Abstract
One of the objectives of the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org/) is to establish a national longitudinal cohort of 9 and 10 year olds that will be followed for 10 years in order to prospectively study the risk and protective factors influencing substance use and its consequences, examine the impact of substance use on neurocognitive, health and psychosocial outcomes, and to understand the relationship between substance use and psychopathology. This article provides an overview of the ABCD Study Substance Use Workgroup, provides the goals for the workgroup, rationale for the substance use battery, and includes details on the substance use module methods and measurement tools used during baseline, 6-month and 1-year follow-up assessment time-points. Prospective, longitudinal assessment of these substance use domains over a period of ten years in a nationwide sample of youth presents an unprecedented opportunity to further understand the timing and interactive relationships between substance use and neurocognitive, health, and psychopathology outcomes in youth living in the United States.
Keywords: Adolescent Brain Cognitive Development Study, Adolescent, Child, Substance use, Alcohol, Cannabis, Marijuana, Nicotine, Longitudinal, Methods, Assessment, Drug use, Prescription drug use, Inhalants
1. ABCD study substance use workgroup: introduction & overview
One of the objectives of the NIH-initiative, the Adolescent Brain Cognitive Development (ABCD) Study, is to establish a national, multisite, longitudinal cohort study to prospectively examine the youth from childhood (ages 9–10) through adolescence to examine the risk and protective factors influencing the trajectories of substance use and its consequences, examine the impact of detailed patterns of substance use on neurocognitive development, health and psychosocial outcomes, and to study the interactive relationship between substance use and psychopathology in youth (https://abcdstudy.org/). The goal of this article is to provide an overview of the ABCD Study Substance Use Workgroup goals, rationale for the substance use battery, and detailed methods of the battery in order for the scientific community to achieve improved harmonization in substance use assessment, which have varied widely, especially in measurement of frequency/quantity patterns of use (Conway et al., 2014).
The Substance Use module was developed for the ABCD Study by the Substance Use Workgroup, comprised of experts on assessment of substance use quantity and frequency patterns, SUD diagnostic interviews, influences on substance use risk, and dimensional assessment of substance use problems and consequences in adolescents. The Substance Use Workgroup Co-Chairs are Drs. Mary Heitzeg (University of Michigan) and Krista Lisdahl (University of Wisconsin-Milwaukee). The Substance Use Workgroup members include Drs. Kevin Conway (National Institute on Drug Abuse), Sarah Feldstein Ewing (Oregon Health and Science University), Raul Gonzalez (Florida International University), Sara Jo Nixon (University of Florida), Devin Prouty (SRI International), Kenneth Sher, (University of Missouri), Susan Tapert (University California San Diego), and Gordon Willis (National Cancer Institute).
In determining methods and constructs to measure, the workgroup considered the ABCD Study aims and requested methodology outlined by the ABCD Study NIH funding opportunity announcement (https://grants.nih.gov/grants/guide/rfa-files/RFA-DA-15-015.html). The workgroup met weekly or biweekly and identified three primary areas to be measured: 1) factors impacting risk of substance use; 2) assessment of detailed substance use patterns; and 3) consequences of substance use. Constructs within these domains were identified by the workgroup utilizing member input, literature review, and consultation with instrument authors and external experts. During the process of finalizing the battery the workgroup prioritized instruments that demonstrated sound psychometric properties, fit the longitudinal design, were developmentally appropriate, reduced participant burden, were open-access, and could be administered by computer. In order to improve cross-study harmonization, if an instrument fit these criteria, priority was given to instruments provided by PhenX (https://www.phenxtoolkit.org/) Patterns of Substance Use module for adolescents (module #510301), the Monitoring the Future (MTF) Study (http://www.monitoringthefuture.org/) (Institute for Social Research and U.o.M. Monitoring the Future, 2010), the Population Assessment of Tobacco and Health (PATH) Study (https://pathstudyinfo.nih.gov/UI/HomeMobile.aspx) (Hyland et al., 2016), and the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) Study (http://ncanda.org/) (Brown et al., 2015) (see Table 1 for instrument overlap identification). Further, great care and consideration was put into organizing a gating structure to avoid exposing non- or low-using children to novel substances (see “heard of” section below and Table 1 for gating details). After developing the draft protocol, the workgroup received and integrated feedback from the NIH advisors and the ABCD Study Coordinating Center, and piloted the protocol at multiple sites with 9–10 year olds to ensure youth comprehension, confirm data quality and timing. Consistent with the goals of the ABCD Study, curated data and detailed data dictionaries, including all the substance use measures, will be released yearly to the NIMH Data Archive (NDA; https://data-archive.nimh.nih.gov/) (for further data sharing details, see https://abcdstudy.org/scientists_data_sharing.html and the ABCD Study overview paper included in this special issue).
Table 1.
ABCD Substance Use Module Measures Overview (by Youth- and Parent-Administered Measures).
| Youth Measures | Construct | Gating | Drugs Covered |
|---|---|---|---|
| Lifetime and Recent Substance Use Patterns | |||
| Lifetime Use Interview (Lisdahl and Price, 2012) | Lifetime patterns of use (ever used, lifetime quantity, first and regular use, length of abstinence) | If heard of substance | All drug categories |
| Web-based TLFB (Sobell and Sobell, 1996, Robinson et al., 2014) | Past 6-month detailed patterns of substance use (baseline); during follow-up years, will cover time since last assessment | If heard of substance and used in past 6 months | All drug categories |
| PLUS form (Brown et al., 2015)* | Hours since last use of nicotine, caffeine or prescription medication (up to 24 h) | If used in lifetime; administered each session | Nicotine, caffeine, OTC, prescription medications |
| Supplemental Beverage Questionnaire | Average caffeine use per week during last 6 months, maximum caffeine dose | If heard of caffeinated beverages | Caffeine |
| iSay Sip Inventory (Jackson et al., 2015) | Alcohol low-level use (sipping) | If heard of alcohol and if endorsed sipping alcohol | Alcohol (first sip) |
| Cannabis low-level use | Cannabis low level use (first puff or taste of marijuana) | If heard of cannabis and if endorsed puff or taste | Cannabis (first puff or taste) |
| Nicotine low-level use | Nicotine low-level use (first puff nicotine, first dip smokeless tobacco) | If heard of nicotine and if endorsed puff or dip | Nicotine (first use of cigarette, e-cigarette or smokeless tobacco) |
| Factors Impacting Substance Use Risk | |||
| Intention to Use (Hyland et al., 2016) + | Extent that youth are curious about or intent to use substances | If heard of alcohol, nicotine and/or cannabis, but have not initiated use | Alcohol, cannabis, nicotine |
| Peer Substance Use (Johnston et al., 2017) *,^ | Youth reports substance use in their peer group | For each question, if heard of substance | Alcohol, cannabis, cigarettes, e-cigarettes, inhalants, other drugs (e.g., cocaine, downers, LSD) |
| Peer Tolerance (Johnston et al., 2017) ^ | Youth’s report on their peer’s tolerance of substance use | For each question, if heard of substance | Alcohol, cannabis, cigarettes, e-cigarettes, smokeless tobacco, inhalants, and prescription drugs, cocaine, heroin or methamphetamine |
| Perceived Harm (Johnston et al., 2017) ^ | Youth’s report on perceived harm of various substances | For each question, if heard of substance | Alcohol, cannabis, cigarettes, e-cigarettes, smokeless tobacco, inhalants, and prescription drugs, cocaine, heroin or methamphetamine |
| AEQ-AB (Stein et al., 2007) * | Youth’s expectancies about alcohol | If heard of alcohol | Alcohol |
| MEEQ-B (Torrealday et al., 2008) | Youth’s expectancies about cannabis | If heard of cannabis | Cannabis |
| ASCQ-modified (Lewis-Esquerre et al., 2005) | Youth’s expectancies about nicotine | If heard of nicotine | Nicotine |
| SRE (Schuckit et al., 2008) * | Youth’s acute subjective response to alcohol (first use, last 3 months, heaviest period of use) | If heard of alcohol and used once in lifetime. | Alcohol |
| Acute Subjective Response to Marijuana scale (Agrawal et al., 2014) | Youth’s acute subjective response to first cannabis exposure | If heard of cannabis and used once in lifetime. | Cannabis |
| Acute Subjective Responses to Tobacco (Trinidad et al., 2018) + | Youth’s acute subjective response to first nicotine exposure | If heard of nicotine and used once in lifetime. | Nicotine (cigarettes, e-cigarettes, or smokeless tobacco) |
| Consequences of Substance Use | |||
| HSS (Slutske et al., 2003) * | Cumulative symptoms of hangover from alcohol use | If heard of alcohol and if used on 2+ (9–11 year olds) or 3+ (12+ years) occasions in past 6 months | Alcohol |
| RAPI (White and Labouvie, 1989) | Cumulative problem symptoms due to alcohol use | If heard of alcohol and if used on 2+ (9–11 year olds) or 3+ (12+ years) occasions in past 6 months | Alcohol |
| MPI (Johnson and White, 1989, Simons et al., 1998) | Cumulative problem symptoms due to cannabis use | If heard of cannabis and if used on 2+ (9–11 year olds) or 3+ (12+ years) occasions in past 6 months | Cannabis |
| Nicotine Dependence (Hyland et al., 2016) + | Cumulative symptoms of nicotine dependence | If heard of nicotine and if used on 2+ (9–11 year olds) or 3+ (12+ years) occasions in past 6 months | Nicotine |
| DPI (Johnson and White, 1989, Caldwell, 2002, Kingston et al., 2011) | Cumulative problem symptoms due to other illicit drug use (excluding cannabis) | If heard of other illicit drug and if used on 2+ (9–11 year olds) or 3+ (12+ years) occasions in past 6 months | Any other illicit drug (excluding cannabis) |
| Parent Measures | Construct | Gating | Drugs Covered |
|---|---|---|---|
| Recent Substance Use | |||
| PLUS form (Brown et al., 2015) * | Hours since last use of caffeine or prescription medication (up to 24 h) | None | Caffeine, OTC, prescription medications |
| Factors Impacting Substance Use Risk | |||
| Availability of Substances (Johnston et al., 2017) ^ | Parent reports how easily youth may access substances in their environment. | None | Alcohol, cannabis, cigarettes, e-cigarettes, other drugs (e.g., cocaine, LSD or amphetamines), medical cannabis |
| Parent Rules (Arthur et al., 2007) | Parental rules on drinking, cigarettes, and cannabis use | None | Alcohol, cannabis, nicotine |
Notes: Gating means certain questions must be answered positively or negatively in order for the youth to receive that question. OTC = over the counter; AEQ-AB = Alcohol Expectancy Questionnaire- Adolescent, Brief; MEEQ-B = Marijuana Effect Expectancy Questionnaire- Brief; ASCQ = Adolescent Smoking Consequences Questionnaire; SRE = Self-Rating of the Effects of Alcohol; HSS = Hangover Symptom Scale; RAPI = Rutgers Alcohol Problem Index; MPI = Marijuana Problem Index; DPI = Drug Problem Index. “All drug categories” include alcohol, cannabis and cannabinoids (smoked cannabis, edible cannabis, cannabis concentrations, cannabis-infused alcohol, cannabis tinctures, synthetic cannabinoids), nicotine (tobacco cigarettes, electronic cigarettes, smokeless tobacco, cigars, hookah, tobacco pipe, nicotine replacement), cocaine or crack cocaine, cathinones, methamphetamine, MDMA (ecstasy), ketamine, gamma hydroxybutyrate, heroin, hallucinogens (lysergic acid diethylamide, phencyclidine, peyote, mescaline, N-dimethyltryptamine, alpha-methyltryptamine, or 5-methoxy-N,N-diisopropyltryptamine), psilocybin, salvia, anabolic steroids, inhalants, prescription stimulants, prescription sedatives, prescription opioids, and OTC cough or cold medicine. Harmonization note: instruments overlap with the * National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) Study (http://ncanda.org/) (Brown et al., 2015), +Monitoring the Future (MTF) Study (http://www.monitoringthefuture.org/) (Institute for Social Research and U.o.M. Monitoring the Future, 2010) and ^ Population Assessment of Tobacco and Health (PATH) Study (https://pathstudyinfo.nih.gov/UI/HomeMobile.aspx) (Hyland et al., 2016).
2. Background and rationale
2.1. Substance use: initiated during adolescence
One of the goals of the ABCD Study is to characterize youth prior to the initiation of significant substance use. Adolescence is a period of ongoing neurodevelopment that is linked with an increase in risk-taking behaviors, including the onset of substance use (Casey et al., 2008; Eaton et al., 2006; Gardner and Steinberg, 2005; Casey et al., 2000; Giedd et al., 1996; Gogtay et al., 2004; Lenroot and Giedd, 2006; Sowell et al., 2004, Sowell et al., 1999, Sowell et al., 2002; Mills et al., 2014; Schmitt et al., 2014; Houston et al., 2014). Initiation of drinking alcohol (beyond a sip) and use of most illicit substances typically begins in the early teen years, although high-risk demographic communities report initiating use during the elementary and early middle school years (Feldstein Ewing et al., 2015). In the U.S., among 8th graders (13–14 year olds), lifetime use of alcohol (22.8%), electronic cigarettes (17.5%), cannabis (12.8%), tobacco cigarettes (9.8%) inhalant (7.7%), prescription amphetamines (5.7%) and prescription tranquilizers (3.0%) are the most commonly used substances (Johnston et al., 2017). Data is unavailable for 8th graders, but an alarming 18% of 12th graders have used any prescription drug and 7.8% of 12th graders report non-medical use of prescription pain relievers (OxyContin, Percocet, Vicodin, Fentanyl) (Johnston et al., 2017). The latter is a particularly important area, given increase risk of developing an opiate use disorder associated with adolescent exposure, significant barriers to treatment, and alarming rate of overdose deaths in adolescents (Liebling et al., 2016; McCabe et al., 2016; Rudd et al., 2016). Caffeine use is very common in youth, with 73.9% of 6–11 year olds consuming caffeinated food or beverage on any given day within the past week and adolescents (aged 12–17 years old) consuming an average of 50 mg per day (Ahluwalia et al., 2014; Ahluwalia and Herrick, 2015).
It is notable that detailed data on substance use patterns in 9- and 10- year olds is less frequently reported, as the youngest age US national surveys assess is 12 or 13 years old [e.g., the MTF (Johnston et al., 2017; Institute P.P.R., 2012) begins the assessment in 8th grade (typically 13–14 years old) while the National Survey on Drug Use and Health (Quality C.f.B.H.S.a., 2014) begins at age 12]. Data that are available for youth younger than 12 comes from state assessments (Donovan, 2007), such as the Texas School Survey of Drug and Alcohol Use, which measures substance use in youth attending grades 4–6 (Institute P.P.R., 2012). This survey reports lifetime use for the following drug categories in 4th graders: alcohol (12.7%), nicotine (2.8%), cannabis (0.8%), and inhalants (liquids, sprays and gases that people sniff or inhale to get high) (11.1%) − other drug categories were not assessed. This survey also revealed that a significant portion of 4th graders report that they never heard of cannabis (26.1%), inhalants (16%), nicotine (6%), and alcohol (3.6%). Taken together, data suggests that youth may initiate first sipping or trying substances in late childhood (as young as 9), and incidents of substance use initiation increase from late childhood into early adolescence. Notably, although some youth may be sipping alcohol or trying tobacco, the vast majority of 9 and 10 year olds are substance-naïve and indeed may not have heard of several drug categories. Thus, studies assessing this age group need to avoid exposing substance-naïve youth to new substance use concepts.
2.2. Factors impacting substance use risk in youth
As stated above, one area identified by the Substance Use Workgroup to measure is factors that influence risk of substance use initiation, substance use trajectories, and substance use consequences, such as early sipping alcohol or puffing tobacco, acute initial subjective response, drug curiosity and intentions to use, peer substance use, parental rules, and availability of substances.
Community samples have shown that up to a third of 8- and 9-year olds report sipping alcohol (Donovan and Molina, 2013; Jackson et al., 2013), demonstrating that very early substance experimentation begins in late childhood. Studies have found that early sipping predicted drinking onset (i.e., consuming full alcohol drinks) by age 14 (Donovan and Molina, 2011). Similarly, Jackson and colleagues (Jackson et al., 2015) found that sipping alcohol prior to 6th grade predicted drinking a full drink, getting drunk, and drinking heavily (i.e., 3 or more drink equivalents on an occasion) by 9th grade, even after controlling for a range of etiologically relevant environmental and individual difference covariates. In contrast to the literature on alcohol, which sometimes operationalizes determinants of a sip and having a full drink as distinct, the field of nicotine has not tended to make this distinction. With few exceptions (Okoli et al., 2008; Alexander et al., 1999), studies rarely distinguish between having had a puff and having had one or more cigarettes and data are generally missing on interim levels of progression from a puffs to first cigarette or to regular smoking. Even less is known about the progression of trying a puff or taste of cannabis to more regular experimentation. Closely assessing initial tobacco or cannabis use could help to both characterize the progression of substance use across substances as well as help to determine variables key to such progression.
Another important factor to measure is individual acute subjective response to early substance experimentation, such as level of response to alcohol, which has been found to predict risk of developing alcohol related consequences in teens (Schuckit et al., 2008) and alcohol use disorders in adulthood (Trim et al., 2009). Early experiences with tobacco are also thought to be indicators for the development of substance use disorders, thus representing an important area of youth and adolescent research (Haberstick et al., 2011; Perkins et al., 2009; Pomerleau et al., 1998). Therefore, if one wants to chart the course of drinking or drug use developmentally, starting with the earliest experiences of alcohol and drug use experimentation provides important information about substance related risk.
