Abstract
Background:
Exposure to endocrine-disrupting chemicals (EDCs) during gestation influences development of the F1 generation offspring and can result in disease and dysfunction in adulthood. Limited evidence suggests consequences on the F2 generation, exposed as germ cells within the F1 fetus. These F2s provide a unique window into the programming effects of EDCs.
Objective:
This study assessed intergenerational effects of EDC exposure on adult physiology and behavior in Sprague-Dawley rats.
Methods:
Pregnant rats were exposed to either a polychlorinated biphenyl (PCB) mixture, Aroclor 1,221 (A1221), the fungicide vinclozolin (VIN), or the vehicle (VEH) (6% dimethylsulfoxide in sesame oil) alone. A1221 is weakly estrogenic, while VIN is antiandrogenic, enabling us to compare different classes of EDCs. The F1 male and female offspring were bred to generate the paternal- and maternal-lineage F2 generation. This F2 generation was assessed for physiological outcomes, ultrasonic vocalizations (USVs), and sexual behavior in adulthood.
Results:
Each EDC caused phenotypic effects in a sex- and lineage-dependent manner. The most robustly affected group was the paternal-lineage males. F2 VIN paternal male descendants had increased body weight throughout the lifespan, lower concentrations of circulating estradiol, and lower adrenal and testicular indices. Both VIN and A1221 paternal-lineage males also exhibited the greatest number of changes in the characteristics of USVs in response to an opposite-sex animal and changes in sexual behaviors in a mating test.
Conclusion:
Exposure of rats to EDCs at the germ cell stage led to differences in the physiological and behavioral phenotype later in life, especially in males. This finding has implications for multigenerational physiological and reproductive health in wildlife and humans. https://doi.org/10.1289/EHP3550
Introduction
Endocrine-disrupting chemicals (EDCs) are ubiquitous environmental contaminants that alter hormone-dependent functions, including behaviors controlled by the brain’s neuroendocrine systems (Gore et al. 2015). Among EDCs are persistent legacy chemicals from past decades such as polychlorinated biphenyls (PCBs), which are present as contaminants in soil and water and detectable in virtually every living organism (Agency for Toxic Substances and Disease Registry 2000). Other EDC classes include modern-use pesticides such as vinclozolin (VIN), a commercially available fungicide (Wong et al. 1995), as well as plastics and plasticizers, pesticides, and other industrial chemicals.
The timing of EDC exposure is key to determining their outcomes. Both epidemiological and animal studies have shown that EDC exposures during perinatal development lead to increased predisposition for disease and dysfunction later in life, including hormone-sensitive neurodevelopmental outcomes (Gore et al. 2015). For example, exposure to PCBs during the period of brain sexual differentiation perturbed the development and manifestation of learning behavior (Colciago et al. 2009; Piedrafita et al. 2008), juvenile play behavior (Bell et al. 2016), and adult anxiety, social, and sexual behaviors in rodents (Bell et al. 2016; Gillette et al. 2017; Reilly et al. 2015; Steinberg et al. 2007). Although the literature on prenatal effects of VIN on behavior is limited, perinatal administration of VIN altered sexually dimorphic behaviors such as activity, play, and sexual behavior in rats (Colbert et al. 2005).
In utero exposure to EDCs influences the fetus (F1), as well as the developing germ cells of the next generation (F2) that reside within the F1 fetus (Anway et al., 2005; Brieño-Enriquez et al., 2015; Manikkam et al. 2013; Elshenawy and Simmons 2016). The F2 generation provides a unique window into understanding the impact of germ cell exposure to EDCs: it allows distinction between a body burden of EDCs (in the F1 generation) versus programming effects of the chemicals on the gametes that will eventually form the F2 generation. While the mechanism and nature of this transmission is not well understood, EDC exposure during F2 germ cell development in rodents has been shown to alter adult reproductive physiology (Steinberg et al. 2008), disrupt juvenile social behavior (Wolstenholme et al. 2012), and perturb the metabolic phenotype (Chamorro-García et al. 2017; 2013; McBirney et al. 2017; Susiarjo et al. 2015).
In rodents, the communication of affective state, social interest, and social status (Knutson et al. 2002) as well as the display of appropriate appetitive and consummatory sexual behaviors (Pfaus et al. 2012) are necessary for an individual’s social and reproductive success. These are accomplished via ultrasonic vocalizations (USVs), olfactory cues, and the physical interactions that occur during mating. USVs are crucial in conveying the affective state of the individual not only in different social and sexual situations (Willadsen et al. 2014; Wöhr et al. 2008), but also when exposed to rewarding or aversive stimuli (Barker et al. 2010; Brudzynski 2013). Although not widely studied in the EDC realm, prenatal PCBs affected properties of USVs in adolescence (Bell et al. 2016), and adult exposure to phthalates reduced the emission of USVs in mice (Dombret et al. 2017). Other behaviors before (appetitive) and during (consummatory) mating convey information about an individual’s attractiveness to the conspecific, potential fitness as a mate, and ultimately reproductive success (Adler 1969; de Medeiros et al. 2010; Erskine 1985).
In this study, we focused on intergenerational exposure to two classes of EDCs on the sociosexual phenotype. Aroclor 1,221 [A1221; a weakly estrogenic industrial mixture of PCBs (Frame, 1997)] and the fungicide VIN [antiandrogenic (Gray et al., 2001)] were selected because of their different downstream signaling actions, evidence for direct and intergenerational effects, and to make comparisons with past work in our lab. We predicted that these exposures would alter physiological and behavioral outcomes in a sexually dimorphic manner and that transmission of effects would differ in the maternal and paternal lineage due to sex differences in programming of the germline (Reik et al. 2001).
