Abstract
Acute pancreatitis (AP) associated with intravenous administration of propofol has been described with unknown causal relation. We therefore assessed this causality in a systematic review. Multiple databases were searched on 16 August 2017; studies were appraised and selected by two reviewers based on a priori criteria. Propofol causality was evaluated with the Naranjo scale and Badalov classification.
We identified 18 studies from 11 countries with a total of 21 patients, and the majority had adequate methodological quality. The median age was 35 years (range, 4–77) and 10 (48%) were males. Overall, propofol was administrated in 8 patients as sedative along with induction/maintenance of anesthesia in 13 patients; median dose was 200 mg, with intermediate latency (1–30 days) in 14 (67%). Serum triglycerides were >1000 mg/dL in four patients. Severe AP was observed in four patients (19%). AP recurrence occurred in one out of two patients who underwent rechallenge. Mortality related to AP was 3/21(14%). Propofol was the probable cause of AP according to the Naranjo scale in 19 patients (89%).
Propofol-induced AP has a probable causal relation and evidence supports Badalov class Ib. Hypertriglyceridemia is not the only mechanism by which propofol illicit AP. Propofol-induced AP was severe in 19% of patients with a mortality rate related to AP of 14%. Future research is needed to delineate whether this risk is higher if combined with other procedures that portend inherent risk of pancreatitis such as endoscopic retrograde cholangiopancreatography.
Keywords: Acute pancreatitis, drug-induced pancreatitis, propofol, systematic review
Introduction
The diagnosis of drug-induced pancreatitis is often a clinical challenge and it can be suspected when a culprit drug has been ingested after excluding common etiologies of acute pancreatitis (AP), such as gallstones, alcohol ingestion and hypertriglyceridemia, in addition to other less common etiologies [1]. Propofol has been a widely used medication since its recognition by the US Food and Drug Administration in 1989 [1], and it has become a popular anesthetic agent. Propofol is used for conscious sedation in diagnostic and surgical procedures, sedation during intensive care, and induction and maintenance of general anesthesia in daily practice, and it is considered a safe drug. AP associated with propofol has been rarely described and is classified as class II in the Badalov classification of drug-induced AP (a minimum of four cases in published literature, with consistent latency in more than 75% of cases) [2]. Recent literature has implied a definite causal relation [3]; however, to our knowledge, no systematic review has evaluated this topic. Moreover, in the absence of hypertriglyceridemia, the mechanism of propofol-induced AP remains unclear and its incidence is unknown. In this study, we aimed to perform a systematic review of AP associated with intravenous administration of propofol and assess the causal relation.
Methods
A priori study protocol was devised and we relied on guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses to conduct this study [4].
Data sources and search strategies
An exhaustive, language-unrestricted search of multiple databases was conducted from each database’s inception date to 16 August 2017. The databases included the Ovid Cochrane Database of Systematic Reviews, Ovid MEDLINE, Ovid Cochrane Central Register of Controlled Trials, Ovid MEDLINE Epub ahead of Print, Ovid EMBASE, Ovid Medline In-Process & Other Non-Indexed Citations and Scopus. With input from study’s principle investigator (F.B.), an experienced librarian designed and conducted the search strategy. We used controlled vocabulary supplemented with keywords to search for propofol and pancreatitis. The actual strategy is available from the corresponding author (F.B.). The first 300 entries in Google Scholar were searched using the terms ‘acute pancreatitis’ and ‘propofol’ to look for studies not present in the above-mentioned databases. Google Scholar has the ability to capture any related gray literature published on the web even before being deposited in librarian-accessed repositories. Since Google Scholar is not a readily accessible repository for librarian-conducted systematic searches, we decided to search it manually to identify the most recent relevant gray literature related to this study. The number 300 was selected arbitrarily, since it would not be realistic to examine all Google Scholar entries and since our purpose was to identify the most recent and unindexed topics related to our search. To find additional cases, we also reviewed reference lists of relevant publications. For any missing data, we contacted the corresponding authors.
Methodology and study parameters
Our methodology has been previously applied in a similar study to assess causal relation of drug-induced pancreatitis [5].
AP diagnosis is made in accordance with the American College of Gastroenterology (ACG) guidelines [6], when two of the three following criteria were present: (i) characteristic abdominal pain, (ii) amylase and/or lipase serum level more than three times the normal upper limit and (iii) abdominal imaging findings of AP.
The severity of AP is classified as mild, moderately severe, or severe according to the revised Atlanta Classification [7].
Local complications of AP are indicated by the presence of acute peripancreatic fluid collection, acute necrotic collection, walled-off necrosis and pseudocyst formation [7, 8].
Systemic complications of AP are indicated by worsening of pre-existing associated illness, such as heart disease or chronic lung disease, exacerbated by AP [7, 8].
Organ failure is diagnosed when a score is ≥2 in one of the following organ systems (cardiac, renal and respiratory) according to the modified Marshall scoring system [9].
Naranjo probability scale for adverse drug reaction consists of 10 questions with yes or no answers to classify adverse drug reactions into definite, probable, possible and doubtful [10].
Badalov classification of drug-induced AP consists of five classes (Ia, Ib, II, III and IV) based on latency, rechallenge and number of published cases [2].
Latency is the interval between medication commencement and AP induction. Latency is categorized into three groups: short (<24 hours), intermediate (1–30 days) and long (>30 days). Latency is considered consistent when ≥75% of cases belong to one of the three previous groups [2].
Exclusion criteria
Duplicates were excluded along with cases that did not fulfill the ACG diagnostic criteria of AP. AP cases triggered by other potential causes were also excluded (e.g. abdominal or cardiothoracic bypass surgeries and endoscopic retrograde cholangiopancreatography [ERCP]). We excluded cases with recent and concomitant administration of drugs belonging to class Ia, Ib or II of Badalov classification of drug-induced AP [2] or drugs that were proven to cause AP after the publication of Badalov classification in 2007. More exclusion details can be found in the ‘Results’ section.