Risk for the transition from initiating to the emergence of problem substance use in adolescence is influenced by a variety of factors. For example, youth who exhibit susceptibility cognitions (intentions to use or curiosity about drugs) (Pierce et al., 1996) are twice as likely than youth who do not see substance use this way to start smoking cigarettes during adolescence (Choi et al., 2001; Nodora et al., 2014; Strong et al., 2015). Recent research suggests that risk may extend to other tobacco products as well (Trinidad et al., 2018). In addition, parenting, home environment, neighborhood factors such as alcohol and drug availability, and peer influence have all been shown to impact substance use onset and outcomes (Buu et al., 2009; Curran et al., 1997; Dielman et al., 1993; Marshal et al., 2003; Trentacosta et al., 2009). The Culture and Environment module of the ABCD protocol covers many of these potential influences on substance use, including parental monitoring, family environment and conflict, and neighborhood safety and crime. In addition to these domains, the ABCD substance use module captures low level substance use, acute subjective response, intentions to use, peer substance use, parent perception of the availability of substances in the neighborhood, and parent rules about substance use. Beginning at the one-year follow up, additional measures will be added to assess the youth’s perceived harm of substance use, disapproval of substance use, and substance-related expectancies. Thus, the ABCD substance use module that was developed measures important predictors of early substance use and escalation to SUDs.
2.3. Assessment of substance use patterns & impact on neurocognition
Another area of focus the Substance Use Workgroup identified is detailed measurement of substance use patterns, including detailed quantity, frequency, route of administration, and co-use patterns. This information is critical in order to complete one of the aims of the ABCD Study: to characterize the impact of substance exposure on adolescent neurocognitive development. Adolescents demonstrate a greater impact of substance use on neurocognitive outcomes (Volkow et al., 2014). Alcohol has historically been the most commonly used substance in adolescents (Johnston et al., 2017) and converging lines of evidence reflect that repeated alcohol use during adolescence, especially binge drinking, has been associated with poor neurocognitive outcomes such as brain structural and function abnormalities and reduced memory, visuospatial skills, attention, and executive function in adolescents (Lisdahl et al., 2013; Jacobus et al., 2015; Nguyen-Louie et al., 2016; Pfefferbaum et al., 2016; Brumback et al., 2016; Meruelo et al., 2017; Squeglia et al., 2015; Müller-Oehring et al., 2017; Sullivan et al., 2016; Whelan et al., 2014). Alcohol hangover symptoms not only reflect an immediate consequence of excessive consumption that causes distress in the drinker (McKinney, 2010), they also uniquely predict acute cognitive impairment in adults (Ling et al., 2010), relate to worsened neurocognition in adolescents (McQueeny et al., 2009; Squeglia et al., 2009), and prospectively predicts later alcohol use disorder onset in adults (Piasecki et al., 2005). Of particular relevance to ABCD are findings that suggest that the studies examining pathophysiology of hangover in adults reveal that it likely involves a neuroinflammatory process (Penning et al., 2010; Verster, 2008), that may be similar to that observed in alcohol-related brain damage (esp. in frontal and hippocampal regions) in rodent models (Crews and Nixon, 2009). That is, hangover may represent an index of alcohol-related neurotoxicity that is associated with more persistent cognitive deficits.
Cannabis is the second most commonly used drug, with 35.6% of 12th graders using it in the past year (Johnston et al., 2017). Early adolescent cannabis (before age 17) use is strongly correlated with substance use and the abuse of other illicit drug use in youth (Agrawal et al., 2004). While there is still some degree of debate (National Academies of Sciences E. and Medicine, 2017), converging data reflect that at least weekly cannabis use during adolescence has been associated with neurocognitive abnormalities, including abnormal brain morphometry and function, lower IQ, and poorer sustained attention, verbal memory, and executive function, especially in those with an early age of cannabis use onset see (Lisdahl et al., 2013; Batalla et al., 2013; Lisdahl et al., 2014; Meruelo et al., 2017; Jacobus and Tapert, 2014 for reviews). It is notable that there have been challenges to this research in terms of the wide array of metrics, lack of measurement of potency and content of cannabinoids [e.g., tetrahydrocannabinol (THC), cannabidiol (CBD)], lack of control of polysubstance use (especially alcohol and nicotine), and the majority are cross-sectional studies, making it difficult to resolve the temporal sequencing of substance exposure and neurocognitive deficits.
Nicotine is the third most commonly used substance by adolescents and use of electronic cigarettes has become twice as popular as traditional tobacco products (Johnston et al., 2017). Concomitantly, e-cigarettes have been found to increase the risk for transitioning to more traditional tobacco cigarettes (Wills et al., 2016). Although acute administration of nicotine may enhance cognition in teens and young adults, especially memory and attention (Poorthuis et al., 2009), chronic use has been linked with attention and working memory deficits in teens (Goriounova and Mansvelder, 2012; Wagner et al., 2013; Jacobsen et al., 2005; England et al., 2017). Acute withdrawal from nicotine in adolescent users has also been associated with abnormal reward processing (Sweitzer et al., 2016), working memory (Falcone et al., 2014; Merritt et al., 2012), and verbal memory (Jacobsen et al., 2007) fMRI tasks, highlighting the necessity to measure last use of nicotine prior to neurocognitive assessment.
Human and preclinical evidence demonstrates that other illicit substances are linked with neurocognitive deficits in adolescents and young adults, including cocaine (Cannizzaro et al., 2014; Nuijten et al., 2016; Kaag et al., 2016, Kaag et al., 2014; Marhe and Franken, 2014; Rose-Jacobs et al., 2011; Lundqvist, 2010; Meade et al., 2015; Fernández-Serrano et al., 2010; Sim et al., 2007; Rahman and Clarke, 2005; Kober et al., 2016; Verdejo-Garcia et al., 2015; Moreno-López et al., 2015; Ide et al., 2014; Tau et al., 2014; Albein-Urios et al., 2014; Ernst et al., 2000; Mayer et al., 2013), methamphetamine (Buck and Siegel, 2015; Cuzen et al., 2015; King et al., 2010; Scott et al., 2007), MDMA or ecstasy (Medina et al., 2005; Medina and Shear, 2007; Costa et al., 2014; Price et al., 2014; Downey et al., 2015; Halpern et al., 2011; Scholey et al., 2011; McCann et al., 2014, McCann et al., 2008; Jager et al., 2008; de Win et al., 2008), inhalants (Takagi et al., 2011a, Takagi et al., 2011b, Takagi et al., 2014; Scott and Scott, 2012, Scott and Scott, 2014), heroin (Zeng et al., 2013; Lundqvist, 2010; Ornstein et al., 2000; Fernández-Serrano et al., 2010), cathinones (Albertson et al., 2016; Patrick et al., 2016), ketamine (Chen et al., 2015; Nagy et al., 2015; Tang et al., 2013; Sun et al., 2014; Morgan et al., 2012a), gamma hydroxybutyrate (Johansson et al., 2014; Sircar et al., 2008; Youn et al., 2015), hallucinogens (lysergic acid diethylamide, phencyclidine, peyote, mescaline, N,N-dimethyltryptamine, alpha-methyltryptamine, or 5-methoxy-N,N-diisopropyltryptamine, psilocybin, or salvia) (Compton et al., 2011; Noworyta-Sokołowska et al., 2016; Graham et al., 2010, Graham et al., 2012; Halpern et al., 2005; Carstairs and Cantrell, 2010; Fickenscher et al., 2006; Mahendran et al., 2016; Ranganathan et al., 2012), and anabolic steroids (Wallin-Miller et al., 2016; Wallin and Wood, 2015; Hildebrandt et al., 2014; Ramos-Pratts et al., 2013; Hermans et al., 2010). Given the common use of caffeinated beverages in youth as young as two years old (Ahluwalia and Herrick, 2015) and growing concern over health effects and addiction risk associated with excessive caffeine use (Budney and Emond, 2014; Temple et al., 2017a; Temple, 2009), examining caffeine effects on health and neurodevelopment in youth is of increasing concern. Thus far, research has shown that acute caffeine administration is generally linked with improved cognition, although impact of chronic caffeine exposure is not well understood (Curran and Marczinski, 2017). And at least one study reported that increased caffeine consumption is linked with increased risk-taking in adolescents (Temple et al., 2017b). Finally, prescription stimulant medications have been linked with cognitive enhancement (Bagot and Kaminer, 2014; Coghill et al., 2014; Tamminga et al., 2016), while prescription anxiolytics/sedatives (Meador et al., 2011; Loring et al., 2012; Reissig et al., 2015; Ghoneim et al., 1984) and opiates (Allen et al., 2003; Schoedel et al., 2010) negatively impact memory, processing speed and attention. Over the counter (OTC) cough medication containing dextromethorphan has been linked with cortical thickness in adolescents in one study (Qiu et al., 2016), although this outcome has yet been replicated. It is notable that the majority of these aforementioned studies have numerous methodological weaknesses in that they are primarily cross-sectional, have relatively small sample sizes, lack female participants, have low power to disentangle polydrug effects, or have not been validated in younger adolescents. Another issue with this research to date is that polydrug use, which is common in adolescence (Johnston et al., 2017), and co-use of substances is rarely studied. Co-use use can uniquely impact neurocognition (Lisdahl et al., 2013); indeed, preliminary evidence has shown that co-use of alcohol and nicotine (Pennington et al., 2015; Schuster et al., 2016), alcohol and cannabis (Lisdahl et al., 2013; McQueeny et al., 2009; Medina and Shear, 2007; Bava et al., 2009; Jacobus et al., 2009; Elofson et al., 2013; Hanson et al., 2010; Medina et al., 2007a, Medina et al., 2008, Medina et al., 2007b, Medina et al., 2007c), alcohol and cocaine (Medina et al., 2006; Pennings et al., 2002; Bolla et al., 2000; Bondi et al., 1998), cannabis and nicotine (Filbey et al., 2015; Schuster et al., 2016), and cannabis and methamphetamine (Gonzalez et al., 2004) has been associated with unique neurocognitive abnormalities above and beyond single substance effects in adolescents and young adults.
In summary, numerous substances have been linked with neurocognitive outcomes in adolescents. Studies have found that numerous factors can impact findings, including total exposure (quantity/frequency, including binge alcohol measures), potency and content (especially cannabis), route of administration, outcomes such as hangover symptoms, and co-use of substances. Therefore, thorough measurement of substance use patterns and other qualifying factors (i.e., potency, cannabis content, route of administration) across numerous substances categories from childhood through adolescence is an important component of the ABCD Substance Use module. The substance use patterns assessed by the module include alcohol, cannabis and cannabinoids (smoked cannabis, edible cannabis, cannabis concentrations, cannabis-infused alcohol, cannabis tinctures, synthetic cannabinoids), nicotine (tobacco cigarettes, electronic cigarettes, smokeless tobacco, cigars, hookah, tobacco pipe, nicotine replacement), caffeine, cocaine, cathinones, methamphetamine, 3,4-methylenedioxymethamphetamine (MDMA, ecstasy or molly), ketamine, gamma hydroxybutyrate, heroin, hallucinogens (including lysergic acid diethylamide, phencyclidine, peyote, mescaline, N-dimethyltryptamine, alpha-methyltryptamine, or 5-methoxy-N,N-diisopropyltryptamine), psilocybin, salvia, anabolic steroids, inhalants, prescription stimulants, prescription sedatives, prescription opioids, and OTC cough or cold medicine. Further, the Substance Use workgroup will release the substance use patterns assessment tools to the scientific community in an attempt to improve harmonization (see Supplemental Material) (Conway et al., 2014).
2.4. Consequences of substance use
Another aim of the ABCD Study is to examine factors that impact the risk for and trajectory of SUD symptoms and consequences; other workgroups will be measuring the numerous outcomes that may represent substance use consequences (i.e., cognitive, brain structure and function, physical health, psychosocial functioning, and psychopathology). Therefore, the ABCD Substance Use module will also obtain SUD diagnosis and symptoms for alcohol, cannabis, nicotine and other drugs. Several studies have reported that adolescent alcohol exposure is associated with increased lifetime risk for developing an alcohol use disorder (AUD) (DeWit et al., 2000; Winters and Lee, 2008; Grant and Dawson, 1997; Hingson et al., 2006; McGue et al., 2001; Dawson et al., 2008; Robins and Przybeck, 1985). Earlier age of cannabis use has also been associated with increased risk for developing a cannabis use disorder (CUD); 11.5% of adults who reported having tried cannabis prior to age 14 met DSM-5 criteria for CUD as compared to only 2.6% of those who tried cannabis after age 18 (SAMHSA, 2013). The peak risk of developing a nicotine use disorder (NUD) is associated with an onset of regular nicotine use at the young age of 10, and females demonstrate a particularly strong relationship between adolescent age of onset and higher rates of nicotine dependence (Lanza and Vasilenko, 2015). Together, these findings support the hypothesis that adolescence is a vulnerable developmental period of high risk for development of a SUD following early substance use exposure. Therefore, the ABCD Study will assess DSM-5 diagnostic criteria of SUD (see the ABCD Mental Health article in this special issue), as well as symptom counts of AUD, CUD, NUD, and combined other illicit drug use disorder.
3. ABCD substance use battery: baseline measures
3.1. Overview, procedures, and timing of the ABCD substance use module
Youth are administered the ABCD Substance Use module by a trained research assistant on an iPad and all questionnaires were converted for electronic data capture via REDCap (Harris et al., 2009). Parents are also administered three measures (PLUS form, availability of substances, and parental rules about substances) on an iPad using REDCap software. First, youth are introduced to the substance use module, rules of confidentiality are restated, and youth are asked if they have heard of certain substances. At this point, research assistants do not show the youth the iPad screen (see section B for details) in order to reduce potential exposure of novel substances. The rest of the interview utilizes gating, in that certain questions must be answered positively or negatively in order for the youth to receive a follow-up question or measure (e.g., youth are only asked about substances they have heard of and only asked about substance consequences if they have actually used the drug; see Table 1 for gating details for each instrument). Because youth may enter the study with some prior substance use, the baseline battery measures lifetime patterns of substance use [including whether they used a substance, age of first- and regular-use assessment, total lifetime quantity (in standard units), maximum lifetime dose, and length of abstinence] of all major drug categories (see section C.1c below for details within each drug category) (Johnston et al., 2017). Next, recent low level use (first sip of alcohol, first puff of cannabis or nicotine) and detailed 6-month quantity and frequency data for each of the aforementioned substance categories are assessed with a computerized modified Timeline Followback interview (TLFB) (Sobell and Sobell, 1996; Robinson et al., 2014). For the measures assessing substance use patterns among youth actually endorsing using a drug, visual aids are provided to improve accuracy of dosing, product identification, and routes of administration. In subsequent waves, the TLFB interview will be utilized to cover measurement of substance use patterns across all ten years to ensure continuous coverage. After the patterns of use are assessed, measures related to risk for substance use initiation and problematic substance use trajectories (e.g., intention to use, acute subjective response, peer use, peer tolerance of use, perceived harm, expectancies), along with substance use consequences (e.g., alcohol hangover symptoms, symptoms of alcohol, cannabis, nicotine and drug use disorders) are administered. The baseline substance use battery takes an average of approximately 9 min for 9 and 10 year olds to complete; with a range between 2 and 19 min (depending on processing speed, how many substances the youth heard of, and if they were current users) and is administered between the first section of the neuropsychological testing and the mental health module.
It is notable that in addition to self-report of substance use, youth undergo substance toxicology screening to measure recent substance exposure. At baseline and year 1 follow-up sessions, biological breath, saliva, urine and hair samples are collected from youth. This includes a breathalyzer test (Dräger Alcotest) to measure current blood alcohol content. In 10% of the sample or anyone reporting past year drug use, an oral saliva sample (Dräger 5000 Drug Test Unit) is collected to test for recent (past 1–3 days) substance use [qualitative positive/negative results are obtained for amphetamine, benzodiazepines, cannabis (D9-tetrahydrocannabinol), methamphetamine, cocaine, methadone, and 3,4-methylenedioxymethamphetamine (MDMA) are obtained]. If a youth demonstrates a positive breathalyzer or oral saliva drug toxicology result, then the test is repeated; both test results are recorded. Hair is being collected from all participants to provide quantitative information about recent (past 1–3 months) substance exposure. Samples for at-risk youth (defined at baseline and year-1 follow-up as youth reporting intent to use cannabis or youth who have past year use of cannabis, alcohol or nicotine) are sent to Psychemedics for quantitative measurement of alcohol ethyl glucurolide, cannabis (11-Nor-9-carboxy-tetrahydrocannabinol and cannabidiol), methamphetamine, MDMA, amphetamine, opiates (codeine, morphone, hyrdomorph, oxycodone, hydrocodone), and cocaine/benzoylecgonine utilizing gas chromatography-mass spectrometry (GC/MS/MS) and liquid chromatography-mass spectrometry (LC/MS/MS). Starting at the year 1 follow-up session, urine (NicAlert) will be collected from 10% of the sample and in all self-reported nicotine users for semi-quantitative cotinine (principal metabolite of nicotine) levels. All toxicology results are coded and maintained in the data repository. Positive results for the alcohol breath test are exclusionary. If a participant is showing signs of intoxication, whether or not the saliva toxicology test was positive, the participant is rescheduled for their research appointment and informed they cannot participate while under the influence of alcohol or drugs (excluding nicotine). (See the ABCD Biospecimens Workgroup publication in this special issue for further details.)
