Abstract
Assessment of pain in rodents is essential for analgesic development and investigations of fundamental neurobiology of pain. We have previously reported on a modified weight bearing apparatus we call VASIC (voluntarily accessed static incapacitance chamber) enabling unbiased and high throughput assessment of pain in rats. The present report provides a detailed description of the construction of the apparatus with all necessary computer assisted design files for the printed circuit board and the plastic components, and the required software for controlling the data capture and data analysis hosted in an online source file repository to allow assembly of the device in-house at a cost affordable to most academic laboratories. We extend the application of the apparatus to assess weight bearing in mice to enable the use of genetic mice models to study pain.
Keywords: Pain assessment, Weight bearing, Arduino shield, Mus musculus
| Specifications table | |
| Hardware name | VASIC – Voluntary Access Static Incapacitance Chamber |
| Subject area | Medical |
| Neuroscience | |
| Hardware type | Measuring physical properties and in-lab sensors |
| Open Source License | GNU General Public License (GPL) |
| Cost of Hardware | ~$725 |
| Source File Repository | https://osf.io/w7mvj/ (Public Repository) https://osf.io/ytf8c/ (Archived Registration) |
1. Hardware in context
Chronic pathological pain remains an unsolved medical problem and the development of new analgesics is an active endeavor in both academic laboratories and the pharmaceutical industry [1,2]. A major bottleneck in pain research continues to be the assessment of pain in animal models. In rodent pain models, the traditional method of pain assessment largely depends on quantifying the behavioral response of the animals following applied painful stimuli, such as thermal stimulation or mechanical probing [3]. This traditional quantitation of reflexive responses has been criticized as an inaccurate reflection of pain in humans where no external stimulation is required for the subjects to experience pain [4,5]. Furthermore, it is impossible to truly blind the observer during rodent behavioral pain assessment experiments since animals in pain can be readily identified by visual observations of awkward gait or less weight placed on the afflicted limb, which increases the likelihood of operator bias confounding the experimental data. More recently, methods that significantly reduce the likelihood of operator bias by assessing spontaneous pain in rodents without application of painful external stimuli, such as through a conditional place preference paradigm, assessment of facial expression, and quantitation of gait, have been introduced (reviewed [6–8]). Validation of these newer assessment of pain by the scientific community is ongoing, but a wide-spread use of these methods is somewhat limited by the requirement for expensive specialized equipment.
We recently introduced a modification of the traditional weight bearing test we call VASIC (Voluntary Access Static Incapacitance Chamber) as a simple, operator-independent, and high throughput method for assessing pain in rats [9]. In contrast to the traditional weight bearing test, our modification combined a brief water deprivation to encourage rats to seek water in the test chamber where weighing platforms were placed underneath the water spout, thereby eliminating the need to restrain the rats to forcibly place them on the weighing platforms. Rats voluntarily entered the weighing chamber, triggering mass data acquisition by a host computer and producing hundreds of mass measurement data points during a 30 min recording session. The operator plays no role in data gathering except for placing the rodents in the test chamber, essentially eliminating the possibility of an operator bias. Weight bias or shifting of the mass distribution to the uninjured side resulting from a standard nerve injury and an inflammatory pain models were accurately captured by this method. The simplicity of the device enables multiple VASIC devices to be controlled by a single laboratory computer, greatly increasing the throughput of behavioral assessment of pain in rodents and reducing the bottleneck inherent to pain research.
In the present paper, we introduce modifications to the device that extend the applicability of the VASIC device for assessing weight bias in mice. Furthermore, details for constructing the device in-house at a very low cost well within the reach of most academic laboratories are provided. All the necessary computer-aided design (CAD) files for reproducing the hardware and both executable binary and the source code for the software necessary for controlling the microcontroller, data acquisition, and data analysis are provided.
2. Hardware and software description
2.1. Electronic circuit design and printed circuit board (PCB)
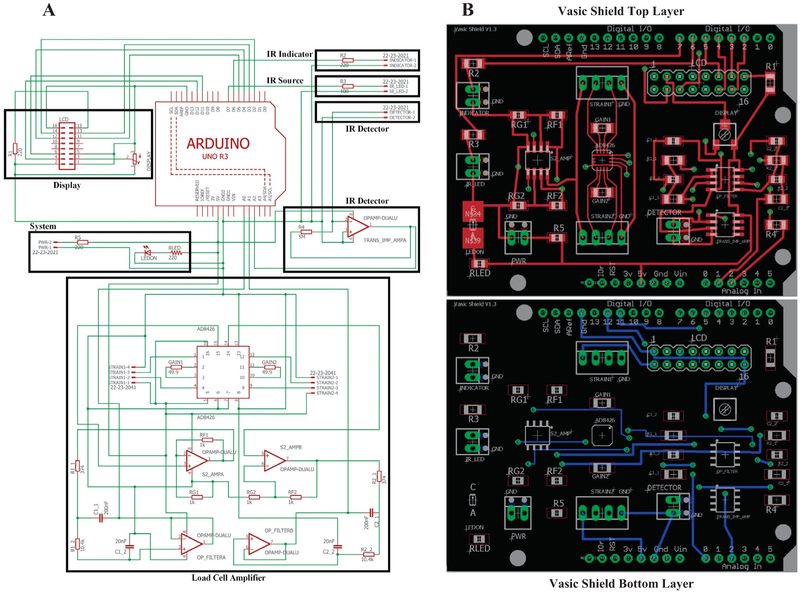
The electronic circuit consists of 3 functional domains: 1. Load cell control, 2. Infrared (IR) sensor control, and 3. Arduino microcontroller interface. A voltage-drop across the load cell, configured as a resistor of a Wheatstone bridge, is sensed by the AD8426 instrumentation amplifier, which amplifies the signal according to the selected gain resistors, and passes it through a second order Bessel low-pass filter for 1 kHz before sending it to the Arduino (version Uno R3) analog pins A0 and A1. The AD8426 instrumentation amplifier also provides a DC voltage offset to the signal which can be adjusted by potentiometers REF1 and REF2 to take advantage of the full dynamic range (5V) of the built-in 10-bit analog to digital converter. The IR sensor utilizes a trans-impedance amplifier circuit to convert small current changes from the photodiode into larger voltage changes that may be read by the Arduino. The value for R4 may be increased to provide greater signal amplification at a slight loss of signal resolution. The voltage signal from the IR sensor is read by the Arduino analog pin A2.