Data extraction and assessment
Data were evaluated and extracted by two independent reviewers (S.H. and R.J.K.). The data included publication format (full-text article, letter to the editors, abstract form), country of origin, publication language, year of publication, type of study (case report, case series, retrospective study, prospective study), patient demographics (age, sex, and medical history, including alcohol consumption, drugs and history of trauma), indication of treatment with propofol and the total administrated dose, clinical presentation of AP with the type of abdominal pain, degree of serum amylase and lipase elevation, serum blood urea nitrogen and creatinine, hypercalcemia and hypertriglyceridemia, interval between start of propofol and AP diagnosis (latency), abdominal imaging modalities (abdominal computed tomography, abdominal ultrasound, abdominal magnetic resonance imaging, ERCP, magnetic resonance cholangiopancreatography, and endoscopic ultrasound), assessment of AP severity, treatment of AP (supportive, mechanical ventilation, hemodialysis, percutaneous or endoscopic procedures, surgical treatment) and time until AP improvement (days), duration of follow-up (days) and final outcome (recovery or death). The two reviewers discussed and resolved disagreements regarding any study.
Assessment of methodological quality of included studies
We have relied on a recently published tool to evaluate the methodological quality [11]. This five-item tool (Table 1) suggests whether the methodological quality is low or not based upon a binary response (yes or no) to the five questions. The quality of the report was considered good when all five criteria were fulfilled, moderate when four were fulfilled and low when three or fewer were fulfilled. Disagreement regarding the methodological quality of the included studies was discussed and resolved by the same two reviewers. This tool revealed consistent results among reviewers in several systematic reviews [11–14].
Table 1.
Tool for methodological quality assessment of case reports and case series [10]
| 1. Did the patient(s) represent the whole case(s) of the medical center? |
| 2. Was the diagnosis correctly made? |
| 3. Were other important diagnoses excluded? |
| 4. Were all important data cited in the report? |
| 5. Was the outcome correctly ascertained? |
Results
Frequency of AP associated with propofol administration
We identified five studies that assessed the frequency of AP associated with propofol administration (Table 2) [15–19]. Three studies were retrospective and two prospective. The duration of propofol administration and its total dose were quite different in these studies. Moreover, these studies did not report in detail the AP diagnosis according to ACG criteria and thus did not meet the eligibility criteria set for this review except for one case from one study [18]. We could not draw definite conclusion from these studies.
Table 2.
Studies assessing the frequency of AP associated with propofol administrationa
| Author, year | Country | Study type | No. of patients | Indication of propofol | Duration of propofol | Patients with AP | % of AP |
|---|---|---|---|---|---|---|---|
| Devlin et al., 2005 [15] | USA | Retrospective | 159 adults | Sedation in ICU | Median: 89 hours | 3 | 1.9% |
| Coleman et al., 2006 [16] | USA | Retrospective | 103 patients | Anesthesiab | NR | 1 | 1% |
| Pradeep et al., 2009 [17] | India | Prospective | 150 adults | Non-abdominal surgery | NR | 0 | 0% |
| Chauhan et al., 2013 [18] | India | Prospective | 60 children | Anesthesia | Median: 1 hour | 0 | 0% |
| Kellock and Perrott, 2016 [19] | Canada | Retrospective | 150 adults | Sedation in ICU | Median: 48 hours | 0 | 0% |
AP, acute pancreatitis; ICU, intensive care unit; NR, not reported.
aNone of these studies fulfilled the eligibility criteria set for this review.
bNo precision for type of surgical interventions.
Study characteristics
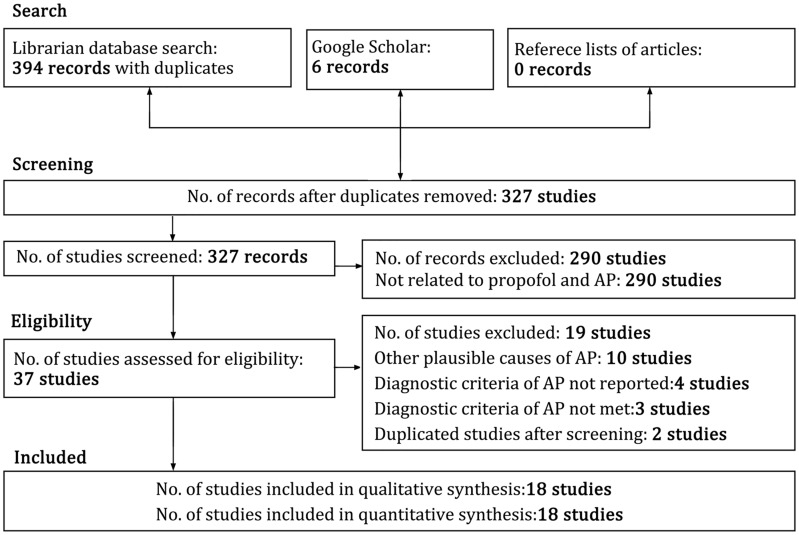
Figure 1 shows the flow diagram for the different phases of this systematic review. The database search revealed 394 publications. After screening for duplicates and excluding studies not related to propofol and AP, 37 studies were retained. We then excluded 19 studies: 2 duplicate studies, 4 studies that omitted ACG criteria details for AP diagnosis, 3 studies that did not satisfy the ACG criteria for AP diagnosis, 10 studies that could have other potential etiologies of AP and 1 patient from the study of Leisure et al. [1] (this study had 4 included patients and 1 excluded patient) who had unexplained recurrence of AP 1 month after the initial episode. A total of 18 publications from 11 countries that fulfilled the selection criteria [1, 3, 16, 20–34] (Table 3) remained. Twelve studies were found by the librarian search and six studies through the Google Scholar database [16, 23, 27, 29–31]. There was one publication each in Japanese [23], Turkish [24], Dutch [3] and Spanish languages [27], whereas the remaining publications were in English. The Google Translate website (translate.google.com) was used to translate studies published in languages other than English. Native speakers were contacted in case of translation difficulty. Primary authors were contacted to solicit more information in case of missing data. Three publications were in abstract form [29–31], four were letters to the editor [25–27, 32] and the remaining were full-text articles. There was 1 case series reporting 4 patients [1] and 17 case reports that reported 1 patient each, for a total of 21 patients.
Figure 1.
Flow chart showing the different phases of the systematic review. AP indicates acute pancreatitis.
Table 3.