The parents and youth are contacted between the baseline and year 1 follow-up assessment for a 6-month follow-up phone interview to capture new onsets of substance use (based on youth report only). Once youth have access to private personal mobile devices (typically around age 12), youth will be contacted directly to complete the on-line 6-month TLFB. See Table 1 for all measures in the baseline and year 1 substance use module. Additional measures (e.g., motives for substance use, cannabis and nicotine acute withdrawal symptoms) will likely be added starting at year 2 follow-up sessions.
3.2. Substance use module introduction and “Heard of” questions (Youth-Administered)
The first portion of the ABCD substance use protocol includes a brief introduction that operationalizes the term “drug use,” repeats that all answers are confidential, and then proceeds to ask the youth if they have heard of specific substances. The description of drug use, confidentiality, and substance use category wording was adapted for 9 and 10 year olds from existing national studies on substance use, including the Collaborative Studies on Genetics of Alcoholism (COGA; https://www.niaaa.nih.gov/research/major-initiatives/collaborative-studies-genetics-alcoholism-coga-study) (Bierut et al., 2002), National Survey on Drug Use and Health (NSDUH; https://nsduhweb.rti.org/) (Quality C.f.B.H.S.a., 2014), the PhenX Patterns of Substance Use module (module #510301) for adolescents, and the MTF Study (Institute for Social Research and U.o.M. Monitoring the Future, 2010).
The intention of the “heard of” substance use section is to ensure the study does not introduce the youth to new substances and to only assess influences on use, substance use patterns, or consequences in youth who are already familiar with each drug category. We ask whether youth have “heard of” alcohol (“alcohol, such as beer, wine or liquor”), cannabis and cannabinoids (“marijuana, weed, pot, blunts, dabs, marijuana drinks or food with marijuana; fake or synthetic marijuana such as K2 or spice”), nicotine (“tobacco products, such as cigarettes, smokeless tobacco, cigars, hookah, pipes, electronic or e-cigarettes), caffeine (‘caffeine, which is found in coffee, tea, energy drinks, and some soda’), inhalants (“sniffing liquids, sprays or other products to get high”), and prescription medications (“taking pills, liquids, or medications to get high in a way that your doctor or parents did not direct you to use them” to assess stimulant, sedative/anxiolytic, and opiate prescription medications and over-the-counter cough or cold medicines). This section ends with a broad substance use question to capture any other non-queried drug categories (“Have you heard of people using anything else to make them feel high, dizzy or different?”). A “yes” response to any of these opens the gating to the next level of query. For the “other” category, research assistants check off any drugs the youth mentions, although they do not read the options to the youth to prevent the mentioning of novel substances [menu of options covers cocaine; cathinones, methamphetamine; MDMA/ecstasy; ketamine; gamma hydroxybutyrate; opiates (heroin, opium); hallucinogens (lysergic acid diethylamide, phencyclidine, peyote, mescaline, N,N-dimethyltryptamine, alpha-methyltryptamine, 5-methoxy-N,N-diisopropyltryptamine in one grouping, psilocybin and salvia separately); and anabolic steroids]. In order to query for over-reporting, we also ask if the youth has heard of drugs in a “bogus” drug category. If the youth says “yes”, then the research assistant will wait a few moments and then remind the youth about confidentiality and the need for accurate responding. If the youth has not heard of a substance, then no questions are asked about that substance moving forward.
3.3. Substance use patterns interview: baseline
3.3.1. Lifetime substance use patterns (Youth-Administered)
At baseline, lifetime patterns of substance use are assessed in detail. This interview was developed in order to comprehensively measure substances that may lead to substance use disorders, or neurocognitive, psychiatric or health consequences in youth. The lifetime interview (created by first author based on a previously published lifetime use interview (Lisdahl and Price, 2012)) assesses whether they ever used each drug. If they used a substance, then follow-up questions are given to assess 1) age of first use, 2) age of first regular use (defined at baseline as at least weekly use for 6 months), 3) lifetime total quantity in standard units, 4) lifetime maximum (max) dose in standard units, and 5) last date of use (to measure length of abstinence). For any drug used in the past 6 months, a detailed Timeline Followback interview is also administered (see below for details). Questions are only administered if the youth has heard of each substance.
3.3.2. Recent (Past 6 months) substance use patterns (Youth-Administered)
At baseline, a 6-months web-based modified Timeline Followback (TLFB) is administered (developed by the first author and Dr. Bartsch). Consistent with the original TLFB (Sobell and Sobell, 1996; Robinson et al., 2014), the ABCD TLFB uses a calendar-based interviewer-administered retrospective report of detailed quantity/frequency substance use patterns during the past 6 months. The TLFB is a psychometrically sound instrument used to measure both alcohol and other drugs (Sobell and Sobell, 1996) (including cannabis, nicotine, cocaine), demonstrating reliability and validity for intervals up to 1 year (360 days) in adolescents and adults (Robinson et al., 2014; Fals-Stewart et al., 2000). For example, reliability indices are high for measuring cannabis use in the past year, including total joints used (0.95), greatest number of joints on any day (.93), and number of joints used per using day (0.94) (Robinson et al., 2014). As with the original TLFB, the ABCD TLFB utilizes memory cues, such as holidays and personal events elicited from the youth that may improve substance use recall (e.g., sleepovers, birthdays, parties, holidays) and these are populated onto the web-based calendar. The site-based research assistant collaborates with the youth to review each week of potential use, and all substance use within that week. (See Fig. 1a–c for pictures of on-line calendar and substance use interface.) To facilitate accurate labeling and dose quantification, if a youth endorses using a substance, the research staff presents visual pictures depicting standard units and modes of use (e.g., standard drink sizes for alcohol; for some substances, “times used” or number of occasions are used as the standard units; research assistants inform participants that this measures separate occasions used per day and that an occasion is defined as a period when you used a drug and then took a break and that the setting may change between occasions (See Supplemental Material for TLFB Standard Unit Visual Slides). As outlined in the next section, follow-up questions assess routes of administration (cannabis concentrates, e-cigarettes, cocaine, methamphetamine, heroin), flavoring (cigarettes), typical dosing (e-cigarettes), product content/potency (smoked cannabis, cannabis concentrate, cannabis-infused alcohol drinks, cannabis tinctures), subjective effects (cannabis), and where youth get their drug (cannabis) (see details below under each substance category).
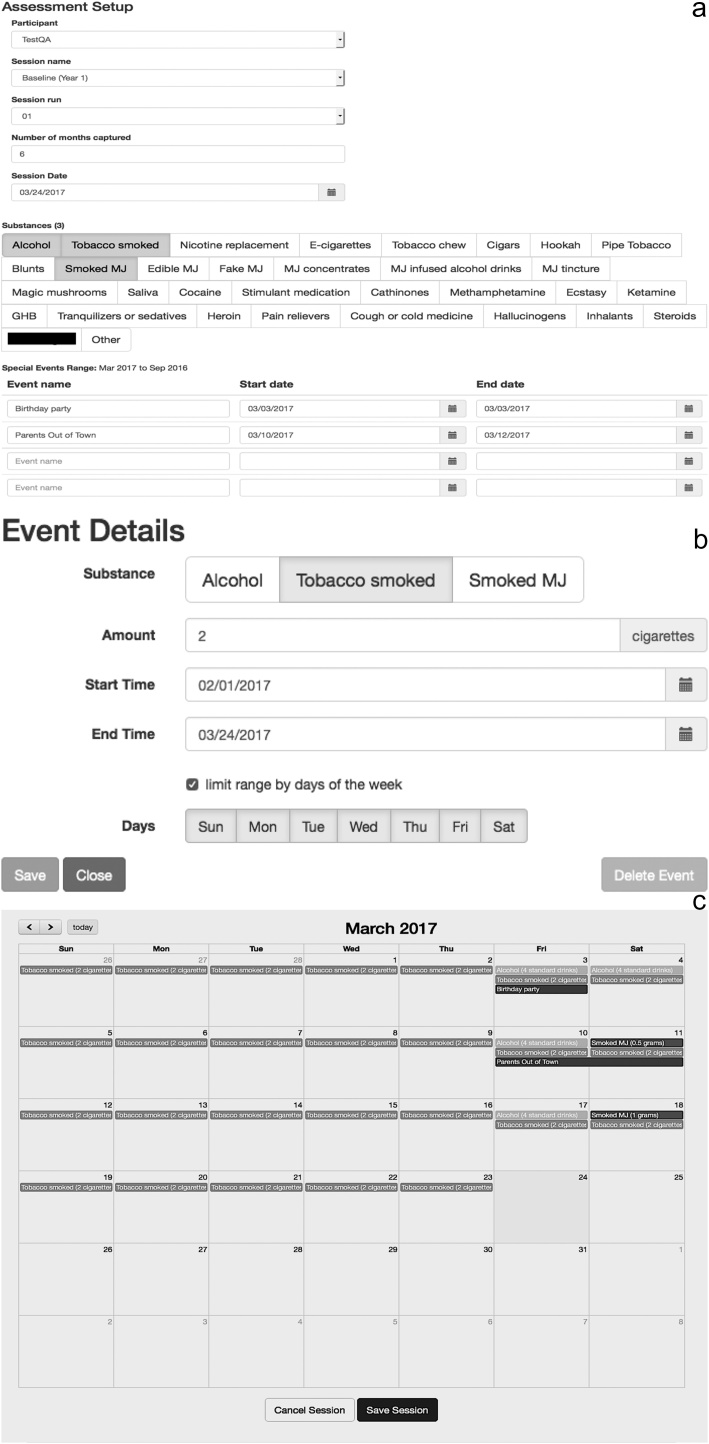
Fig. 1.
a) Picture of initial set-up for the ABCD on-line TLFB interview. Research assistants fill out the participant identification, session name, number of months measured, session run, and session date. Substances used by the youth (in this case, alcohol, tobacco cigarettes, and smoked cannabis “MJ”) are selected. Remembered events, such as “parents out of town” and “birthday party”, are populated onto calendar to aid recall (also see Fig. 1c). b) Individual Substance Use Events: After the TLFB is set up (Fig. 1a), the research assistant fills out each substance-use event into the on-line TLFB interview by noting the substance used, standard units, and dates of use (repeated dates are allowable). This example demonstrates a recurring event of daily tobacco cigarette use, in the standard unit of two cigarettes per day. c) This picture shows a completed month in the on-line ABCD TLFB interview. Example shows daily tobacco cigarette use (two cigarettes a day; see Fig. 1b), weekend alcohol use (reported in standard alcohol drinks), and intermittent smoked cannabis use (“smoked MJ”, reported in grams).
Note: the fake drug name is blacked-out to protect validity of the instrument.
The implementation of the calendar-driven ABCD TLFB instrument was done in JavaScript with the software packages bootstrap for design and the full calendar plugin. The server component of this tool was written in php and interfaces with our electronic data collection system (REDCap) for retrieval of enrolled participant information such as participant ID (pGUID), longitudinal event name and attempt number. The server component is also responsible for the storage of the collected information and the computation of derived scores. Source code of the application is available online in the public ABCD software repository (GitHub.com/ABCD-STUDY/timeline-followback).
For each annual follow-up, the TLFB will cover time since last assessment to ensure continuous coverage of substance use patterns over the longitudinal study. As noted above, once youth have access to mobile technology that they can use in a confidential environment (typically by age 12), a 6-month TLFB interview will be administered by research staff directly with the adolescent via web and/or phone. These data will facilitate generation of overall metrics of adolescent substance use for each endorsed category, total substance used (in standard units), number of binge drinking episodes, drinks per binge episode, age of first use, age of first regular use (how “regular” substance use is defined at each time point can be determined by each scientist/authors accessing the data by altering or creating their own TLFB scoring code, e.g., “regular use” can be defined as using every week, every day, etc. for various time periods such as the past 3, 6, 12 months), dose per occasion, and total number of co-use days (all substance combinations).
3.3.3. Lifetime & 6-month TLFB patterns of use: drug categories assessed (Youth Administered)
Below is a description of each drug use category that is measured during the lifetime patterns of use and past 6-month TLFB interviews. These same drug categories will be assessed each year utilizing the TLFB format. Visual aids are only provided if the youth reported using the drug recently or in their lifetime to assist with quantification and accurate follow-up question on type of product, typical dosing, and route of administration.
-
1.
Alcohol (described as “alcohol such as beer, wine, or liquor – such as rum, vodka, gin, whiskey)”. The unit of measurement for lifetime quantity, max drinks, and 6-month TLFB is in standard drinks (http://rethinkingdrinking.niaaa.nih.gov), defined as 1 12-ounce bottle of beer or wine cooler, 1 5-ounce glass of wine, or 1 shot (1.25 ounces) of 80-proof alcohol. Visual aids with conversations of standard drinks are provided. On the TLFB, by measuring standard drinks, we will also be able to measure binge-drinking episodes (can be defined in several ways based on children’s and adolescents body sizes (Donovan, 2009)), drinks per drinking episode, and days of co-occurring use of other substance categories (e.g., cigarettes, cannabis).
-
2.
Cannabis and cannabinoids. Cannabis is a complex substance to measure, as there are differing potency, product content (e.g., THC and CBD), and routes of administration, which have historically not been well addressed in cannabis research (Feldstein Ewing et al., 2017; Lisdahl et al., 2014). Thus, the ABCD Study will track smoked cannabis (“smoked marijuana, also called pot, grass, weed, ganja”) assessed in grams; blunts (combined nicotine and cannabis) measured in grams; edible cannabis (“marijuana that you eat, such as pot cookies, gummy bears, brownies”) measured in occasions; cannabis concentrates (”marijuana oils or concentrates such as 710, hash oil, BHO/butane hash oil, dabs, shatter, budder, honey oil; CO2 oil, vape pen, Rick Simpson Oil/RSO, phoenix tears”) measured in occasions; cannabis –infused alcohol drinks (e.g., THC-infused wine, beer or liquor) measured in standard drinks; cannabis tinctures measured in ml; and synthetic cannabinoids (“fake marijuana or synthetics such as K2 and spice”) measured in occasions. Visual aids include pictures and descriptions of different types of cannabis, concentrates, edibles, drinks, tinctures, and synthetic cannabinoids, including routes of administration, and typical doses. Follow-up questions on the lifetime and 6-month TLFB interview assess the typical strain of smoked cannabis the youth uses, typical dose of edible cannabis (if known), type of cannabis concentrate (if known) and typical route of administration, potency of smoked and cannabis concentrate (if known), subjective experience of cannabis smoking (extent of feeling “high”), cannabinoid content of cannabis –infused alcohol and tinctures (THC, CBD or both), and source of cannabis to measure possible diversion (Di Forti et al., 2014, Di Forti et al., 2015; Morgan et al., 2012a, Morgan et al., 2012b; Thurstone et al., 2011, Thurstone et al., 2013; Salomonsen-Sautel et al., 2012; Raber et al., 2015; Loflin and Earleywine, 2014; Michaels and Christiansen, 2012; Pierre et al., 2016; Miller et al., 2016; Stogner and Miller, 2015; Politi et al., 2008; Peschel, 2016; Cao et al., 2016; Barrus et al., 2016; Hopfer, 2014). As new cannabis products are likely to emerge over time, the ABCD Substance Use Workgroup will closely attend to and monitor trends in cannabis use, new products, and new routes of administration throughout the longitudinal study (Borodovsky et al., 2016).
-
3.
Nicotine. Nicotine can be used via myriad routes of administration. We will assess the following: tobacco cigarettes, electronic cigarettes (e-cigarettes, vape pens, or e-hookah), smokeless tobacco (chew or snus), cigars (including traditional cigars, little cigars, or cigarillos), hookah, tobacco pipe use, and nicotine replacement (patches, gums, nasal sprays, inhalers and lozenges). Standard of units for the lifetime quantity, max use, and 6-month TLFB include: cigarettes (# of cigarettes), e-cigarettes (# of occasions), smokeless tobacco (# pinches), cigars (# of cigars or cigarellos (Sterling et al., 2016)), hookah (# of hits), pipes (# of hits), nicotine replacement (# of doses); visual aids will be provided for all nicotine categories. Follow-up questions on the lifetime and 6-month TLFB will assess whether youth typically use cigarettes with flavoring (Biswas et al., 2016; Alsharari et al., 2015; Nonnemaker et al., 2013). Additional follow-up questions will determine typical amount of liquid used in e-cigarette, typical dose of nicotine, how often their e-cigarettes contain nicotine, and type of cartridge (disposable versus re-chargeable), which may impact total nicotine exposure (Breland et al., 2016, Breland et al., 2014; Lopez et al., 2016).
-
4.
Cocaine or crack cocaine. Cocaine and crack cocaine will be measured in total occasions (max use in mg). Follow-up questions on the TLFB ask about typical route of administration (smoking, oral ingestion, intranasal/snorting, injecting subcutaneous, injecting intramuscular, injecting intravenous), how often they inject cocaine, how often they use clean needles, and how often they smoke it (Nuijten et al., 2016; Conti et al., 2014; Novak and Kral, 2011). Pictures of various types of cocaine products and typical dosing are provided.
-
5.
Cathinones (“cathinones such as bath salts, drone, M-cat, MDVP or meph”). The unit of measurement is in occasions (max use measured in mg). Pictures of various types of cathinones and information about mgs per package are provided.
-
6.