The circuit schematic (*.sch file extension) and the printed circuit board (PCB) layout (*.brd file extension) were designed in EAGLE (v7.6.0, Autodesk Inc, San Rafael, CA). The final PCB layout can be found in Arduino_Shield.zip in the VASIC Hardware CAD folder of the Source File Repository. A free EAGLE software download can be found at https://www.autodesk.com/compare/eagle-vs-eagle-premium. The files are also saved as KiCAD (http://kicad-pcb.org/), a popular open source PCB design software, compatible files. The *.brd output file from the EAGLE software was sent online to the OSH Park PCB manufacturer (https://oshpark.com/), which offers high quality, lead-free boards (emersion gold finish), manufactured in the USA. A complete list of all electronics parts and vendors can be found in Hardware Parts List.xlsx in the VASIC Hardware CAD folder. The components are soldered onto the PCB via a hot-air reflow station in the laboratory.
2.2. Plastic and metal hardware
Additional CAD files (SolidWorks, Dassault Systems, Waltham, MA) of all other components (outside enclosure, inside chamber, bottom plate, metal enclosure) are provided in Vasic_(1004LC)_SOLIDWORKS.zip in the VASIC Hardware CAD folder. We submit these CAD files to a local plastics manufacturer (Routed 4 U LLC, Sun Prairie, WI, www.laser-4-u.com) for production, except for the metal enclosure which is purchased premade (SC-12101, Bud Industries Inc, Willoughby, OH) with holes cut and markings engraved on the surface by the same local plastics manufacturer. Supplemental Data 1 shows images during the hardware assembly of VASIC with the parts cross referenced to the parts list Excel file.
2.3. Software
There are 3 pieces of software required for operating VASIC: 1. Arduino script uploaded to the microcontroller for controlling local data averaging of analog signals from the load cells reflecting the mass measurements (Supplemental Data 2), 2. C++ data acquisition GUI built using the Qt graphic environment framework (www.1.qt.io/download-open-source/) and run on the host computer. This software controls the calibration of the loadcells, setting data acquisition parameters, receiving the digital mass information, and writing the output to the CSV datafiles (Supplemental Data 3), and 3. A crosscompatible data analysis script for GNU Octave v4.2.1 (https://www.gnu.org/software/octave/) and MATLAB vR2017a (https://www.mathworks.com/products/matlab.html) that reads in the CSV data files, calculates the mass reading distribution, and fits in a normal distribution to arrive at the mean weight bias and error measures (Supplemental Data 4). The data analysis script has 3 input parameters for controlling the post-acquisition data filtering: 1. Readings without a minimum of X (default 2) data points (i.e. unsettled positioning on the platform), 2. Data points where the summation of both platforms is outside of ±X% (default 25%) of the calculated body-mass (i.e. full body-mass is not on the platforms), and 3. Eliminating likely spurious mass readings where the mass measurement on either platform is <X% (default 3%) of the calculated body-mass (i.e. hind limbs are not positioned on both platforms). The data analysis input parameters typically do not have a large effect on the overall mean value of the mass bias, which is the numerical value ultimately quantified as the readout of behavioral pain. The final output of the data analysis script is a results folder created in the source data directory that contains the following: 1. Cumulative and individual access statistics for each rodent, 2. Figures for each rodent on each day consisting of histogram and time-course data plots for the raw data and selected filters, and 3. Absolute and body-mass-normalized mass bias summary statistics for individual rodents and cumulative averages. Please see the README file provided with the data analysis script for more details on the final output.
Instructions for uploading Arduino script to the microcontroller can be found at www.arduino.cc and the C++/Qt GUI was compiled with the Qt Creator IDE (v5.9.3, www.1.qt.io/download-open-source/). Installer for executable binary files for all software can be found in VASIC Data Analysis, VASIC DATA Acquisition GUI, and VASIC Arduino Sketch folders of the Source File Repository. The most recent and historical source files can also be obtained from the following GitHub links: Arduino script (https://github.com/NulliSecundus/VASIC_Arduino_Sketch), Data Acquisition (https://github.com/NulliSecundus/VASIC_Data_Acquisition), and Data Analysis (https://github.com/NulliSecundus/VASIC_Data_Analysis).
2.4. Summary
The VASIC device could be useful in pain research due to the following:
VASIC provides objective, unbiased assessment of pain in rodents, in contrast to most traditional pain assessment techniques.
VASIC offers much greater scalability for larger experiments with more test subjects in comparison to traditional pain assessment techniques.
VASIC requires essentially no training to use in comparison to most traditional pain assessment techniques or devices.
VASIC units are significantly less expensive than most devices used for traditional pain assessment.
3. Design files
3.1. Design files summary
| Design file name | File type | Open source license | Location of the file |
|---|---|---|---|
| Vasic_(1004LC)_SOLIDWORKS.zip | Zip File | GNU GPL | https://osf.io/yg657/ |
| Vasic_(1004LC) – IGES.zip | Zip File | GNU GPL | https://osf.io/yg657/ |
| Vasic_(1004LC) – STEP.zip | Zip File | GNU GPL | https://osf.io/yg657/ |
| Vasic_Arduino_Shield.zip | Zip File | GNU GPL | https://osf.io/yg657/ |
| VASIC_Data_Analysis_Installer_web.exe | Executable | GNU GPL | https://osf.io/fq746/ |
| Vasic Data Analysis (source code) | Source Code | GNU GPL | https://osf.io/fq746/ |
| VasicInstaller_Windows.exe | Executable | GNU GPL | https://osf.io/4vjgt/ |
| Vasic Data Acquisition (source code) | Source Code | GNU GPL | https://osf.io/4vjgt/ |
| VASIC_Arduino_Sketch.zip | Zip File | GNU GPL | https://osf.io/8h6t7/ |
The following is a description of the content of these files:
Vasic_(1004LC)_SOLIDWORKS.zip: Zip file containing all the SolidWorks CAD files for the device in an editable format.
Vasic_(1004LC) – IGES.zip: Zip file containing all the IGES CAD files for the device in an editable format.