Overall results of 21 cases of AP associated with propofol administration
| Author, year | Country | Age, yrs | Sex | Propofol |
Latency | Treatment of AP | Follow-up | Outcome | Naranjo scale | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication | Duration | Total dose, mg | |||||||||
| Leisure et al., 1996 [1] | USA | 34 | Male | Induction of anesthesia | 65 min | 200 | 24 h | Supportive | NR | Recovery | 5 (probable) |
| 43 | Male | Induction of anesthesia | NR | NRa | few d | Supportive | NR | Recovery | 5 (probable) | ||
| 77 | Female | Induction of anesthesia | NR | 100 | 1–2 d | Artificial ventilation | Death | Death | 4 (possible) | ||
| 58 | Female | Induction of anesthesia | NR | 150 | 1–2 d | Hemodialysis | Death | Death | 4 (possible) | ||
| Metkus et al., 1996 [22] | USA | 34 | Female | Sedation | 69 d | NRb | 69 d | Drainage of fluid | 330 d | Recovery | 5 (probable) |
| Fujii et al., 1998 [23] | Japan | 73 | Male | Induction of anesthesia | 140 min | 100 | 1 d | Supportive | NR | Recovery | 5 (probable) |
| Şentürk and Kerman, 1999 [24] | Turkey | 60 | Male | Induction of anesthesia | 140 min | 200 | 2 d | Supportive | 10 d | Recovery | 5 (probable) |
| Bird and Brim, 2000 [25] | UK | 34 | Male | Induction of anesthesia | 15 min | 200 | 1 d | Supportive | 7 d | Recovery | 5 (probable) |
| Betrosian et al., 2001[26] | Denmark | 35 | Male | Induction + maintenance | 15 min | 140 | few h | Hemodialysis | 21 d | Recovery | 7 (probable) |
| Jawaid et al., 2002 [20] | USA | 21 | Female | Induction of anesthesia | 138 min | 150 | 1 d | Supportive- antibiotics | 21 d | Recovery | 7 (probable) |
| Farina Castro et al., 2002 [27] | Spain | 23 | Male | Sedation | 7 d | 103 750 | 6 d | Supportive | 35 d | Recovery | 5 (probable) |
| Manfredi et al., 2004 [21] | Italy | 27 | Female | Sedation | 60 h | 600 | 3 d | Supportive-octreotide | 19 d | Recovery | 7 (probable) |
| Gottschling et al., 2005 [28] | Germany | 12 | Female | Sedation | 45 min | 235 | 2 h | Intensive care | NR | Recovery | 5 (probable) |
| Rake et al., 2006 [29] | USA | 12 | Male | Sedation | <24 h | NR | <24 h | Parenteral nutrition | NR | Recovery | 5 (probable) |
| Coleman et al., 2006 [16] | USA | 42 | Female | Induction of anesthesia | NR | 300 | 1–2 d | Supportive | > 18 d | Recovery | 5 (probable) |
| Gottesman et al., 2007 [30] | USA | 36 | Female | Sedation | NR | NR | 1 d | Supportive | 14 d | Recovery | 7 (probable) |
| Tan et al., 2007 [31] | USA | 4 | Female | Induction + maintenance | 60 min | >45c | 10 h | Parenteral nutrition | 30 d | Recovery | 5 (probable) |
| Ting and Lee, 2012 [32] | Singapore | 14 | Female | Sedation | 2 d | 5043 | 72 h | Supportive | Death | Deathd | 5 (probable) |
| Muniraj and Aslanian, 2012 [33] | USA | 71 | Male | Sedation | 6 d | 8800 | 5 d | Supportive | 17 d | Recovery | 7 (probable) |
| Scholten and Buijs, 2014 [3] | Netherlands | 72 | Male | Anesthesia maintenance | few h | 300 | 2–3 h | Supportive | 365 d | Recovery | 7 (probable) |
| Csomor et al., 2017 [34] | Czech Republic | 57 | Female | Induction + maintenance | NR | 250 | 15 h | Artificial ventilation, vasopressors, 14 operations | Death | Death | 6 (probable) |
| Total: 21 patients | 11 countries | Median35 yrs | 10 males 11 females | Sedation: 8 Anesthesia: 13 | Median 140 min | Median 200 mg | Intermediate: 14 | 20 d | Death: 4 | Possible: 2 Probable: 19 | |
AP, acute pancreatitis; NR, not reported; min, minute; h, hour; d, day; yrs, years.
aThe reported dose was 1–1.5 mg/kg without reporting the weight and the duration.
bThe reported dose was 30–140 µg/kg/min for 69 days without reporting patient weight.
cThe total dose was 45 mg for induction and not mentioned for maintenance of anesthesia.
dPatient had viral pneumonia, bacterial tracheitis and septic shock before sedation with propofol and died from bronchopneumonia 12 days after the onset of mild AP onset.
Patient characteristics
A total of 21 patients with AP associated with propofol administration were included (Table 3). The median age of patients was 35 years (range, 4–77) and 10 (48%) patients were males. The indication of propofol was sedation in 8 patients and induction with or without maintenance of anesthesia in 13 patients. The median propofol dosage was 200 mg (range, 100–103 750 mg) and the median duration of its administration was 2.33 hours (range, 15 minutes to 69 days).
The criteria of ACG for the diagnosis of AP were satisfied in all patients (Table 4). Biliary, alcoholic and traumatic etiologies of AP were excluded in all but one patient for whom biliary imaging was not reported. Serum calcium was normal in six patients and not reported in the remaining patients. Serum triglycerides level was <150, 150–1000 and >1000 mg/dL in five, three and three patients, respectively (range, 73–4274 mg/dL) and was not reported in the remaining patients. Two patients had pre-existing hypertriglyceridemia [28, 31] and one of them had glycogen storage disease type IA [31]. Other drugs were administered in addition to propofol in 20 patients, but were considered unlikely to induce AP for different reasons: drugs that do not cause AP according to Badalov classification, absence of clear temporal relationship between propofol administration and occurrence of AP, drugs belonging to class III or IV of Badalov classification, previous exposure to a drug known to induce AP without subsequent sequelae and recurrence of AP after rechallenge with propofol despite withdrawal of the suspected drug (Table 5). The latency between propofol administration and AP diagnosis was short (<24 hours), intermediate (1–30 days) and long (>30 days) in 28, 67 and 5% of patients, respectively. No drug reaction with eosinophilia and systemic symptoms (DRESS) was reported.
Table 4.
