Abstract
BACKGROUND:
Heart size and function in children with single right ventricle (RV) anomalies may be influenced by shunt type at the Norwood procedure. We sought to identify shunt-related differences during early childhood after staged surgical palliations using echocardiography.
METHODS AND RESULTS:
We compared echocardiographic indices of RV, neo-aortic, and tricuspid valve size and function at 14 months, pre-Fontan and 6 years in 241 subjects randomized to a Norwood procedure using either the modified Blalock-Taussig shunt (MBTS) or right ventricle-to-pulmonary artery shunt (RVPAS). At 6 years, the shunt groups did not differ significantly in any measure except for increased indexed neo-aortic area in the MBTS. RV ejection fraction (EF) improved between pre-Fontan and 6 years in the RVPAS group, but was stable in the MBTS group. For the entire cohort, RV diastolic and systolic size and functional indices were improved at 6 years compared to earlier measurements, and indexed tricuspid and neo-aortic annular area decreased from 14 months to 6 years. The prevalence of ≥moderate tricuspid and neo-aortic regurgitation was uncommon, and did not vary by group or time period. Diminished RVEF at the 14-month study was predictive of late death/transplant; the hazard of late death/transplant when RVEF was <40% was tripled (HR=3.18, 95% CI 1.41–7.17).
CONCLUSIONS:
By 6 years after staged palliation, shunt type has not impacted RV size and function, and RV and valvar size and function show beneficial remodeling. Poor RV systolic function at 14 months predicts worse late survival independent of the initial shunt type.
Keywords: congenital heart disease; echocardiography, cardiovascular surgery; single ventricle; Norwood procedure
CLINICAL SUMMARY
Initial shunt type at the Norwood procedure does not impact echocardiographic indices of cardiac size and function in survivors with single right anomalies enrolled in the Single Ventricle Reconstruction trial at 6 years of age. Prior concerns about deteriorating right ventricular systolic function in SVR subjects with an right ventricular to pulmonary artery shunt are not apparent at 6 years, and indices of right ventricular and valvar size and function suggest beneficial remodeling after the Fontan procedure for the cohort. The best choice of initial shunt type in infants requiring the Norwood procedure for single right ventricle anomalies remains unclear based on current data from the Single Ventricle Reconstruction trial. Longitudinal follow-up of the Single Ventricle Reconstruction cohort is ongoing and will likely provide further insights into the long-term effect of initial shunt type on clinical outcome and echocardiographic indices of cardiac size and function.
INTRODUCTION
The optimal strategy for initial surgical palliation of hypoplastic left heart syndrome and other single right ventricle anomalies remains unclear. The Pediatric Heart Network’s1 Single Ventricle Reconstruction (SVR) trial compared outcomes in 549 infants undergoing a Norwood procedure for single right ventricle (RV) anomalies randomly assigned to either a modified Blalock-Taussig shunt (MBTS) or right ventricle-to-pulmonary artery shunt (RVPAS) at 15 North American centers.2 Although better one-year transplant-free survival was found for those who received a RVPAS compared with those who had a MBTS,3 the survivor benefit was no longer statistically significant at 3 years4 and 6 years5 of age. Similarly, the early advantage seen in RV systolic function diminished over time. Specifically, RV ejection fraction (EF) was better early after Norwood palliation in those who received a RVPAS, but had become similar between the shunt groups at 14 months after shunt removal and stage II palliation.6 By 3 years of age, RVEF was significantly worse in the RVPAS compared to the MBTS.7 These observations suggested that important differences between the shunt groups might emerge with longer-term follow-up.
The Pediatric Heart Network’s SVR Extension Study was undertaken to provide longitudinal surveillance of the cohort, including echocardiography within 6 months of Fontan palliation7 and at 6 years of age. In this analysis, we compared 2-dimensional and Doppler indices of RV size and function as well as neo-aortic and tricuspid valve annulus dimensions and function, between shunt groups at 14 months of age, prior to the Fontan procedure, and at 6 years. We investigated within-patient changes over time in these measures and sought to identify echocardiographic RV systolic indices at 14 months that might predict clinical outcomes at latest follow-up.
METHODS
The data, analytic methods, and study materials will be/have been made available to other researchers for purposes of reproducing the results or replicating the procedure. The public use dataset for the SVR trial is available on the public website: http://www.pediatricheartnetwork.org/ForResearchers/PHNPublicUseDatasets.aspx. We anticipate that the Single Ventricle Reconstruction Extension trial datasets will be available for public use at the same website in the next 12 months
Subjects
The entry criteria for the SVR trial2 have been reported previously. In the current study, we included SVR trial survivors who, within one year of their 6th birthday, underwent a transthoracic echocardiogram that was submitted to the SVR core echocardiography lab for interpretation. We excluded subjects who underwent cardiac transplantation (n=44), biventricular repair (n=3) or did not consent to the SVR extension trial (n=34). The study was approved by an institutional review board at each clinical site where the echocardiographic studies were performed, and the participants’ parents gave written informed consent.
Study Design
Earlier publications have described the SVR study design2 and outcomes at ages of 12 months3,8–10 and 3 years4 and 6 years5,11,12 after randomization. We have also characterized echocardiographic markers of outcome for the SVR trial, including 14-month6 and pre-Fontan7 indices of RV function, cardiac and vascular dimensions, valve annulus dimensions and function, and neo-aortic flow patterns13 in previous publications.
Echocardiographic Analysis
An echocardiography core laboratory at the Medical College of Wisconsin reviewed 2D/Doppler echocardiograms performed at each clinical center within 1 year of a subject’s 6th birth date. The echocardiogram was performed at SVR-designated clinical sites when possible, but also could be performed under the supervision of the local primary cardiologist. Core lab procedures for image analysis and data management have been previously described.6,7 Echocardiographic measures are summarized in supplemental Tables 1 and 2. The core lab was blinded to the initial shunt type once the shunt was removed at the Stage II operation for all 14 month, pre-Fontan, and 6-year echocardiograms.
Statistical Analysis
Summary descriptive statistics for echocardiographic indices are presented by assigned shunt type for the Norwood procedure. Continuous measures were compared between assigned shunt types with either Student’s or Welch’s t-test or a Wilcoxon rank sum test as appropriate. Categorical measures were compared between shunt types with a Fisher exact test; ordinal measures were compared with a Fisher exact test and the Mantel-Haenszel test for trend. In addition, we used paired t-tests to compare changes in these indices from 14 months of age (end of SVR study visit from previously published data6) to the 6-year study, as well as from the pre-Fontan study7 to the 6-year study when protocol echocardiograms were available within the respective time periods. Comparisons of variables were paired, i.e, within subject, and only subjects who were alive at 6 years were included in the analyses of changes over time. We also performed a longitudinal analysis across all three time-points among subjects with an echo at 6 years, using repeated measures ANOVA for continuous measures and Fisher exact tests for categorical measures. Study inferences were similar using both methods. Finally, we performed Cox regression analyses, conditional on survival to the 14-month echocardiogram (time 0), to explore the associations of RVEF and RV % fractional area change (FAC) at 14 months with transplant-free survival using all available data. In all analyses, a p-value≤0.05 was accepted for statistical significance. No formal adjustment was made for multiple testing, as many of the echo indices are correlated. If all 26 were independent, one would expect 1–2 significant comparisons due to chance alone. However, many variables were not independent and the degree of correlation among echocardiographic parameters is highly variable. Therefore, we did not adjust for multiple testing. Rather, based upon the principle in the ASA’s Statement on p=values, we report the number of hypotheses explored during the study, all data collection decisions, all statistical analyses conducted, and all p-values computed.14 Analyses were performed in SAS Version 9.4 (SAS Institute, Cary NC).