Vasic_(1004LC) – STEP.zip: Zip file containing all the STEP CAD files for the device in an editable format.
Vasic_Arduino_Shield.zip: Zip file containing the Autodesk Eagle formatted files for PCB design.
Vasic_Data_Analysis.exe: Compiled executable file (installer) for the Vasic Data Analysis software.
Vasic Data Analysis: Contains the source code for the Vasic Data Analysis software.
Vasic_Data_Acquisition.exe: Compiled executable file (installer) for the Vasic Data Acquisition software.
Vasic Data Acquisition: Contains the source code for the Vasic Data Acquisition software.
VASIC_Arduino_Sketch.zip: Zip file containing the source code and requisite libraries for the Arduino script.
These files are available via the source file repository link (see Specifications Table).
3.2. Bill of materials
| Designator | Component | Number | Cost per unit -USD |
Total cost-USD |
Source of materials |
Material type |
|---|---|---|---|---|---|---|
| GAIN1, GAIN2 | Resistor SMD 49.9 ohms | 2 | 0.10 | 0.20 | Mouser | Non-specific |
| R3 | Resistor SMD 100 ohms | 1 | 0.10 | 0.10 | Mouser | Non-specific |
| R1, R2, RLED, R5 | Resistor SMD 220 ohms | 4 | 0.10 | 0.40 | Mouser | Non-specific |
| R1_1, R2_1 | Resistor SMD 374 ohms | 2 | 0.80 | 1.60 | Mouser | Non-specific |
| RG1, RG2, RF1, RF2 | Resistor SMD 1 K ohms | 4 | 0.30 | 1.20 | Mouser | Non-specific |
| R1_2, R2_2 | Resistor SMD 10.4 K ohms | 2 | 0.94 | 1.88 | Mouser | Non-specific |
| R4 | Resistor SMD 5 M ohms | 1 | 1.61 | 1.61 | Mouser | Non-specific |
| DISPLAY | Trimmer Resistor SMD 10 K ohms | 1 | 2.08 | 2.08 | Mouser | Non-specific |
| TRANS_IMP_AMP, OP_REF, OP_FILTER | Op Amp Dual Rail-to-Rail | 3 | 0.87 | 2.61 | Mouser | Non-specific |
| LEDON | Green LED SMD | 1 | 0.37 | 0.37 | Mouser | Non-specific |
| AD8426 | Instrumentation Amplifier | 1 | 5.75 | 5.75 | Mouser | Non-specific |
| STRAIN1, STRAIN2 | 4P Fixed Terminal Blocks | 2 | 3.02 | 6.04 | Mouser | Non-specific |
| INDICATOR, IR_LED, DETECTOR | 2P Fixed Terminal Blocks | 3 | 1.51 | 4.53 | Mouser | Non-specific |
| C1_2, C2_2 | Capacitor SMD 0.02uF | 2 | 0.36 | 0.72 | Mouser | Ceramic |
| C1_1, C2_1 | Capacitor SMD 0.2uF | 2 | 0.51 | 1.02 | Mouser | Ceramic |
| LCD | 2 × 8 Male Pin Header | 2 | 0.69 | 1.38 | Jameco | Non-specific |
| ARD_10 | 1 × 10 Arduino Pin Header | 1 | 0.79 | 0.79 | Jameco | Non-specific |
| ARD_8 | 1 × 8 Arduino Pin Header | 2 | 0.59 | 1.18 | Jameco | Non-specific |
| ARD_6 | 1 × 6 Arduino Pin Header | 1 | 0.55 | 0.55 | Jameco | Non-specific |
| LC | Load Cell 0.6 kg | 2 | 51.28 | 102.56 | Sensor Techniques Ltd | Metal |
| GLED | Green LED | 1 | 1.26 | 1.26 | Mouser | Non-specific |
| RLED | Red LED | 1 | 1.67 | 1.67 | Mouser | Non-specific |
| LCD_DISP | LCD Display | 1 | 11.10 | 11.10 | Mouser | Non-specific |
| PD | IR Photodiode | 1 | 0.72 | 0.72 | Mouser | Non-specific |
| IR | IR LED | 1 | 0.05 | 0.05 | Chanzon | Non-specific |
| MS_1 | 6–32 1/2″ MACHINE SCREW PAN PHILLIPS | 4 | 0.0773 | 0.31 | Digikey | Metal |
| MS_2 | 6–32 5/16″ MACHINE SCREW PAN PHILLIPS | 8 | 0.0306 | 0.24 | Digikey | Metal |
| HS_1 | 6–32 1–3/4″ HEX STANDOFF | 4 | 1.28 | 5.12 | Digikey | Metal |
| HS_2 | 6–32 3/8″ HEX STANDOFF | 4 | 0.57 | 2.28 | Digikey | Metal |
| MS_3 | M3 MACHINE SCREW PAN PHILLIPS | 4 | 0.63 | 2.52 | Digikey | Metal |
| HS_3 | M2.5 HEX STANDOFF ALUMINUM 10MM | 4 | 0.54 | 2.16 | Digikey | Metal |
| HN_1 | M2.5 HEX NUT 0.197″ STEEL | 4 | 0.15 | 0.60 | Digikey | Metal |
| MS_4 | M2.5 MACHINE SCREW PAN SLOTTED | 4 | 0.38 | 1.52 | Digikey | Metal |
| BOX | BOX ALUM BLK/WHT 10″L X 11.93″W | 1 | 64.40 | 64.40 | Digikey | Metal |
| MS_5 | M3 35 mm Machine Screw Phillips | 4 | 0.15 | 0.60 | uxcell | Metal |
| INSERT | 6–32 Brass Heat-Set Insert Thermoplastic | 12 | 0.1538 | 1.85 | McMASTER-CARR | Metal |
| FLEXARM | Threaded Fitting Arm | 2 | 21.80 | 43.6 | Moffatt | Non-specific |
| CABLE | 8×2 Flat Ribbon Cable | 1 | 2.53 | 2.53 | Amazon | Non-specific |
| ARDUINO | Elegoo UNO R3 (Arduino clone) | 1 | 10.90 | 10.90 | Amazon | Non-specific |
| BOARD | Custom PCB | 1 | 9.45 | 9.45 | OSH Park | Non-specific |
| Custom routing and engraving of BOX | 1 | 75.00 | 75.00 | Routed-4-U LLC. | Metal | |
| Custom Acrylic Components | 1 | 350.00 | 350.00 | Routed-4-U LLC. | Polymer |
Note: Custom fabricated components, such as the PCB, enclosure routing/engraving, and acrylic pieces, are subject to different rates depending on preference and availability of manufacturers. The Custom Acrylic Components listing includes the following individual pieces (see Design Files section): Plastic Base, Right Footpad, Left Footpad, 2 × LC Attachment Blocks, LC Mounting Platform, Lid, Outer Container, Top Frame, Water Bottle Insert. With access to an applicable 3D printer, many of these acrylic pieces can be 3D printed for a further reduction in cost.
