison of protein-DNA interfaces: why is there no simple code for recognition? J Mol Biol 301: 597–624. [DOI] [PubMed] [Google Scholar]
- 30.Bell AC, West AG, Felsenfeld G. 1999. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell 98: 387–96. [DOI] [PubMed] [Google Scholar]
- 31.Bell AC, Felsenfeld G. 2000. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 405: 482–5. [DOI] [PubMed] [Google Scholar]
- 32.Chao W, Huynh KD, Spencer RJ, et al. 2002. CTCF, a candidate transacting factor for X-inactivation choice. Science 295: 345–7. [DOI] [PubMed] [Google Scholar]
- 33.Barski A, Cuddapah S, Cui K, et al. 2007. High-resolution profiling of histone methylations in the human genome. Cell 129: 823–37. [DOI] [PubMed] [Google Scholar]
- 34.Cuddapah S, Jothi R, Schones DE, et al. 2009. Global analysis of the insulator binding protein CTCF in chromatin barrier regions reveals demarcation of active and repressive domains. Genome Res 19: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Xu H, Yuan P, et al. 2008. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133: 1106–17. [DOI] [PubMed] [Google Scholar]
- 36.Holohan EE, Kwong C, Adryan B, et al. 2007. CTCF genomic binding sites in Drosophila and the organisation of the bithorax complex. PLoS Genet 3: e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wendt KS, Yoshida K, Itoh T, et al. 2008. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 451: 796–801. [DOI] [PubMed] [Google Scholar]
- 38.Bartkuhn M, Straub T, Herold M, et al. 2009. Active promoters and insulators are marked by the centrosomal protein 190. EMBO J 28: 877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi H, Nesvizhskii AI, Ghosh D, et al. 2009. Hierarchical Hidden Markov Model with Application to Joint Analysis of ChIP-chip and ChIP-seq data. Bioinformatics 25: 1715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teytelman L, Ozaydin B, Zill O, et al. 2009. Impact of chromatin structures on DNA processing for genomic analyses. PLoS One 4: e6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bao L, Zhou M, Cui Y. 2008. CTCFBSDB: a CTCF-binding site database for characterization of vertebrate genomic insulators. Nucleic Acids Res 36 (Database issue): D83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukhopadhyay R, Yu W, Whitehead J, et al. 2004. The binding sites for the chromatin insulator protein CTCF map to DNA methylation-free domains genome-wide. Genome Res 14: 1594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jothi R, Cuddapah S, Barski A, et al. 2008. Genome-wide identification of in vivo protein-DNA binding sites from ChIP-Seq data. Nucleic Acids Res 36: 5221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renaud S, Loukinov D, Bosman FT, et al. 2005. CTCF binds the proximal exonic region of hTERT and inhibits its transcription. Nucleic Acids Res 33: 6850–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim M, Li D, Cui Y, et al. 2006. Regulatory factor interactions and somatic silencing of the germ cell-specific ALF gene. J Biol Chem 281: 34288–98. [DOI] [PubMed] [Google Scholar]
- 46.Renaud S, Pugacheva EM, Delgado MD, et al. 2007. Expression of the CTCF-paralogous cancer-testis gene, brother of the regulator of imprinted sites (BORIS), is regulated by three alternative promoters modulated by CpG methylation and by CTCF and p53 transcription factors. Nucleic Acids Res 35: 7372–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filippova GN, Fagerlie S, Klenova EM, et al. 1996. An exceptionally conserved transcriptional repressor, CTCF, employs different combinations of zinc fingers to bind diverged promoter sequences of avian and mammalian c-myc oncogenes. Mol Cell Biol 16: 2802–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Filippova GN, Qi CF, Ulmer JE, et al. 2002. Tumor-associated zinc finger mutations in the CTCF transcription factor selectively alter tts DNA-binding specificity. Cancer Res 62: 48–52. [PubMed] [Google Scholar]