Diagnosis of AP and exclusion of other causes of pancreatitis
| Author, year | Diagnosis of AP (ACG criteria) |
Exclusion of other causes of AP |
||||||
|---|---|---|---|---|---|---|---|---|
| Pain | Amylase/lipase > 3 ULN | Pancreatic imaging (US-CT-MRI) | Biliary | Alcohol | Trauma | Hypercalcemia | Hypertriglyceridemiaa | |
| Leisure et al., 1996 [1] | Present | Present | US: mild pancreatitis | neg | neg | neg | NR | NR |
| Present | Present | CT: pancreatic inflammation | neg | neg | neg | NR | NR | |
| Present | Present | CT: pancreatic inflammation | neg | neg | neg | NR | NR | |
| Present | Present | CT: pancreatic inflammation | neg | neg | neg | NR | NR | |
| Metkus et al., 1996 [22] | Present | Absent | CT: peripancreatic fat stranding & edema | neg | neg | neg | NR | pos |
| Fujii et al., 1998 [23] | Present | Present | CT: minimal inflammation of pancreas | neg | neg | neg | NR | NR |
| Şentürk and Kerman, 1999 [24] | Present | present | CT: mild pancreatic edema | neg | neg | neg | NR | NR |
| Bird and Brim, 2000 [25] | Present | Present | CT: prominent & inflammatory pancreas | neg | neg | neg | NR | NR |
| Betrosian et al., 2001 [26] | Present | Present | CT: confirmed AP | neg | neg | neg | neg | neg |
| Jawaid et al., 2002 [20] | Present | Present | CT: enlarged edematous pancreas—peripancreatic fluid | neg | neg | neg | neg | neg |
| Farina Castro et al., 2002 [27] | Present | NR | CT: necrosis of 40% of pancreas | neg | neg | neg | NR | pos |
| Manfredi et al., 2004 [21] | Absent | Present | US & CT: head edema—increase volume | neg | neg | neg | neg | neg |
| Gottschling et al., 2005 [28] | Present | Present | MRI: heterogeneous- tail swelling | neg | neg | neg | neg | neg |
| Rake et al., 2006 [29] | Present | Present | NR | NR | neg | neg | NR | neg |
| Coleman et al., 2006 [16] | Present | Present | CT: thickened bowel wall—bilateral pleural effusion | neg | neg | neg | NR | neg |
| Gottesman et al., 2007 [30] | Present | Present | CT: peripancreatic fluid | neg | neg | neg | neg | pos |
| Tan et al., 2007 [31] | Present | Present | US: normal pancreas | neg | neg | neg | NR | neg |
| Ting and Lee, 2012 [32] | Present | Present | CT: bulky pancreatic body—peripancreatic fluid | neg | neg | neg | NR | neg |
| Muniraj and Aslanian, 2012 [33] | Present | Present | CT: edematous pancreatitis | neg | neg | neg | NR | neg |
| Scholten and Buijs, 2014 [3] | Present | Absent | CT: infiltration & edema around head | neg | neg | neg | NR | NR |
| Csomor et al., 2017 [34] | Present | present | CT: confirmed AP | neg | neg | neg | neg | neg |
| Total: 21 patients | Present 20 Absent 1 | Present: 18 Absent: 2 NR: 1 | Signs of AP: 20 NR: 1 | Neg: 20 NR: 1 | Neg: 21 | Neg: 21 | Neg: 6 NR: 15 | Neg: 10 Pos: 3 NR: 8 |
AP, acute pancreatitis; ACG, American College of Gatroenterology; ULN, upper limit of normal; US, ultrasound; CT, computed tomography; MRI, magnetic resonance imaging; neg, negative; pos, positive; NR, not reported.
aHypertriglyceridemia >1000 mg/dL at the time of AP diagnosis.
Table 5.
Other drugs administrated with propofol
| Author, year | Drugs administrated with propofol |
||||
|---|---|---|---|---|---|
| Drugs not known to induce acute pancreatitis | Absence of clear temporal relationship | Drugs belonging to Badalov class III/IV | Previous exposure without complications | Positive rechallenge without suspected drug | |
| Leisure et al., 1996 [1] | Fentanyl—gentamicin—isoflurane—lidocaine—mezlocillin—nitrous oxide—succinylcholine—tubocurarin | – | – | – | – |
| FDA 1st patient [1] | Alfentanil—isoflurane—nitrous oxide | – | – | – | – |
| FDA 3th patient [1] | Desflurane—diltiazem—fentanyl—levothyroxine midazolam—succinylcholine | – | – | – | – |
| FDA 4th patient [1] | Alfentanil—cefazolin—desflurane—fentanil—succinylcholine—xylocaine—rocuronium | – | – | – | – |
| Metkus et al., 1996 [22] | Lorazepam—morphine—vecuronium | – | – | – | – |
| Fujii et al., 1998 [23] | Diazepam—famotidine—fentanyl—nitrous oxide—pentazocine—vecuronium—prophosporus | Acarbose—glibenclamide | Diclofenac (class IV) | ||
| Şentürk and Kerman, 1999 [24] | Atracurium—diazepam—fentanyl—isoflurane—succinylcholine | – | – | – | – |
| Bird and Brim, 2000 [25] | Alfentanil—desflurane | – | Ketorolac (class III) | – | – |
| Betrosian et al., 2001 [26] | Alfentanyl—amoxycillin/clavulanate—tobramycin | – | – | – | – |
| Jawaid et al., 2002 [20] | Amlodipine—cefotetan—fentanyl—isoflurane—lidocaine—midazolam—morphine—nitrous oxide | Ethinyl estradiol/norgestamine (class Ib) | Ketorolac (class III) | – | – |
| Farina Castro et al., 2002 [27] | – | Cannabis (class Ia)—cocaine | – | – | – |
| Manfredi et al., 2004 [21] | Chloramphenicol | – |
|
– | – |
| Gottschling et al., 2005 [28] | Thyroxin—trimethoprim (class Ib) | Carboplatine—desmopressin etoposide—hydrocortisone (class Ib)—ifosphamide (class Ib) | – | Gadolinium diethylene triamine pentaacetic acid | – |
| Rake et al., 2006 [29] | Opiate—benzodizepine | – | – | – | – |
| Coleman et al., 2006 [16] | Fentanyl—vecuronium—sevoflurane | – | – | – | – |
| Gottesman et al., 2007 [30] | – | – | – | – | – |
| Tan et al., 2007 [31] | Fentanyl—nitrous oxide—rocuronium—remifentanil | – | – | – | – |
| Ting and Lee, 2012 [32] | Fentanyl—ketamine | – | – | – | |
| Muniraj and Aslanian, 2012 [33] | Eptifibatide | – | – | – | – |
| Scholten and Buijs, 2014 [3] | Calcium carbonate—cefazoline—ephedrine—morphine—pantoprazole—phenylephrine—ondansetron | Atorvastatin (class III) Diclofenac (class IV) Hydrochlorthiazide (class II) | – | – | Acetaminophen (class II) |
| Csomor et al., 2017 [34] | Isradipine—theophylline | Hydrochlorthiazide (class II) Losartan (class Ib) | – | – | – |
Severity of AP
Different scoring systems were employed to assess the severity of AP in the published studies, such as BISAP, Ranson criteria, Balthazar score and APACHE II. The retrospective assessment of the AP severity based on Revision of Atlanta Classification revealed mild, moderately severe and severe AP in 11, 6 and 4 patients, respectively.