4. Build instructions
Prior to assembling a VASIC unit, some custom components must be fabricated, such as the plastic pieces and the VASIC Arduino shield PCB. We utilize a local plastics manufacturer (www.laser-4-u.com) to fabricate the custom plastic pieces, but many of the components can be 3D printed to reduce the total build cost. Regarding the VASIC Arduino shield, we utilize the online PCB manufacturer OSH Park (https://oshpark.com/) for fabrication based on the board designed in Autodesk’s Eagle software. OSH Park’s 2-layer boards cost $5 per square inch and ships in under 12 calendar days from ordering. Another benefit of OSH Park is that the online interface accepts Eagle files (*.brd) directly, which negates the need to use a CAM processor for conversion to Gerber files, and processes the layers into a set of image depictions of the board, so one can verify that everything is as intended before ordering. Additionally, the sloped metal enclosure box must be drilled and cut according to the specifications. We utilize the same local plastics manufacturer for drilling, cutting, and engraving this enclosure, but the total cost of the device can be reduced by manually performing this step given sufficient tools.
Following fabrication of the requisite parts, the VASIC Arduino shield PCB should be soldered. Surface-mounted components should be soldered on first via a hot-air rework station or other suitable tool for surface-mount soldering. Next, the pin headers should be hand soldered on, starting with the 8 × 2 LCD pin header and then the Arduino pin headers. Lastly, the 4-pin and 2-pin screw terminals should be hand soldered on. The plastic housing of the screw terminals has the lowest melting point out of the PCB components, so they should be soldered last to avoid damage. Separately, another 8 × 2 pin-header must be hand soldered to the LCD screen’s board. The assembled PCB is shown on Supplemental Data 1 Fig. a, b.
Following soldering, the plastic base plate should be screwed into place on top of the metal enclosure. Flip the enclosure over so that the underside of the metal enclosure is shown. Begin assembling the interior of the VASIC base, using Supplemental Data 1 Fig. e as a guideline and referring to Supplemental Data 1 Fig. c, d for part identification noted by bold alphabets. Place the Arduino into position on the base plate and screw into place before plugging in the VASIC Arduino shield. Using the M2.5 hex-standoffs, screws, and nuts [D], screw the LCD screen into position on the inner side of the metal enclosure. Separately, attach the two load cells [L] to the load cell mounting platform [Q]. Using the 1–3/4-inch hex standoffs [V], attach the load cell mounting platform [Q] to the plastic base plate and screw into place. Place the green and red indicator LEDs into the Power and IR Sensor drilled holes on the front panel of the metal enclosure, respectively. Connect these indicator LEDs to the VASIC Arduino shield through their respective screw terminals (GND labeled). Connect the load cell wires to the left (STRAIN1) and right (STRAIN2) 4-pin load cell screw terminals on the VASIC Arduino shield. Each load cell wiring color should be in order of Green (PWR), Red, White, Black (GND). Using the 8 × 2 Flat Ribbon Cable [J], connect the LCD screen to the LCD pin headers on the VASIC Arduino shield.
Flip the enclosure over so that the plastic base is shown on top. The device should now look like Supplemental Data 1 Fig. f. Solder 25-inch wire to the leads of the IR diode [H] and photodiode [I]. Line the bottom ridge of the diodes with a small amount of glue or adhesive and place into the diode covers [G]. Firmly press the assembled diode covers with diodes embedded onto one end of the IR flex-arms [A]. Note that the diode-cover/flex-arm assembly is a friction fit and not screw on. Screw the assembled IR flex-arms into place as shown in Supplemental Data 1 Fig. g. The remaining length of wire from the diodes should be passed through the drilled holes to the underside of the metal enclosure. Connect the wires from the IR emitter diode and IR photodiode to the IR_LED and DETECTOR screw terminals (GND labeled) of the VASIC Arduino shield, respectively. Using the 35 mm M3 machine screws [R] and [S], attach the left [T] and right [U] weighing platforms to the top of the load cells, with the load cell spacers [O] placed in between. Screw on the bottom piece of the metal enclosure to complete the assembly of the VASIC base components.
5. Operation instructions
5.1. VASIC device setup
To setup the VASIC device for collecting data, place a rodent water bottle containing at least 50 mL of 6% sucrose water into the VASIC’s water bottle tower. The height of the bottle should be adjusted such that the rodent is not able to sit while accessing water but must be able to reach the water without standing on one leg. Adjust the IR sensors on each side of the water bottle tower so that they are below the bottle’s nozzle by approximately 3 cm for mice and 6 cm for rats, and positioned in front of the nozzle by approximately 1.5 cm. Placing the sensors in this way reduces the chance that data will be recorded from improper positioning (e.g. standing backwards) and ensures that the sensor is broken with the larger part of the rodent’s head. This sensor arrangement prevents minor movements during an access event from repeatedly triggering the sensor and generally results in more consistent segments of data collection.