To adjust for the effect of somatic growth and age on the linear, area and volumetric dimensions of cardiac structures and Doppler flow patterns, z-scores relative to BSA were used when normative data were available.15 Z-scores from the normal right ventricle in a biventricular circulation were used for these comparisons. The dimensions of the neo-aortic valve annulus were expressed as z-scores using normal values for the dimensions of the native aortic valve in a normal population.
All Core Lab echocardiographic parameters were measured by a single dedicated pediatric sonographer trained in pediatric echocardiography and confirmed by a single experienced pediatric echocardiographer. Reproducibility of echocardiographic measurements has been previously published for the SVR trial using this core lab with good intraclass correlations and percent agreement for continuous and dichotomous variables.7
RESULTS
Of the 269 SVR survivors who had research protocol echocardiographic studies submitted as part of their 6-year assessment, 241 (90%; 116 MBTS and 125 RVPAS) were acceptable for core-lab analysis. The mean age at the time of 6-year echocardiogram was 71.1 months (2 subjects were >1 year past their 6th birthday at the time of the echocardiogram). Among the 241 subjects, 208 (86%; 102 MBTS and 125 RVPAS) had also undergone a pre-Fontan echocardiogram at a mean age of 34.3 ± 10.4 months. The mean duration between the pre-Fontan and 6-year echocardiograms was 36.7 ± 11.2 months. Among the 241 survivors with acceptable 6-year exams, 228 (110 MBTS and 118 RVPAS) also had an acceptable exam at 14 months (mean 14.1 ± 1.2 months).
Echocardiographic Assessment
Right ventricular systolic size and function
At the 6-year echocardiogram, shunt types were similar with respect to all RV indices of size and systolic function, including RV indexed systolic volume, RVEF, indexed systolic area, FAC, and peak tricuspid annular systolic velocity (Table 1). In the overall cohort, at the 6-year compared with the pre-Fontan study, RV systolic volumes and indexed areas decreased significantly, and RVEF, FAC, and peak tricuspid annular systolic velocity increased significantly (Table 2). When compared with the 14- month studies for the entire cohort, 6-year RV systolic volume was also significantly decreased (p=0.006), peak tricuspid annular systolic velocity increased (p<0.001), and RVEF increased non-significantly (p=0.06), but RV indexed areas and FAC were similar between those stages (Table 3). The change in RVEF and FAC over time for the cohort are summarized in Figure 1. In the combined cohort, there was no significantly difference in the percentage of subjects who had an RVEF <40% at age 14 months compared with age 6 years (21.6% vs 20.5%; p>0.99). When comparing interval changes in measures between pre-Fontan and 6 years by assigned shunt group, RVEF (+3.90 for the RVPAS vs. +0.10 for the MBTS; p=0.015) and FAC both significantly increased in the RVPAS compared to the MBTS group (Figure 2; supplemental Table 3). No other significant changes in systolic indices were identified from the pre-Fontan study to 6 years, and all systolic indices were similar for both shunt groups when comparing the 14-month and 6-year echocardiograms (supplemental Table 4).
TABLE 1.
Summary Statistics for 6-year Echocardiographic Measures by Initial Shunt Group
| MBTS | RVPAS | ||||
|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | p Value | |
| RV systolic size and function | |||||
| End systolic volume, ml/BSA1.3 | 55 | 45.16±11.55 | 61 | 48.27±18.72 | 0.28 |
| End systolic area, cm2/BSA0.8 | 90 | 15.51±3.69 | 101 | 15.98±4.24 | 0.42 |
| Ejection fraction, biplane pyramidal, % | 55 | 45.34±6.07 | 62 | 45.81±6.38 | 0.68 |
| Ejection fraction, biplane pyramidal, % | 55 | 62 | >.99 | ||
| ≤ 40%, n (%) | 11 (20.0%) | 12 (19.4%) | |||
| > 40%, n (%) | 44 (80.0%) | 50 (80.6%) | |||
| Fractional area change, % | 90 | 32.47±5.70 | 102 | 33.26±5.69 | 0.34 |
| Peak systolic velocity, cm/sec | 65 | 6.06±1.23 | 75 | 5.99±1.21 | 0.76 |
| Peak systolic velocity for age, z-score | 65 | −1.68±0.79 | 75 | −1.74±0.75 | 0.65 |
| Isovolumic contraction acceleration, cm/sec/sec | 55 | 31.44±9.90 | 59 | 29.63±9.55 | 0.32 |
| RV diastolic size and function | |||||
| End diastolic volume/BSA1.3 | 55 | 82.52±18.40 | 62 | 88.02±28.61 | 0.21 |
| End diastolic area/BSA0.8 | 90 | 22.90±4.76 | 102 | 23.77±5.54 | 0.25 |
| RV sphericity index | 90 | 1.28±0.30 | 103 | 1.25±0.28 | 0.41 |
| Tricuspid valve peak early velocity, m/sec | 98 | 0.87±0.25 | 106 | 0.85±0.24 | 0.67 |
| Tricuspid valve peak early velocity for age, z-score | 98 | −0.21±1.34 | 106 | −0.29±1.27 | 0.66 |
| Tricuspid valve peak atrial velocity, m/sec | 84 | 0.65±0.22 | 91 | 0.61±0.20 | 0.17 |
| Tricuspid valve peak atrial velocity for age, z-score | 84 | 1.60±1.80 | 91 | 1.25±1.61 | 0.18 |
| E/A ratio | 84 | 1.42±0.52 | 91 | 1.48±0.54 | 0.44 |
| E/A ratio for age, z-score | 84 | −1.06±0.82 | 91 | −0.97±0.85 | 0.47 |
| Peak early diastolic annular velocity, cm/sec | 65 | 9.43±3.19 | 75 | 9.23±2.93 | 0.70 |
| Peak early diastolic annular velocity for age, z-score | 65 | −2.68±1.16 | 75 | −2.77±1.05 | 0.64 |
| Peak atrial diastolic annular velocity, cm/sec | 56 | 5.88±1.80 | 64 | 5.44±1.91 | 0.20 |
| Peak atrial diastolic annular velocity for age, z-score | 56 | 0.04±1.14 | 64 | −0.23±1.20 | 0.20 |
| E/E' ratio | 63 | 10.28±4.52 | 72 | 10.36±3.94 | 0.91 |
| E/E' ratio for age, z-score | 63 | 2.95±2.83 | 72 | 3.01±2.47 | 0.90 |
| Flow reversal during atrial systole | 97 | 113 | 0.55 | ||
| Yes, n (%) | 4 (4.1%) | 7 (6.2%) | |||
| No, n (%) | 93 (95.9%) | 106 (93.8%) | |||
| Duration of flow reversal during atrial systole, msec | 4 | 123.75±15.54 | 7 | 104.86±21.09 | 0.16 |
| RV global function | |||||
| MPI, Inflow Doppler/ET calculation | 77 | 0.49±0.18 | 84 | 0.47±0.19 | 0.37 |
| MPI DTI calculation | 56 | 0.63±0.20 | 67 | 0.65±0.22 | 0.70 |