Methods
Endocrine-Disrupting Chemical Treatment
A1221 (C-221N-50MG, 083-166; AccuStandard) and VIN (N-13,745-250MG, 4,054,200; Chem Service Inc.), each at , were dissolved in a vehicle (VEH) of 6% dimethylsulfoxide (Sigma number D4540; Sigma) in sesame oil. This dose of VIN is below the acceptable daily intake for human health, estimated at (Alyea et al. 2014). Furthermore, in rats, a NOAEL level was estimated at (Hellwig et al. 2000). Previous studies have estimated that maternal–fetal transfer of this dose of PCBs results in fetal concentrations of (Takagi et al. 1986), which is relevant to circulating levels in humans (Fitzgerald et al. 2008). Although there is disagreement regarding the best route of EDC exposure, intraperitoneal injections are reliable, effective, and not toxic to the pregnant dam or the litter, and most importantly, they allow us to compare current to past work with these chemicals (Bell et al. 2016; Reilly et al. 2015; Steinberg et al. 2008). Notably, both VIN and lightly chlorinated PCBs such as A1221 have short half-lives () and are unlikely to persist beyond the exposure period (Sierra-Santoyo et al. 2008; Sundström et al. 1976). The timeframe of exposure from E8 to E18 encompasses primordial germ cell migration and reprogramming (Smallwood and Kelsey 2012), as well as the beginning of brain sexual differentiation due to sex differences in gonadal steroids in rats (Lenz et al. 2012).
Animal Husbandry and Physiological Measures
All animal protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin. Sprague-Dawley rats purchased from Harlan Laboratories were housed in a temperature- and humidity-controlled room with a 10:14 partially reversed light:dark cycle (lights off at 11:30 a.m.) and maintained at . Animals (two to three) were housed together in polycarbonate cages () with aspen bedding (Sani-Chips®; PJ Murphy Forest Products), along with a polyvinyl chloride tube for enrichment. Cages were changed weekly. Rats were fed low-phytoestrogen Harlan Teklad Global Diet (Harlan Teklad) ad libitum. Rats were handled once a week for 5 min to acclimate them to the experimenters and their new housing conditions. Mating began 2 wk after their arrival.
Daily vaginal smears were taken to monitor estrous cyclicity, and virgin female rats (3–4 mo old) were mated with sexually experienced male rats ( old). The day of mating was designated as embryonic day zero (E0). The morning after mating, vaginal smears were checked for the presence of sperm, and sperm-positive dams were single housed. From E8 to E18, dams were weighed daily and injected intraperitoneally with (depending on weight) of VEH, A1221, or VIN ( prior to the onset of the dark stage). These dams were weighed weekly to monitor growth. After the last injection, dams were provided with nesting material through parturition () and into lactation. On the day after birth, postnatal day 1 (P1), the F1 litters were culled to 10 pups of equal sex ratio, or as close as possible (de Medeiros et al. 2010). To do this, we culled those rats with extreme anogenital index measures [; (Gillette et al. 2017)] in order to remove extremes and to have a litter most representative of the treatment. After weaning on P21, individuals were housed with same-sex littermates. The F1 rats were weighed and monitored weekly.
In adulthood (), one to two F1 females and one to two F1 males per litter were bred to untreated stimulus Sprague-Dawley rats (purchased from Harlan; Figure 1A). At of age, after litters had been weaned, a subset of these latter F1 rats (; subset from rats shown in Figure 1a) were euthanized by decapitation at prior to lights off. Blood was collected and serum stored as described below for use in hormone assays. The litters that had been generated (F2) were the primary focus of this experimental design (Figure 1B). For these F2 rats, BW and anogenital distance was measured weekly for each individual until weaning on P21, after which pups were housed with same-sex littermates (two to three per cage). We continued to monitor BW weekly for the rest of each animal’s life. Females were checked daily for vaginal opening beginning on P28, while males were checked daily for preputial separation beginning on P35. The individuals in each litter were weighed and handled for 5 mins weekly, and the focal F2 individuals underwent behavioral testing in adulthood (between ). Approximately 2 wk after behavioral characterization was completed, experimental males were weighed and euthanized. Maternal-lineage F2 females, which were used to generate an F3 generation for another study, were euthanized after parturition and weaning of their F3 offspring. Paternal-lineage F2 females were euthanized on day 18 of their gestation because their F3 offspring would have a paternal–maternal mixed lineage that was not part of the current study. While the latter F3 offspring are potentially interesting, the large scope of work led us to focus exclusively on animals derived from the pure maternal and pure paternal lineages.
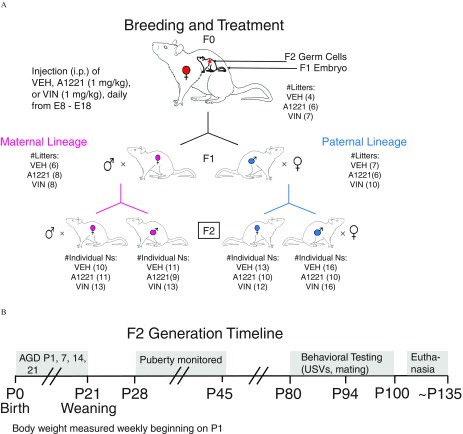
Figure 1.
(A) The breeding and treatment paradigm is shown. Pregnant rats were exposed to vinclozolin (VIN), Aroclor 1,221 (A1221), or the vehicle (VEH) dimethylsulfoxide (DMSO) daily from E8–E18. The F1 fetus was exposed directly, and F2 generation was exposed as developing germ cells, as indicated within the F0 dam. The numbers of F0 litters is indicated (four VEH, six A1221, seven VIN). From these litters, to generate a maternal lineage, six VEH, eight A1221, and eight VIN F1 females were allowed to mature and breed with untreated male Sprague-Dawley rats. To generate the paternal lineage, seven VEH, six A1221, and 10 VIN F1 males were allowed to mature and were bred with untreated female Sprague-Dawley rats. The F2 male and female pups of each lineage were the focal experimental animals, with numbers of individuals used for behavioral phenotyping indicated in parentheses and no more than two pups of a sex were used per litter. (B) Timeline of developmental monitoring and behavioral characterization in the F2 generation is shown. Note: AGD, anogenital distance; i.p., intraperitoneal; P, postnatal day; USV, ultrasonic vocalization.
Litter Usage and Sample Size
Figure 1 shows breeding and the allocation of offspring. The study began with 17 F0 dams exposed during their pregnancies (four VEH, six A1221, seven VIN). From each litter of F1 pups, one to two females and one to two males were assigned to this study and used for breeding the maternal and paternal lineages. Littermates of these F1 animals were assigned to a different study. For breeding in the current study (Figure 1A), six VEH, eight A1221, and eight VIN F1 females were bred to generate maternal-lineage F2 litters. Similarly, seven VEH, six A1221, and 10 VIN F1 males were bred to generate the paternal F2 lineage. From each F2 litter, one to two female and one to two male F2 pups were run through the entire timeline shown in Figure 1B, with n’s for the F2 generation shown in Figure 1A. For birth outcomes and developmental milestones, because of the availability of the rest of the F2 littermates, we used up to five animals per sex per litter when available, resulting in the following sample size for these endpoints: maternal females: , , ; maternal males: , , ; paternal females: , , ; paternal males: , , . Litter was used as a covariate in statistical analyses of each measure, and no effects of litter were found.