- 49.Pant V, Kurukuti S, Pugacheva E, et al. 2004. Mutation of a single CTCF target site within the H19 imprinting control region leads to loss of Igf2 imprinting and complex patterns of de novo methylation upon maternal inheritance. Mol Cell Biol 24: 3497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pugacheva EM, Tiwari VK, Abdullaev Z, et al. 2005. Familial cases of point mutations in the XIST promoter reveal a correlation between CTCF binding and pre-emptive choices of X chromosome inactivation. Hum Mol Genet 14: 953–65. [DOI] [PubMed] [Google Scholar]
- 51.Wallace JA, Felsenfeld G. 2007. We gather together: insulators and genome organization. Curr Opin Genet Dev 17: 400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szabo PE, Tang SH, Reed MR, et al. 2002. The chicken beta-globin insulator element conveys chromatin boundary activity but not imprinting at the mouse Igf2/H19 domain. Development 129: 897–904. [DOI] [PubMed] [Google Scholar]
- 53.Vostrov AA, Quitschke WW. 1997. The zinc finger protein CTCF binds to the APBbeta domain of the amyloid beta-protein precursor promoter. Evidence for a role in transcriptional activation. J Biol Chem 272: 33353–9. [DOI] [PubMed] [Google Scholar]
- 54.Baniahmad A, Steiner C, Kohne AC, et al. 1990. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell 61: 505–14. [DOI] [PubMed] [Google Scholar]
- 55.Egloff S, Al-Rawaf H, O’Reilly D, et al. 2009. Chromatin structure is implicated in ‘‘late’’ elongation checkpoints on the U2 snRNA and beta-actin genes. Mol Cell Biol 29: 4002–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho DH, Thienes CP, Mahoney SE, et al. 2005. Antisense transcription and heterochromatin at the DM1 CTG repeats are constrained by CTCF. Mol Cell 20: 483–9. [DOI] [PubMed] [Google Scholar]
- 57.Libby RT, Hagerman KA, Pineda VV, et al. 2008. CTCF cis-regulates trinucleotide repeat instability in an epigenetic manner: a novel basis for mutational hot spot determination. PLoS Genet 4: e1000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lutz M, Burke LJ, LeFevre P, et al. 2003. Thyroid hormone-regulated enhancer blocking: cooperation of CTCF and thyroid hormone receptor. EMBO J 22: 1579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanduri M, Kanduri C, Mariano P, et al. 2002. Multiple nucleosome positioning sites regulate the CTCF-mediated insulator function of the H19 imprinting control region. Mol Cell Biol 22: 3339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu Y, Sinha M, Peterson CL, et al. 2008. The insulator binding protein CTCF positions 20 nucleosomes around its binding sites across the human genome. PLoS Genet 4: e1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lefevre P, Witham J, Lacroix CE, et al. 2008. The LPS-induced transcriptional upregulation of the chicken lysozyme locus involves CTCF eviction and noncoding RNA transcription. Mol Cell 32: 129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davey C, Fraser R, Smolle M, et al. 2003. Nucleosome positioning signals in the DNA sequence of the human and mouse H19 imprinting control regions. J Mol Biol 325: 873–87. [DOI] [PubMed] [Google Scholar]
- 63.Pant V, Mariano P, Kanduri C, et al. 2003. The nucleotides responsible for the direct physical contact between the chromatin insulator protein CTCF and the H19 imprinting control region manifest parent of origin-specific long-distance insulation and methylation-free domains. Genes Dev 17: 586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schoenherr CJ, Levorse JM, Tilghman SM. 2003. CTCF maintains differential methylation at the Igf2/H19 locus. Nat Genet 33: 66–9. [DOI] [PubMed] [Google Scholar]
- 65.Han L, Lee DH, Szabo PE. 2008. CTCF is the master organizer of domain-wide allele-specific chromatin at the H19/Igf2 imprinted region. Mol Cell Biol 28: 1124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caiafa P, Zlatanova J. 2009. CCCTC-binding factor meets poly(ADP-ribose) polymerase-1. J Cell Physiol 219: 265–70. [DOI] [PubMed] [Google Scholar]