Methamphetamine (“meth or crystal meth”). Methamphetamine is measured in total occasions (max use in mg). Pictures of various types of methamphetamine products and information about typical dosing are provided. Follow-up questions on the TLFB ask about typical route of administration (smoking, oral ingestion, intranasal/snorting, injecting subcutaneous, injecting intramuscular, injecting intravenous), how often they inject methamphetamine, how often they use clean needles, and how often they smoke it (Novak and Kral, 2011; Cunningham et al., 2015; Hadland et al., 2010; Marshall et al., 2012; Al-Tayyib et al., 2014).
-
7.
3,4-methylenedioxymethamphetamine (“ecstasy, molly or MDMA”). MDMA is measured in number of tablets (max use, TLFB). Pictures of forms of MDMA/ecstasy and information about typical dose per pill or powder are provided.
-
8.
Ketamine (“ketamine or special K”) is measured in occasions (max use in mg). Pictures of forms of ketamine and information about dosing are provided.
-
9.
Gamma hydroxybutyrate (GHB, “liquid G, or Georgia home boy”) is measured in occasions (max use in grams) and pictures about forms of GHB and information about dosing are provided.
-
10.
Heroin (“heroin, opium, or junk, smack or dope”) is measured in occasions (max use in mg). Pictures of forms of heroin and information about dosing are provided. Follow-up questions on the TLFB ask about typical route of administration (smoking, oral ingestion, intranasal/snorting, injecting subcutaneous, injecting intramuscular, injecting intravenous), how often they inject heroin, and how often they use clean needles (Novak and Kral, 2011; Hadland et al., 2010; Al-Tayyib et al., 2014).
-
11.
Hallucinogens (described as “hallucinogen drugs that cause people to see or experience things that are not real, such as LSD or acid, PCP or angel dust, peyote, mescaline, DMT, AMT, or Foxy”). The ABCD study assesses tryptamine hallucinogens, including lysergic acid diethylamide (LSD), N,N-dimethyltryptamine (DMT), and ayahuasca (Callaway et al., 1999; McKenna et al., 1984; McKenna and Riba, 2015), synthetically produced tryptamine hallucinogens, such as alpha-methyltryptamine (AMT), or 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DIPT), which have similar effects (Araújo et al., 2015; Meatherall and Sharma, 2003), phencyclidine (PCP), mescaline, and peyote (Halpern et al., 2005; Carstairs and Cantrell, 2010). Unit of measurement is in occasions; visual aids are provided to note what types of hallucinogens they have used.
-
12.
Hallucinogen psilocybin (“described as magic mushrooms or shrooms”). Use of psilocybin appears more common than other hallucinogens (Hardaway et al., 2016; Hendricks et al., 2014; Hallock et al., 2013); psilocybin is also important to track because of the increased attention to use of psilocybin as a form of addiction treatment (Bogenschutz, 2017). The unit of measurement is occasions (max in grams); visual aids are provided to assist with dosing and identification of the drug.
-
13.
Hallucinogen salvia divinorum (“salvia”) is measured in occasions; visual aids are provided to assist in identifying salvia and measuring max use (in mg).
-
14.
Anabolic steroids (“arnolds, pumpers or roids”) is measured in occasions; visual aids assist in identifying anabolic steroids and measuring max use (in mg).
-
15.
Inhalants (“liquids, sprays and gases that people sniff or inhale to get high, this includes substances like poppers, correction fluid, gasoline, glue, shoe polish, spray paints, or nitrious oxide or whippits””). Inhalants are measured in occasions; visual aids assist in identifying which type of inhalant used (noted in a follow-up question, options include: poppers, correction fluid, gasoline, glue, shoe polish, spray paint, nitrious oxide or whippits, other.)
-
16.
Prescription stimulants (described as “stimulant drugs such as amphetamine, Ritalin, Adderall, ephedrine in a way a doctor did not direct you to use them”). The unit of measurement is in number of pills (mg for max use); visual aids assist in identifying types of prescription stimulants and typical dosing.
-
17.
Prescription sedative drugs (“prescription anxiolytics, tranquilizers or sedatives in a way your doctor did not direct you to use them, such as Xanax, Ativan, Valium, Rohypol, or sleeping pills”). Unit if measurement is in number of pills (mg for max use); visual aids assist in identifying types of prescription sedatives and typical dosing.
-
18.
Prescription opioid pain relievers. (“prescription pain relievers such as Vicodin, Lortab, Norco, Hydrocodone, OxyContin or Percocet that you used in a way your doctor did not direct you to use them (this does not include OTC pain relievers such as aspirin, Tylenol or Advil).” Unit if measurement is in number of pills (mg for max use); visual aids assist in identifying types of prescription opiates and typical dosing.
-
19.
Cough or cold medicine containing dextromethorphan (DXM) (“Over the counter cough or cold medicine or DXM used to get high”). Hundreds of OTC cough and cold remedies contain DXM (Schwartz, 2005), and in 2016 4% of 12th graders reported purposefully abusing OTC DXM products (Johnston et al., 2017). Cough medicine will be measured in occasions (max use in ml) and visual aids with various types of OTC cough or cold medicine will be provided to assist with max dose information.
-
20.
Over-reporting Validity Check. During the “ever used” and 6-month TLFB questions, we added a bogus drug category with two made-up substances to assess potential over-responding. If a youth answers “yes” to the bogus category, the research assistant will give them a few moments, and then remind the youth about confidentiality and the need for accurate responding. Youth are allowed to alter any information after this prompt.
3.4. Assessment of low level use and number of sips or puffs of cannabis and nicotine (Youth-Administered)
3.4.1. Alcohol low level use (iSay Sip Inventory)
In order to characterize participants’ earliest sipping experience, we adapted items used by Jackson (Jackson et al., 2015) to determine the extent and context of sipping/tasting, which has been linked with heavy drinking in later adolescence (Jackson et al., 2015). Specifically, at baseline we assessed the number of times the child has sipped alcohol, whether or not such sipping was part of a religious ceremony, the age of onset of sipping, the context in which this occurred, the type of alcohol it was, and whether or not the alcohol was offered to the child or whether it was taken without permission. We also query whether or not a full drink was consumed. The first sip qualitative information will only be collected once per youth, although total number of past year sips (religious and non-religious) will be collected for each youth who has not consumed a full drink for each assessment period.
3.4.2. Cannabis and nicotine low level use
In a similar way, we query initial experiences with cannabis (first puff or taste of marijuana) and nicotine products (e.g., first puff of a combustible cigarette or e-cigarette, first dip of smokeless tobacco), where they obtained the substance, when these experiences occurred, and whether it led to further use. For cannabis, subjective experience of feeling “high” and estimated potency (THC) is also assessed. These measures are given if the youth heard of each substance, and reported sipping alcohol, puffing or tasting cannabis, or puffing or chewing nicotine products.
3.5. Very recent (24 h) use of caffeine, nicotine, and prescription medications (youth- and parent- administered)
The PLUS Form will be administered to parents and youth on each day that there is a cognitive or MRI assessment to collect detailed information on length of abstinence from nicotine, caffeine, OTC and prescription medications (modified from NCANDA study (Brown et al., 2015)), which may acutely impact neurocognitive performance. Parents and youth are asked if the youth had taken any caffeine, OTC or prescription medications within the last 24 h, and if so, hours since last use. Youth are also asked time since last nicotine use within the past 24 h. OTC and prescription medication names and dosing are also collected.
3.6. Assessment of caffeine use (Youth-Administered)
Given the availability of caffeinated beverages that are available and consumed by youth (e.g., caffeinated sodas; caffeinated coffee drinks; energy drinks), there is growing concern over excessive caffeine use and development of caffeine use disorders in youth (Budney and Emond, 2014; Cotter et al., 2013; Temple, 2009); thus, patterns of recent caffeine consumption will be assessed in the ABCD Study via modified Supplemental Beverage Questions (2004, Fred Hutchinson Cancer Research Center shared documents). If a youth endorses hearing of any caffeinated beverages, they are then asked the typical number of caffeinated drinks they have per week in the past 6-months (covering the categories of coffee, espresso, tea with caffeine, soda with caffeine, and energy drinks). Typical serving sizes are provided (coffee = 8 oz; espresso = 1 shot; tea = 8 oz, soda = 12 oz; energy drink = 5 oz or 2 oz for 5-h energy drink). Maximum dose (largest amount of a caffeinated beverage consumed in one day in the past 6 months) is also obtained in ounces.
3.7. Factors impacting substance use risk: baseline
3.7.1. Intention to use (Youth-Administered)
As sated above, youth who demonstrate curiousity about trying substances soon are more likely to start using substances during adolescence (Choi et al., 2001; Nodora et al., 2014; Strong et al., 2015). Thus, the ABCD substance use module includes a 9-item instrument to measure the extent to which substance-naïve youth are likely to start using alcohol, cannabis, or nicotine. The three nicotine items were used from the Population Assessment of Tobacco and Health (PATH) Study (Hyland et al., 2016), which expanded Pierce’s (Pierce et al., 1996) well-established measure of cigarette susceptibility to other tobacco products in youth and adults (ages 12 and older). The ABCD Study Substance Use workgroup also added questions of the same format for alcohol and cannabis to assess potential risk for initiating use across each of these primary categories of substance exploration in this age group. For each substance, respondents answer three questions to indicate the extent to which they are curious about the substance, will try the substance soon, and would use the substance if it were offered by a friend. The item responses appear on a 4-point Likert scale from “very curious,” “somewhat curious,” “a little curious,” to “not at all curious.” The remaining scales include “definitely yes,” “probably yes,” “probably not,” and “definitely not.” Alcohol was defined as “alcohol”; cannabis was defined as “marijuana”; tobacco was defined as “a tobacco product such as cigarettes, e-cigarettes, hookah, or cigars”. Youth complete this form if they have heard of the individual substance, but have not yet tried the substance.
3.7.2. Peer substance use (Youth-Administered)
Peer substance use and deviance is linked with risk for adolescent substance use (Trinidad et al., 2018; Buu et al., 2009). The ABCD protocol includes a 9-item Peer Group Deviance instrument that was modified from the PhenX (https://www.phenxtoolkit.org/) peer substance use questionnaire, which include items from the MTF study (Johnston et al., 2015, Johnston et al., 1988). For each item, participants are asked the initial stem: “how many of your friends…?” with queries for drink alcohol (full beer, wine or liquor), get drunk, have problems with alcohol or other drugs, use cannabis, smoke cigarettes, use inhalants (gas, glue, nitrous oxide), use other drugs like cocaine, downers or LSD, and sell or give drugs to others. The item responses are on a 5-point scale: “none,” “a few,” “some,” “most” or “all.” One additional question was added to address the rising use of alternative nicotine delivery devices: use other tobacco products like e-cigarettes, pipes or hookah (Breland et al., 2016, Breland et al., 2014; Lopez et al., 2016). Youth are only asked questions about peer substance use for those substances they endorsed having heard of at the beginning of the substance use interview.
3.7.3. Acute subjective effects (Youth-Administered)
As outlined above, acute subject effects experienced following initial substance exposure predicts risk for developing SUD (Trim et al., 2009; Haberstick et al., 2011; Perkins et al., 2009; Pomerleau et al., 1998).
3.7.3.1. Alcohol
The PhenX (https://www.phenxtoolkit.org/) Acute Subjective Responses to Alcohol, based on the Self-Rating of the Effects of Alcohol (SRE) for (Schuckit et al., 2008), measures youths’ subjective effects to alcohol use following the participant’s first 5 times of drinking, recent 3 months, and over their heaviest period of use. The SRE has been shown to be a robust and reliable measure with regards to the development of alcohol problems (Ray et al., 2011; Schuckit et al., 2008). In Ray et al.’s 2011 paper, SRE scores account for as much as 25% of the variance in Alcohol Use Disorders Identification Test scores and highly correlate (r = 0.70–0.80) with interview format scores. The self-administered questionnaire asks participants to report about their drinking behaviors during these time periods (first 5 times drinking, recent 3 months, and period of heaviest use) and to list the number of standard drinks (10–12 g of ethanol) required for the experience of each potential effect of alcohol, including feelings of intoxication, slurred speech, feeling unsteady or developing a stumbling gait, and unwanted falling asleep (5).
3.7.3.2. Cannabis
The Acute Subjective Response to Marijuana scale, developed for adolescents in the Cannabis Twin Study (Agrawal et al., 2014, Agrawal et al., 2013), was selected to measure the participant’s subjective response to cannabis following the youth’s first or second exposure. Early subjective response has been linked with onset of cannabis use disorder and cannabinoid genetics in adolescents and young adults (Agrawal et al., 2014). The assessment asks the participant to report his/her subjective responses on a scale from 1 “not at all,” 2 “somewhat,” 3 “a little,” to 4 “a lot,” including 11 questions about taste, coughing, dizziness, feeling relaxed, headache, heart racing, muscle tremble or feeling jittery, burning in throat, feeling confused, nausea, and sensations of pleasure (i.e. rush or buzz). Youth are only queried about each of these categories if they endorsed hearing of the substance and using the substance at least once in their lifetime.
3.7.3.3. Nicotine
The Acute Subjective Responses to Tobacco (PhenX (https://www.phenxtoolkit.org/) instrument has been modified from the adult version for purposes of this ABCD Study. The self-administered instrument assesses the participant’s subjective response to tobacco cigarette following first exposure. The assessment has been shown to be efficacious in capturing early smoking experiences (Trinidad et al., 2018), subjective dizziness and related genetic influences (Haberstick et al., 2011) and nicotinic sensitivity (Perkins et al., 2009) in adolescents and young adults. The ABCD-adapted version additionally captures respondents’ subjective experiences surrounding first use of e-cigarettes or e-hookahs, smokeless tobacco (i.e. snus or chew). The questionnaire assesses pleasant (pleasurable buzz or rush; relaxed, dizzy) and unpleasant (e.g., did you feel nausea, did you cough) experiences surrounding first-time tobacco use, rating sensations on a scale from 1 “none” to 4 “intense”.
3.7.4. Availability of substances (Parent-Administered)
Increased availability of substances in the environment has previously been linked with risk for substance use (Strong et al., 2015). This 9-item parent-administered instrument was modified from the PhenX (https://www.phenxtoolkit.org/) community risk and protective factors questionnaire, based on the MTF study (Arthur et al., 2007). Only questions regarding how easily youth may access substances in the environment are given; these include questions regarding alcohol, cannabis, cigarettes and other drugs. Due to the rise of electronic cigarette, vape pen and hookah use among adolescents (Singh et al., 2016), a question was added regarding availability of these devices. Because the original instrument was not validated on children as young as 9–10 years, it was modified for administration to parents. The final instrument asks, “if your child wanted to get <substance>, how easy would it be for her/him to get some?” Five questions cover the following substances: alcohol (“beer, wine, or hard liquor”); marijuana; cigarettes, e-cigarettes, vape pens or e-hookah; and other drugs (“a drug like cocaine, LSD, or amphetamines”). Responses are on a 4-point Likert scale from “very hard,” “sort of hard,” “sort of easy” to “very easy”. In order to determine the impact of legalized medical cannabis on cannabis availability, an additional four questions are asked: “Is medical marijuana (marijuana prescribed by a doctor) legal in your state?” If yes, follow up questions are asked regarding how many friends or family have a prescription; how easy it would be for the child to get a prescription for medical marijuana; or how easy it would be to get medical marijuana from someone with a prescription. All parents fill out this instrument.
3.7.5. Parent rules regarding use (Parent-Administered)
Parental monitoring and enforcing rules against substance use serve as a protective factor for adolescent substance use (Dishion et al., 2003; Dishion and Kavanagh, 2003); therefore, the ABCD Study uses a 9-item parent-administered instrument covers parent rules about the use of substances in the home and enforcement of family rules. The instrument was modified from the Rules on Drinking and Smoking Questionnaire (Dishion et al., 2003; Dishion and Kavanagh, 2003) used in the Internet Surveys about you (iSay) study – a prospective study on alcohol initiation and progression in adolescents (Jackson et al., 2015, Jackson et al., 2014); this was expanded to include rules about cannabis for the ABCD Study. Three questions are asked of the parent for the three primary substances of experimentation for this age group (alcohol, cannabis, cigarettes), beginning with a question about the rules for their child’s use. Five response options range from “my child is not allowed to drink/use marijuana/smoke cigarettes under any circumstances” to “I do not set rules about my child’s drinking/marijuana use/smoking cigarettes” with an additional response option of “I have not made rules yet about my child drinking/marijuana use/smoking cigarettes.” If rules have been set, two follow-up questions are designed to determine whether penalties are enforced for violating the rules (“Are these the same rules for all family members; Do you enforce penalties for violating family rules about using marijuana/smoking cigarettes”). All parents fill out this instrument.
3.8. Consequences of substance use: baseline
3.8.1. Alcohol hangover symptoms (Youth-Administered)
Hangover symptoms are linked with excessive alcohol consumption and uniquely predict neurocognitive outcomes (McKinney, 2010; McQueeny et al., 2009; Squeglia et al., 2009). For the ABCD Study, we selected the Hangover Symptom Scale (HSS) (Slutske et al., 2003); this contains 13 items surveying typical symptoms of alcohol hangover including physical (e.g., tiredness, headache, sleep disturbance) and cognitive/psychological symptoms (e.g., difficulty concentrating, anxiety, depressed mood). Factor analysis indicates that covariation among these symptoms are well represented by a single factor with good internal consistency (α = 0.84). As reported by 248, total scores was associated with both alcohol-related consequences and parental alcohol problems even after controlling for multiple consumption measures, indicating HSS scores were indexing more than simply “amount consumed.” Additional evidence of the validity of this measure comes from an electronic diary study where alcohol hangover scores were found to predict the occurrence of hangover the morning after drinking in a sample of 404 recent drinkers, even after controlling for amount consumed on the prior day (Robertson et al., 2012). Youth who report using alcohol twice over the past 6 months will fill out this measure.