Physiology
Serum hormones.
A trunk blood sample was collected from all F2 animals at decapitation, conducted before the onset of the dark phase. A subset of F1 progenitors of the F2 generation () had the same serum hormone assays run to assess the F1 dams and sires’ physiological status as a potential contributor to F2 outcomes. In all cases, blood was centrifuged at for 5 min. Serum was collected and stored at until analysis by radioimmunoassay (RIA). Corticosterone, estradiol (), and testosterone were selected as the primary targets for this study based on previous work highlighting their sexually dimorphic disruption by VIN and A1221 (Gillette et al. 2014; Reilly et al. 2015). For all assays, standards and samples were processed in duplicate samples from individual rats, all run in a single assay per hormone. Concentrations were calculated using the average of the two duplicates based on the standard curves. Serum corticosterone was measured in serum (Corticosterone 125I RIA, RCBK1708; MP Biomedicals), with intra-assay coefficient of variation (CV) 2.1% and assay sensitivity . Serum was measured in serum (Estradiol 125I RIA, 17052C; Beckman Coulter), with intra-assay CV 2.4% and assay sensitivity . Serum testosterone was measured in serum (Testosterone 125I RIA, 07-189,102; MP Biomedicals), with intra-assay CV 2.03% and assay sensitivity .
Adrenosomatic and gonadosomatic index.
At euthanasia, adrenals and gonads were removed from all behaviorally characterized experimental animals (Ns in Figure 1A), cleaned of fat, and weighed. The adrenosomatic index (ASI) was calculated as , and the gonadosomatic index as (Crews et al. 2012; Reilly et al. 2015).
Behavior
All behavioral tests were run in the same order, at least 2 h after lights-out under dim red light, in rats aged 80–100 d of age. Adult F2 generation females were vaginally swabbed daily for 1 wk prior to testing and throughout the testing period to establish the phase of estrous cycle; all behavioral tests were conducted when females were sexually receptive.
Ultrasonic vocalizations.
On the evening of proestrus, experimental F2 females were tested with sexually experienced but untreated males to confirm receptivity. Only receptive females proceeded through the USV paradigm. Experimental males were tested at similar ages to the females.
USVs were recorded in a Plexiglas chamber () using a CM16/CMPA microphone (Avisoft) suspended above the floor of the chamber (Bell et al. 2016; Reno et al. 2013). Baseline USVs were recorded from experimental males and females in a 5-min pretest session. A plastic mesh barrier was introduced, partitioning the chamber into two equal compartments. Opposite-sex stimulus rats unexposed to EDCs were used. For experimental males, the stimulus animal was an ovariectomized Sprague-Dawley rat, implanted with a Silastic capsule (Dow Corning, inner diameter, ; outer diameter, ; length, ) containing 5% estradiol and 95% cholesterol. On the day of the test, the female was injected with progesterone (, subcutaneous) 3–5 h prior to the test to induce receptivity. For experimental females, the stimulus animal was a sexually experienced Sprague-Dawley male rat. In each case, the opposite-sex stimulus rat was introduced on the other side of the barrier from the experimental rat (Figure 2A). Then, the stimulus animal and barrier were removed, and USVs were recorded from the experimental animal left alone in the chamber for 5 min (Figure 2B).
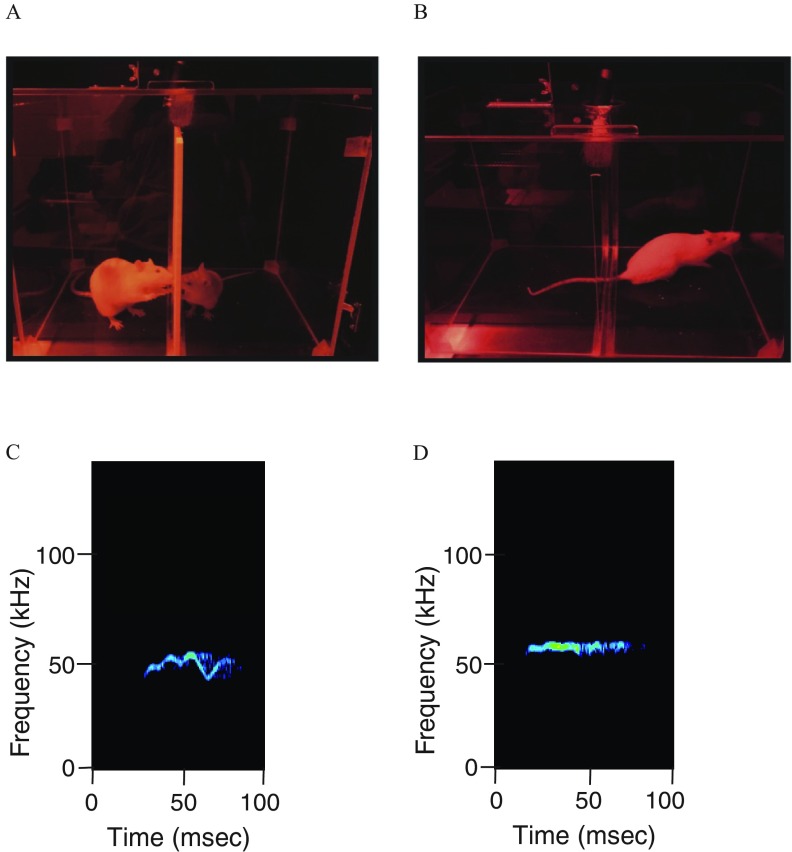
Figure 2.
(A) Photograph of an ultrasonic vocalization (USV) testing session shows an experimental and a stimulus animal interacting across a plastic grid. (B) Photograph showing the experimental rat alone in the chamber after removal of the stimulus rat. Representative spectrograms of (C) FM, and (D) flat USVs are shown. Note: FM, frequency modulated.