- 67.Guelen L, Pagie L, Brasset E, et al. 2008. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453: 948–51. [DOI] [PubMed] [Google Scholar]
- 68.Li T, Hu JF, Qiu X, et al. 2008. CTCF regulates allelic expression of Igf2 by orchestrating a promoter-polycomb repressive complex 2 intrachro-mosomal loop. Mol Cell Biol 28: 6473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gondor A, Ohlsson R. 2009. Chromosome crosstalk in three dimensions. Nature 461: 212–7. [DOI] [PubMed] [Google Scholar]
- 70.de Laat W, Grosveld F. 2007. Inter-chromosomal gene regulation in the mammalian cell nucleus. Curr Opin Genet Dev 17: 456–64. [DOI] [PubMed] [Google Scholar]
- 71.Ohlsson R 2007. Genetics. Widespread monoallelic expression. Science 318: 1077–8. [DOI] [PubMed] [Google Scholar]
- 72.Bushey AM, Dorman ER, Corces VG. 2008. Chromatin insulators: regulatory mechanisms and epigenetic inheritance. Mol Cell 32: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hark AT, Schoenherr CJ, Katz DJ, et al. 2000. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 405: 486–9. [DOI] [PubMed] [Google Scholar]
- 74.Kanduri C, Pant V, Loukinov D, et al. 2000. Functional association of CTCF with the insulator upstream of the H19 gene is parent of origin-specific and methylation-sensitive. Curr Biol 10: 853–6. [DOI] [PubMed] [Google Scholar]
- 75.Chung JH, Whiteley M, Felsenfeld G. 1993. A 5’ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell 74: 505–14. [DOI] [PubMed] [Google Scholar]
- 76.Kurukuti S, Tiwari VK, Tavoosidana G, et al. 2006. CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc Natl Acad Sci U S A 103: 10684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Recillas-Targa F, Pikaart MJ, Burgess-Beusse B, et al. 2002. Position-effect protection and enhancer blocking by the chicken beta-globin insulator are separable activities. Proc Natl Acad Sci U S A 99: 6883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Majumder P, Gomez JA, Chadwick BP, et al. 2008. The insulator factor CTCF controls MHC class II gene expression and is required for the formation of long-distance chromatin interactions. J Exp Med 205: 785–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tolhuis B, Palstra RJ, Splinter E, et al. 2002. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol Cell 10: 1453–65. [DOI] [PubMed] [Google Scholar]
- 80.Splinter E, Heath H, Kooren J, et al. 2006. CTCF mediates long-range chromatin looping and local histone modification in the beta-globin locus. Genes Dev 20: 2349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bender MA, Byron R, Ragoczy T, et al. 2006. Flanking HS-62.5 and 3’ HS1, and regions upstream of the LCR, are not required for beta-globin transcription. Blood 108: 1395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoon YS, Jeong S, Rong Q, et al. 2007. Analysis of the H19ICR insulator. Mol Cell Biol 27: 3499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qiu X, Vu TH, Lu Q, et al. 2008. A complex deoxyribonucleic acid looping configuration associated with the silencing of the maternal Igf2 allele. Mol Endocrinol 22: 1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaffer CR, Srivastava M, Park KY, et al. 2000. A transcriptional insulator at the imprinted H19/Igf2 locus. Genes Dev 14: 1908–19. [PMC free article] [PubMed] [Google Scholar]
- 85.Engel N, Raval AK, Thorvaldsen JL, et al. 2008. Three-dimensional conformation at the H19/Igf2 locus supports a model of enhancer tracking. Hum Mol Genet 17: 3021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao Z, Tavoosidana G, Sjolinder M, et al. 2006. Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions. Nat Genet 38: 1341–7. [DOI] [PubMed] [Google Scholar]