Patient management
Thirteen patients improved with conservative management (Table 3). Artificial ventilation, hemodialysis, intensive care and total parenteral nutrition were needed in the remaining patients. One patient underwent 14 operations for infected pancreatic necrosis and died [34]. AP was improved with a median duration of 7 days (range, 3–35 days). Twelve of 17 AP patients who recovered were followed up for a median duration of 20 days (range, 7–365 days). During the follow-up period, no recurrence of AP was reported.
Rechallenge with propofol
Two patients underwent rechallenge with propofol and one was reported positive according to ACG criteria with the exclusion of some but not all other causes of AP [3] (Table 6). Propofol was discontinued 24 hours after rechallenge in the second patient because serum amylase and lipase levels were elevated more than three times the upper limit of normal [33].
Table 6.
Rechallenge with propofol
| Author, year | Rechallenge | Drugs with propfol | Latency | Diagnosis of AP (ACG criteria) |
Improvement | ||
|---|---|---|---|---|---|---|---|
| Pain | Amylase/lipase > 3 ULN | Pancreatic imaging (US-CT-MRI) | |||||
| Muniraj and Aslanian, 2012 [33] | Several days | None | Several hours | NR | Present | NR | 1 day |
| Scholten and Buijs, 2014 [3] | 1 year | Sufentanyl—lidocaine—rocuronium sevofluran—morphine—cefazolin | Several hours | Present | Present | NR | 2 days |
| Total: 2 patients | 1 confirmed AP | ||||||
AP, acute pancreatitis; US, ultrasound; CT, computed tomography; MRI, magnetic resonance imaging; ULN, upper limit of normal; NR, not reported.
Final outcome
Mortality caused by to AP was reported in 3 of 21 patients (14%). The cause of death was acute respiratory distress syndrome in one patient [1], severe metabolic acidosis and renal failure in another patient [1] and multiple organ failure with septic shock in a third patient [34]. One patient had viral pneumonia, bacterial tracheitis and septic shock before sedation with propofol and died from bronchopneumonia 12 days after the onset of mild AP [32]. The overall mortality rate was 19% (4/21 patients).
Assessment of the methodological quality of included studies
Table 7 summarizes the assessment of the methodological quality of included studies. Five studies had good, 15 moderate and 1 low methodological quality. Regarding Question 1 (Table 1), the authors of included studies did not mention whether they reported all cases seen in their center and we assumed that they included all of their case(s) because of the rarity of this association.
Table 7.
Assessment of methodological quality of included studiesa
| Author, year | No. of patients | Question 1 |
Question 2 |
Question 3 |
Question 4 |
Question 5 |
Methodological quality | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |||
| Leisure et al., 1996 [1] | 4 | Yes | Yes | No | Yes | Yes | Moderate | |||||
| Yes | Yes | No | Yes | Yes | Moderate | |||||||
| Yes | Yes | No | Yes | Yes | Moderate | |||||||
| Yes | Yes | No | Yes | Yes | Moderate | |||||||
| Metkus et al., 1996 [22] | 1 | Yes | Yes | No | Yes | Yes | Moderate | |||||
| Fujii et al., 1998 [23] | 1 | Yes | Yes | Yes | Yes | Yes | Moderate | |||||
| Şentürk and Kerman, 1999 [24] | 1 | Yes | Yes | No | Yes | Yes | Moderate | |||||
| Bird and Brim, 2000 [25] | 1 | Yes | Yes | No | Yes | Yes | Moderate | |||||
| Betrosian et al., 2001 [26] | 1 | Yes | Yes | Yes | Yes | Yes | Good | |||||
| Jawaid et al., 2002 [20] | 1 | Yes | Yes | Yes | Yes | Yes | Good | |||||
| Farina Castro et al., 2002 [27] | 1 | Yes | Yes | No | Yes | Yes | Moderate | |||||
| Manfredi et al., 2004 [21] | 1 | Yes | Yes | Yes | Yes | Yes | Good | |||||
| Gottschling et al., 2005 [28] | 1 | Yes | Yes | No | Yes | Yes | Moderate | |||||
| Rake et al., 2006 [29] | 1 | Yes | Yes | No | No | Yes | Low | |||||
| Coleman et al., 2006 [16] | 1 | Yes | Yes | Yes | Yes | Yes | Good | |||||
| Gottesman et al., 2007 [30] | 1 | Yes | Yes | Yes | Yes | Yes | Good | |||||
| Tan et al., 2007 [31] | 1 | Yes | No | Yes | Yes | Moderate | ||||||
| Ting and Lee, 2012 [32] | 1 | Yes | Yes | No | Yes | Yes | Moderate | |||||
| Muniraj and Aslanian, 2012 [33] | 1 | Yes | Yes | No | Yes | Yes | Moderate | |||||
| Scholten and Buijs, 2014 [3] | 1 | Yes | Yes | No | Yes | Yes | Moderate | |||||
| Csomor et al., 2017 [34] | 1 | Yes | Yes | Yes | Yes | Yes | Good | |||||
| Total | 21 | 21 | 0 | 21 | 0 | 7 | 14 | 20 | 1 | 21 | 0 | Good: 5 Moderate: 15 Low: 1 |
aSee Table 1 for the contents of Questions 1–5.
Assessment of causal relation
According to the Naranjo probability scale of adverse drug reaction, 19 AP cases (90.5%) were classified as probable and 2 cases (9.5%) as possible (Table 3). Propofol belongs to class Ib of the Badalov classification of drug-induced pancreatitis given that we have one patient with positive rechallenge after the exclusion of most, but not all, other causes of AP.