USVs were recorded with UltraSoundGate hardware and software (Avisoft) and analyzed with the decision tree machine learning algorithm, WAV-file Automated Analysis of Vocalizations Environment Specific (WAAVES) (Reno et al. 2013). Calls detected within the range of 30 to were considered high-frequency calls [often referred to as “positive affect” calls because they are emitted in rewarding situations (Burgdorf et al. 2011)], while those in the range of 20 to were considered low-frequency calls [referred to as “negative affect” because they are emitted in aversive situations (Knutson et al. 2002)]. The calls were further categorized into frequency modulated (FM) and non-FM (flat) calls (Figure 2C, D). Other spectrogram measures from WAAVES are detailed in Table 1. WAAVES identifies “objects” (potential USV calls) within each sound file and separates them from background noise using the criteria described in Reno et al. (2013). To optimize the software for our apparatus and paradigm, the following modifications were made:
Table 1.
Ultrasonic vocalization (USV) spectrogram measures.
| Measure | Description |
|---|---|
| Mean frequency | Mean pitch of each call |
| Duration | Length of each call, in milliseconds |
| Power | Average amplitude of each call, in negative decibels |
| Bandwidth | Range between minimum and maximum pitch of each call |
The minimum call duration for calls was set at .
Flat calls in the range were designated as those varying between per interval, whereas USVs with kHz variations over across the same intervals were classified as FM calls.
Mating behavior.
F2 male mating behavior.
Five to seven days after the USV recordings, experimental males were observed for their mating behavior. Males were habituated to the Plexiglas chamber () for 5 min prior to each test. A description of behaviors is in Table 2. Observations began once a gonadally intact and receptive stimulus female (untreated by EDCs) was introduced into the chamber. Trials were concluded after 1 h of observation or until the male had ejaculated twice. Males who did not ejaculate within this timeframe were given two additional mating trial opportunities following at least a 1-d waiting period between trials. Males who did not engage in any sexual activity in all three mating trials were excluded from the analyses (two VEH maternal lineage, one A1221 maternal lineage; five VEH paternal lineage, three VIN paternal lineage). Trials were recorded on videotape, and behaviors were scored at a later date by the experimenter, who was blind to the subjects’ ancestral treatment. Males were observed for number of and latency to first mount, intromission, and ejaculation. After the trial, the male and female were housed together overnight in a home cage and allowed to continue mating to ensure pregnancy.
Table 2.
Mating behaviors of male rats.
| Behavioral category | Endpoint measured | Description |
|---|---|---|
| Male-initiated behaviors | Mount/intromission frequency | Frequency of mounts/intromissions displayed (per minute) |
| Mount/intromission/ejaculation latency | Latency to first mount/intromission/ejaculation (seconds) | |
| Female-initiated behaviors | Hops/darts elicited | Frequency of hops & darts displayed by the female (per minute) |
| Lordosis quotient (LQ) elicited | ||
| Lateral kicks elicited | Frequency of lateral kicks by the female to the face of the male (per minute) |
Paced mating behavior of F2 females.
Similar to the experimental males, experimental F2 females were tested 5–7 d after the USV recordings, on the evening of proestrus. These females were tested for sexual behaviors using a paced mating paradigm (Coopersmith and Erskine 1994; Erskine 1985; Martínez and Paredes 2001; Paredes and Vazquez 1999; Steinberg et al. 2007). Behavior was videotaped in a Plexiglas aquarium () bisected lengthwise by a clear Plexiglas divider. Two -diameter openings were constructed at the base of the divider, allowing the female to move freely between the two compartments while restricting the male to one side, due to his size. The side with the male is hereby referred to as the mating chamber, while the other side is referred to as the escape chamber. Females were observed for proceptive and receptive behaviors, as well as mating trial pacing (Table 3). After the trial, the male and female were housed together overnight in a home cage.
Table 3.
Paced mating behaviors of female rats.
| Behavioral category | Endpoint measured | Description |
|---|---|---|
| Mating trial pacing | Mounts/intromissions received | Frequency of mounts/intromissions received (per minute) |
| Latency to enter mating chamber | Latency for female to first enter mating chamber (seconds) | |
| Latency to first mount/intromission/ejaculation received | Latency to first mount/intromission/ejaculation after female’s first entry into mating chamber (seconds) | |
| Proximity behaviors | Percent time in mating chamber | |
| Proceptive behaviors | Hops/darts frequency | No. of hops & darts in the mating chamber (per min) |
| Kick frequency | No. of lateral kicks to the face of the male in the mating chamber (per min) | |
| Receptive behaviors | Lordosis quotient (LQ) | |
| Mean lordotic intensity (MLI) | Value of each lordosis score (0, 1 or 2) summed and divided by the total number of mounts (Benedict and Williams 1993; Pfaff and Sakuma 1979) |
Statistical Analyses
Physiological and behavioral data were analyzed within each sex and lineage combination. Using the Grubb’s test, at most, two outliers were removed per group per dependent variable. Because we had unexpectedly large effects of lineage (maternal vs. paternal) on a number of variables, and because most behaviors and physiological measures were specific to each sex, we conducted one-way analysis of variance (ANOVA) within each sex and lineage combination to test for effects of treatment. If a significant main effect () or trend () was found, Tukey’s HSD was used for posthoc analyses, as it corrects for multiple statistical comparisons. The data were tested for normality and homoscedasticity using the Shapiro-Wilk and Bartlett’s tests, respectively. If the data failed these assumptions, they were transformed (log, square, or square root) and analyzed with a similar one-way ANOVA. If the transformations did not resolve these issues, the data were analyzed using the nonparametric Kruskal-Wallis test (KW) to determine a main effect of EDC treatment and lineage. Since this latter test does not determine an interaction effect, we followed up on any main effect by pairwise comparisons using the Steel-Dwass method. Multiple comparisons were accounted for using the Bonferroni correction. For ANOVAs, effect sizes were calculated as partial eta-squared (), which represents the proportion of variance explained by the factor being tested. An of 0.14 or greater is considered large, whereas a between 0.06 and 0.13 is considered medium. For KW tests, effect sizes were calculated as epsilon squared (), using similar parameters for large, medium, and small effect sizes. Significance was defined as .
Principal components analysis.
Due to the large number of variables assessed, we applied a principal components analysis (PCA) to a select subset of measures to identify which accounted for most of the variance in the data. Each measure was selected, scaled, and rotated to determine the principal components, then analyzed for the magnitude and direction of correlation (loading) upon the first two principal components. Eigenvalues from each principal component were then analyzed using a one-way ANOVA within each sex and lineage combination to determine the effect of treatment. Three separate PCAs were conducted based on the following variables:
Serum Hormones. Included all three serum hormones (estradiol, testosterone, and corticosterone).