- 87.Ling JQ, Li T, Hu JF, et al. 2006. CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science 312: 269–72. [DOI] [PubMed] [Google Scholar]
- 88.Yusufzai TM, Tagami H, Nakatani Y, et al. 2004. CTCF tethers an insulator to subnuclear sites, suggesting shared insulator mechanisms across species. Mol Cell 13: 291–8. [DOI] [PubMed] [Google Scholar]
- 89.Ottaviani A, Schluth-Bolard C, Rival-Gervier S, et al. 2009. Identification of a perinuclear positioning element in human subtelomeres that requires A-type lamins and CTCF. EMBO J 28: 2428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu H, Huang J, Wang J, et al. 2008. Transvection mediated by the translocated cyclin D1 locus in mantle cell lymphoma. J Exp Med 205: 1843–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu N, Donohoe ME, Silva SS, et al. 2007. Evidence that homologous X-chromosome pairing requires transcription and Ctcf protein. Nat Genet 39: 1390–6. [DOI] [PubMed] [Google Scholar]
- 92.Donohoe ME, Zhang LF, Xu N, et al. 2007. Identification of a Ctcf cofactor, Yy1, for the X chromosome binary switch. Mol Cell 25: 43–56. [DOI] [PubMed] [Google Scholar]
- 93.Rippe K 2001. Making contacts on a nucleic acid polymer. Trends Biochem Sci 26: 733–40. [DOI] [PubMed] [Google Scholar]
- 94.Ishihara K, Oshimura M, Nakao M. 2006. CTCF-dependent chromatin insulator is linked to epigenetic remodeling. Mol Cell 23: 733–42. [DOI] [PubMed] [Google Scholar]
- 95.Parelho V, Hadjur S, Spivakov M, et al. 2008. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell 132: 422–33. [DOI] [PubMed] [Google Scholar]
- 96.Stedman W, Kang H, Lin S, et al. 2008. Cohesins localize with CTCF at the KSHV latency control region and at cellular c-myc and H19/Igf2 insulators. EMBO J 27: 654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shintomi K, Hirano T. 2007. How are cohesin rings opened and closed? Trends Biochem Sci 32: 154–7. [DOI] [PubMed] [Google Scholar]
- 98.Hadjur S, Williams LM, Ryan NK, et al. 2009. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature 460: 410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spilianakis CG, Lalioti MD, Town T, et al. 2005. Interchromosomal associations between alternatively expressed loci. Nature 435: 637–45. [DOI] [PubMed] [Google Scholar]
- 100.Degner SC, Wong TP, Jankevicius G, et al. 2009. Cutting edge: developmental stage-specific recruitment of cohesin to CTCF sites throughout immunoglobulin loci during B lymphocyte development. J Immunol 182: 44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kang H, Lieberman PM. 2009. Cell cycle control of Kaposi’s sarcoma-associated herpesvirus latency transcription by CTCF-cohesin interactions. J Virol 83: 6199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chernukhin I, Shamsuddin S, Kang SY, et al. 2007. CTCF interacts with and recruits the largest subunit of RNA polymerase II to CTCF target sites genome-wide. Mol Cell Biol 27: 1631–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Klenova EM, Chernukhin IV, El-Kady A, et al. 2001. Functional phos-phorylation sites in the C-terminal region of the multivalent multifunctional transcriptional factor CTCF. Mol Cell Biol 21: 2221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Docquier F, Kita GX, Farrar D, et al. 2009. Decreased poly(ADP-ribosyl)ation of CTCF, a transcription factor, is associated with breast cancer phenotype and cell proliferation. Clin Cancer Res 15: 5762–71. [DOI] [PubMed] [Google Scholar]
- 105.Yu W, Ginjala V, Pant V, et al. 2004. Poly(ADP-ribosyl)ation regulates CTCF-dependent chromatin insulation. Nat Genet 36: 1105–10. [DOI] [PubMed] [Google Scholar]
- 106.Dejardin J, Kingston RE. 2009. Purification of proteins associated with specific genomic Loci. Cell 136: 175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cai S, Lee CC, Kohwi-Shigematsu T. 2006. SATB1 packages densely looped, transcriptionally active chromatin for coordinated expression of cytokine genes. Nat Genet 38: 1278–88. [DOI] [PubMed] [Google Scholar]