| Neo-aortic valve size and function | |||||
| Neo-aortic annular area/BSA | 76 | 4.06±1.06 | 90 | 3.74±0.94 | 0.042 |
| Neo-aortic annular area, z-score | 76 | 6.39±3.16 | 90 | 5.44±2.79 | 0.040 |
| Severity of neoaortic valve regurgitation | 116 | 124 | 0.40 | ||
| None, n (%) | 72 (62.1%) | 67 (54.0%) | |||
| Mild, n (%) | 36 (31.0%) | 49 (39.5%) | |||
| Moderate, n (%) | 8 (6.9%) | 8 (6.5%) | |||
| Severe, n (%) | 0 (0.0%) | 0(0.0%) | |||
| ≥Moderate neo-aortic valve regurgitation | 116 | 124 | >.99 | ||
| 8 (6.9%) | 8 (6.5%) | ||||
| 108 (93.1%) | 116 (93.5%) | ||||
| Tricuspid valve size and function | |||||
| Tricuspid indexed annular area/BSA | 110 | 5.81±1.42 | 115 | 5.77±1.69 | 0.84 |
| Tricuspid indexed annular area for BSA, z-score | 110 | 2.26±1.75 | 115 | 2.21±2.08 | 0.83 |
| Severity of tricuspid valve regurgitation | 116 | 125 | 0.22 | ||
| None, n (%) | 6 (5.2%) | 7 (5.6%) | |||
| Mild, n (%) | 90 (77.6%) | 97 (77.6%) | |||
| Moderate, n (%) | 18 (15.5%) | 13 (10.4%) | |||
| Severe, n (%) | 2 (1.7%) | 8 (6.4%) | |||
| Multiple, n (%) | 0 (0.0%) | 0 (0.0%) | |||
| ≥Moderate tricuspid valve regurgitation | 116 | 125 | >.99 | ||
| Yes, n (%) | 20 (17.2%) | 21 (16.8%) | |||
| No, n (%) | 96 (82.8%) | 104 (83.2%) | |||
| Neo-aortic size and patency | |||||
| Ascending neoaortic maximal diameter, cm | 98 | 2.18±0.44 | 108 | 2.14±0.48 | 0.57 |
| Ascending neoaortic maximal diameter for BSA, z-score | 98 | 3.14±2.43 | 108 | 2.96±2.69 | 0.60 |
| Distal arch to descending aorta CWD peak velocity, m/sec | 92 | 1.78±0.48 | 99 | 1.84±0.52 | 0.43 |
BSA = body surface area; CWD = continuous wave Doppler; DTI = Doppler tissue imaging; MPI=myocardial performance index; RV=right ventricle; SD = standard deviation.
Table 2.
Summary Statistics for Pre-Fontan and 6-Year Echocardiographic Measures for the Entire Cohort
| Pre-Fontan | 6 Year | |||
|---|---|---|---|---|
| n | Mean ± SD | Mean ± SD | p Value | |
| RV systolic size and function | ||||
| End systolic volume, ml/BSA1.3 | 76 | 51.55±15.10 | 45.79±12.18 | <.001 |
| End systolic area, cm2/BSA0.8 | 150 | 16.37±3.82 | 15.28±3.57 | <.001 |
| Ejection fraction, biplane pyramidal, % | 76 | 43.81±6.00 | 45.86±5.92 | 0.011 |
| Ejection fraction, biplane pyramidal, % | 76 | 0.12 | ||
| ≤ 40%, n (%) | 22 (28.9%) | 13 (17.1%) | ||
| > 40%, n (%) | 54 (71.1%) | 63 (82.9%) | ||
| Fractional area change, % | 150 | 31.48±5.74 | 33.39±5.53 | <.001 |
| Peak systolic velocity, cm/sec | 80 | 5.55±1.39 | 5.98±1.20 | 0.020 |
| Peak systolic velocity for age, z-score | 80 | −1.58±1.02 | −1.73±0.77 | 0.22 |
| Isovolumic contraction acceleration, cm/sec/sec | 44 | 32.95±9.99 | 31.42±9.91 | 0.41 |
| RV diastolic size and function | ||||
| End diastolic volume, mL/BSA1.3 | 76 | 91.64±25.19 | 84.31±19.50 | 0.011 |
| End diastolic area, cm2/BSA0.8 | 150 | 23.80±4.98 | 22.90±4.83 | 0.043 |
| RV sphericity index | 152 | 1.28±0.30 | 1.26±0.30 | 0.52 |
| Tricuspid valve peak early velocity, m/sec | 154 | 0.78±0.22 | 0.86±0.24 | <.001 |
| Tricuspid valve peak early velocity for age, z-score | 154 | −0.54±1.18 | −0.28±1.30 | 0.013 |
| Tricuspid valve peak atrial velocity, m/sec | 115 | 0.68±0.21 | 0.62±0.21 | 0.006 |
| Tricuspid valve peak atrial velocity for age, z-score | 115 | 1.71±1.73 | 1.36±1.73 | 0.05 |
| E/A ratio | 115 | 1.16±0.40 | 1.49±0.56 | <.001 |
| E/A ratio for age, z-score | 115 | −1.23±0.63 | −0.95±0.88 | <.001 |
| Peak early diastolic annular velocity, cm/sec | 80 | 8.77±2.60 | 9.57±2.87 | 0.038 |
| Peak early diastolic annular velocity for age, z-score | 80 | −2.42±0.96 | −2.64±1.04 | 0.10 |
| Peak atrial diastolic annular velocity, cm/sec | 60 | 6.95±2.32 | 5.86±2.08 | <.001 |
| Peak atrial diastolic annular velocity for age, z-score | 60 | 0.69±1.49 | 0.03±1.31 | 0.002 |
| E/E' ratio | 70 | 9.36±3.94 | 9.94±3.79 | 0.27 |
| E/E' ratio for age, z-score | 70 | 2.11±2.46 | 2.75±2.38 | 0.05 |
| Flow reversal during atrial systole | 129 | 0.60 | ||
| Yes, n (%) | 6 (4.7%) | 9 (7.0%) | ||
| No, n (%) | 123 (95.3%) | 120 (93.0%) | ||
| RV global function | ||||
| MPI, Inflow Doppler/ET calculation | 108 | 0.43±0.17 | 0.47±0.18 | 0.07 |
| MPI DTI calculation | 51 | 0.60±0.21 | 0.64±0.19 | 0.35 |
| Neo-aortic valve size and function | ||||
| Neo-aortic annular area/BSA | 126 | 3.75±0.94 | 3.83±0.99 | 0.31 |
| Neo-aortic annular area for BSA, z-score | 126 | 5.29±2.74 | 5.72±2.94 | 0.10 |
| Severity of neo-aortic valve regurgitation | 205 | 0.40 | ||
| None, n (%) | 119 (58.0%) | 120 (58.5%) | ||
| Mild, n (%) | 77 (37.6%) | 71 (34.6%) | ||
| Moderate, n (%) | 8 (3.9%) | 14 (6.8%) | ||
| Severe, n (%) | 1 (0.5%) | 0 (0.0%) | ||
| ≥ Moderate neo-aortic valve regurgitation | 205 | 0.39 | ||
| Yes, n (%) | 9 (4.4%) | 14 (6.8%) | ||
| No, (%) | 196 (95.6%) | 191 (93.2%) | ||
| Tricuspid valve size and function | ||||
| Tricuspid indexed annular area/BSA | 183 | 5.75±1.70 | 5.70±1.54 | 0.69 |
| Tricuspid indexed annular area for BSA, z-score | 183 | 2.16±2.07 | 2.13±1.90 | 0.85 |
| Severity of tricuspid valve regurgitation | 208 | 0.044 | ||
| None, n (%) | 27 (13.0%) | 12 (5.8%) | ||
| Mild, n (%) | 141 (67.8%) | 162 (77.9%) | ||
| Moderate, n (%) | 31 (14.9%) | 28 (13.5%) | ||
| Severe, n (%) | 9 (4.3%) | 6 (2.9%) | ||
| Multiple, n (%) | 0 (0.0%) | 0 (0.0%) | ||
| ≥Moderate tricuspid valve regurgitation | 208 | 0.52 | ||
| Yes, n (%) | 40 (19.2%) | 34 (16.3%) | ||
| No, n (%) | 168 (80.8%) | 174 (83.7%) | ||
| Neo-aortic size and patency | ||||
| Ascending neoaortic maximal diameter, cm | 157 | 1.94±0.39 | 2.18±0.45 | <.001 |
| Ascending neoaortic maximal diameter for BSA, z-score | 157 | 3.35±2.31 | 3.16±2.52 | 0.28 |
| Distal arch to descending aorta CWD peak velocity, m/sec | 131 | 1.73±0.53 | 1.79±0.54 | 0.07 |
BSA = body surface area; CWD = continuous wave Doppler; DTI = Doppler tissue imaging; MPI=myocardial performance index; RV=right ventricle; SD = standard deviation
TABLE 3.