5.2. VASIC data acquisition program
Begin the data collection process by opening the VASIC Data Acquisition GUI application. Upon first opening the program, the user should choose “Select Port”, which will display a list of available devices connected to the computer that can interact with the program. Users should select the desired VASIC device from the drop-down menu before clicking “Apply”. If the program is unable to connect to the device, an error message will be displayed on screen. This program also includes a required input label “Name”, as well as two optional input labels “Option 1” and “Option 2” for any set of data. These labels direct the program’s automatic file naming feature, which generates names for the output data files in the format <Date>_ <Name>_<Option 1>_<Option 2>, omitting the optional labels if left unfilled. Users can choose to not utilize the automatic naming feature by selecting “Advanced” and entering a custom filename. After entering the labels, users should select the “Change Folder” option to specify the destination directory for the output data file. Users can also opt to change the default data averaging time from 1 s by choosing “Select Time”. Once a new averaging time is chosen, users should select “Send” to update the device’s saved averaging time variable. Note that any such saved variables will remain even after powering down the device. Once setup is completed, carefully place the rodent in the VASIC device, making sure to avoid unnecessary stress while handling the rodent, and click “Start” in the session control box to begin the data collection period. After the time for the researcher’s experiment has elapsed, click “Stop” in the session control box to end data acquisition.
5.3. Calibration, maintenance, and troubleshooting
If the device requires calibration, users should select the “Calibrate” button in the load cell setup box. The threshold for when calibration is required is dependent on user preference; however, we have found that calibrating the device when measurements deviate from a known mass by more than 0.5 g results in reliable and accurate data. Choose either the left or right load cell and select “Start” to begin calibration. Ensure that weighing platforms are clear of debris or liquid and click “Read Empty Weight”. Place a known mass (maximum of 0.5 kg) on the end of the selected weight platform and click “Read Test Weight”. Enter the known mass used and click “Send Test Weight”. Select the next load cell and repeat the calibration steps for the other side. Verify that calibration was successful by measuring the known mass once more after exiting the calibration window. Additionally, if known mass readings are within tolerance limits, but the zero-mass reading exceeds tolerance limits, users can select “Tare” to recalibrate the zero-mass measurement without repeating the entire calibration process.
Proper maintenance of the VASIC includes, at minimum, a superficial cleaning of the device’s exterior after each use to eliminate any rodent urine or feces. If general cleanliness of the device is not maintained, it is possible for the weight platforms to accumulate enough debris/residue such that they begin to stick to one another, which can lead to inaccurate mass readings. Occasionally, some of the sucrose water or rodent urine residue can accumulate on the load cells within the interior of the VASIC and solidify, which can temporarily alter the resistive properties of the Wheatstone bridge configuration of the load cells. If such is the case, the user may notice some inaccuracies in the reported mass values following a full calibration. Proper mass readings can be confirmed by observing the front panel numerical readout when different masses are placed on the platforms. If the device is inaccurately reporting values after calibration, the load cells and/or weight platform should be disassembled and thoroughly cleaned to eliminate any dried residue before repeating the full calibration process.
If general communication issues between the VASIC and host computer occur at any point during device operation, simply restarting the device and data acquisition program is typically sufficient to fix the issue. For issues that persist, please contact jyang75@wisc.edu with a description of the problem.
5.4. Experimental design
Prior to experimental data collection, the rodents must be subjected to a short conditioning period in which they learn the necessary water-accessing behavior. This conditioning period involves a standard 3-h water deprivation directly before 30 min of data acquisition, once per day, over a series of days without gaps in between. We have found that the minimum conditioning period is typically 2–3 days for rats and 5–6 days for mice, and we recommend adding at least 1 additional day to the minimum to ensure stability of the learned behavior. No adverse effects on the mice due to daily brief water deprivation could be discerned as assessed by weight gain, normal activity, and normal grooming. Generally, we consider the behavior to be successfully learned once the average total access time surpasses 100 s for rats and 50 s for mice; however, this is based on our observations and has not been exhaustively studied. Note that if the rodents are not exhibiting sufficient motivation to access water, the length of water deprivation can be increased by 1–2 h during the conditioning period after proper approval by the local animal use committee to increase their water access.
Following the conditioning period, the experimental data collection period can proceed. Each day of data collection should begin with a standard 3-h water deprivation directly before 30 min minimum of data acquisition. The timing of the experimental period is flexible, and we have not observed significant extinction of the learned water-accessing behavior after up to 2 weeks without data collection. After completion of the experimental period, the VASIC Data Analysis software can be used to process and filter the collection of data (see Section 2.3 Software), which generates both cumulative and individual reports for the data.
6. Validation and characterization
6.1. Mice experiments
6.1.1. Spared nerve injury pain model
Animal studies were approved (Protocol M05883) by the University of Wisconsin institutional animal care use committee, and mice were treated in accordance with published NIH standards. Male mice (5–6 wks old) weighing ~30 g, purchased from Envigo Lab (Madison, WI), were used for this study. General anesthesia was induced by delivery of 3–4% isoflurane in oxygen at 3 L/m in an induction chamber. Once the animal was unconscious, it was placed on the surgical pad and 1.5% isoflurane in oxygen was introduced at a rate of 2 L/m via a nose cone to maintain anesthesia during the surgery. Upon clipping the hair on the dorsal aspect of the left hind leg, the surgical area was cleaned with 70% ethanol and povidone–iodine scrub solution. An incision of 1.5–2 cm was made dorsal to the pelvis where the biceps femoris and left gluteus superficialis are separated. A small incision was made between the two muscle bellies to expose the sciatic nerve and distally followed to identify the tibial and common peroneal nerves. These two terminal branches of the sciatic nerve were tied with a strand of a silk suture and cut distally as described [10]. The incision was infiltrated with 1 mL of 0.5% bupivacaine for local analgesia and closed by surgical staples. Mice exhibited normal exploratory activity almost immediately upon awakening from general anesthesia. Post-operatively, no mice exhibited evidence of extreme pain such as autotomy, poor grooming, or lack of normal weight gain.
6.1.2. Behavioral assessment protocol
Prior to a single behavioral assessment session in VASIC, the mice were subjected to 3-h of water deprivation in their housing cage. The behavioral assessment protocol for mice was identical to the protocol previously described for rats [9] except the data collection period lasted 45 min instead of 30 min. During behavioral assessment, the period of water deprivation was ended and the animals were given free access to 6% sucrose in water from the bottle within the weighing chamber. The mice repeatedly and voluntarily entered the weighing chamber to access water during this period, which initiated data collection from the left and right weight platforms. Following the 45 min of behavioral assessment, the mice were returned to their housing with restored access to water.