- 108.Dekker J, Rippe K, Dekker M, et al. 2002. Capturing chromosome conformation. Science 295: 1306–11. [DOI] [PubMed] [Google Scholar]
- 109.Simonis M, Klous P, Splinter E, et al. 2006. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C). Nat Genet 38: 1348–54. [DOI] [PubMed] [Google Scholar]
- 110.Gondor A, Rougier C, Ohlsson R. 2008. High-resolution circular chromosome conformation capture assay. Nat Protoc 3: 303–13. [DOI] [PubMed] [Google Scholar]
- 111.Klenova EM, Nicolas RH, Paterson HF, et al. 1993. CTCF, a conserved nuclear factor required for optimal transcriptional activity of the chicken c-myc gene, is an 11-Zn-finger protein differentially expressed in multiple forms. Mol Cell Biol 13: 7612–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Burke LJ, Hollemann T, Pieler T, et al. 2002. Molecular cloning and expression of the chromatin insulator protein CTCF in Xenopus laevis. Mech Dev 113: 95–8. [DOI] [PubMed] [Google Scholar]
- 113.Moon H, Filippova G, Loukinov D, et al. 2005. CTCF is conserved from Drosophila to humans and confers enhancer blocking of the Fab-8 insulator. EMBO Rep 6: 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pugacheva EM, Kwon YW, Hukriede NA, et al. 2006. Cloning and characterization of zebrafish CTCF: Developmental expression patterns, regulation of the promoter region, and evolutionary aspects of gene organization. Gene 375: 26–36. [DOI] [PubMed] [Google Scholar]
- 115.Hore TA, Deakin JE, Marshall Graves JA. 2008. The evolution of epigenetic regulators CTCF and BORIS/CTCFL in amniotes. PLoS Genet 4: e1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Risinger JI, Chandramouli GV, Maxwell GL, et al. 2007. Global expression analysis of cancer/testis genes in uterine cancers reveals a high incidence of BORIS expression. Clin Cancer Res 13: 1713–9. [DOI] [PubMed] [Google Scholar]
- 117.Hoffmann MJ, Muller M, Engers R, et al. 2006. Epigenetic control of CTCFL/BORIS and OCT4 expression in urogenital malignancies. Bio-chem Pharmacol 72: 1577–88. [DOI] [PubMed] [Google Scholar]
- 118.D’Arcy V, Pore N, Docquier F, et al. 2008. BORIS, a paralogue of the transcription factor, CTCF, is aberrantly expressed in breast tumours. Br J Cancer 98: 571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jelinic P, Stehle JC, Shaw P. 2006. The testis-specific factor CTCFL cooperates with the protein methyltransferase PRMT7 in H19 imprinting control region methylation. PLoS Biol 4: e355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hong JA, Kang Y, Abdullaev Z, et al. 2005. Reciprocal binding of CTCF and BORIS to the NY-ESO-1 promoter coincides with derepression of this cancer-testis gene in lung cancer cells. Cancer Res 65: 7763–74. [DOI] [PubMed] [Google Scholar]
- 121.Defossez PA, Kelly KF, Filion GJ, et al. 2005. The human enhancer blocker CTC-binding factor interacts with the transcription factor Kaiso. J Biol Chem 280: 43017–23. [DOI] [PubMed] [Google Scholar]
- 122.De La Rosa-Velazquez IA, Rincon-Arano H, Benitez-Bribiesca L, et al. 2007. Epigenetic regulation of the human retinoblastoma tumor suppressor gene promoter by CTCF. Cancer Res 67: 2577–85. [DOI] [PubMed] [Google Scholar]
- 123.Donohoe ME, Silva SS, Pinter SF, et al. 2009. The pluripotency factor Oct4 interacts with Ctcf and also controls X-chromosome pairing and counting. Nature 460: 128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chernukhin IV, Shamsuddin S, Robinson AF, et al. 2000. Physical and functional interaction between two pluripotent proteins, the Y-box DNA/ RNA-binding factor, YB-1, and the multivalent zinc finger factor, CTCF. J Biol Chem 275: 29915–21. [DOI] [PubMed] [Google Scholar]
- 125.Lutz M, Burke LJ, Barreto G, et al. 2000. Transcriptional repression by the insulator protein CTCF involves histone deacetylases. Nucleic Acids Res 28: 1707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