Summary Statistics for 14-Month and 6-year Echocardiographic Measures for Entire Cohort
| 14 months | 6 Year | |||
|---|---|---|---|---|
| n | Mean ± SD | Mean ± SD | p Value | |
| RV systolic size and function | ||||
| End systolic volume/BSA1.3 | 88 | 49.93±15.61 | 45.47±13.05 | 0.006 |
| End systolic area/BSA0.8 | 174 | 15.79±4.06 | 15.54±3.92 | 0.44 |
| Ejection fraction, biplane pyramidal, % | 88 | 43.99±6.86 | 45.62±6.05 | 0.06 |
| Ejection fraction, biplane pyramidal, % | 88 | >.99 | ||
| ≤ 40%, n (%) | 19 (21.6%) | 18 (20.5%) | ||
| > 40%, n (%) | 69 (78.4%) | 70 (79.5%) | ||
| Fractional area change, % | 174 | 32.98±6.62 | 32.97±5.55 | >.99 |
| Peak systolic velocity, cm/sec | 125 | 5.48±1.46 | 6.04±1.25 | <.001 |
| Peak systolic velocity for age, z-score | 125 | −1.09±1.21 | −1.70±0.79 | <.001 |
| Isovolumic contraction acceleration, cm/sec/sec | 61 | 34.39±11.02 | 29.50±9.37 | 0.009 |
| RV diastolic size and function | ||||
| End diastolic volume/BSA1.3 | 88 | 88.66±23.50 | 83.37±20.64 | 0.028 |
| End diastolic area/BSA0.8 | 174 | 23.46±4.94 | 23.12±5.18 | 0.40 |
| RV sphericity index | 174 | 1.27±0.29 | 1.27±0.30 | 0.92 |
| Tricuspid valve peak early velocity, m/sec | 185 | 0.86±0.25 | 0.87±0.24 | 0.66 |
| Tricuspid valve peak early velocity for age, z-score | 185 | −0.02±1.30 | −0.21±1.30 | 0.08 |
| Tricuspid valve peak atrial velocity, m/sec | 95 | 0.74±0.22 | 0.61±0.20 | <.001 |
| Tricuspid valve peak atrial velocity for age, z-score | 95 | 2.02±1.79 | 1.29±1.66 | <.001 |
| E/A ratio | 95 | 1.10±0.45 | 1.52±0.61 | <.001 |
| E/A ratio for age, z-score | 95 | −1.10±0.69 | −0.89±0.97 | 0.046 |
| Peak early diastolic annular velocity, cm/sec | 126 | 8.32±3.79 | 9.39±3.07 | 0.008 |
| Peak early diastolic annular velocity for age, z-score | 126 | −1.99±1.43 | −2.70±1.11 | <.001 |
| Peak atrial diastolic annular velocity, cm/sec | 67 | 7.03±2.09 | 5.88±1.77 | <.001 |
| Peak atrial diastolic annular velocity for age, z-score | 67 | 0.70±1.36 | 0.04±1.12 | 0.001 |
| E/E' ratio | 117 | 11.60±4.48 | 10.42±4.09 | 0.017 |
| E/E' ratio for age, z-score | 117 | 3.19±2.83 | 3.05±2.57 | 0.65 |
| Flow reversal during atrial systole | 182 | 0.83 | ||
| Yes, n (%) | 12 (6.6%) | 10 (5.5%) | ||
| No, n (%) | 170 (93.4%) | 172 (94.5%) | ||
| Duration of flow reversal during atrial systole, msec | 3 | 112.00±51.68 | 103.00±28.16 | 0.86 |
| RV global function | ||||
| MPI, Inflow Doppler/ET calculation | 140 | 0.42±0.16 | 0.48±0.19 | 0.002 |
| MPI DTI calculation | 67 | 0.60±0.20 | 0.64±0.20 | 0.17 |
| Neo-aortic valve size and function | ||||
| Neoaortic annular area/BSA | 149 | 4.32±1.18 | 3.83±0.99 | <.001 |
| Neoaortic annular area for BSA, z-score | 149 | 6.73±3.29 | 5.72±2.95 | <.001 |
| Severity of neoaortic valve regurgitation | 226 | 0.002 | ||
| None, n (%) | 106 (46.9%) | 130 (57.5%) | ||
| Mild, n (%) | 115 (50.9%) | 82 (36.3%) | ||
| Moderate, n (%) | 5 (2.2%) | 14 (6.2%) | ||
| Severe, n (%) | 0 (0.0%) | 0 (0.0%) | ||
| ≥ Moderate neoaortic valve regurgitation | 226 | 0.06 | ||
| Yes, n (%) | 5 (2.2%) | 14 (6.2%) | ||
| No, n (%) | 221 (97.8%) | 212 (93.8%) | ||
| Tricuspid valve size and function | ||||
| Tricuspid indexed annular area/BSA | 209 | 6.39±2.00 | 5.81±1.60 | <.001 |
| Tricuspid indexed annular area for BSA, z-score | 209 | 2.88±2.39 | 2.27±1.97 | <.001 |
| Severity of tricuspid valve regurgitation | 227 | 0.004 | ||
| None, n (%) | 35 (15.4%) | 13 (5.7%) | ||
| Mild, n (%) | 147 (64.8%) | 175 (77.1%) | ||
| Moderate, n (%) | 34 (15.0%) | 31 (13.7%) | ||
| Severe, n (%) | 11 (4.8%) | 8 (3.5%) | ||
| Multiple, n (%) | 0 (0.0%) | 0 (0.0%) | ||
| ≥Moderate tricuspid valve regurgitation | 227 | 0.55 | ||
| Yes, n (%) | 45 (19.8%) | 39 (17.2%) | ||
| No, n (%) | 182 (80.2%) | 188 (82.8%) | ||
| Neo-aortic size and patency | ||||
| Ascending neoaortic maximal diameter, cm | Not collected at 14 months | |||
| Ascending neoaortic maximal diameter for BSA, z-score | Not collected at 14 months | |||
| Distal arch to descending aorta CWD peak velocity, m/sec | 141 | 1.68±0.54 | 1.81±0.52 | 0.001 |
BSA = body surface area; CWD = continuous wave Doppler; DTI = Doppler tissue imaging; MPI=myocardial performance index; RV=right ventricle; SD = standard deviation.