3.8.2. Symptoms of substance use disorders (Youth-Administered)
The ABCD Study Substance Use module includes symptom measures of alcohol, nicotine, cannabis, and other drug use disorder.
3.8.2.1. Alcohol
The Rutgers Alcohol Problem Index (RAPI) is a 23-item, self-report measure designed to assess adverse consequences of alcohol consumption in adolescents (White and Labouvie, 1989). Prior to the development of the RAPI, existing measures included consequences that may only manifest after lengthy and chronic history of alcohol consumption among adults (e.g., medical complications, loss of employment); such problems would be less likely among adolescent alcohol users. In addition, the RAPI offers well-established and desirable psychometrics, relatively brief administration time, developmental appropriateness, and the availability of analogue measures assessing other substances (see MPI and DPI below), which will facilitate comparisons of problems across substances. Principal component analyses resulted in a unidimensional scale consisting of 23 items that were found to be as informative as the full set of 52 items and that showed high internal consistency upon initial (.92) and follow-up visits (0.93). Three-year stability coefficients for the total sample was adequate (0.40), particularly when considering that use trajectories may not yet have stabilized during adolescence. Test-retest reliability among a college-aged sample is reported to be much higher (e.g., 0.88 at 1 year) in other studies (Miller et al., 2002). The ABCD Study employs a shorter, 18-item version of the RAPI, which is reported to correlate very highly (0.99) with the 23-item version (White and Labouvie, 2000, White and Labouvie, 2018). Participants are instructed to indicate how often they experienced specific problems “within the past 6 months” because of their alcohol drinking (e.g., “Neglected your responsibilities”) using a 4-point Likert scale: 0 times; 1–2 times; 3–5 times; or 5+ times.
3.8.2.2. Marijuana and Other Drugs.
The Marijuana Problem Index (MPI) (Johnson and White, 1989; Simons et al., 1998) and the Drug Problem Index (DPI) (Johnson and White, 1989; Caldwell, 2002; Kingston et al., 2011) are parallel versions of the RAPI designed with the same goals in mind, but focus on problems from cannabis use and other drug use, respectively. The MPI has high internal consistency (>0.85) based on several studies (Simons et al., 1998; Simons and Carey, 2002, Simons and Carey, 2006). Detailed psychometric data for the DPI have not been reported. The MPI and DPI used in ABCD consist of the same instructions and 18-items as the RAPI, but any references to “alcohol” or “drinking” are replaced with “smoking or marijuana use” or “use” for the MPI and DPI, respectively. Both rely on a 5-point Likert scale to indicate the number of times that a participant has experienced a given problem: 0 times; 1–2 times; 3–5 times; 6–10 times, 10+ times. The DPI is filled out considering all illicit drug use (aside from cannabis).
3.8.2.3. Nicotine
The ABCD Study substance use module includes 10 items from the nicotine-dependence section of the Population Assessment of Tobacco and Health (PATH) Study (Hyland et al., 2016), which were adopted from existing well-validated and reliable surveys designed to assess different domains of nicotine dependence (including the Fagerström Test for Nicotine Dependence (Pomerleau et al., 1994; Heatherton et al., 1991), PhenX (https://www.phenxtoolkit.org/), National Youth Tobacco Survey or NYTS (Margolis et al., 2016; Bunnell et al., 2015), Brief Wisconsin Inventory of Smoking Motives or B-WISDM (Adkison et al., 2016; Smith et al., 2010). These included “How soon after you awake do you want to use tobacco?” (within 5 min, from 6 to 30 min, from more than 30 min to 1 h, after more than 1 h but less than 24 h, and I rarely want to use tobacco; (Fagerström/PhenX/NYTS), “Do you ever have strong cravings to use tobacco?” (yes/no), “Have you ever felt like you really needed to use tobacco?” (yes/no), and seven items adapted from the B-WISDM. For these items, respondents indicated on a 5-point Likert scale (from “not true of me at all” to “extremely true of me”) their level of agreement for each of the following statements: “I frequently crave tobacco,” “My tobacco use is out of control,” “Using tobacco really helps me feel better if I’ve been feeling down,” “Using tobacco helps me think better,” “I would feel alone without my tobacco,” and “I usually want to use tobacco right after I wake up.” Recent analyses reported by Strong et al. Strong (Strong et al., 2018) supports the psychometric validity of the nicotine dependence measure among adolescent and adult users in the PATH Study. Youth fill out the RAPI, MPI, DPI and Nicotine Dependence Severity questionnaires if they heard of each substance and have used each substance two or more occasions in the past 6 months. This threshold increases to three or more occasions if a participant is 12 years of age or older. It is also important to note that the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADs) assesses several major past and present substance use disorder diagnostic criteria [including alcohol, cannabis, stimulant (amphetamine-type), cocaine sedative, opioid, hallucinogen, phencyclidine (PCP), and inhalant use disorders] (see the ABCD Mental Health Workgroup article in this special issue).
3.9. 6- And 18- month follow-up to catch new onset of use
As briefly described above, until youth have access to private personal mobile devices (typically around age 12), youth and their parents will be called every six months (so at 6 months, and 18 months). First the youth are reminded about confidentiality and asked if they are in a private, quiet place where no one else can hear the conversation. Next, “heard of” questions are repeated for alcohol, cannabis products, nicotine products, inhalants, prescription anxiolytics, prescription stimulants, prescription opiates, OTC cough medication, and “anything else to make you feel high”. If they have heard of a substance, they are asked if they have used that substance within the past 6-months, past month or past week to track recent onset of new substances between in-person assessments. Because youth are likely to be using parent phones, response options are limited to yes/no format to maximize privacy. Once youth have access to mobile technology that they can use privately (typically by age 12), the initial 6-month and 18-month phone interview will be replaced with a more detailed 6-month TLFB interview (covering all aforementioned drug categories in section C.3) will be administered by research staff directly with the adolescent via web and/or personal cell phone.
4. ABCD substance use battery: future follow-up assessments
Beginning at year one follow-up, additional questionnaires will be added to the ABCD Substance Use module to supplement the aforementioned baseline measures. These will include measures to assess additional constructs that may influence substance use initiation and onset of SUD in youth.
4.1. Factors impacting substance use risk: year 1 follow-up
Additional inventories will be added to the baseline interview at year 1 follow-up:
4.1.1. Peer tolerance of substance use (Youth-Administered)
This 16-item instrument is from the PhenX (https://www.phenxtoolkit.org/) peer substance tolerance of substance use, which is based on items from the MTF study (Johnston et al., 2015, Johnston et al., 1988) and modified for the ABCD Study to include additional low-level use items and additional items measuring cannabis, e-cigarettes, smokeless tobacco, inhalants, and prescription drugs (most commonly used substances in youth (Johnston et al., 2017) as well as an “other drug” category (cocaine, heroin or methamphetamine). For each item, participants are asked the initial stem question, “How do you think your CLOSE FRIENDS feel (or would feel) about YOU doing each of the following things”. The instrument covers trying alcohol (one or two drinks, taking one or two drinks nearly every day, having five or more drinks once or twice each weekend), cannabis (trying marijuana once or twice, smoking marijuana occasionally, smoking marijuana regularly), cigarettes (trying one or two, smoking occasionally, smoking one or more packs of cigarettes per day), e-cigarettes (using regularly), smokeless tobacco (using regularly), inhalants (trying once or twice), trying prescription pain, sedative, and stimulants in a way a doctor did not direct you (once or twice), and trying “other drugs like cocaine, heroin or methamphetamine”. Youth are administered individual items if they have heard of the substance.
4.1.2. Perceived harm of substance use (Youth-Administered)
This 16-item instrument was modified the Perceived Harm of Substance Use survey, which is based on items from the MTF study and correlated with adolescent substance use (Johnston et al., 2015; Johnston et al., 1988) and modified for the ABCD Study to include additional low-level use items and additional items measuring cannabis, e-cigarettes, smokeless tobacco, inhalants, and prescription drugs (most commonly used substances in youth (Johnston et al., 2017)). For each item, participants are asked the initial stem question, “The next questions ask for YOUR opinions on the effects of using certain drugs and other substances. How much do you think people risk harming themselves (physically or in other ways) if they…”. The instrument covers the same substances and dosing as outlined above (Peer Tolerance of Substance Use). Youth are administered individual items if they have heard of the substance.
4.1.3. Expectancies (Youth-Administered)
4.1.3.1. Alcohol
Alcohol expectancies are assessed with the Alcohol Expectancy Questionnaire- Adolescent, Brief (AEQ-AB (Brown et al., 1987; Stein et al., 2007)). This 7-item questionnaire was based on the AEQ-A, demonstrates two factors (general positive and general negative; accounting for 46.2% of the variance), has good internal consistency (0.49–0.51), and demonstrated validity in predicting adolescent drinks per week and average number of drinks per heavy drinking day (Stein et al., 2007).
4.1.3.2. Cannabis
The Marijuana Effect Expectancy Questionnaire- Brief (MEEQ-B (Torrealday et al., 2008)) is used to measure cannabis related expectancies in youth and is based on the original MEEQ (Schafer and Brown, 1991). Some wording modification was conducted (removing the sexual item) due to the young age of the ABCD cohort. This 6-item measure has demonstrated two overall factors (positive effects and negative effects) accounting for 52% of the variance, good internal consistency (0.42.60), and predictive validity for cannabis use in youth (Torrealday et al., 2008).
4.1.3.3. Nicotine
The ABCD Study uses the Adolescent Smoking Consequences Questionnaire (ASCQ) to assess nicotine expectancies, which was developed on youth aged 11–19 years old, demonstrating excellent reliability, a latent factor structure of seven factors, and predictive validity for intention to smoke and smoking behavior (Lewis-Esquerre et al., 2005). The ASCQ was modified to create a short version of 7-items, choosing items that loaded most heavily (0.70–0.91) on six factors [boredom reduction factor (“during the day, smoking can help kill time if there is nothing to do”), negative affect reduction factor (“cigarettes help with concentration”, “when someone is sad, smoking helps him or her feel better”), negative physical feelings factor (“smoking will make a person cough”), negative social impression factor (“smoking makes people look ridiculous or silly”), social facilitation factor (“parties are more enjoyable when a person is smoking”), taste/sensorimotor manipulation factor (“the look and feel of a cigarette in the mouth is good”)] that significantly predicted adolescent smoking (Lewis-Esquerre et al., 2005).
5. Conclusions
The purpose of this article was to provide an overview and detailed account of the ABCD Study (https://abcdstudy.org/) Substance Use module. The Substance Use module is administered to youth and parents at the baseline research session, 6-month phone interviews, and in-person year 1 follow-up sessions. The module covers detailed substance use patterns, factors impacting substance use risk, and consequences of substance use in youth. In assembling the battery for ABCD, we attempted to balance a number of competing demands including subject burden, psychometric properties, comprehensiveness, developmental appropriateness, timing, nonreactivity (e.g., not have our assessment procedures affect future substance use behaviors), and use in other studies. The battery presented above reflects those considerations with the recognition that one of the ABCD Study’s focus is on the effects of substance use on brain development and neurocognition with the attendant need to assess drug exposures in as much detail as possible with respect to the specific substance, quantity/frequency, dosage, potency, and route of administration. Consequently, considerable time was spent developing a detailed lifetime use and TLFB interviews that took these parameters into consideration. However, we are mindful of the fact that at the initial stages of the ABCD Study, drug exposures are likely to be minimal in this young cohort; thus, we included measures designed to be sensitive to low-level exposures (e.g., sipping alcohol, first puffs of cannabis and nicotine) in order to be able to track the trajectories of substance involvement for all participants. Similarly, substance use does not arise in a vacuum so we are attentive to those social/interpersonal (drug availability, descriptive norms, family rules) and individual (e.g., outcome expectancies) that presage initial and later use. At the same time, for that small minority of participants who do engage in early substance use (i.e., assessed at baseline), we are poised to capture both acute drug effects (e.g., subjective effects), various harms (e.g., individual, neurocognitive, and social consequences) as well as SUD symptomatology and diagnosis (see ABCD Mental Health module). In this way, we hope to be able to portray substance use and its correlates as comprehensively as possible and track its course developmentally in combination with brain development and other important variables.
In summary, detailed, on-going prospective, longitudinal assessment of these domains over a period of ten years in a diverse, nationwide sample of youth in the United States presents an unprecedented opportunity to examine the risk and protective factors influencing the onset and sequela of substance use and its consequences, the impact of detailed patterns of substance use (including co-use and polydrug use) on neurocognitive development, health and psychosocial outcomes, and to further understand the timing and interactive relationship between substance use and psychopathology in youth that live in the United States.