Generalizability of the results
The generalizability of the results of this review to all patients with propofol-induced AP is limited because of the small number of documented cases. However, the included patients belonged to different ethnicities, had different comorbidities and the quality assessment of most included studies was moderate to good.
Discussion
Difficulty still exists in establishing the true incidence of drug-induced AP, considering the lack of prospective clinical trials as well as diagnostic uncertainties in complex clinical scenarios. However, the literature suggests that drug-induced AP accounts for 3–5% of all AP cases [35] and it is usually thought to be the third most common cause of AP, after excluding alcoholic and gallstones pancreatitis [36].
Our current clinical knowledge regarding this entity is surmised mainly from case series and case reports to the local committees or peer-reviewed academic journals. Clearly, such data may be inaccurate [37]. The true incidence, severity and etiology of drug-induced AP necessitate a framework of population-based research, which may not be available for numerous medications.
More than 100 drugs have been reported to cause AP. Strong causality evidence is not established by many published studies because of either lack of sufficient criteria to establish the diagnosis of AP, lack of rechallenge test or the inability to exclude other etiologies of AP [2]. In this systematic review, ACG criteria for AP diagnosis were met in all patients, major etiologies of AP (gallstones, alcohol, trauma) were excluded in all but one patient for whom the status of the gallbladder was not reported, and rechallenge with propofol was performed in two patients and was positive in one of them. We strongly adhered to our eligibility criteria and we excluded 17 studies reporting 26 cases of AP for different reasons. Four of these studies that reported 11 patients did not mention sufficient data to fulfill the ACG criteria and may have been appropriate for inclusion if more details were provided. However, we opted to apply highly rigorous and specific criteria to be able to confidently assess the role of propofol as pancreatitis-causative agent.
To determine the best evidence for drug-induced AP, multiple attempts have been made by critically reviewing the literature and six large studies have been published [2, 38–42]. We applied Badalov classification on included cases in this systematic review because it is based on the presence of rechallenge, latency and burden of reports to develop an evidence-based classification on the one hand and because it is a widely used tool on the other.
There is a lack of evidence in the literature to assess the frequency of AP associated with propofol administration. The five published studies assessing this frequency did not meet our eligibility criteria [15, 19]. Given that propofol is frequently administered in quotidian practice and the rarity of published cases of AP associated with its administration, this frequency seems to be very rare. The Danish drug information estimated this frequency at less than 0.01% [43].
Direct toxic injury, indirect injury by inducing hypertriglyceridemia and idiosyncratic reaction are various mechanisms of drug-induced pancreatitis [44]. Some observations support an idiosyncratic reaction of propofol as a potential mechanism [33]. Administration of propofol can result in AP due to severe hypertriglyceridemia, a well-established cause of AP [15], though it is not necessarily the only mechanism. Some authors advised against the use of propofol in patients with pre-existing hypertriglyceridemia for general anesthesia or elective sedation due to an increased risk of AP following its administration. In this review, there were three cases of hypertriglyceridemia >1000 mg/dL at the time of AP diagnosis, whereas, in five other patients, the serum level of triglycerides was normal.
DRESS rarely coincides with drug-induced AP, although they are established drug-reaction manifestations. In the French pharmacovigilance database, there were 10 cases of DRESS among 1151 reported cases of drug-induced pancreatitis (0.9%) [40]. No cases of DRESS associated with AP were seen in this systematic review.
Most patients in our review were treated with other drugs that could induce AP. The probability of these drugs to induce AP is low for different reasons (Table 5). According to Badalov classification, data that link class III and IV drugs to cause AP are weak and we did not consider the administration of these drugs as a potential cause of AP [2]. However, we cannot exclude an additional synergistic role for the drugs given concomitantly with propofol in inducing AP. A dose-dependent association between risk of AP and increasing polypharmacy is shown by, in a recent study, the odds ratio 1.69 (95% confidence interval [CI], 1.55–1.86) for patients taking 1 or 2 drugs and the odds ratio 4.57 (95% CI, 4.12–5.06) for patients taking 10 or more drugs [45].
The role of propofol compared with other sedating agents to induce AP in patients undergoing ERCP is controversial. One study showed a protective effect of propofol compared to conventional sedation for the development of post-ERCP pancreatitis in univariate analysis and no effect in multivariate analysis [46]. No statistically significant difference was seen in patients who received propofol versus patients who received other sedating agents in two additional studies [47, 48]. Another study showed increased incidence of AP in patients sedated with propofol for esophagogastroduodenoscopy and ERCP (P < 0.0001) [49]. In this systematic review, we excluded ERCP patients sedated with propofol and who developed post-ERCP pancreatitis in order to reduce the effect of this confounding factor.
A drug reaction is considered to cause a disease in the presence of a reasonable temporal sequence, amelioration of symptoms after drug withdrawal (de-challenge) and re-emergence of symptoms following rechallenge. Rechallenge is attempted when medication is not suspected to cause AP, which was seen in one patient in this review [3]. Rechallenge could also be attempted when the benefit of the drug outweighs the risk of another attack of AP, which was seen in a second patient in this review [33]. The absence of ACG criteria fulfillment for the diagnosis of AP after rechallenge in this patient could be due to the early discontinuation of propofol.
A consistent latency between drug initiation and the onset of AP may also place the drug under suspicion when rechallenge is absent and such latency implies a common underlying mechanism of action [37]. In this review, despite the absence of consistent latency, 67% of patients showed intermediate latency (1–30 days), which could indicate a common mechanism of action.
Adverse drug reactions causality can be established with the help of scoring systems. In this review, we used the Naranjo probability scale to establish the causal relationship, since it is a valid and widely applied tool in clinical practice [9]. The majority of cases in this review were classified as probable. Some questions in the Naranjo scale could not be considered (e.g. it is not ethical to increase the dose or to repeat the administration of the drug) and, for this reason, a higher score is difficult to achieve [50].
It is difficult to establish a causal relation between development of AP and a drug. We have to consider the presence of one or several confounding factors: bias, the ethical limitations of rechallenge and, in certain cases, the presence of acute idiopathic pancreatitis [35].