USV measures. Included USV acoustic characteristics of mean frequency, power, bandwidth, and duration.
Male sex behavior. Included all behavioral measures [latency to and frequency of mounts, intromission and ejaculations, hops/darts, kicks, lordosis quotient (LQ), and mean lordotic intensity (MLI)].
Functional landscapes.
We utilized functional landscapes to visually represent the directionality and magnitude of treatment and lineage effects across selected measures of interest (Reilly et al. 2015; Scarpino et al. 2014). Since the majority of effects were observed in male rats, we created functional landscapes for this sex only. To construct the landscapes, we used a combination of z-scaled measures. Eigenvalues from the following measures were used for the landscape: serum hormone module (PC1), USV properties module (PC1), latency to engage in sex behavior module (PC1), female-elicited sex behavior module (PC2), and z-scaled total call counts. The median eigenvalues for each treatment group were normalized to VEH to determine the magnitude of change between EDC and VEH within each lineage. Using a linear discriminant analysis (LDA), we examined whether treatment was a determining factor in how the principal components clustered within each lineage.
Results
F2 Birth Outcomes and Body Weight
The average litter size () and sex ratio (M:F) at birth for F2 litters was: maternal VEH, (1.03); paternal VEH, (1.09); maternal A1221, (1.06); paternal A1221, (1.13); maternal VIN, (1.14); and paternal VIN, (1.15).
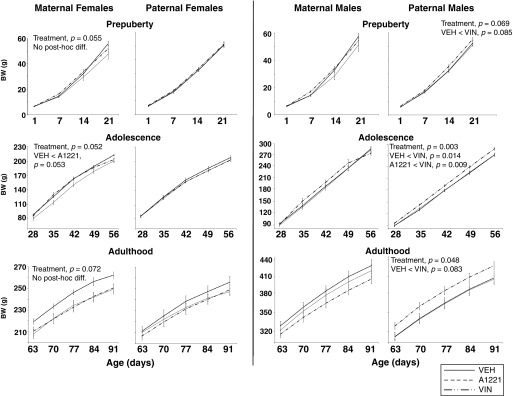
Because BW curves change through postnatal development, we analyzed BW subdivided into three distinct life phases: prepuberty (P1–P21), adolescence (P28–P56), and adulthood (P63–P91), and ran a repeated-measures ANOVA within each life stage (Gillette et al. 2017). Treatment affected BW in particular life stages (Figure 3). Females from the maternal lineage tended to be heavier in the A1221 than VEH groups at the three life stages, but this did not attain significance. In males from the paternal lineage, significant main effects of treatment were found in adolescence and adulthood () with a trend in prepuberty (). For all, the VIN males were heavier than VEH males.
Figure 3.
Body weight (BW; grams) is shown from birth through adulthood for F2 females and F2 males during three phases of development: prepuberty (P1–P21), adolescence (P28–P56), and adulthood (P63–P91). Note: A1221, Aroclor 1,221; VEH, vehicle; VIN, vinclozolin. For each curve, p-values were determined by repeated-measures analysis of variance (ANOVA) followed by Tukey’s HSD for posthoc analysis. Data are shown as .
F2 Age at Puberty
The age at vaginal opening and preputial separation were analyzed by one-way ANOVA within each sex and lineage combination. In paternal females, both A1221 and VIN females displayed vaginal opening on average d earlier than their VEH counterparts (; Table 4). In males, no significant treatment effects were found within each sex and lineage combination.
Table 4.
Statistical outcomes for physiological data in F2 female and male rats.
| Measure | Treatment | N | F (df) for ANOVA; Chi square (df) for Kruskal-Wallis | p-Value | Effect sizes: partial-eta-square (ANOVA); Episilon-square (Kruskal-Wallis) | Post hocs | Outliers (n, if any) | ||
|---|---|---|---|---|---|---|---|---|---|
| Vaginal opening (days of age) | |||||||||
| Maternal females | VEH | 13 | 0.46 | 0.04 | 2 | ||||
| A1221 | 12 | ||||||||
| VIN | 17 | ||||||||
| Paternal females | VEH | 17 | 0.001 | 0.27 |
|
2 | |||
| A1221 | 16 | ||||||||
| VIN | 15 | ||||||||
| Preputial separation (days of age) | |||||||||
| Maternal males | VEH | 13 | 0.36 | 0.05 | 2 | ||||
| A1221 | 13 | ||||||||
| VIN | 16 | 2 | |||||||
| Paternal males | VEH | 19 | 0.60 | 0.02 | |||||
| A1221 | 16 | 2 | |||||||
| VIN | 22 | ||||||||
| Adrenosomatic index | |||||||||
| Maternal females | VEH | 11 | 0.09 | 0.13 | No post hoc differences | 2 | |||
| A1221 | 10 | ||||||||
| VIN | 18 | ||||||||
| Paternal females | VEH | 11 | 0.46 | 0.06 | |||||
| A1221 | 10 | ||||||||
| VIN | 9 | ||||||||
| Maternal males | VEH | 10 | 0.02 | 0.23 |
|
1 | |||
| A1221 | 9 | ||||||||
| VIN | 13 | 1 | |||||||
| Paternal males | VEH | 13 | 0.001 | 0.32 |
|
||||
| A1221 | 12 | ||||||||
| VIN | 15 | ||||||||
| Gonadosomatic index | |||||||||
| Maternal females | VEH | 10 | 0.89 | 0.01 | |||||
| A1221 | 12 | ||||||||
| VIN | 18 | ||||||||
| Paternal females | VEH | 10 | 0.92 | 0.01 | |||||
| A1221 | 10 | ||||||||
| VIN | 9 | ||||||||
| Maternal males | VEH | 11 | 0.20 | 0.10 | 1 | ||||
| A1221 | 9 | ||||||||
| VIN | 14 | ||||||||
| Paternal males | VEH | 13 | 0.03 | 0.18 | , | 2 | |||
| A1221 | 11 | 1 | |||||||
| VIN | 15 | ||||||||
| Corticosterone () | |||||||||
| Maternal females | VEH | 10 | 0.31 | 0.08 | |||||
| A1221 | 9 | ||||||||
| VIN | 11 | ||||||||
| Paternal females | VEH | 11 | 0.81 | 0.02 | |||||
| A1221 | 9 | ||||||||
| VIN | 9 | 1 | |||||||
| Maternal males | VEH | 10 | 0.41 | 0.07 | |||||
| A1221 | 9 | ||||||||
| VIN | 10 | ||||||||