Conflict of Interest
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.dcn.2018.02.007.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Adkison S.E., Rees V.W., Bansal-Travers M., Hatsukami D.K., O'Connor R.J. Psychometric characteristics of the brief wisconsin inventory of smoking dependence motives among a nonclinical sample of smokers. Nicotine Tob. Res. 2016;18:470–476. doi: 10.1093/ntr/ntv113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A., Neale M.C., Prescott C.A., Kendler K.S. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychol. Med. 2004;34:1227–1237. doi: 10.1017/s0033291704002545. [DOI] [PubMed] [Google Scholar]
- Agrawal A., Madden P.A., Martin N.G., Lynskey M.T. Do early experiences with cannabis vary in cigarette smokers? Drug Alcohol Depend. 2013;128:255–259. doi: 10.1016/j.drugalcdep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A., Madden P.A., Bucholz K.K., Heath A.C., Lynskey M.T. Initial reactions to tobacco and cannabis smoking: a twin study. Addiction. 2014;109:663–671. doi: 10.1111/add.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia N., Herrick K. Caffeine intake from food and beverage sources and trends among children and adolescents in the United States: review of national quantitative studies from 1999 to 2011. Adv. Nutr. 2015;6:102–111. doi: 10.3945/an.114.007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia N., Herrick K., Moshfegh A., Rybak M. Caffeine intake in children in the United States and 10-y trends: 2001–2010. Am. J. Clin. Nutr. 2014;100:1124–1132. doi: 10.3945/ajcn.113.082172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tayyib A.A., Rice E., Rhoades H., Riggs P. Association between prescription drug misuse and injection among runaway and homeless youth. Drug Alcohol Depend. 2014;134:406–409. doi: 10.1016/j.drugalcdep.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albein-Urios N. Re-appraisal of negative emotions in cocaine dependence: dysfunctional corticolimbic activation and connectivity. Addict. Biol. 2014;19:415–426. doi: 10.1111/j.1369-1600.2012.00497.x. [DOI] [PubMed] [Google Scholar]
- Albertson T.E., Chenoweth J.A., Colby D.K., Sutter M.E. The changing drug culture: use and misuse of cognition-enhancing drugs. FP Essent. 2016;441:25–29. [PubMed] [Google Scholar]
- Alexander C.S., Allen P., Crawford M.A., McCormick L.K. Taking a first puff: cigarette smoking experiences among ethnically diverse adolescents. Ethn. Health. 1999;4:245–257. doi: 10.1080/13557859998038. [DOI] [PubMed] [Google Scholar]
- Allen G.J. Cognitive and motor function after administration of hydrocodone bitartrate plus ibuprofen, ibuprofen alone, or placebo in healthy subjects with exercise-induced muscle damage: a randomized, repeated-dose, placebo-controlled study. Psychopharmacology (Berl.) 2003;166:228–233. doi: 10.1007/s00213-002-1358-x. [DOI] [PubMed] [Google Scholar]
- Alsharari S.D. Effects of menthol on nicotine pharmacokinetic, pharmacology and dependence in mice. PLoS One. 2015;10:e0137070. doi: 10.1371/journal.pone.0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo A.M., Carvalho F., Bastos M.e.L., Guedes de Pinho P., Carvalho M. The hallucinogenic world of tryptamines: an updated review. Arch. Toxicol. 2015;89:1151–1173. doi: 10.1007/s00204-015-1513-x. [DOI] [PubMed] [Google Scholar]
- Arthur M.W. Measuring risk and protection in communities using the communities that care youth survey. Eval. Program Plann. 2007;30:197–211. doi: 10.1016/j.evalprogplan.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Bagot K.S., Kaminer Y. Efficacy of stimulants for cognitive enhancement in non-attention deficit hyperactivity disorder youth: a systematic review. Addiction. 2014;109:547–557. doi: 10.1111/add.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrus D.G. Methods Rep RTI Press; 2016. Tasty THC: Promises and Challenges of Cannabis Edibles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalla A. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8:e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S. Altered white matter microstructure in adolescent substance users. Psychiatry Res. 2009;173:228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut L.J. Defining alcohol-related phenotypes in humans. The collaborative study on the genetics of alcoholism. Alcohol Res. Health. 2002;26:208–213. [PMC free article] [PubMed] [Google Scholar]
- Biswas L. Enhancing effect of menthol on nicotine self-administration in rats. Psychopharmacology (Berl.) 2016;233:3417–3427. doi: 10.1007/s00213-016-4391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz M.P. It's time to take psilocybin seriously as a possible treatment for substance use disorders. Am. J. Drug Alcohol Abuse. 2017;43:4–6. doi: 10.1080/00952990.2016.1200060. [DOI] [PubMed] [Google Scholar]
- Bolla K.I., Funderburk F.R., Cadet J.L. Differential effects of cocaine and cocaine alcohol on neurocognitive performance. Neurology. 2000;54:2285–2292. doi: 10.1212/wnl.54.12.2285. [DOI] [PubMed] [Google Scholar]
- Bondi M.W., Drake A.I., Grant I. Verbal learning and memory in alcohol abusers and polysubstance abusers with concurrent alcohol abuse. J. Int. Neuropsychol. Soc. 1998;4:319–328. [PubMed] [Google Scholar]
- Borodovsky J.T., Crosier B.S., Lee D.C., Sargent J.D., Budney A.J. Smoking, vaping, eating: is legalization impacting the way people use cannabis? Int. J. Drug Policy. 2016;36:141–147. doi: 10.1016/j.drugpo.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland A.B., Spindle T., Weaver M., Eissenberg T. Science and electronic cigarettes: current data, future needs. J. Addict. Med. 2014;8:223–233. doi: 10.1097/ADM.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland A. Electronic cigarettes: what are they and what do they do? Ann. N. Y. Acad. Sci. 2016;1394(1):5–30. doi: 10.1111/nyas.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.A., Christiansen B.A., Goldman M.S. The alcohol expectancy questionnaire: an instrument for the assessment of adolescent and adult alcohol expectancies. J. Stud. Alcohol. 1987;48:483–491. doi: 10.15288/jsa.1987.48.483. [DOI] [PubMed] [Google Scholar]
- Brown S.A. The national consortium on alcohol and NeuroDevelopment in adolescence (NCANDA): a multisite study of adolescent development and substance use. J. Stud. Alcohol Drugs. 2015;76:895–908. doi: 10.15288/jsad.2015.76.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumback T.Y. Neural predictors of alcohol use and psychopathology symptoms in adolescents. Dev. Psychopathol. 2016;28:1209–1216. doi: 10.1017/S0954579416000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck J.M., Siegel J.A. The effects of adolescent methamphetamine exposure. Front. Neurosci. 2015;9:151. doi: 10.3389/fnins.2015.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney A.J., Emond J.A. Caffeine addiction? Caffeine for youth? Time to act! Addiction. 2014;109:1771–1772. doi: 10.1111/add.12594. [DOI] [PubMed] [Google Scholar]
- Bunnell R.E. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: national Youth Tobacco Survey, 2011–2013. Nicotine Tob. Res. 2015;17:228–235. doi: 10.1093/ntr/ntu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buu A. Parent, family, and neighborhood effects on the development of child substance use and other psychopathology from preschool to the start of adulthood. J. Stud. Alcohol Drugs. 2009;70:489–498. doi: 10.15288/jsad.2009.70.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell P.E. Drinking levels, related problems and readiness to change in a college sample. Alcohol. Treat. Q. 2002;20:1–15. [Google Scholar]
- Callaway J.C. Pharmacokinetics of Hoasca alkaloids in healthy humans. J. Ethnopharmacol. 1999;65:243–256. doi: 10.1016/s0378-8741(98)00168-8. [DOI] [PubMed] [Google Scholar]
- Cannizzaro D.L., Elliott J.C., Stohl M., Hasin D.S., Aharonovich E. Neuropsychological assessment battery-screening module (S-NAB): performance in treatment-seeking cocaine users. Am. J. Drug Alcohol Abuse. 2014;40:476–483. doi: 10.3109/00952990.2014.916718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D., Srisuma S., Bronstein A.C., Hoyte C.O. Characterization of edible marijuana product exposures reported to United States poison centers. Clin. Toxicol. (Phila) 2016;54:840–846. doi: 10.1080/15563650.2016.1209761. [DOI] [PubMed] [Google Scholar]
- Carstairs S.D., Cantrell F.L. Peyote and mescaline exposures: a 12-year review of a statewide poison center database. Clin. Toxicol. (Phila) 2010;48:350–353. doi: 10.3109/15563650903586745. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Giedd J.N., Thomas K.M. Structural and functional brain development and its relation to cognitive development. Biol. Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. The adolescent brain. Dev. Rev. 2008;28(62):2–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Wang L.J., Lin S.K., Chen C.K. Neurocognitive profiles of methamphetamine users: comparison of those with or without concomitant ketamine use. Subst. Use Misuse. 2015;50:1778–1785. doi: 10.3109/10826084.2015.1050110. [DOI] [PubMed] [Google Scholar]
- Choi W.S., Gilpin E.A., Farkas A.J., Pierce J.P. Determining the probability of future smoking among adolescents. Addiction. 2001;96:313–323. doi: 10.1046/j.1360-0443.2001.96231315.x. [DOI] [PubMed] [Google Scholar]
- Coghill D.R. Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: evidence from a systematic review and a meta-analysis. Biol. Psychiatry. 2014;76:603–615. doi: 10.1016/j.biopsych.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Compton D.M., Dietrich K.L., Selinger M.C., Testa E.K. 5-Methoxy-N,N-di(iso)propyltryptamine hydrochloride (Foxy)-induced cognitive deficits in rat after exposure in adolescence. Physiol. Behav. 2011;103:203–209. doi: 10.1016/j.physbeh.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Conti C.L., Moscon J.A., Fregni F., Nitsche M.A., Nakamura-Palacios E.M. Cognitive related electrophysiological changes induced by non-invasive cortical electrical stimulation in crack-cocaine addiction. Int. J. Neuropsychopharmacol. 2014;17:1465–1475. doi: 10.1017/S1461145714000522. [DOI] [PubMed] [Google Scholar]
- Conway K.P. Data compatibility in the addiction sciences: an examination of measure commonality. Drug Alcohol Depend. 2014;141:153–158. doi: 10.1016/j.drugalcdep.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa G., Simola N., Morelli M. MDMA administration during adolescence exacerbates MPTP-induced cognitive impairment and neuroinflammation in the hippocampus and prefrontal cortex. Psychopharmacology (Berl.) 2014;231:4007–4018. doi: 10.1007/s00213-014-3536-z. [DOI] [PubMed] [Google Scholar]
- Cotter B.V. Energy drink and other substance use among adolescent and young adult emergency department patients. Pediatr. Emerg. Care. 2013;29:1091–1097. doi: 10.1097/PEC.0b013e3182a6403d. [DOI] [PubMed] [Google Scholar]
- Crews F.T., Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham E.B. Methamphetamine injecting is associated with phylogenetic clustering of hepatitis C virus infection among street-involved youth in Vancouver, Canada. Drug Alcohol Depend. 2015;152:272–276. doi: 10.1016/j.drugalcdep.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran C.P., Marczinski C.A. Taurine, caffeine, and energy drinks: reviewing the risks to the adolescent brain. Birth Defects Res. 2017;109:1640–1648. doi: 10.1002/bdr2.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran P.J., Stice E., Chassin L. The relation between adolescent alcohol use and peer alcohol use: a longitudinal random coefficients model. J. Consult. Clin. Psychol. 1997;65:130–140. doi: 10.1037//0022-006x.65.1.130. [DOI] [PubMed] [Google Scholar]
- Cuzen N.L., Koopowitz S.M., Ferrett H.L., Stein D.J., Yurgelun-Todd D. Methamphetamine and cannabis abuse in adolescence: a quasi-experimental study on specific and long-term neurocognitive effects. BMJ Open. 2015;5:e005833. doi: 10.1136/bmjopen-2014-005833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D.A., Goldstein R.B., Chou S.P., Ruan W.J., Grant B.F. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol. Clin. Exp. Res. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Win M.M. Neurotoxic effects of ecstasy on the thalamus. Br. J. Psychiatry. 2008;193:289–296. doi: 10.1192/bjp.bp.106.035089. [DOI] [PubMed] [Google Scholar]
- DeWit D.J., Adlaf E.M., Offord D.R., Ogborne A.C. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am. J. Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Di Forti M. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr. Bull. 2014;40:1509–1517. doi: 10.1093/schbul/sbt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Forti M. Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: a case-control study. Lancet Psychiatry. 2015;2:233–238. doi: 10.1016/S2215-0366(14)00117-5. [DOI] [PubMed] [Google Scholar]
- Dielman T.E., Butchart A.T., Shope J.T. Structural equation model tests of patterns of family interaction, peer alcohol use, and intrapersonal predictors of adolescent alcohol use and misuse. J. Drug Educ. 1993;23:273–316. doi: 10.2190/8YXM-K9GB-B8FD-82NQ. [DOI] [PubMed] [Google Scholar]
- Dishion T.J., Kavanagh K. The Guilford Press; New York, NY: 2003. Intervening in Adolescent Problem Behavior: A Family-centered Approach. [Google Scholar]
- Dishion T.J., Nelson S.E., Kavanagh K. The family check-up with high-risk young adolescents: preventing early-onset substance use by parent monitoring. Behav. Ther. 2003;34:553–571. [Google Scholar]
- Donovan J.E., Molina B.S. Childhood risk factors for early-onset drinking. J. Stud. Alcohol Drugs. 2011;72:741–751. doi: 10.15288/jsad.2011.72.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan J.E., Molina B.S. Types of alcohol use experience from childhood through adolescence. J. Adolesc. Health. 2013;53:453–459. doi: 10.1016/j.jadohealth.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan J.E. Really underage drinkers: the epidemiology of children's alcohol use in the United States. Prev. Sci. 2007;8:192–205. doi: 10.1007/s11121-007-0072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan J.E. Estimated blood alcohol concentrations for child and adolescent drinking and their implications for screening instruments. Pediatrics. 2009;123:e975–981. doi: 10.1542/peds.2008-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey L.A. Reduced memory skills and increased hair cortisol levels in recent Ecstasy/MDMA users: significant but independent neurocognitive and neurohormonal deficits. Hum. Psychopharmacol. 2015;30:199–207. doi: 10.1002/hup.2474. [DOI] [PubMed] [Google Scholar]
- Eaton D.K. Youth risk behavior surveillance–United states, 2005. MMWR Surveill. Summ. 2006;55:1–108. [PubMed] [Google Scholar]
- Elofson J., Gongvatana W., Carey K.B. Alcohol use and cerebral white matter compromise in adolescence. Addict. Behav. 2013;38:2295–2305. doi: 10.1016/j.addbeh.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England L.J. Developmental toxicity of nicotine: a transdisciplinary synthesis and implications for emerging tobacco products. Neurosci. Biobehav. Rev. 2017;72:176–189. doi: 10.1016/j.neubiorev.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T., Chang L., Oropilla G., Gustavson A., Speck O. Cerebral perfusion abnormalities in abstinent cocaine abusers: a perfusion MRI and SPECT study. Psychiatry Res. 2000;99:63–74. doi: 10.1016/s0925-4927(00)00056-1. [DOI] [PubMed] [Google Scholar]
- Falcone M. Age-related differences in working memory deficits during nicotine withdrawal. Addict. Biol. 2014;19:907–917. doi: 10.1111/adb.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fals-Stewart W., O'Farrell T.J., Freitas T.T., McFarlin S.K., Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J. Consult. Clin. Psychol. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing S.W., Filbey F.M., Loughran T.A., Chassin L., Piquero A.R. Which matters most? Demographic, neuropsychological, personality, and situational factors in long-term marijuana and alcohol trajectories for justice-involved male youth. Psychol. Addict. Behav. 2015;29:603–612. doi: 10.1037/adb0000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing S.W., Lovejoy T.I., Choo E.K. How has legal recreational cannabis affected adolescents in your state? A window of opportunity. Am. J. Public Health. 2017;107:246–247. doi: 10.2105/AJPH.2016.303585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Serrano M.J., Pérez-García M., Perales J.C., Verdejo-García A. Prevalence of executive dysfunction in cocaine, heroin and alcohol users enrolled in therapeutic communities. Eur. J. Pharmacol. 2010;626:104–112. doi: 10.1016/j.ejphar.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Fickenscher A., Novins D.K., Manson S.M. Illicit peyote use among American Indian adolescents in substance abuse treatment: a preliminary investigation. Subst. Use Misuse. 2006;41:1139–1154. doi: 10.1080/10826080600692142. [DOI] [PubMed] [Google Scholar]
- Filbey F.M., McQueeny T., Kadamangudi S., Bice C., Ketcherside A. Combined effects of marijuana and nicotine on memory performance and hippocampal volume. Behav. Brain Res. 2015;293:46–53. doi: 10.1016/j.bbr.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M., Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Dev. Psychol. 2005;41:625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Ghoneim M.M., Hinrichs J.V., Mewaldt S.P. Dose-response analysis of the behavioral effects of diazepam: I. Learning and memory. Psychopharmacology (Berl.) 1984;82:291–295. doi: 10.1007/BF00427672. [DOI] [PubMed] [Google Scholar]
- Giedd J.N. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb. Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gogtay N. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R. Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug Alcohol Depend. 2004;76:181–190. doi: 10.1016/j.drugalcdep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Goriounova N.A., Mansvelder H.D. Short- and long-term consequences of nicotine exposure during adolescence for prefrontal cortex neuronal network function. Cold Spring Harb. Perspect. Med. 2012;2:a012120. doi: 10.1101/cshperspect.a012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D.L., Herring N.R., Schaefer T.L., Vorhees C.V., Williams M.T. Glucose and corticosterone changes in developing and adult rats following exposure to (+/−)-3,4-methylendioxymethamphetamine or 5-methoxydiisopropyltryptamine. Neurotoxicol. Teratol. 2010;32:152–157. doi: 10.1016/j.ntt.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D.L. Electroencephalographic and convulsive effects of binge doses of (+)-methamphetamine, 5-methoxydiisopropyltryptamine, and (±)-3, 4-methylenedioxymethamphetamine in rats. Open Neuropsychopharmacol. J. 2012;5:1–8. doi: 10.2174/1876523801205010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B.F., Dawson D.A. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J. Subst. Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Haberstick B.C. Common and drug-specific genetic influences on subjective effects to alcohol, tobacco and marijuana use. Addiction. 2011;106:215–224. doi: 10.1111/j.1360-0443.2010.03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland S.E. Non-injection drug use patterns and history of injection among street youth. Eur. Addict. Res. 2010;16:91–98. doi: 10.1159/000279767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock R.M., Dean A., Knecht Z.A., Spencer J., Taverna E.C. A survey of hallucinogenic mushroom use, factors related to usage, and perceptions of use among college students. Drug Alcohol Depend. 2013;130:245–248. doi: 10.1016/j.drugalcdep.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Halpern J.H., Sherwood A.R., Hudson J.I., Yurgelun-Todd D., Pope H.G. Psychological and cognitive effects of long-term peyote use among Native Americans. Biol. Psychiatry. 2005;58:624–631. doi: 10.1016/j.biopsych.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Halpern J.H. Residual neurocognitive features of long-term ecstasy users with minimal exposure to other drugs. Addiction. 2011;106:777–786. doi: 10.1111/j.1360-0443.2010.03252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K.L. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict. Behav. 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway R., Schweitzer J., Suzuki J. Hallucinogen use disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2016;25:489–496. doi: 10.1016/j.chc.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Harris P.A. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T.F., Kozlowski L.T., Frecker R.C., Fagerström K.O. The fagerström test for nicotine dependence: a revision of the fagerström tolerance questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hendricks P.S., Clark C.B., Johnson M.W., Fontaine K.R., Cropsey K.L. Hallucinogen use predicts reduced recidivism among substance-involved offenders under community corrections supervision. J. Psychopharmacol. 2014;28:62–66. doi: 10.1177/0269881113513851. [DOI] [PubMed] [Google Scholar]