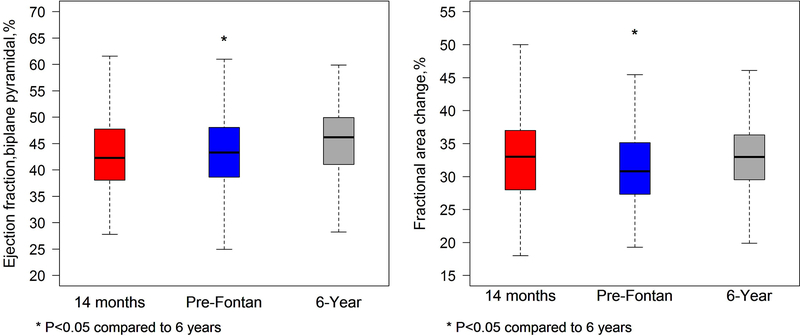
Figure 1.
Graph comparing right ventricular ejection fraction (left panel) and fractional area change (right panel) for the cohort with paired studies at the 14 months, pre-Fontan, and 6-year intervals. There was a significant increase in both the ejection fraction and fractional area change from the pre-Fontan to the 6-year echocardiogram. There was a trend toward increased right ventricular ejection fraction from 14 months to 6 years (p=0.06), but fractional area change was similar between those stages.
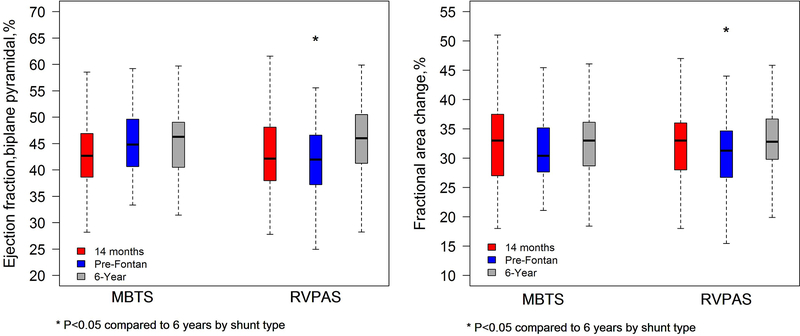
Figure 2.
Similar to Figure 1, this graph compares right ventricular ejection fraction (left panel) and fractional area change (right panel) between the modified Blalock-Taussig shunt (MBTS) and RV-to-pulmonary artery shunt (RVPAS) groups who had paired studies at the 14 months, pre-Fontan, and 6-year intervals. From the pre-Fontan to the 6-year echocardiogram, both ejection fraction and fractional area change significantly increased in the RVPAS when compared to the interval change in the MBTS group. Ejection fraction and fractional area change were similar for both shunt groups when comparing the 14 months and 6-year studies.
Right ventricular diastolic size and function
At the 6-year echocardiogram, all diastolic indices were similar between the shunt groups. These included RV indexed end-diastolic volume, indexed end-diastolic area, RV sphericity index, tricuspid peak E and A velocities and E/A ratio, tricuspid annular E’ and A’ velocities, E/E’ ratio, and presence of pulmonary vein flow reversal during atrial systole (Table 1). The presence of pulmonary vein flow reversal with atrial systole was rarely identified in either group (4.1% in MBTS vs 6.2 % in RVPAS; p=0.55). The shunt groups were combined and again comparisons were made between the pre-Fontan and 6-year exams (Table 2). At 6 years, there was a significant decrease in RV indexed end-diastolic volumes and areas (Figure 3), an increase in E velocities and E’ velocities with a decrease in A and A’ velocities and a resultant increased E/A ratio. Similar changes in diastolic indices occurred between the 14-month and 6-year echocardiograms (Table 3). When comparing interval changes between shunt groups at the pre-Fontan and 6-year studies, the MBTS group had a significant increase in E’ velocities that was not seen in the RVPAS group, while the RVPAS had a significant decrease in the A’ velocities not seen in the MBTS (supplemental Table 3). The same analysis was done looking at interval change between 14 months and 6 years, stratified by shunt type, and there were no significant differences. (supplemental Table 4).
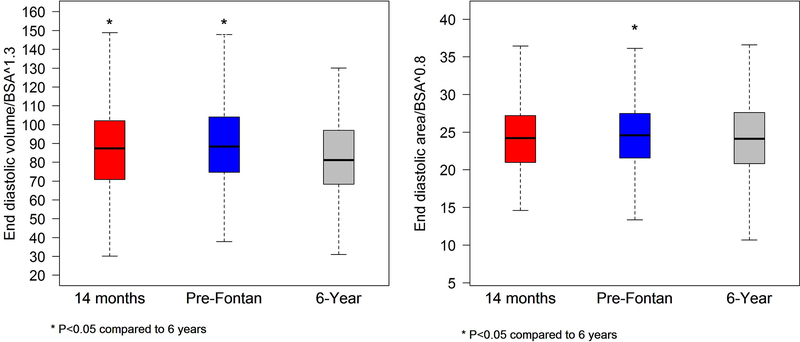
Figure 3.
Graph comparing indexed right ventricular end-diastolic volume (left panel) and end-diastolic area (right panel) for the cohort with paired studies at the 14 months, pre-Fontan, and 6-year intervals. There was a significant decrease in both the end-diastolic volume and area from the pre-Fontan to the 6-year echocardiogram. End-diastolic volume was also decreased when comparing the 14 months and 6-year studies, but end-diastolic areas were similar.
Right ventricular global function
The myocardial performance index (MPI), as assessed by both blood flow Doppler and annular Doppler tissue imaging, was not significantly different between the shunt groups at the 6-year study (Table 1). In addition, there was no significant change in the index as calculated by either method from the pre-Fontan study to 6 years for entire cohort (Table 2). There was a significant increase in MPI as calculated by blood flow Doppler (0.48±0.19 vs 0.42±0.16; p=0.002) from the 14-month to the 6-year study echocardiogram for the entire cohort that was not seen when calculated by annular Doppler (Table 3). When comparing interval changes between shunt groups pre-Fontan and at 6 years, the MBTS group had a significant increase in MPI by blood flow Doppler that was not seen in the RVPAS group (supplemental Table 3); no changes in MPI by either method were seen between shunt groups from the 14-month to 6-year study (supplemental Table 4).