The mice were subjected to a conditioning period of 13 days to evaluate potential differences in learning between the mice strains; however, 5 days appeared sufficient for conditioning of the FVB/N mice as it was for rats. The physical VASIC device used by a given mouse was rotated over subsequent days to reduce potential device-dependent bias in the behavior. The brief water deprivation before behavioral assessment had no apparent ill-effect on the health of the mice as evaluated by general grooming and daily weight measurements.
6.2. Results
We provide details of the VASIC device, which incorporates updates from our previous report. The external aspect of the improved VASIC looks the same except for an additional insert placed in the central chamber allowing for assessing behavior in mice (Fig. 1). In contrast to the OEM electronic circuitry used in the original version, the internal hardware in the present device was greatly simplified and the production cost was significantly reduced using a commercially available Arduino microcontroller and custom Arduino Shield PCB designed in-house. The operation of the VASIC device remains the same from our original report in that a rodent seeking water enters the central chamber with their hind feet placed on the weighing platforms. The rodent reaching for the water spout breaks the infrared sensor, which indicates correct positioning and triggers data acquisition. The left and right mass of the rodent, as recorded by the weighing platforms, are averaged over a user specified duration and the data is sent to a host computer along with a time stamp for each averaged data point over the duration of the sensor break. We define the total duration of sensor breakage and water access by the rodent as an “access event”. As the rodent explores the environment and voluntarily enters and leaves the central chamber, the number of access events over the data collection period increases. The typical behavior of a mouse in VASIC can be seen in a video clip (Ex.Post-SNI-edit.mp4 in Vasic Samples Folder). The data capture continues for the user specified duration of the experiment and is output as a comma separated value (CSV) formatted datafile upon completion of the experiment. A typical experiment of 45 min duration consists of numerous access events with a total access time of >100 s and hundreds of time-averaged mass data points. One computer can host several VASIC devices without loss of data communication, which greatly increases the efficiency of behavioral data capture. We have hosted up to 8 VASIC devices for simultaneous data acquisition on a single computer, but do not know the upper limit of the number of devices that can be hosted without incurring clashing and loss of data. Fig. 2 shows the circuit diagram separated into the three functional components of load cell amplifier, IR sensor, and a microcontroller. Computer aided design (CAD) files for production of the printed circuit board, the plexiglass enclosure, acrylic pieces, and metal base box can be found in the VASIC Hardware Folder.
Fig. 1.
Views of the assembled VASIC device. A. Front view with mice standing on the weighing platform drinking water. Note the recorded mass of a mouse 5 days after surgery on the LCD screen showing asymmetric mass distribution with 15.97 g (right) and 7.87 g (left). The IR sensor LED is “off” indicating correct positioning of the mouse and that the data is being recorded. B. Inside view of the device with the panel mounted LCD screen, Arduino and the stacked shield in the left-middle, and the two load cells in the middle. More inside views during the assembly process can be found in Supplemental Data 1. C. Top view with the weighing platforms and the IR sensor flexible arms removed showing the load cells through the opening in the platform.
Fig. 2.
Circuit diagram and PC board layout. A. Wiring diagram of the circuit in functional blocks of Display (top left), IR detector (top right), and Arduino and load cell amplifier (middle). B. Arduino shield printed circuit board design with conductors and mounting holes for the various components. A CAD file of the shield is provided in the online repository and a view of the fully assembled shield with all the components mounted can be found in Supplemental Data 1.
Table 1 lists the main features of the software and Supplemental Data 2–4 further details the program flow. The analysis software allows for several post-acquisition optional levels of filtering the data. The first filtering level excludes access events with less than 2 s of data (i.e. 4 data points with a data averaging duration of 0.5 s) to reduces inclusion of mass measurements from unstable positioning of the rodent on the platforms that can occur when the rodent only briefly breaks the sensor. The second data filtering level eliminates data points where the summation of both weighing platforms (i.e. the total detected mass) is outside of ±25% of the calculated body-mass. The elimination of these outliers reduces inclusion of data where the rodent’s body is not fully positioned on the weighing platforms, which could result in inaccurate weight bias representation. The third filtering level considers the infrequent but incorrect positioning of the rodent on the platforms such that both hind limbs are on a single platform. We exclude these measurements by eliminating data with >97% of the total mass of the rodent placed on one platform. In general, we have found that these filtering parameters typically do not change the average weight bias in a comparison between raw and filtered data, provided that the rodent has sufficient total access time (>50 s) during the experiment. The script for the device’s Arduino microcontroller can be obtained from the Github file repository link provided above and needs to be uploaded to the device upon a first-time setup.
Table 1.
Main features of the 3 programs. Further details of the flow of the programs can be found in Supplemental Data 2–4.
| Arduino setup (Arduino Script) |
| Main loop-set IR LED, display LCD, Check for command from the host computer |
| Time Mode-read and set signal averaging time |
| Collection Mode-set up read/write timer, check IR status |
| Tare Mode-set up load cell tare routine, read 0 weight values |
| Calibration Mode-set up calibration routine, read calibration values |
| Data Acquisition (QT Creator) |
| Main window-set default values, initiate event listener |
| GUI interface-Calibration, Tare, Avg Time Selection, Settings Dialog |
| Data Processing (MATLAB) |
| Main loop-read raw data, calculate access time, append calculated data, create output file |
| Filter 1-filter data by duration per access, retain valid data |
| Filter 2-filter data by total weight, retain valid data |
| Filter 3-filter by minimum weight per foot pad, retain valid data |
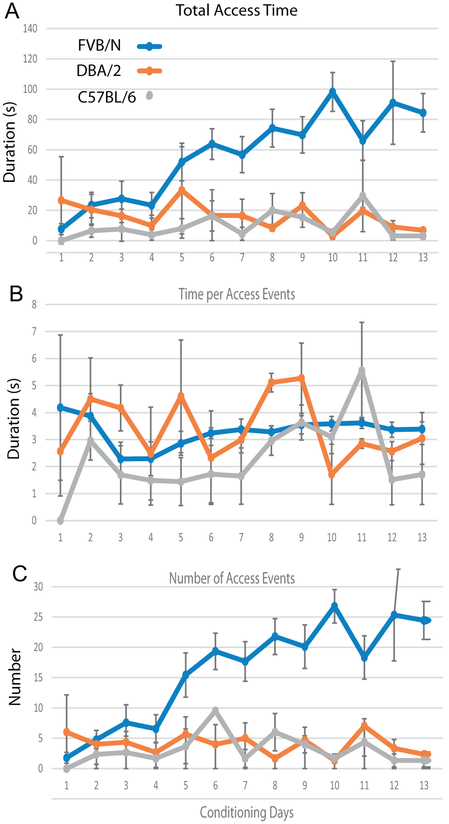
We determined that a simple insert placed within the central chamber to restrict the physical space allowed the use of VASIC designed for rats for weight bias determination in mice. Preliminary experiments with 3 strains of mice (FVB/N, DBA/2J, C57BL/6) demonstrated a difference in their ability to learn water access. Fig. 3 shows the behavior of mice during the conditioning phase with 3 h of water deprivation immediately preceding the behavioral assessment, which follows the protocol we established for the Sprague Dawley rats. FVB/N mice demonstrated an increase in the number of access events and total access time with conditioning like what we observed with rats. Five days of conditioning resulted in FVB/N mice providing >50 s of total access time during a single 45 min experimental session with a gradual increase in total access time with further conditioning. In contrast, DBA/2J and C57BL/6 performance did not improve with increasing days of conditioning up to 13 days. However, even the poor performers gave 5–10 s of total access time, allowing for assessment of the weight bias albeit with greater variability due to the smaller amount of data that was acquired.