This systematic review has certain inevitable shortcomings. First, it is not a population-based study; rather, it is based on case series and case reports, which do not conclude a definitive causation but can be viewed as hypothesis-generating tools [51]. In the absence of higher evidence, evidence from case series becomes more significant [11]. Second, the sample size of included patients is small and it is difficult to exclude a selection bias with the report of more severe cases, which impedes the full understanding of propofol-induced AP prognosis and natural history. This issue, however, does not interfere with evaluation of causality. Third, hypertriglyceridemia and hypercalcemia were not explicitly reported to be excluded in some patients. Fourth, several drugs were administered concomitantly with propofol in most patients. The available evidence to implicate these drugs is low for the different reasons mentioned above. Fifth, for most patients, the follow-up after occurrence of AP was of short duration. Lastly, the quality of case reports and case series assessed by our proposed tool has not been validated. This tool was previously applied with good consistency among reviewers.
To our knowledge, this is the first systematic review to assess the causal relationship between propofol administration and occurrence of AP. A comprehensive literature review was conducted by an experienced librarian to identify relevant articles, manually reviewing references of relevant papers, establishing strict selection criteria and assessing their eligibility, extracting data and assessing the methodological quality of included studies by pairs of independent reviewers with a good level of agreement.
Physicians, surgeons, anesthesiologists and gastroenterologists should consider this rare and possibly life-threatening deleterious effect, due to the wide use of propofol in everyday practice. Future research is required to determine the frequency of AP associated with propofol administration and whether using this medication in patients who are either at high risk for developing AP or for procedures that have an inherent increased risk of pancreatitis (e.g. ERCP) increases the rate of propofol-induced AP.
In conclusion, AP associated with propofol is a rarely reported phenomenon and, according to the Naranjo scale, it has a probable causal relationship. The available evidence places propofol in class Ib of the Badalov classification. Hypertriglyceridemia is not the only route by which propofol may cause AP. This form of drug-induced AP is severe in 19% of patients, with a 14% mortality rate related to AP.
Acknowledgments
We highly appreciate Dr Mary M Joseph’s contribution for providing information on her study of AP associated with propofol administration. We also would like to thank Mr Larry J. Prokop for his unstinting librarian support.
Funding
No funding was provided for this manuscript.
Conflict of interest statement: none declared.
References
- 1. Leisure GS, O’flaherty J, Green L. et al. Propofol and postoperative pancreatitis. Anesthesiology 1996;84:224–7. [DOI] [PubMed] [Google Scholar]
- 2. Badalov N, Baradarian R, Iswara K. et al. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol 2007;5:648–61. [DOI] [PubMed] [Google Scholar]
- 3. Scholten J, Buijs E. [ Acute pancreatitis after propofol administration]. Ned Tijdschr Geneeskd 2014;158:A7115. [PubMed] [Google Scholar]
- 4. Moher D, Liberati A, Tetzlaff J. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bazerbachi F, Haffar S, Hussain MT. et al. Systematic review of acute pancreatitis associated with interferon-α or pegylated interferon-α: possible or definitive causation? Pancreatology 2018;18:691–99. [DOI] [PubMed] [Google Scholar]
- 6. Tenner S, Baillie J, DeWitt J. et al. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013;108:1400–15. [DOI] [PubMed] [Google Scholar]
- 7. Banks PA, Bollen TL, Dervenis C. et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11. [DOI] [PubMed] [Google Scholar]
- 8. Sureka B, Bansal K, Patidar Y. et al. Imaging lexicon for acute pancreatitis: 2012 Atlanta Classification revisited. Gastroenterol Rep (Oxf) 2016;4:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marshall JC, Cook DJ, Christou NV. et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995;23:1638–52. [DOI] [PubMed] [Google Scholar]
- 10. Naranjo C, Busto U, Sellers E. et al. Naranjo ADR probability scale. Clin Pharmacol Ther 1981;30:239–45. [DOI] [PubMed] [Google Scholar]
- 11. Murad MH, Sultan S, Haffar S. et al. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018;23:60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bazerbachi F, Sawas T, Vargas EJ. et al. Metal stents versus plastic stents for the management of pancreatic walled-off necrosis: a systematic review and meta-analysis. Gastrointest Endosc 2018;87:30–42.e15. [DOI] [PubMed] [Google Scholar]
- 13. Haffar S, Bazerbachi F, Prokop L. et al. Frequency and prognosis of acute pancreatitis associated with fulminant or non-fulminant acute hepatitis A: a systematic review. Pancreatology 2017;17:166–75. [DOI] [PubMed] [Google Scholar]
- 14. Bazerbachi F, Leise MD, Watt KD. et al. Systematic review of mixed cryoglobulinemia associated with hepatitis E virus infection: association or causation? Gastroenterol Rep (Oxf) 2017;5:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Devlin JW, Lau AK, Tanios MA.. Propofol-associated hypertriglyceridemia and pancreatitis in the intensive care unit: an analysis of frequency and risk factors. Pharmacotherapy 2005;25:1348–52. [DOI] [PubMed] [Google Scholar]
- 16. Coleman L, Zhang S, Joseph MM (eds). Propofol and Postoperative Pancreatitis—A Case Report. In: 44th Western Anesthesia Residents Conference, 2006.