Neo-aortic valve size and function
Indexed neo-aortic annular areas were larger in the MBTS group compared with the RVPAS group at the 6-year study, with marked neo-aortic annular dilatation in both groups, as evidenced by mean z-scores of 6.4±3.4 for MBTS vs. 5.4±2.8 for RVPAS, p=0.04 (Table 1). At 6 years, >mild regurgitation was rare and similar between groups (6.9% of the MBTS and 6.5% of the RVPAS; p=0.4), and no subject had severe regurgitation. Indexed neo-aortic valve areas were similar at the pre-Fontan and 6-year studies for the entire cohort (Table 2) and decreased significantly between 14 months and 6 years (Figure 4; Table 3). No differences were noted between the shunt groups when comparing 14-month (supplemental Table 4) and pre-Fontan studies (supplemental Table 3) with measures at 6 years. The degree of neo-aortic regurgitation was similar in the two groups and did not vary significantly between time points.
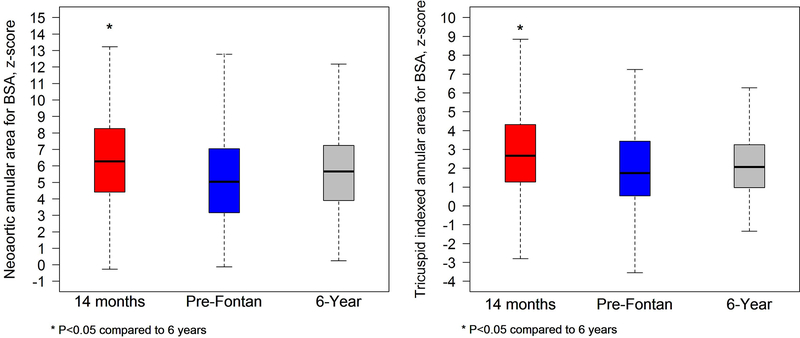
Figure 4.
Graph comparing indexed neo-aortic valve annular area z-score (left panel) and tricuspid valve annular area z-score (right panel) for the cohort with paired studies at the 14 months, pre-Fontan, and 6-year intervals. The annular z-score decreased significantly for both the neo-aortic and tricuspid valves between 14 months and 6 years for the cohort with no differences in either valve z-score between pre-Fontan and 6 year studies.
Tricuspid valve size and function
Indexed tricuspid annular areas and z-scores were similar with mild annular dilatation in both groups at the 6-year study as evidenced by mean z-scores of 2.3±1.8 for MBTS and 2.2±2.1 for RVPAS (p=0.86; Table 1). At 6 years, ≥moderate tricuspid regurgitation occurred in 17% of subjects in each shunt group. Among all survivors to 6 years, indexed tricuspid valve areas and z-scores were similar to pre-Fontan study values (Table 2), in contrast to the significant decrease seen in both indexed areas and z-scores from 14 months to 6 years, with z-scores approaching normal in both groups at 6 years (Figure 4; Table 3). The degree of change over time was similar for both shunt groups when comparing the 6-year study with the pre-Fontan (supplemental Table 3) and 14-month studies (supplemental Table 4). The degree of tricuspid regurgitation was also stable and similar for the two groups at all time intervals.
Aortic size and patency
The ascending aortic diameter was similar in both shunt groups at the 6-year study with mild ascending aortic dilatation, as evidenced by mean z-scores of 3.1±2.4 for MBTS and 2.9±2.7 for RVPAS; p=0.57 (Table 1). Absolute values for the ascending aorta increased from the pre-Fontan to 6-year study for the entire cohort, but z-scores were similar. The size of the ascending aorta was not recorded at the 14-month study, so information about that interval change was not available. The average peak velocity through the distal aortic arch was similar between the shunt groups at the 6-year study (1.8±0.5 for MBTS vs 1.8±0.5 m/s for RVPAS; p=0.43). In the overall cohort, the peak velocity was stable when compared with the pre-Fontan assessment, with average peak velocities <2 m/s for both groups at both intervals (Table 2). A statistically significant increase in peak velocity was found at 6-year compared with the 14-month study, but this difference was clinically insignificant (1.81±0.52 at 6 years vs 1.68±0.54 at 14 months; p=0.001; Table 3). The shunt groups did not vary in the distal arch velocities over time (supplemental Table 3 and 4).
Right ventricular systolic function and transplant-free survival:
The 14-month RVEF and RV FAC values were evaluated for their relationship to late death/transplant using a conditional analysis (with time zero equal to 14 months). There were 43 deaths/transplants in the 337 subjects (13%) who had 14-month echocardiograms. RVEF was evaluated both as a continuous variable and as a categorical variable (<40% vs ≥40%). In both models, decreased RVEF at 14 months was predictive of late outcome (both p=0.03). When RVEF was <40% the hazard ratio for death/transplant was 3.18 (95% CI 1.41–7.17). However, the effect of shunt type on late death/transplant did not depend on RVEF (interaction p=0.5). The same analysis was done using FAC. Similarly, higher risk of late death/transplant associated with lower RV FAC (p=0.01) when it was evaluated as a continuous variable. Categorical values for FAC (<30% vs. ≥30%) were not predictive of late outcome. The effect of shunt type on late death/transplant did not depend on FAC.
DISCUSSION
The SVR trial sought to answer a critical question in surgical management of infants with single RV anomalies undergoing a Norwood procedure: which shunt (the RVPAS or MBTS) was associated with better clinical outcomes? After 6 years of longitudinal follow-up, that answer remains unclear. Better transplant-free survival at 1 year in the SVR cohort3 was not sustained at 3 years, when the RVPAS group had lower RVEF7 and more frequent catheter-based interventions.4 This report summarizing the echocardiographic characteristics of the SVR cohort at 6 years continues to add to the longitudinal assessment of this unique population and supports the notion that neither shunt is unequivocally superior, with no shunt-related survivor benefit at 6 years of age.5 Specifically, among transplant-free survivors at 6 years, we found that initial shunt type did not significantly impact echocardiographic indices of RV, neo-aortic, and tricuspid valve size and function. Overall, these data suggest that there is beneficial remodeling of RV size, systolic and diastolic function, and neo-aortic and tricuspid valve size over time in this cohort, regardless of shunt type.
The last report summarizing echocardiographic findings for the SVR cohort was based on observations from serial studies collected during infancy, at 14 months, and prior to Fontan palliation7. Most concerning was the finding that, prior to Fontan, RVEF was worse in the RVPAS group compared with the MBTS, a finding that was supported by other investigations16–19 showing poorer RV function after RVPAS. In the current study using paired echocardiograms, we did not see further decline in RVEF from the pre-Fontan- to the 6-year exam, suggesting a period of stable RV function early after Fontan palliation. Despite concerns that the ventriculotomy required for the RVPAS could result in myocardial injury/scarring that would eventually affect ventricular performance,20,21 our data suggest that systolic indices do not differ between the two shunt types at 6 years. When comparing the shunt groups with respect to within-patient interval changes between the pre-Fontan and 6-year studies, RVEF and FAC both significantly increased in the RVPAS compared with the MBTS group, reversing the trend seen between 14 months and the pre-Fontan studies for the RVPAS.7 For the entire cohort from the pre-Fontan study to 6 years, indexed RV volumes/areas declined and RVEF and FAC improved, suggesting beneficial remodeling during this time period. Diastolic indices also improved for the cohort compared with the 14-month and pre-Fontan study, although it is important to recognize that Doppler-derived filling indices, particularly E/A ratio and E/E’, have little supporting validation in the Fontan group when compared to normative data from children with a biventricular circulation.