| Paternal males | VEH | 10 | 0.46 | 0.06 | |||||
| A1221 | 10 | ||||||||
| VIN | 8 | 2 | |||||||
| Estradiol () | |||||||||
| Maternal females | VEH | 10 | 0.94 | 0.00 | |||||
| A1221 | 8 | 1 | |||||||
| VIN | 10 | 1 | |||||||
| Paternal females | VEH | 11 | 0.59 | 0.04 | |||||
| A1221 | 10 | ||||||||
| VIN | 10 | ||||||||
| Maternal males | VEH | 10 | 0.37 | 0.08 | |||||
| A1221 | 8 | 1 | |||||||
| VIN | 10 | ||||||||
| Paternal males | VEH | 10 | 0.04 | 0.22 | , | ||||
| A1221 | 10 | ||||||||
| VIN | 9 | 1 | |||||||
| Testosterone () | |||||||||
| Maternal males | VEH | 8 | 0.60 | 0.04 | 2 | ||||
| A1221 | 9 | ||||||||
| VIN | 10 | ||||||||
| Paternal males | VEH | 10 | 0.01 | 0.28 |
|
||||
| A1221 | 9 | 1 | |||||||
| VIN | 10 | ||||||||
Note: Data are shown as . Statistics are presented with F-(ANOVA) or Chi-square values (Kruskal-Wallis). Degrees of freedom (df) are shown parenthetically. An effect size of 0.14 is large, and between 0.06 and 0.13 is medium. Numbers of outliers in a group (up to 2 calculated by the Grubb’s test) are indicated. Adrenosomatic index was calculated as: ; gonadosomatic index was calculated as: . A1221, Aroclor 1221; VEH, vehicle; VIN, vinclozolin.
F2 Serum Hormones
Treatment had a significant effect on serum concentrations only in males from the paternal lineage (), with F2 VIN males significantly lower than VEH males (; Figure 4; Table 4). In females, it should be noted that serum concentrations differed between maternal and paternal lineages because of differing physiological status at euthanasia (maternal-lineage females on proestrus; paternal-lineage females on day 18 of pregnancy). The resulting concentrations reflected this expected difference (concentrations on proestrus, and in late pregnancy). However, there were no treatment effects in either lineage of females. Serum testosterone (Figure 4; Table 4), measured only in males, differed significantly between treatment groups in the paternal lineage (; ; ), with A1221 males lower than VIN () or VEH () males. Serum corticosterone did not differ between treatment groups in either sex or lineage (Table 4).
Figure 4.
Serum estradiol (A) and testosterone (B) concentrations are shown for F2 rats. (A) Serum estradiol in female and male rats. Maternal-lineage females were euthanized as adults on proestrus, whereas paternal females were euthanized on day 18 of pregnancy, indicated as E18. All male rats were euthanized between 4–4.5 mo of age. (B) Serum testosterone in the same adult male rats. Note: A1221, Aroclor 1,221; MAT, maternal; PAT, paternal; VEH, vehicle; VIN, vinclozolin. *; #, as determined using ANOVA followed by Tukey’s HSD posthoc analysis. Data shown are .
A PCA on the three serum hormones in males revealed that and testosterone loaded heavily onto PC1, which accounted for 44% of the variability in the data. There was a trend toward a significant difference between the eigenvalues of the EDC treatments within the paternal-lineage males, with A1221 males lower than VEH males (; ; ).
F2 Adreno- and Gonadosomatic Indices
ASI (Table 4) differed between treatments in both maternal and paternal-lineage males. In maternal-lineage males, A1221 animals had a higher ASI than VEH or VIN (), whereas in paternal-lineage males, VIN was lower than both A1221 and VEH animals (). The ovarian index did not vary among treatment groups in females, but the testicular index in paternal-lineage males differed significantly in that VIN males were lower than their VEH counterparts (; Table 4). Both adrenosomatic and ovarian index varied between the maternal- and paternal-lineage females, which is likely attributable to the differential physiological status at euthanasia, as mentioned earlier.
F2 Ultrasonic Vocalization Results
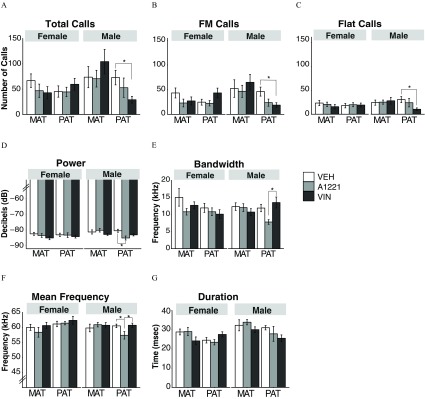
Few calls were detected; therefore, all analyses and results are focused on calls in the range. The only group affected by treatment was the paternal male lineage. For this group, total calls were emitted at significantly lower rates in VIN than their VEH counterparts (; Figure 5A), an outcome that was also seen when calls were subdivided into FM vs. flat calls (FM: ; flat: , Figure 5B, C). By contrast, A1221 affected the paternal male lineage in certain USV acoustic characteristics. These A1221 male animals had lower power/intensity than their VEH counterparts (; Figure 5D), narrower bandwidth than the VIN group (; Figure 5E), and lower mean frequency than both the VEH and VIN paternal males (; Figure 5F). Call duration did not differ significantly by treatment (Figure 5G).
Figure 5.
Ultrasonic vocalization (USV) results are shown for calls emitted by adult female and male rats of the maternal and paternal lineages. (A) Total, (B) FM, and (C) flat USVs are shown, as well as (D) call power, (E) bandwidth, (F) mean frequency, and (G) duration. Note: A1221, Aroclor 1,221; MAT, maternal; PAT, paternal; VEH, vehicle; VIN, vinclozolin. * as determined by analysis of variance (ANOVA) followed by Tukey’s HSD posthoc analysis. Data shown are .