Fig. 3.
Time course of conditioning for 3 mice strains. Total access time (time the mice spent on the platform drinking water) (A), time per access event (time the mice spent on the platform each time the IR sensor was triggered (B), and number of access events (number of times the mice triggered the IR sensor during the recording session (C) plotted for 13 days of conditioning. Data from 3 mice for each strain (FVB/N n = 9, DBA/2n = 3, C57BL/6n = 3). Note the excellent learning with a steady increase in total access time over the duration of conditioning for FVB/N.
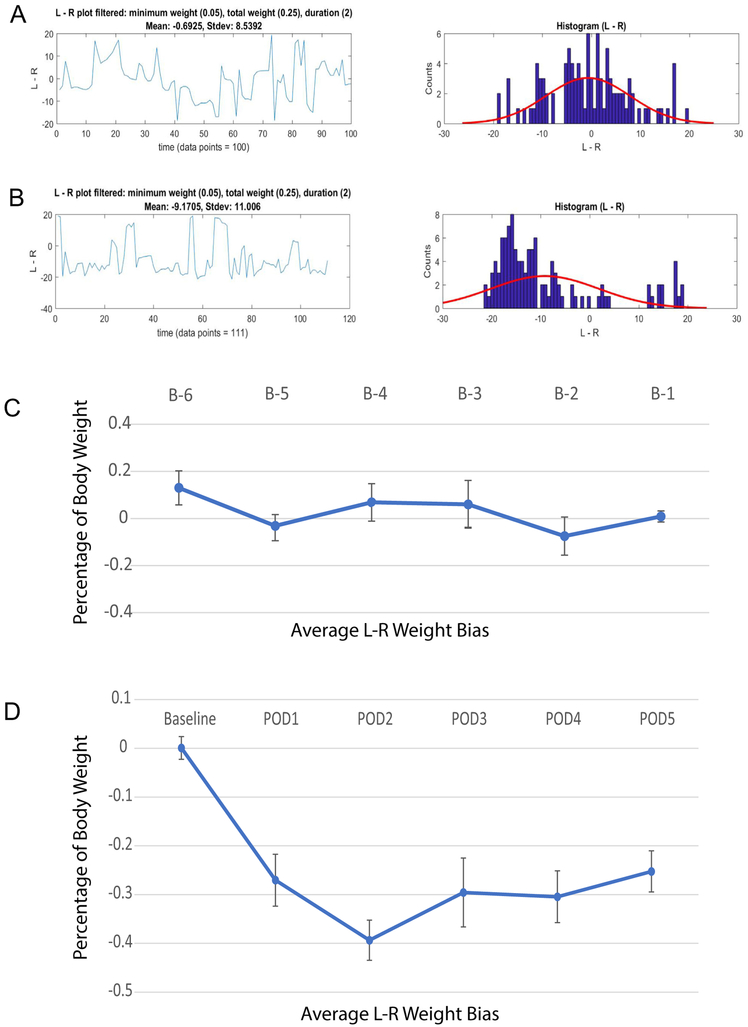
Fig. 4 shows the weight bias in FVB/N mice after a spared nerve injury (SNI) to the left sciatic nerve. VASIC assessment demonstrated a consistent 30–40% (body-mass) weight bias favoring the uninjured leg, immediately notable at postoperative day (POD) #1 and remaining stable for the 5-day observation period. In our previously published data with rats [9], we have followed the rodents for up to 6 weeks post-surgery (SNI model) without an observable extinction of the weight bias (30–40% body-mass). Sample mice raw data file from the behavioral assay at D1 and D5 during conditioning prior to surgery (Conditioning_D1.csv, Conditioning_D5.CSV) and D1 after SNI surgery (SNI-POD1.csv) can be found in the VASIC Samples Folder. Furthermore, once conditioned to seek water after a brief water deprivation, the mice retained the learned water-access behavior, thus provided weight-bias data, with little evidence of behavioral extinction even after 1 week of no conditioning. This retention of the conditioned behavior eliminates the necessity for continued conditioning with implications on experimental designs extending for a period requiring weeks to months. However, we have not systematically examined how long this behavior is maintained.
Fig. 4.
Weight bias measurements before and after SNI surgery. A. Left-Right weight bias (g) vs. time (s) for a FVB/N mouse at one day before surgery (left) and the same data shown as a histogram (right) with a best fitting normal distribution (red) with a mean = −0.6925 g and SD = 8.5392 g based on 100 data points obtained during a recording session. B. Same as A but at 2 days post-surgery. The weight bias distribution showed a mean = −9.1705 g and SD = 11.006 g from 111 data points. C. Time course of weight bias for a cohort of FVB/N (n = 9) over 6 days before SNI surgery plotted as percentage of body mass (mean ± S.E.M.). D. Time course of weight bias of the same cohort of FVB/N (n = 9) over 5 days after SNI surgery plotted as percentage of body mass (mean ± S.E.M.). The baseline data point (from B-1 pre-surgery) is shown for comparison of the post- to pre-surgery data.