The reasons for improvement in RV systolic function in the RVPAS group after Fontan are not clear. Follow-up studies of the SVR cohort, focusing on outcomes at 3 years4 and 6 years,5 show no significant differences in transplant-free survival at those ages between the 2 shunt groups. Of note, the RVPAS group had a lower hazard of death or transplant up until the Stage II procedure, after which the shunt effect on transplant-free survival was not significant. However, the RVPAS group had a somewhat higher hazard from Stage II until the Fontan procedure, then a somewhat lower hazard of death or transplant after that procedure. These findings mirror changes in RVEF between the 2 groups, with a higher EF prior to stage II palliation in the RVPAS6, similar EF at 14 months, and worse RVEF prior to Fontan7. The RVPAS also had a significantly higher incidence of catheter-based interventions at both 3 years4 (balloon angioplasty, stent, and coiling). It is possible that these more frequent interventions, when coupled with the anticipated improvement in systemic oxygen saturations after Fontan, may have significantly lessened the hemodynamic burden for the RV in the RVPAS subjects, with a resultant improvement in RVEF.
Similarly, the neo-aortic and tricuspid valves also showed no signs of significant deterioration at 6 years. It was interesting to note that, despite having increased neo-aortic valve size, our cohort had appropriate rate of growth in annulus size over time with little clinically significant valve dysfunction. The same was true for the tricuspid valve. Though other studies have shown progressive valve dysfunction in older children, our study suggests that at these ages, the valves can function well in the systemic circulation. Neo-aortic valve size and integrity are critical to this cohort over time, given reports of significant neo-aortic root dilatation22–24 and neo-aortic valve regurgitation25 later in childhood after Norwood palliation. Our findings are consistent with a “honeymoon” period for both cardiac valves during early childhood in survivors with single RV anomalies after completion of staged surgical palliations.
We found that, independent of shunt type, poor RV systolic function at 14 months using RVEF or FAC was predictive of late death/transplant. Indeed, the hazard of late death/transplant when RVEF was <40% was tripled. This risk of a worse outcome with poorer RV function as identified using these systolic indices should warrant closer surveillance/consideration for increased supportive therapies, as these findings are consistent with previous reports that identified impaired RV systolic function after second stage palliation as a risk factor for later death/need for transplantation.25,26
STUDY LIMITATIONS
Our analyses of changes in echocardiographic parameters between 14 months and 6 years were performed among patients who survived to at least age 6 years. These findings thus could be subject to survivor bias. This limitation was not true, however, for the assessment of the effect of 14-month RVEF on transplant-free survival, because we did not require that subjects survive to later studies to be included in that analysis. Unlike the left ventricle, 2D echocardiographic tools used to assess RV volume and systolic function are limited by the chamber’s complex geometry. Analysis of regional RV wall motion was not performed as part of this protocol because of limited frame rates used for image capture and poor characterization of RV circumferential motion, so the impact of focal scarring/dyskinesis was not specifically assessed; deformation techniques to assess RV function may provide additional information about risk of adverse outcomes.27 Careful training in protocol image acquisition was provided and reinforced at all sites; however, the 6-year studies were performed as part of routine clinical care and thus submitted not only by participating PHN sites but also by local cardiologists providing clinical care. Image acquisition was inadequate to achieve all measurements in the majority of studies. In particular, this limitation impacted the characterization of RV function, as seen in the low rate of measurable RVEF for the cohort, as the imaging necessary to calculate RVEF is difficult to obtain in this age group even in the most skilled hands. Because of this small sample size, we were unable to identify specific risk factors for reduced systolic RV function or more than mild tricuspid regurgitation within subgroups of age and shunt type. To optimize assessments of RV function going forward, continued follow-up in this cohort now underway at age 10–12 years includes a cardiac MRI.
CONCLUSIONS
Among survivors of staged palliation for single RV anomalies at 6 years in the SVR trial, initial surgical shunt type does not impact echocardiogram-derived indices of RV size and function. Beneficial remodeling of RV size, systolic and diastolic function, and neo-aortic and tricuspid size are apparent over time, without progressive valvular regurgitation during early childhood. Poor RV systolic function at 14 months predicts worse late survival/need for cardiac transplantation independent of the initial shunt type.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by grants (HL068269, HL068270, HL068279, HL068281, HL068285, HL068288, HL068290, HL068292, and HL085057) from the National Heart, Lung, and Blood Institute (NHLBI). This work is solely the responsibility of the authors and do not necessarily represent the official views of NHLBI or NIH.
Footnotes
Disclosures
None
CLINICAL TRIAL REGISTRATION INFORMATION— Unique Identifier: NCT00115934 URL: http://clinicaltrials.gov
References
- 1.Mahony L, Sleeper LA, Anderson PA, Gersony WM, McCrindle BW, Minich LL, Newburger JW, Saul JP, Vetter VL, Pearson GD. The Pediatric Heart Network: a primer for the conduct of multicenter studies in children with congenital and acquired heart disease. Pediatr Cardiol. 2006;27:191–198. [DOI] [PubMed] [Google Scholar]
- 2.Ohye RG, Gaynor JW, Ghanayem NS, Goldberg CS MD,Laussen PC Frommelt PC Newburger JW, Pearson GD MD, Tabbutt S MD, Wernovsky G, Wruck LM, Atz AM, Colan SD MD, Jaggers J, McCrindle BW, Prakash A MD, Puchalski MJ, Sleeper LA, Stylianou MP Mahony M, Pediatric Heart Network Investigators. Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2003;136:968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar-Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW, Pediatric Heart Network Investigators. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newburger JW, Sleeper LA, Frommelt PC, Pearson GD, Mahle WT, Chen S, Dunbar-Masterson C, Mital S, Williams IA, Ghanayem NS, Goldberg CS, Jacobs JP, Krawczeski CD, Lewis AB, Pasquali SK, Pizarro C, Gruber PJ, Atz AM, Khaikin S, Gaynor JW, Ohye RG, Pediatric Heart Network Investigators. Transplant-free survival and interventions at three years in the single ventricle reconstruction trial. Circulation 2014;129:2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newburger JW, Sleeper LA, Gaynor JW, Hollenbeck-Pringle D, Frommelt PC, Li JS, Mahle WT, Williams IA, Atz AM, Burns KM, Chen S, Cnota J, Dunbar-Masterson C, Ghanayem NS, Goldberg CS, Jacobs JP, Lewis AB, Mital S, Pizarro C, Eckhauser A, Stark P, Ohye RG, Pediatric Heart Network Investigators. Transplant-free survival and interventions at six years in the single ventricle reconstruction trial. Circulation 2018;137: 2246–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frommelt PC, Guey LT, Minich LL, Bhat M, Bradley TJ, Colan SD, Ensing G, Gorentz J, Heydarian H, John JB, Lai WW, Levine JC, Mahle WT, Miller SG, Ohye RG, Pearson GD, Shirali GS, Wong PC, Cohen MS, Pediatric Heart Network Investigators. Does Initial Shunt Type for the Norwood Procedure Impact Echocardiographic Measures of Cardiac Size and Function during Infancy? The Single Ventricle Reconstruction Trial. Circulation 2012;125:2630–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frommelt PC, Gerstenberger E, Baffa J, Border WL, Bradley TJ, Colan S, Gorentz J, Heydarian H, John JB, Lai WW, Levine J, Lu JC, McCandless RT, Miller S, Nutting A, Ohye RG, Pearson GD, Wong PC, Cohen MS, Pediatric Heart Network Investigators. Impact of initial shunt type on cardiac size and function in children with single right ventricle anomalies before the Fontan procedure: the single ventricle reconstruction extension trial. J Am Coll Cardiol. 2014;64:2026–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghanayem NS, Allen KR, Tabbutt S, Atz AM, Clabby ML, Cooper DS, Eghtesady P, Frommelt PC, Gruber PJ, Hill KD, Kaltman JR, Laussen PC, Lewis AB, Lurito KJ, Minich LL, Ohye RG, Schonbeck JV, Schwartz SM, Singh RK, Goldberg CS, Pediatric Heart Network Investigators. Interstage mortality after the Norwood procedure: Results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabbutt S, Ghanayem N, Cooper DS, Frank DU, Lu M, Frommelt PC, Lai WW, Goldberg CS, Graham EM, Dent-Krawczeski D, Mahony L, Ravishankar C, Sleeper LA, Kirsch JA, Lewis A, Lodge A, Ohye R, Pizarro C, Simsic J, Spurrier E, Stylianou M, Laussen P, Pediatric Heart Network Investigators. Risk factors for hospital morbidity and mortality after the Norwood procedure: A report from the Pediatric Heart Network Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012. October;144:882–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tweddell JS, Sleeper LA, Ohye RG, Williams IA, Mahony L, Pizarro C, Pemberton VL, Frommelt PC, Bradley SM, Cnota JF, Hirsch J, Kirshbom PM, Li JS, Pike N, Puchalski M, Ravishankar C, Jacobs JP, Laussen PC, McCrindle BW, Pediatric Heart Network Investigators. Intermediate-term mortality and cardiac transplantation in infants with single-ventricle lesions: Risk factors and their interaction with shunt type. J Thorac Cardiovasc Surg. 2012;144:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burch PT, Ravishankar C, Newburger JW, Lambert LM, Pemberton VL, Granger S, Floh AA, Anderson JB, Hill GD, Hill KD, Oster ME, Lewis AB, Schumacher KR, Zyblewski SC, Davies RR, Jacobs JP, Lai WW, Minich LL, Pediatric Heart Network Investigators. Assessment of Growth 6 Years after the Norwood Procedure. J Pediatr. 2017;180:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahle WT, Hu C, Trachtenberg F, Menteer J, Kindel SJ, Dipchand AI, Richmond ME, Daly KP, Henderson HT, Lin KY, McCulloch M, Lal AK, Schumacher KR, Jacobs JP, Atz AM, Villa CR, Burns KM, Newburger JW, Pediatric Heart Network Investigators. Pediatric Heart Network Investigators. Heart failure after the Norwood procedure: An analysis of the Single Ventricle Reconstruction Trial. J Heart Lung Transplant. 2018;37:879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frommelt PC, Gerstenberger E, Baffa J, Border WL, Bradley TJ, Colan S, Gorentz J, Heydarian H, John JB, Lai WW, Levine J, Lu JC, McCandless RT, Miller S, Nutting A, Ohye RG, Pearson GD, Wong PC, Cohen MS, Pediatric Heart Network Investigators. Doppler flow patterns in the right ventricle-to-pulmonary artery shunt and neo-aorta in infants with single right ventricle anomalies: impact on outcome after initial staged palliations. J Am Soc Echocardiogr. 2013;26:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wasserstein RL, Lazar NA. The ASA’s Statement on p-values: Context, process, and purpose. The American Statistician 2016:70:2;129–133. [Google Scholar]
- 15.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–457. [DOI] [PubMed] [Google Scholar]
- 16.Ballweg JA, Dominguez TE, Ravishankar C, Gaynor JW, Nicolson SC, Spray TL, Tabbutt S. A contemporary comparison of the effect of shunt type in hypoplastic left heart syndrome on the hemodynamics and outcome at Fontan completion. J Thorac Cardiovasc Surg. 2010;140:537–544. [DOI] [PubMed] [Google Scholar]
- 17.Graham EM, Zyblewski SC, Phillips JW, Shirali GS, Bradley SM, Forbus GA, Bandisode VM, Atz AM. Comparison of Norwood shunt types: do the outcomes differ 6 years later? Ann Thorac Surg 2010;90:31–35. [DOI] [PubMed] [Google Scholar]
- 18.Tanoue Y, Kado H, Shiokawa Y, Ishikawa S. Midterm Ventricular Performance After Norwood Procedure With Right Ventricular–Pulmonary Artery Conduit. AnnThorac Surg. 2004;78:1965–1971. [DOI] [PubMed] [Google Scholar]
- 19.Padalino MA, Castellani C, Toffoli S, Della Barbera M, Milanesi O, Thiene G, Stellin G, Angelini A Pathological changes and myocardial remodeling related to the mode of shunting following surgical palliation for hypoplastic left heart syndrome. Cardiol Young. 2008;18:415–422. [DOI] [PubMed] [Google Scholar]
- 20.Menon SC, Minich LL, Casper TC, Puchalski MD, Hawkins JA, Tani LY. Regional myocardial dysfunction following Norwood with right ventricle to pulmonary artery conduit in patients with yypoplastic left heart syndrome. J Am Soc Echocardiogr. 2011;24:826–833. [DOI] [PubMed] [Google Scholar]
- 21.Hill GD, Frommelt PC, Stelter J, Saudek D. Impact of Initial Norwood Shunt Type on Right Ventricular Deformation: The Single Ventricle Reconstruction Trial. J Am Soc Echocardiogr 2015;28:517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima T, Kuwata S, Kurishima C, Iwamoto Y, Saiki H, Ishido H, Masutani S, Senzaki H. Aortic root dilation and aortic stiffness in patients with single ventricle circulation. Circ J. 2014;78:2507–2511. [DOI] [PubMed] [Google Scholar]
- 23.Dasi LP, Sundareswaran KS, Sherwin C, de Zelicourt D, Kanter K, Fogel MA, Yoganathan AP. Larger aortic reconstruction corresponds to diminished left pulmonary artery size in patients with single-ventricle physiology. J Thorac Cardiovasc Surg. 2010;139:557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen MS, Marino BS, McElhinney DB, Robbers-Visser D, van der Woerd W, Gaynor JW, Spray TL, Wernovsky G Neo-aortic root dilatation and valve regurgitation up to 21 years after staged reconstruction for hypoplastic left heart syndrome. J Am Coll Cardiol. 2003;42:533–540. [DOI] [PubMed] [Google Scholar]
- 25.Kotani Y, Kasahara S, Fujii Y, Yoshizumi K, Oshima Y, Otsuki S, Akagi T, Sano S. Clinical outcome of the Fontan operation in patients with impaired ventricular function. Eur J Cardiothorac Surg. 2009;36:683–687. [DOI] [PubMed] [Google Scholar]
- 26.Friedman KG, Salvin JW, Wypij D, Gurmu Y, Bacha EA, Brown DW, Laussen PC, Scheurer MA. Risk factors for failed staged palliation after bidirectional Glenn in infants who have undergone stage one palliation. Eur J Cardiothorac Surg. 2011;40:1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghelani SJ, Harrild DM, Gauvreau K, Geva T, Rathod RH. Comparison Between Echocardiography and Cardiac Magnetic Resonance Imaging in Predicting Transplant-Free Survival After the Fontan Operation. Am J Cardiol 2015;116:1132–1138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.