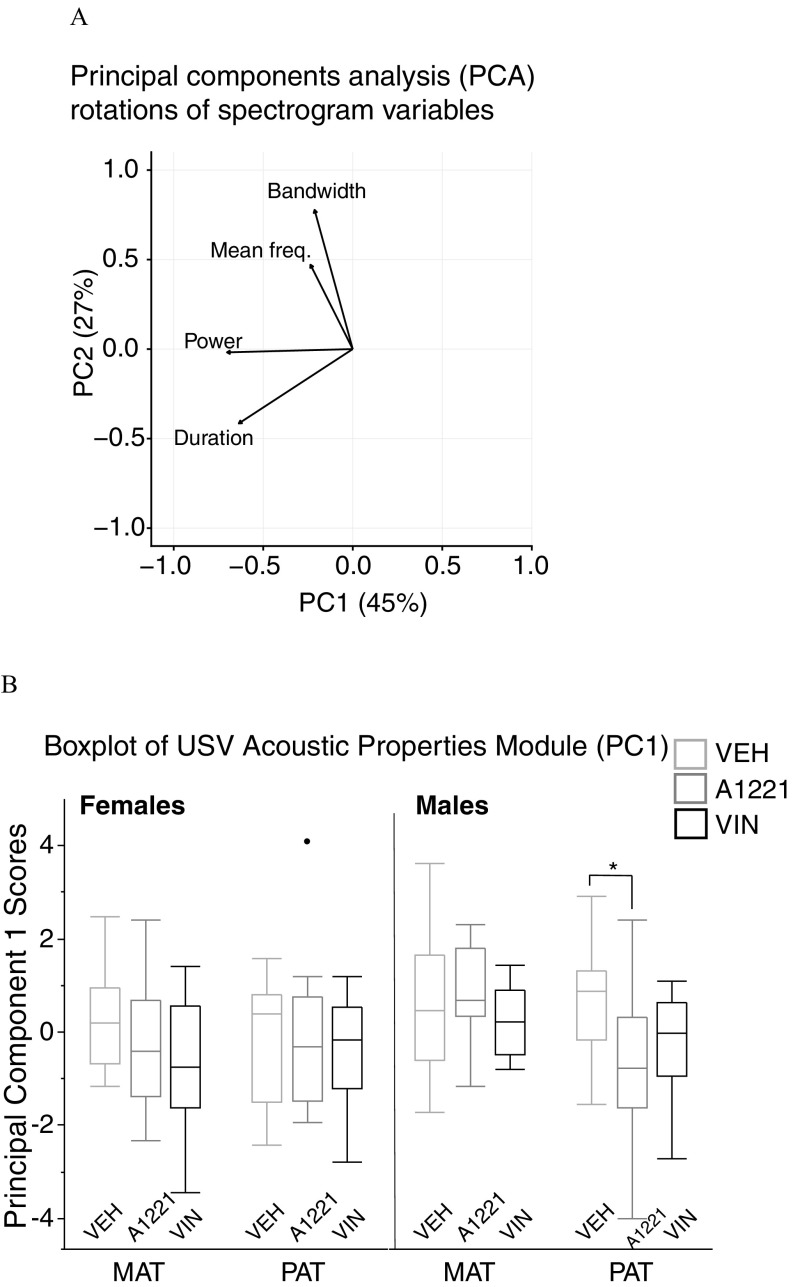
A PCA was conducted on USV spectrographic measures of mean frequency, bandwidth, power, and duration (Reno et al. 2013). Figure 6A displays the directional loading of each variable onto the first two PCs, which, combined, explained 72% of the variability in the data. Power and duration loaded heavily onto PC1, while bandwidth and mean frequency loaded onto PC2. We analyzed the differences between groups with the eigenvalues of PC1, which we named “USV Acoustic Properties Module.” A one-way ANOVA conducted on this module revealed a significant difference in paternal males between the VEH and A1221 groups (; ; ; Figure 6B).
Figure 6.
(A) Principal components analysis (PCA) with spectrogram measures, and (B) boxplots of USV Acoustic Properties Module scores from principal component 1 (PC1). Arrows indicate the directionality of loading of each variable onto each PC. (B) Boxplot data are shown as median (line), with the 25th percentile and 75th percentile indicated by the upper and lower quartiles of the box. Whiskers represent the furthest points within interquartile range (IQR) of the limits of the box (75th quartile minus 25th quartile). A single datapoint (black circle) in the paternal A1221 females was a statistical outlier. Note: A1221, Aroclor 1,221; MAT, maternal; PAT, paternal; VEH, vehicle; VIN, vinclozolin. * determined by one-way analysis of variance (ANOVA).
F2 Sex Behavior in Males
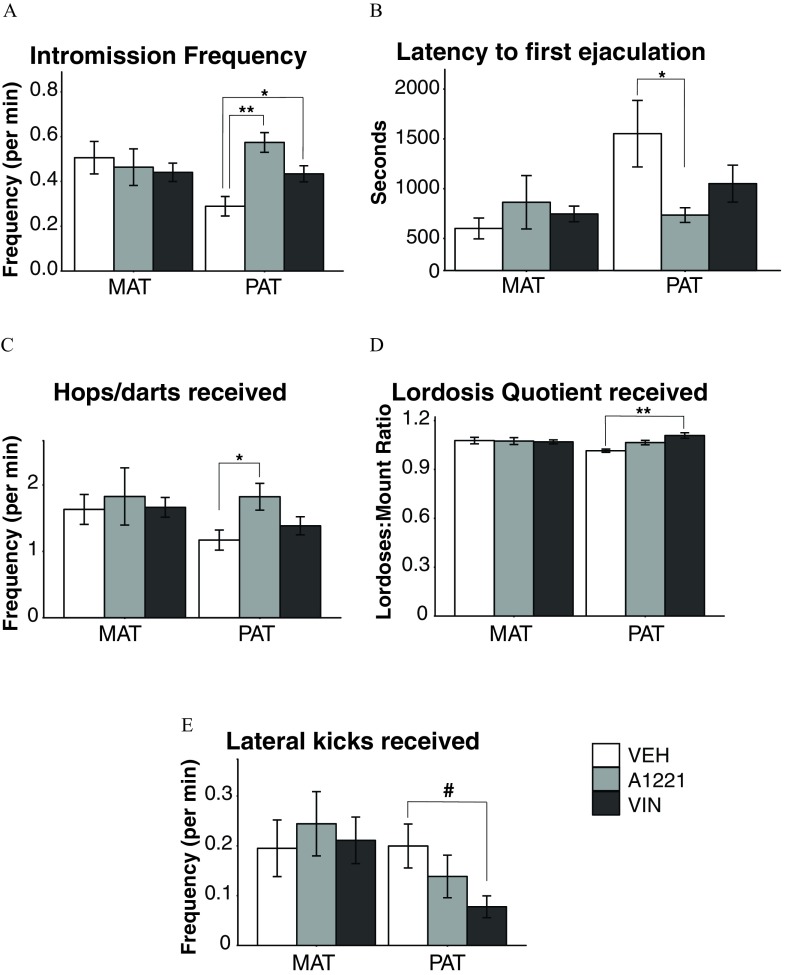
Behaviors in males were altered exclusively in the paternal lineage (Figure 7). Paternally derived A1221 males had higher frequencies of intromission (; ; Figure 7A), shorter latencies to first ejaculation (; ; Figure 7B), and received more hops and darts from females (; ; Figure 7C). VIN males also had higher frequencies of intromissions (; ), received higher lordosis quotients from the females (; ; Figure 7D), and had a trend for receiving fewer lateral kicks to the face (; ; Figure 7E).