- Hermans E.J. Effects of exogenous testosterone on the ventral striatal BOLD response during reward anticipation in healthy women. Neuroimage. 2010;52:277–283. doi: 10.1016/j.neuroimage.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T. Exercise reinforcement, stress, and β-endorphins: an initial examination of exercise in anabolic-androgenic steroid dependence. Drug Alcohol Depend. 2014;139:86–92. doi: 10.1016/j.drugalcdep.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R.W., Heeren T., Winter M.R. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch. Pediatr. Adolesc. Med. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Hopfer C. Implications of marijuana legalization for adolescent substance use. Subst. Abus. 2014;35:331–335. doi: 10.1080/08897077.2014.943386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston S.M., Herting M.M., Sowell E.R. The neurobiology of childhood structural brain development: conception through adulthood. Curr. Top. Behav. Neurosci. 2014;16:3–17. doi: 10.1007/7854_2013_265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland A. Design and methods of the population assessment of tobacco and health (PATH) study. Tob. Control. 2016;26(4):371–378. doi: 10.1136/tobaccocontrol-2016-052934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide J.S. Cerebral gray matter volumes and low-frequency fluctuation of BOLD signals in cocaine dependence: duration of use and gender difference. Drug Alcohol Depend. 2014;134:51–62. doi: 10.1016/j.drugalcdep.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute P.P.R . Texas A&M University; College Station, TX: 2012. Texas School Survey of Drug and Alcohol Use: 2012. State: Grades 4–6. [Google Scholar]
- Institute for Social Research, U.o.M. Monitoring the Future . Institute for Social Research, University of Michigan; Ann Arbor, MI: 2010. Combined Forms- Part B; p. 2010. [Google Scholar]
- Jackson C., Ennett S.T., Dickinson D.M., Bowling J.M. Attributes that differentiate children who sip alcohol from abstinent peers. J. Youth Adolesc. 2013;42:1687–1695. doi: 10.1007/s10964-012-9870-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson K.M. Willingness to drink as a function of peer offers and peer norms in early adolescence. J. Stud. Alcohol Drugs. 2014;75:404–414. doi: 10.15288/jsad.2014.75.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson K.M., Barnett N.P., Colby S.M., Rogers M.L. The prospective association between sipping alcohol by the sixth grade and later substance use. J. Stud. Alcohol Drugs. 2015;76:212–221. doi: 10.15288/jsad.2015.76.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen L.K. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol. Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jacobsen L.K., Mencl W.E., Constable R.T., Westerveld M., Pugh K.R. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology (Berl.) 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Jacobus J., Tapert S.F. Effects of cannabis on the adolescent brain. Curr. Pharm. Des. 2014;20:2186–2193. doi: 10.2174/13816128113199990426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol. Teratol. 2009;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J. Neuropsychological performance in adolescent marijuana users with co-occurring alcohol use: a three-year longitudinal study. Neuropsychology. 2015;29(6):829–843. doi: 10.1037/neu0000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G. Assessment of cognitive brain function in ecstasy users and contributions of other drugs of abuse: results from an FMRI study. Neuropsychopharmacology. 2008;33:247–258. doi: 10.1038/sj.npp.1301415. [DOI] [PubMed] [Google Scholar]
- Johansson J., Grönbladh A., Hallberg M. Gamma-hydroxybutyrate (GHB) induces cognitive deficits and affects GABAB receptors and IGF-1 receptors in male rats. Behav. Brain Res. 2014;269:164–174. doi: 10.1016/j.bbr.2014.04.034. [DOI] [PubMed] [Google Scholar]
- Johnson V., White H.R. An investigation of factors related to intoxicated driving behaviors among youth. J. Stud. Alcohol. 1989;50:320–330. doi: 10.15288/jsa.1989.50.320. [DOI] [PubMed] [Google Scholar]
- Johnston L., Bachman J.G., O'Malley P.M. National Institute on Drug Abuse: Alcohol, Drug Abuse, and Mental Health Administration; Rockville, Maryland: 1988. Illicit Drug Use, Smoking and Drinking by America's High School Studtents, College Students, and Young Adults, 1975–1987. [Google Scholar]
- Johnston L.D., O'Malley P.M., Bachman J.G., Schulenberg J.E. Institute for Social Research, The University of Michigan; Ann Arbor: 2015. Monitoring the Future National Survey Results on Drug Use, 1975–2014: Volume I, Secondary School Students. [Google Scholar]
- Johnston L.D., O'Malley P.M., Miech R.A., Bachman J.G., Schulenberg J.E. Institute for Social Research, The University of Michigan; Ann Arbor, MI: 2017. Monitoring the Future National Survey Results on Drug Use, 1975–2016: Overview, Key Findings on Adolescent Drug Use. (113 pp.) [Google Scholar]
- Kaag A.M. Relationship between trait impulsivity and cortical volume, thickness and surface area in male cocaine users and non-drug using controls. Drug Alcohol Depend. 2014;144:210–217. doi: 10.1016/j.drugalcdep.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Kaag A.M. White matter alterations in cocaine users are negatively related to the number of additionally (ab)used substances. Addict. Biol. 2016;22(4):1048–1056. doi: 10.1111/adb.12375. [DOI] [PubMed] [Google Scholar]
- King G., Alicata D., Cloak C., Chang L. Neuropsychological deficits in adolescent methamphetamine abusers. Psychopharmacology (Berl.) 2010;212:243–249. doi: 10.1007/s00213-010-1949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston J., Clarke S., Ritchie T., Remington B. Developing and validating the composite measure of problem behaviors. J. Clin. Psychol. 2011;67:736–751. doi: 10.1002/jclp.20802. [DOI] [PubMed] [Google Scholar]
- Kober H. Brain activity during cocaine craving and gambling urges: an fMRI study. Neuropsychopharmacology. 2016;41:628–637. doi: 10.1038/npp.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza S.T., Vasilenko S.A. New methods shed light on age of onset as a risk factor for nicotine dependence. Addict. Behav. 2015;50:161–164. doi: 10.1016/j.addbeh.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lewis-Esquerre J.M., Rodrigue J.R., Kahler C.W. Development and validation of an adolescent smoking consequences questionnaire. Nicotine Tob. Res. 2005;7:81–90. doi: 10.1080/14622200412331328475. [DOI] [PubMed] [Google Scholar]
- Liebling E.J. Access to substance use treatment among young adults who use prescription opioids non-medically. Subst. Abuse Treat. Prev. Policy. 2016;11:38. doi: 10.1186/s13011-016-0082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J., Stephens R., Heffernan T.M. Cognitive and psychomotor performance during alcohol hangover. Curr. Drug Abuse Rev. 2010;3:80–87. doi: 10.2174/1874473711003020080. [DOI] [PubMed] [Google Scholar]
- Lisdahl K.M., Price J.S. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J. Int. Neuropsychol. Soc. 2012;18:678–688. doi: 10.1017/S1355617712000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl K.M., Gilbart E.R., Wright N.E., Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl K.M., Wright N.E., Kirchner-Medina C., Maple K.E., Shollenbarger S. Considering cannabis the effects of regular cannabis use on neurocognition in adolescents and young adults. Curr. Addict. Rep. 2014;1:144–156. doi: 10.1007/s40429-014-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loflin M., Earleywine M. A new method of cannabis ingestion: the dangers of dabs? Addict. Behav. 2014;39:1430–1433. doi: 10.1016/j.addbeh.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Lopez A.A. Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco cigarette smokers: a preliminary report. Nicotine Tob. Res. 2016;18:720–723. doi: 10.1093/ntr/ntv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring D.W., Marino S.E., Parfitt D., Finney G.R., Meador K.J. Acute lorazepam effects on neurocognitive performance. Epilepsy Behav. 2012;25:329–333. doi: 10.1016/j.yebeh.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Lundqvist T. Imaging cognitive deficits in drug abuse. Curr. Top. Behav. Neurosci. 2010;3:247–275. doi: 10.1007/7854_2009_26. [DOI] [PubMed] [Google Scholar]
- Müller-Oehring E.M. Influences of age, sex, and moderate alcohol drinking on the intrinsic functional architecture of adolescent brains. Cereb. Cortex. 2017;1–15 doi: 10.1093/cercor/bhx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahendran R., Lim H.A., Tan J.Y., Chua S.M., Winslow M. Salvia divinorum: an overview of the usage, misuse, and addiction processes. Asia Pac. Psychiatry. 2016;8:23–31. doi: 10.1111/appy.12225. [DOI] [PubMed] [Google Scholar]
- Margolis K.A., Nguyen A.B., Slavit W.I., King B.A. E-cigarette curiosity among U.S. middle and high school students: findings from the 2014 national youth tobacco survey. Prev. Med. 2016;89:1–6. doi: 10.1016/j.ypmed.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhe R., Franken I. Error-related brain activity as a biomarker for cocaine relapse. Neuropsychopharmacology. 2014;39:241. doi: 10.1038/npp.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshal M.P., Molina B.S., Pelham W.E., Jr. Childhood ADHD and adolescent substance use: an examination of deviant peer group affiliation as a risk factor. Psychol. Addict. Behav. 2003;17:293–302. doi: 10.1037/0893-164X.17.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B.D. Frequent methamphetamine injection predicts emergency department utilization among street-involved youth. Public Health. 2012;126:47–53. doi: 10.1016/j.puhe.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.R., Wilcox C.E., Teshiba T.M., Ling J.M., Yang Z. Hyperactivation of the cognitive control network in cocaine use disorders during a multisensory Stroop task. Drug Alcohol Depend. 2013;133:235–241. doi: 10.1016/j.drugalcdep.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe S.E., Veliz P., Schulenberg J.E. Adolescent context of exposure to prescription opioids and substance use disorder symptoms at age 35: a national longitudinal study. Pain. 2016;157:2173–2178. doi: 10.1097/j.pain.0000000000000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann U.D. Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (+/−)3, 4-methylenedioxymethamphetamine (ecstasy) users: relationship to cognitive performance. Psychopharmacology (Berl.) 2008;200:439–450. doi: 10.1007/s00213-008-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann M., Higgins K., Perra O., McCartan C., McLaughlin A. Adolescent ecstasy use and depression: cause and effect, or two outcomes of home environment? Eur. J. Public Health. 2014;24:845–850. doi: 10.1093/eurpub/cku062. [DOI] [PubMed] [Google Scholar]
- McGue M., Iacono W.G., Legrand L.N., Malone S., Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol. Clin. Exp. Res. 2001;25:1156–1165. [PubMed] [Google Scholar]
- McKenna D., Riba J. New world tryptamine hallucinogens and the neuroscience of ayahuasca. Curr. Top. Behav. Neurosci. 2015 doi: 10.1007/7854_2015_368. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- McKenna D.J., Towers G.H., Abbott F. Monoamine oxidase inhibitors in South American hallucinogenic plants: tryptamine and beta-carboline constituents of ayahuasca. J. Ethnopharmacol. 1984;10:195–223. doi: 10.1016/0378-8741(84)90003-5. [DOI] [PubMed] [Google Scholar]
- McKinney A. A review of the next day effects of alcohol on subjective mood ratings. Curr. Drug Abuse Rev. 2010;3:88–91. doi: 10.2174/1874473711003020088. [DOI] [PubMed] [Google Scholar]
- McQueeny T. Altered white matter integrity in adolescent binge drinkers. Alcohol. Clin. Exp. Res. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade C.S., Towe S.L., Skalski L.M., Robertson K.R. Independent effects of HIV infection and cocaine dependence on neurocognitive impairment in a community sample living in the southern United States. Drug Alcohol Depend. 2015;149:128–135. doi: 10.1016/j.drugalcdep.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador K.J., Gevins A., Leese P.T., Otoul C., Loring D.W. Neurocognitive effects of brivaracetam, levetiracetam, and lorazepam. Epilepsia. 2011;52:264–272. doi: 10.1111/j.1528-1167.2010.02746.x. [DOI] [PubMed] [Google Scholar]
- Meatherall R., Sharma P. Foxy. a designer tryptamine hallucinogen. J. Anal. Toxicol. 2003;27:313–317. doi: 10.1093/jat/27.5.313. [DOI] [PubMed] [Google Scholar]
- Medina K.L., Shear P.K. Anxiety, depression, and behavioral symptoms of executive dysfunction in ecstasy users: contributions of polydrug use. Drug Alcohol Depend. 2007;87:303–311. doi: 10.1016/j.drugalcdep.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina K.L., Shear P.K., Corcoran K. Ecstasy (MDMA) exposure and neuropsychological functioning: a polydrug perspective. J. Int. Neuropsychol. Soc. 2005;11:753–765. doi: 10.1017/S1355617705050915. [DOI] [PubMed] [Google Scholar]
- Medina K.L., Shear P.K., Schafer J. Memory functioning in polysubstance dependent women. Drug Alcohol Depend. 2006;84:248–255. doi: 10.1016/j.drugalcdep.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Medina K.L. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J. Int. Neuropsychol. Soc. 2007;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina K.L., Nagel B.J., Park A., McQueeny T., Tapert S.F. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J. Child Psychol. Psychiatry. 2007;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina K.L., Schweinsburg A.D., Cohen-Zion M., Nagel B.J., Tapert S.F. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol. Teratol. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina K.L. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol. Clin. Exp. Res. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt P.S., Cobb A.R., Cook G.I. Sex differences in the cognitive effects of tobacco abstinence: a pilot study. Exp. Clin. Psychopharmacol. 2012;20:258–263. doi: 10.1037/a0027414. [DOI] [PubMed] [Google Scholar]
- Meruelo A.D., Castro N., Cota C.I., Tapert S.F. Cannabis and alcohol use, and the developing brain. Behav. Brain Res. 2017;325(Pt A):44–50. doi: 10.1016/j.bbr.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels D., Christiansen E. Red Eye Express; 2012. Green A Field Guide to Marijuana. [Google Scholar]
- Miller E.T. Test-retest reliability of alcohol measures: is there a difference between internet-based assessment and traditional methods? Psychol. Addict. Behav. 2002;16:56–63. [PubMed] [Google Scholar]
- Miller B.L., Stogner J.M., Miller J.M. Exploring butane hash oil use: a research note. J. Psychoactive Drugs. 2016;48:44–49. doi: 10.1080/02791072.2015.1118173. [DOI] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.L., Clasen L.S., Giedd J.N., Blakemore S.J. The developmental mismatch in structural brain maturation during adolescence. Dev. Neurosci. 2014;36:147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Moreno-López L. Cocaine use severity and cerebellar gray matter are associated with reversal learning deficits in cocaine-dependent individuals. Addict. Biol. 2015;20:546–556. doi: 10.1111/adb.12143. [DOI] [PubMed] [Google Scholar]
- Morgan C.J. Neurocognitive function and schizophrenia-proneness in individuals dependent on ketamine, on high potency cannabis ('skunk') or on cocaine. Pharmacopsychiatry. 2012;45:269–274. doi: 10.1055/s-0032-1306310. [DOI] [PubMed] [Google Scholar]
- Morgan C.J. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol. Med. 2012;42:391–400. doi: 10.1017/S0033291711001322. [DOI] [PubMed] [Google Scholar]
- Nagy L.R., Featherstone R.E., Hahn C.G., Siegel S.J. Delayed emergence of behavioral and electrophysiological effects following juvenile ketamine exposure in mice. Transl. Psychiatry. 2015;5:e635. doi: 10.1038/tp.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences E. and Medicine . National Academies Press; Washington, DC: 2017. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. [PubMed] [Google Scholar]
- Nguyen-Louie T.T. Learning and memory in adolescent moderate, binge, and extreme-binge drinkers. Alcohol. Clin. Exp. Res. 2016;40:1895–1904. doi: 10.1111/acer.13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodora J. Curiosity predicts smoking experimentation independent of susceptibility in a US national sample. Addict. Behav. 2014;39:1695–1700. doi: 10.1016/j.addbeh.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonnemaker J. Initiation with menthol cigarettes and youth smoking uptake. Addiction. 2013;108:171–178. doi: 10.1111/j.1360-0443.2012.04045.x. [DOI] [PubMed] [Google Scholar]
- Novak S.P., Kral A.H. Comparing injection and non-injection routes of administration for heroin, methamphetamine, and cocaine users in the United States. J. Addict. Dis. 2011;30:248–257. doi: 10.1080/10550887.2011.581989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noworyta-Sokołowska K., Kamińska K., Kreiner G., Rogóż Z., Gołembiowska K. Neurotoxic effects of 5-MeO-DIPT: a psychoactive tryptamine derivative in rats. Neurotox. Res. 2016;30:606–619. doi: 10.1007/s12640-016-9654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuijten M., Blanken P., Van den Brink W., Goudriaan A.E., Hendriks V.M. Impulsivity and attentional bias as predictors of modafinil treatment outcome for retention and drug use in crack-cocaine dependent patients: results of a randomised controlled trial. J. Psychopharmacol. 2016;30:616–626. doi: 10.1177/0269881116645268. [DOI] [PubMed] [Google Scholar]
- Okoli C.T., Richardson C.G., Ratner P.A., Johnson J.L. Adolescents' self-defined tobacco use status, marijuana use, and tobacco dependence. Addict. Behav. 2008;33:1491–1499. doi: 10.1016/j.addbeh.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Ornstein T.J. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- Patrick M.E. Novel psychoactive substance use by US adolescents: characteristics associated with use of synthetic cannabinoids and synthetic cathinones. Drug Alcohol Rev. 2016;35:586–590. doi: 10.1111/dar.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning R., van Nuland M., Fliervoet L.A., Olivier B., Verster J.C. The pathology of alcohol hangover. Curr. Drug Abuse Rev. 2010;3:68–75. doi: 10.2174/1874473711003020068. [DOI] [PubMed] [Google Scholar]