- 17. Pradeep K, Wig J, Panda N. et al. Dose-related effect of propofol on pancreatic enzymes and triglyceride levels in patients undergoing non-abdominal surgery. Anaesth Intensive Care 2009;37:27–31. [DOI] [PubMed] [Google Scholar]
- 18. Chauhan M, Garg A, Bharadwaj A.. Effect of short-term propofol administration on pancreatic enzymes and lipid biochemistry in children between 1 month and 36 months. Paediatr Anaesth 2013;23:355–9. [DOI] [PubMed] [Google Scholar]
- 19. Kellock N, Perrott J (eds). Assessment of the frequency of propofol-associated hypertriglyceridemia and pancreatitis in an adult intensive care unit at a large tertiary care centre In: Pharmacy Practice Residency Program Research Project. University of British Columbia, 2016. http://www.lmpsresidency.com/research/past-projects/2015-2016-projects/. [Google Scholar]
- 20. Jawaid Q, Presti ME, Neuschwander-Tetri BA. et al. Acute pancreatitis after single-dose exposure to propofol: a case report and review of literature. Dig Dis Sci 2002;47:614–8. [DOI] [PubMed] [Google Scholar]
- 21. Manfredi R, Dentale N, Fortunato L. et al. Pancreotoxicity of propofol sedation during purulent meningitis: what is the role for octreotide? Clin Drug Investig 2004;24:181–3. [DOI] [PubMed] [Google Scholar]
- 22. Metkus AP, Trabulsy PP, Schlobohm RS. et al. A firefighter with pancreatitis. Lancet 1996;348:1702.. [DOI] [PubMed] [Google Scholar]
- 23. Fujii Y, Ohta S, Ueda N. et al. [ A case of postoperative acute pancreatitis]. J Jpn Soc Clin Anesth 1998;18:784–6. [Google Scholar]
- 24. Şentürk Z, Kerman M. [ Acute postoperative pancreatitis]. Turk Anesteziyoloji ve Reanimasyon 1999;27:209–11. [Google Scholar]
- 25. Bird H, Brim V.. Propofol and postoperative pancreatitis. Anaesthesia 2000;55:506–7. [DOI] [PubMed] [Google Scholar]
- 26. Betrosian AP, Balla M, Papanikolaou M. et al. Post-operative pancreatitis after propofol administration. Acta Anaesthesiol Scand 2001;45:1052.. [DOI] [PubMed] [Google Scholar]
- 27. Farina Castro R, Monzon Rubio E, Ojeda Betancor N. et al. [ Necro-hemorrhagic pancreatitis after prolonged propofol perfusion]. Rev Esp Anestesiol Reanim 2002;49:558–9. [PubMed] [Google Scholar]
- 28. Gottschling S, Larsen R, Meyer S. et al. Acute pancreatitis induced by short‐term propofol administration. Pediatr Anesth 2005;15:1006–8. [DOI] [PubMed] [Google Scholar]
- 29. Rake AJ, Bart R, Abou-Zamzam A.. Propofol-induced pancreatitis in a pediatric ICU: a case report. Crit Care Med 2006;34:A167. [Google Scholar]
- 30. Gottesman A, Saifan C, Graziano C.. Acute pancreatitis after propofol administration. J Hosp Med 2007;2 https://www.shmabstracts.com/abstract/acute-pancreatitis-after-propofol-administration/. [Google Scholar]
- 31. Tan P, Youssef G, Niezgoda J.. Acute pancreatitis in a four year old following a sixty minute propofol infusion. Society for Pediatric Anesthesia Newsletter 2007. (case report no. 26). [Google Scholar]
- 32. Ting TW, Lee JH.. Acute pancreatitis after propofol infusion in a teenage patient. Anaesth Intensive Care 2012;40:561–2. [PubMed] [Google Scholar]
- 33. Muniraj T, Aslanian HR.. Hypertriglyceridemia independent propofol-induced pancreatitis. JOP 2012;13:451–3. [DOI] [PubMed] [Google Scholar]
- 34. Csomor J, Murínová I, Broulíková K. et al. Propofol‐induced acute pancreatitis. J Clin Pharm Ther 2017;42:495–8. [DOI] [PubMed] [Google Scholar]
- 35. Tenner S. Drug induced acute pancreatitis: does it exist? World J Gastroenterol 2014;20:16529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nitsche C, Maertin S, Scheiber J. et al. Drug-induced pancreatitis. Curr Gastroenterol Rep 2012;14:131–8. [DOI] [PubMed] [Google Scholar]
- 37. Tenner S. Drug-Induced Acute Pancreatitis: Underdiagnosis and Overdiagnosis. Dig Dis Sci 2010;55:2706–08. [DOI] [PubMed] [Google Scholar]
- 38. Lankisch P, Dröge M, Gottesleben F.. Drug induced acute pancreatitis: incidence and severity. Gut 1995;37:565–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eland IA, Rasch MC, Sturkenboom MJ. et al. Acute pancreatitis attributed to the use of interferon alfa-2b. Gastroenterology 2000;119:230–3. [DOI] [PubMed] [Google Scholar]
- 40. Chebane L, Bagheri H, Hillaire-Buys D. et al. [Drug-induced pancreatitis: a review of French spontaneous reports]. Rev Med Interne 2015;36:573–8. [DOI] [PubMed] [Google Scholar]
- 41. Andersen V, Sonne J, Andersen M.. Spontaneous reports on drug-induced pancreatitis in Denmark from 1968 to 1999. Eur J Clin Pharmacol 2001;57:517–21. [DOI] [PubMed] [Google Scholar]
- 42. Trivedi CD, Pitchumoni C.. Drug-induced pancreatitis: an update. J Clin Gastroenterol 2005;39:709–16. [DOI] [PubMed] [Google Scholar]
- 43. Lange K, Rostgaard-Knudsen M, Rasmussen B.. [Propofol-induced pancreatitis after surgery for thyroid carcinoma]. Ugeskrift Laeger 2014;176:2–3. [PubMed] [Google Scholar]
- 44. Kumar AN, Schwartz DE, Lim KG.. Propofol-induced pancreatitis: recurrence of pancreatitis after rechallenge. Chest 1999;115:1198–9. [DOI] [PubMed] [Google Scholar]
- 45. Razavi D, Lindblad M, Bexelius T. et al. Polypharmacy and risk of acute pancreatitis. Pharmacoepidemiol Drug Saf 2016;25:1337–41. [DOI] [PubMed] [Google Scholar]
- 46. Zhou W, Li Y, Zhang Q. et al. Risk factors for postendoscopic retrograde cholangiopancreatography pancreatitis: a retrospective analysis of 7, 168 cases. Pancreatology 2011;11:399–405. [DOI] [PubMed] [Google Scholar]
- 47. Fernandes AM, Tieng A, Weinstein L. et al. Does the use of propofol sedation increase the incidence of post-ERCP pancreatitis? Gastrointest Endosc 2005;61:AB205. [Google Scholar]
- 48. Li N, Tieng A, Novak S. et al. Effects of medications on post-endoscopic retrograde cholangiopancreatography pancreatitis. Pancreatology 2010;10:238–42. [DOI] [PubMed] [Google Scholar]
- 49. Goudra B, Nuzat A, Singh PM. et al. Association between type of sedation and the adverse events associated with gastrointestinal endoscopy: an analysis of 5 years’ data from a tertiary center in the USA. Clin Endosc 2017;50:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Silva JD, Giroldi SB, Basso FDO. et al. Acute pancreatitis during interferon-alpha and ribavirin treatment for hepatitis C. BMJ Case Rep 2009;2009. Doi: 10.1136/bcr.09.2008.0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goldman SA. Limitations and strengths of spontaneous reports data. Clin Ther 1998;20:C40–4. [DOI] [PubMed] [Google Scholar]