6.3. Conclusions
The main contribution of this work is in documenting that VASIC can be used to assess weight bias in mice, allowing for unbiased high throughput evaluation of pain. Although many of the currently utilized genetic mice models for pain research is not created in the FVB/N background strain, future genetic models could be created in this background to take advantage of the excellent learning exhibited by this strain. Non-genetic pain models such as inflammatory or surgical models could be readily implemented in the FVB/N mice. All CAD files and information required for reproducing the VASIC hardware, in-house, and all source code for the software are provided for any interested investigator to implement this device. The cost for fabricating the plastic parts and the metal enclosure is ~$500 with an additional ~$200 for the electronics and load cells for a total cost of $724. Soldering of the electronic components onto the PCB does require practice and access to tools for surface-mount components (i.e. hot-air reflow station), but the entire assembly of the hardware takes little over 3 h in our laboratory. The executable binary files of the software should run without modifications on most Windows computers. However, the source code is also provided for custom modification of the software if desired. Multiple devices can be created and behavioral pain assessment in rodents can be performed in parallel all well within the budget of most academic laboratories. Aside from the clear benefit of reducing time required for the hitherto bottle-neck behavioral assessment in pain research, the standardized weight bearing by VASIC with minimal chances of operator bias should produce results comparable from one laboratory to another.
Mice tended to explore the environment more than rats and, even after conditioning, the mice climbed onto the flex-arm shaft supporting the infrared LED and photodiode during the recording sessions. These activities did not trigger the IR sensor and did not interfere with the mass measurement data collection. However, even with the additional plastic insert placed inside the inner recording chamber to limit mice movement, the mice exhibited a greater frequency of misalignment and bad positioning on the weighing platforms in comparison to rats. The typical hundreds of weight-bias data points provided by VASIC, in contrast to 10 s of data points available at best in most behavioral pain assays, allowed for post-acquisition filtering of data to eliminate these spurious values. Unbiased and unsupervised collection of mass data results in capturing some data where the mice are mispositioned on the weight platform. These incorrect mass data points were filtered during data analysis, but overall the post-acquisition filtering does not typically change the overall average weight bias value since the spurious data are few in comparison to the large number of total data captured. Overall, we believe the VASIC assessment of weight bias worked well for mice once they were conditioned to seek water after a brief water deprivation.
The mice strain difference in their conditioning ability documented in the current study is consistent with the reported strain divergence in a range of behaviors reported [11,12]. Significant behavioral differences exist even among mouse sublines relatively recently derived from the same parent strain [13]. Of the three mice strains studied, FBV/N showed the best conditioning and produced the most abundant weight bias data. The other two strains examined also showed some water-seeking behavior and provided sufficient observations to assess weight bearing bias, although with less reliability due to the small number of observations obtained during the 45-min experimental duration. The design of the VASIC device and the ability to follow weight bias over time in freely roaming rodents by incorporating weight bias measurement capability in a home cage environment should allow a longitudinal study of diurnal variation in pain.
We believe the ability to build the device in-house at a low cost from the detailed information provided will open the door for a more wide-spread evaluation of this device and incorporation of VASIC into creative studies of pain.
Supplementary Material
Acknowledgment
This work was supported by grants from the National Institutes of Health (RO1 GM105665 and GM107054) and the Bamforth Endowment Fund from the Department of Anesthesiology, UW Madison.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ohx.2018.e00031.
References
- [1].Woolf CJ, Overcoming obstacles to developing new analgesics, Nat. Med. 16 (2010) 1241–1247, 10.1038/nm.2230. [DOI] [PubMed] [Google Scholar]
- [2].Borsook D, Hargreaves R, Bountra C, Porreca F, Lost but making progress-where will new analgesic drugs come from?, Sci Transl. Med. 6 (2014), 10.1126/scitranslmed.3008320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA, an overview of animal models of pain: disease models and outcome measure, Pain 14 (2013) 1255–1269, 10.1016/j.jpain.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mogil JS, Animal models of pain: progress and challenges, Nat. Rev. Neurosci. 10 (2009) 283–294, 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- [5].Tappe-Theodor A, Kuner R, Studying ongoing and spontaneous pain in rodents-challenges and opportunities, Eur. J. Neurosci. 39 (2014) 1881–1890, 10.1111/ejn.12643. [DOI] [PubMed] [Google Scholar]
- [6].Li JX, The application of conditioning paradigms in the measurement of pain, Eur. J. Pharmacol. 716 (2013) 158–168, 10.1016/j.ejphar.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Whittaker AL, Howarth GS, Use of spontaneous behavior measures to assess pain in laboratory rats and mice: how are we progressing?, Appl Anim. Behav. Sci. 51 (2013) 1–12, 10.3109/00365521.2013.812142. [DOI] [Google Scholar]
- [8].Barrett JE, The pain of pain: challenges of animal behavior models, Eur. J. Pharmacol. 753 (2015) 183–190, 10.1016/j.ejphar.2014.11.046. [DOI] [PubMed] [Google Scholar]
- [9].Kim HT, Uchimoto K, Duellman T, Yang J, Automated assessment of pain in rats using a voluntarily accessed static weight-bearing test, Physiol. Behav. 151 (2015) 139–146, 10.1016/j.physbeh.2015.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Decostered I, Woolf CJ, Spared nerve injury: an animal model of persistent peripheral neuropathic pain, Pain 87 (2000) 149–158. [DOI] [PubMed] [Google Scholar]
- [11].Crabbe JC, Wahlsten D, Dudek BC, Genetics of mouse behavior: interactions with laboratory environment, Science 284 (1999) 1670–1672. [DOI] [PubMed] [Google Scholar]
- [12].Volkar V, Koks S, Vasar E, Rauvala H, Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies, Phys. Behav. 72 (2001) 271–281. [DOI] [PubMed] [Google Scholar]
- [13].Bryant CD, Zhang NN, Sokoloff G, Fanselow MS, Ennes HS, Palmer AA, McRoberts JA, Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies, J. Neurogenet 22 (2008) 315–331, 10.1080/01677060802357388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.