- Pennings E.J., Leccese A.P., Wolff F.A. Effects of concurrent use of alcohol and cocaine. Addiction. 2002;97:773–783. doi: 10.1046/j.1360-0443.2002.00158.x. [DOI] [PubMed] [Google Scholar]
- Pennington D.L. Alcohol use disorder with and without stimulant use: brain morphometry and its associations with cigarette smoking, cognition, and inhibitory control. PLoS One. 2015;10:e0122505. doi: 10.1371/journal.pone.0122505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins K.A. Variability in initial nicotine sensitivity due to sex, history of other drug use, and parental smoking. Drug Alcohol Depend. 2009;99:47–57. doi: 10.1016/j.drugalcdep.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel W. Quality control of traditional cannabis tinctures: pattern, markers, and stability. Sci. Pharm. 2016;84:567–584. doi: 10.3390/scipharm84030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A. Adolescent development of cortical and white matter structure in the NCANDA sample: role of sex, ethnicity, puberty, and alcohol drinking. Cereb. Cortex. 2016;26:4101–4121. doi: 10.1093/cercor/bhv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki T.M., Sher K.J., Slutske W.S., Jackson K.M. Hangover frequency and risk for alcohol use disorders: evidence from a longitudinal high-risk study. J. Abnorm. Psychol. 2005;144:223–234. doi: 10.1037/0021-843X.114.2.223. [DOI] [PubMed] [Google Scholar]
- Pierce J.P., Choi W.S., Gilpin E.A., Farkas A.J., Merritt R.K. Validation of susceptibility as a predictor of which adolescents take up smoking in the United States. Health Psychol. 1996;15:355–361. doi: 10.1037//0278-6133.15.5.355. [DOI] [PubMed] [Google Scholar]
- Pierre J.M., Gandal M., Son M. Cannabis-induced psychosis associated with high potency wax dabs. Schizophr. Res. 2016;172:211–212. doi: 10.1016/j.schres.2016.01.056. [DOI] [PubMed] [Google Scholar]
- Politi M. Direct NMR analysis of cannabis water extracts and tinctures and semi-quantitative data on delta9-THC and delta9-THC-acid. Phytochemistry. 2008;69:562–570. doi: 10.1016/j.phytochem.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Pomerleau C.S., Carton S.M., Lutzke M.L., Flessland K.A., Pomerleau O.F. Reliability of the fagerstrom tolerance questionnaire and the fagerstrom test for nicotine dependence. Addict. Behav. 1994;19:33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Pomerleau O.F., Pomerleau C.S., Namenek R.J. Early experiences with tobacco among women smokers, ex-smokers, and never-smokers. Addiction. 1998;93:595–599. doi: 10.1046/j.1360-0443.1998.93459515.x. [DOI] [PubMed] [Google Scholar]
- Poorthuis R.B., Goriounova N.A., Couey J.J., Mansvelder H.D. Nicotinic actions on neuronal networks for cognition: general principles and long-term consequences. Biochem. Pharmacol. 2009;78:668–676. doi: 10.1016/j.bcp.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Price J.S., Shear P., Lisdahl K.M. Ecstasy exposure & gender: examining components of verbal memory functioning. PLoS One. 2014;9:e115645. doi: 10.1371/journal.pone.0115645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y.W. Potential gray matter unpruned in adolescents and young adults dependent on dextromethorphan-containing cough syrups: evidence from cortical and subcortical study. Brain Imaging Behav. 2016;11(5):1470–1478. doi: 10.1007/s11682-016-9628-0. [DOI] [PubMed] [Google Scholar]
- Quality C.f.B.H.S.a . Substance Abuse and Mental Health Services Administration; Rockville, MD: 2014. National Survey on Drug Use and Health (NSDUH): CAI Specifications for Programming (English Version) [Google Scholar]
- Raber J.C., Elzinga S., Kaplan C. Understanding dabs: contamination concerns of cannabis concentrates and cannabinoid transfer during the act of dabbing. J. Toxicol. Sci. 2015;40:797–803. doi: 10.2131/jts.40.797. [DOI] [PubMed] [Google Scholar]
- Rahman Q., Clarke C.D. Sex differences in neurocognitive functioning among abstinent recreational cocaine users. Psychopharmacology (Berl.) 2005;181:374–380. doi: 10.1007/s00213-005-2257-8. [DOI] [PubMed] [Google Scholar]
- Ramos-Pratts K., Rosa-González D., Pérez-Acevedo N.L., Cintrón-López D., Barreto-Estrada J.L. Sex-specific effect of the anabolic steroid, 17α-methyltestosterone, on inhibitory avoidance learning in periadolescent rats. Behav. Processes. 2013;99:73–80. doi: 10.1016/j.beproc.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M. Dose-related behavioral, subjective, endocrine, and psychophysiological effects of the (opioid agonist Salvinorin A in humans. Biol. Psychiatry. 2012;72:871–879. doi: 10.1016/j.biopsych.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L.A., Hart E.J., Chin P.F. Self-rating of the effects of alcohol (SRE): predictive utility and reliability across interview and self-report administrations. Addict. Behav. 2011;36:241–243. doi: 10.1016/j.addbeh.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Reissig C.J., Harrison J.A., Carter L.P., Griffiths R.R. Inhaled vs. oral alprazolam: subjective, behavioral and cognitive effects, and modestly increased abuse potential. Psychopharmacology (Berl.) 2015;232:871–883. doi: 10.1007/s00213-014-3721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B.M. Validity of the hangover symptoms scale: evidence from an electronic diary study. Alcohol. Clin. Exp. Res. 2012;36:171–177. doi: 10.1111/j.1530-0277.2011.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins L.N., Przybeck T.R. Age of onset of drug use as a factor in drug and other disorders. NIDA Res. Monogr. 1985;56:178–192. [PubMed] [Google Scholar]
- Robinson S.M., Sobell L.C., Sobell M.B., Leo G.I. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol. Addict. Behav. 2014;28:154–162. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- Rose-Jacobs R. Early adolescent executive functioning, intrauterine exposures and own drug use. Neurotoxicol. Teratol. 2011;33:379–392. doi: 10.1016/j.ntt.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd R.A., Seth P., David F., Scholl L. Increases in drug and opioid-involved overdose deaths – United States, 2010–2015. MMWR. Morb. Mortal. Wkly. Rep. 2016;65:1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- SAMHSA . 2013. National Survey on Drug Use and Health (NSDUH). Table 5.5A—Substance Dependence or Abuse in the Past Year among Persons Aged 12–17, by Demographic Characteristics: Numbers in Thousands, 2012 and 2013. Available at: https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabsPDFWHTML2013/Web/HTML/NSDUH-DetTabsSect5peTabs1to56-2013.htm-tab5.5a (2013) [Google Scholar]
- Salomonsen-Sautel S., Sakai J.T., Thurstone C., Corley R., Hopfer C. Medical marijuana use among adolescents in substance abuse treatment. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:694–702. doi: 10.1016/j.jaac.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J., Brown S.A. Marijuana and cocaine effect expectancies and drug use patterns. J. Consult. Clin. Psychol. 1991;59:558–565. doi: 10.1037//0022-006x.59.4.558. [DOI] [PubMed] [Google Scholar]
- Schmitt J.E. The dynamic role of genetics on cortical patterning during childhood and adolescence. Proc. Natl. Acad. Sci. U. S. A. 2014;111:6774–6779. doi: 10.1073/pnas.1311630111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoedel K.A., McMorn S., Chakraborty B., Zerbe K., Sellers E.M. Reduced cognitive and psychomotor impairment with extended-release oxymorphone versus controlled-release oxycodone. Pain Physician. 2010;13:561–573. [PubMed] [Google Scholar]
- Scholey A.B. Hair MDMA samples are consistent with reported ecstasy use: findings from a study investigating effects of ecstasy on mood and memory. Neuropsychobiology. 2011;63:15–21. doi: 10.1159/000321833. [DOI] [PubMed] [Google Scholar]
- Schuckit M.A. The self-rating of the effects of alcohol questionnaire as a predictor of alcohol-related outcomes in 12-year-old subjects. Alcohol Alcohol. 2008;43:641–646. doi: 10.1093/alcalc/agn077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster R.M., Mermelstein R.J., Hedeker D. Ecological momentary assessment of working memory under conditions of simultaneous marijuana and tobacco use. Addiction. 2016;111(8):1466–1476. doi: 10.1111/add.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R.H. Adolescent abuse of dextromethorphan. Clin. Pediatr. (Phila.) 2005;44:565–568. doi: 10.1177/000992280504400702. [DOI] [PubMed] [Google Scholar]
- Scott K.D., Scott A.A. An examination of information-processing skills among inhalant-using adolescents. Child Care Health Dev. 2012;38:412–419. doi: 10.1111/j.1365-2214.2011.01277.x. [DOI] [PubMed] [Google Scholar]
- Scott K.D., Scott A.A. Adolescent inhalant use and executive cognitive functioning. Child Care Health Dev. 2014;40:20–28. doi: 10.1111/cch.12052. [DOI] [PubMed] [Google Scholar]
- Scott J.C. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol. Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Sim M.E. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Simons J.S., Carey K.B. Risk and vulnerability for marijuana use problems: the role of affect dysregulation. Psychol. Addict. Behav. 2002;16:72–75. [PubMed] [Google Scholar]
- Simons J.S., Carey K.B. An affective and cognitive model of marijuana and alcohol problems. Addict. Behav. 2006;31:1578–1592. doi: 10.1016/j.addbeh.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Simons J., Correia C.J., Carey K.B., Borsari B.E. Validating a five-factor marijuana motives measure: relations with use, problems, and alcohol motives. J. Counsel. Psychol. 1998;45:265–273. [Google Scholar]
- Singh T. Tobacco use among middle and high school students–United states, 2011–2015. MMWR. Morb. Mortal. Wkly. Rep. 2016;65:361–367. doi: 10.15585/mmwr.mm6514a1. [DOI] [PubMed] [Google Scholar]
- Sircar R., Basak A., Sircar D. Gamma-hydroxybutyric acid-induced cognitive deficits in the female adolescent rat. Ann. N. Y. Acad. Sci. 2008;1139:386–389. doi: 10.1196/annals.1432.044. [DOI] [PubMed] [Google Scholar]
- Slutske W.S., Piasecki T.M., Hunt-Carter E.E. Development and initial validation of the hangover symptoms scale: prevalence and correlates of hangover symptoms in college students. Alcohol. Clin. Exp. Res. 2003;27:1442–1450. doi: 10.1097/01.ALC.0000085585.81711.AE. [DOI] [PubMed] [Google Scholar]
- Smith S.S. Development of the brief wisconsin inventory of smoking dependence motives. Nicotine Tob. Res. 2010;12:489–499. doi: 10.1093/ntr/ntq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L.C., Sobell M.B. Addiction Research Foundation; Toronto, Ontario: 1996. Timeline FollowBack. A Calendar Method for Assessing Alcohol and Drug Use. User's Guide. [Google Scholar]
- Sowell E.R., Thompson P.M., Holmes C.J., Jernigan T.L., Toga A.W. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat. Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Trauner D.A., Gamst A., Jernigan T.L. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev. Med. Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Sowell E.R. Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L.M., Spadoni A.D., Infante M.A., Myers M.G., Tapert S.F. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol. Addict. Behav. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L.M. Brain development in heavy-drinking adolescents. Am. J. Psychiatry. 2015;172:531–542. doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L.A. Validity and reliability of the alcohol expectancy questionnaire-adolescent, brief. J. Child Adolesc. Subst. Abuse. 2007;16:115–127. doi: 10.1300/J029v16n02_06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling K.L., Fryer C.S., Pagano I., Fagan P. Little cigars and cigarillos use among young adult cigarette smokers in the United States: understanding risk of concomitant use subtypes. Nicotine Tob. Res. 2016;18:2234–2242. doi: 10.1093/ntr/ntw170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stogner J.M., Miller B.L. The dabbing dilemma: a call for research on butane hash oil and other alternate forms of cannabis use. Subst. Abuse. 2015;36:393–395. doi: 10.1080/08897077.2015.1071724. [DOI] [PubMed] [Google Scholar]
- Strong D.R. Predictive validity of the expanded susceptibility to smoke index. Nicotine Tob. Res. 2015;17:862–869. doi: 10.1093/ntr/ntu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong, D., et al. Indications of dependence for different types of tobacco product users: Descriptive findings from wave 1 (2013–2014) of the population assessment of tobacco and health (PATH) study. (Under review). [DOI] [PubMed]
- Sullivan E.V. Cognitive, emotion control, and motor performance of adolescents in the NCANDA study: contributions from alcohol consumption, age, sex, ethnicity, and family history of addiction. Neuropsychology. 2016;30:449–473. doi: 10.1037/neu0000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L. Chronic ketamine exposure induces permanent impairment of brain functions in adolescent cynomolgus monkeys. Addict. Biol. 2014;19:185–194. doi: 10.1111/adb.12004. [DOI] [PubMed] [Google Scholar]
- Sweitzer M.M. Blunted striatal response to monetary reward anticipation during smoking abstinence predicts lapse during a contingency-managed quit attempt. Psychopharmacology (Berl.) 2016;233:751–760. doi: 10.1007/s00213-015-4152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M. Verbal memory, learning, and executive functioning among adolescent inhalant and cannabis users. J. Stud. Alcohol Drugs. 2011;72:96–105. doi: 10.15288/jsad.2011.72.96. [DOI] [PubMed] [Google Scholar]
- Takagi M. Executive control among adolescent inhalant and cannabis users. Drug Alcohol Rev. 2011;30:629–637. doi: 10.1111/j.1465-3362.2010.00256.x. [DOI] [PubMed] [Google Scholar]
- Takagi M.J. A signal detection analysis of executive control performance among adolescent inhalant and cannabis users. Subst. Use Misuse. 2014;49:1920–1927. doi: 10.3109/10826084.2014.935793. [DOI] [PubMed] [Google Scholar]
- Tamminga H.G., Reneman L., Huizenga H.M., Geurts H.M. Effects of methylphenidate on executive functioning in attention-deficit/hyperactivity disorder across the lifespan: a meta-regression analysis. Psychol. Med. 2016;46:1791–1807. doi: 10.1017/S0033291716000350. [DOI] [PubMed] [Google Scholar]
- Tang W.K., Liang H.J., Lau C.G., Tang A., Ungvari G.S. Relationship between cognitive impairment and depressive symptoms in current ketamine users. J. Stud. Alcohol Drugs. 2013;74:460–468. doi: 10.15288/jsad.2013.74.460. [DOI] [PubMed] [Google Scholar]
- Tau G.Z. Neural correlates of reward-based spatial learning in persons with cocaine dependence. Neuropsychopharmacology. 2014;39:545–555. doi: 10.1038/npp.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple J.L. The safety of ingested caffeine: a comprehensive review. Front. Psychiatry. 2017;8:80. doi: 10.3389/fpsyt.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple J.L., Ziegler A.M., Graczyk A.M., Crandall A. Effects of acute and chronic caffeine on risk-taking behavior in children and adolescents. J. Psychopharmacol. 2017;31:561–568. doi: 10.1177/0269881117691568. [DOI] [PubMed] [Google Scholar]
- Temple J.L. Caffeine use in children: what we know, what we have left to learn, and why we should worry. Neurosci. Biobehav. Rev. 2009;33:793–806. doi: 10.1016/j.neubiorev.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurstone C., Lieberman S.A., Schmiege S.J. Medical marijuana diversion and associated problems in adolescent substance treatment. Drug Alcohol Depend. 2011;118:489–492. doi: 10.1016/j.drugalcdep.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurstone C., Tomcho M., Salomonsen-Sautel S., Profita T. Diversion of medical marijuana: when sharing is not a virtue. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52:653–654. doi: 10.1016/j.jaac.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Torrealday O. Validation of the marijuana effect expectancy questionnaire-brief. J. Child Adolesc. Subst. Abuse. 2008;17:1–17. doi: 10.1080/15470650802231861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentacosta C.J., Hyde L.W., Shaw D.S., Cheong J. Adolescent dispositions for antisocial behavior in context: the roles of neighborhood dangerousness and parental knowledge. J. Abnorm. Psychol. 2009;118:564–575. doi: 10.1037/a0016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trim R.S., Schuckit M.A., Smith T.L. The relationships of the level of response to alcohol and additional characteristics to alcohol use disorders across adulthood: a discrete-time survival analysis. Alcohol. Clin. Exp. Res. 2009;33:1562–1570. doi: 10.1111/j.1530-0277.2009.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinidad, D., et al. Susceptibility to tobacco product use among youth in wave 1 on the population assessment of tobacco and health (PATH) study. (Under review). [DOI] [PMC free article] [PubMed]
- Verdejo-Garcia A. Neural substrates of cognitive flexibility in cocaine and gambling addictions. Br. J. Psychiatry. 2015;207:158–164. doi: 10.1192/bjp.bp.114.152223. [DOI] [PubMed] [Google Scholar]
- Verster J.C. The alcohol hangover–a puzzling phenomenon. Alcohol Alcohol. 2008;43:124–126. doi: 10.1093/alcalc/agm163. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Baler R.D., Compton W.M., Weiss S.R. Adverse health effects of marijuana use. N. Engl. J. Med. 2014;370:2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M. Neurocognitive impairments in non-deprived smokers–results from a population-based multi-center study on smoking-related behavior. Addict. Biol. 2013;18:752–761. doi: 10.1111/j.1369-1600.2011.00429.x. [DOI] [PubMed] [Google Scholar]
- Wallin K.G., Wood R.I. Anabolic-androgenic steroids impair set-shifting and reversal learning in male rats. Eur. Neuropsychopharmacol. 2015;25:583–590. doi: 10.1016/j.euroneuro.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin-Miller K., Li G., Kelishani D., Wood R.I. Anabolic-androgenic steroids decrease dendritic spine density in the nucleus accumbens of male rats. Neuroscience. 2016;330:72–78. doi: 10.1016/j.neuroscience.2016.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185–189. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H.R., Labouvie E.W. Towards the assessment of adolescent problem drinking. J. Stud. Alcohol. 1989;50:30–37. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]
- White H.R., Labouvie E.W. Longitudinal trends in problem drinking as measured by the Rutgers Alcohol Problem Index. Alcohol.: Clin. Exp. Res. 2000;24(76A) [Google Scholar]
- White, H.R., Labouvie, E.W., Rutgers Alcohol Problem Index (RAPI).
- Wills T.A., Sargent J.D., Knight R., Pagano I., Gibbons F.X. E-cigarette use and willingness to smoke: a sample of adolescent non-smokers. Tob. Control. 2016;25:e52–e59. doi: 10.1136/tobaccocontrol-2015-052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters K.C., Lee C.Y. Likelihood of developing an alcohol and cannabis use disorder during youth: association with recent use and age. Drug Alcohol Depend. 2008;92:239–247. doi: 10.1016/j.drugalcdep.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn S.C. Comprehensive application of time-of-flight secondary ion mass spectrometry (TOF-SIMS) for ionic imaging and bio-energetic analysis of club drug-induced cognitive deficiency. Sci. Rep. 2015;5:18420. doi: 10.1038/srep18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H. Impulsivity, cognitive function, and their relationship in heroin-dependent individuals. J. Clin. Exp. Neuropsychol. 2013;35:897–905. doi: 10.1080/13803395.2013.828022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.