Figure 7.
Males were observed for sexual behavior with an untreated receptive female. (A) Frequency of intromissions, (B) latency to ejaculate, (C) numbers of hops/darts received, (D) lordosis quotient, and (E) lateral kicks received are shown. Note: A1221, Aroclor 1,221; MAT, maternal; PAT, paternal; VEH, vehicle; VIN, vinclozolin. Data are shown as . #; *; ** as determined by analysis of variance (ANOVA) followed by Tukey’s HSD posthoc analysis.
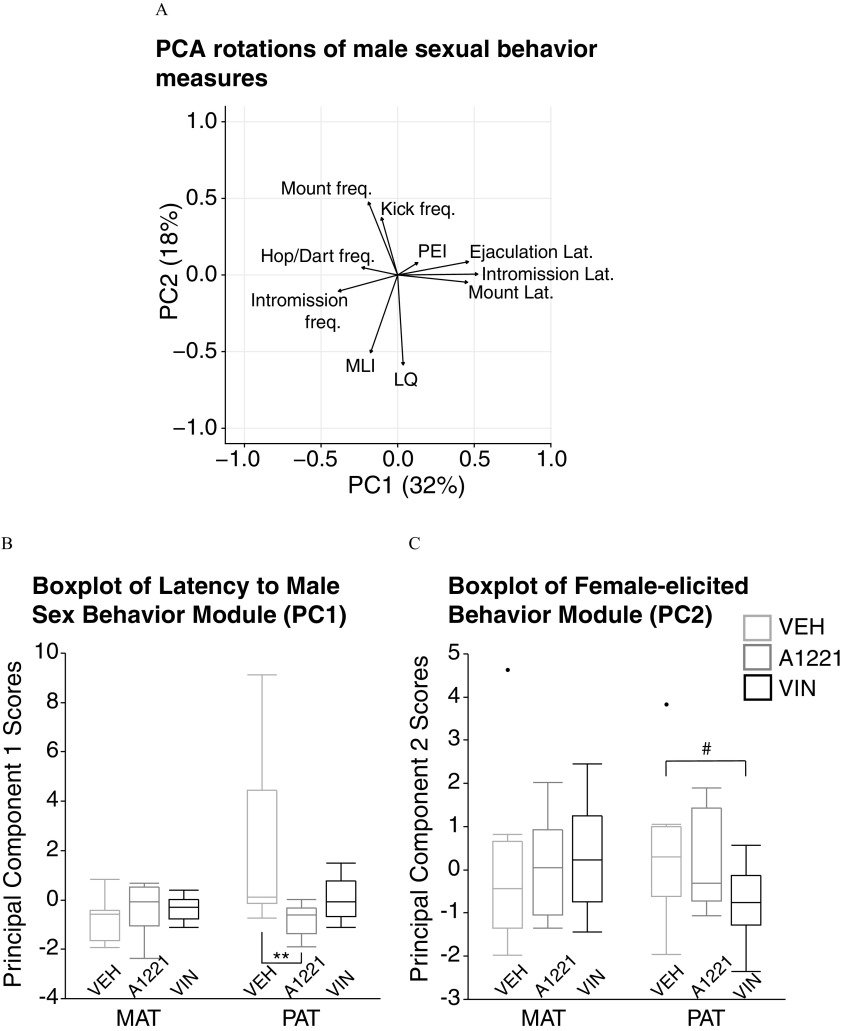
To identify the underlying principal components of these behaviors and determine the overall influence of treatment in males, we conducted a PCA similar to that done for the USV properties. Figure 8A displays the directional loadings of each variable onto the two primary PCs that, together, explained 50% of the variability in the data. PC1 comprised measures describing the males’ latency to perform behaviors such as mounting, intromitting, and ejaculating (“Latency to Male Sex Behavior Module”), while PC2 comprised behaviors elicited from the female such as LQ, MLI, and kick frequency (“Female-elicited Sex Behavior Module”). Using the eigenvalues, we ran a one-way ANOVA on each PC to determine the effect of treatment within a lineage. Males from the paternal line differed significantly from one another in the latency to behavior module (; ; ; Figure 8B), with A1221 animals having shorter latencies than their control counterparts (). Similarly, paternal-lineage males tended to differ in the female-elicited behavior module (; ; ; Figure 8C), with VIN males eliciting fewer behaviors from females than controls ().
Figure 8.
(A) Principal components analysis (PCA) with male sexual behavior measures, (B) boxplots of measures associated with PC1 [Latency to Male Sex Behavior Module (PC1)], and (C) measures associated with PC2 [Female-elicited Behavior Module (PC2)] are shown. A) Arrows indicate the directionality and magnitude of variable loading onto each PC. (B, C) Boxplot data are shown as median (line), with the 25th percentile and 75th percentile indicated by the upper and lower quartiles of the box. Whiskers represent the furthest points within . Two datapoints (black circle), one each in the maternal vehicle male and the paternal vehicle male groups, were statistical outliers. Note: A1221, Aroclor 1,221; MAT, maternal; PAT, paternal; VEH, vehicle; VIN, vinclozolin. #; ** as determined by one-way analysis of variance (ANOVA).
F2 Paced Mating Behaviors in Females
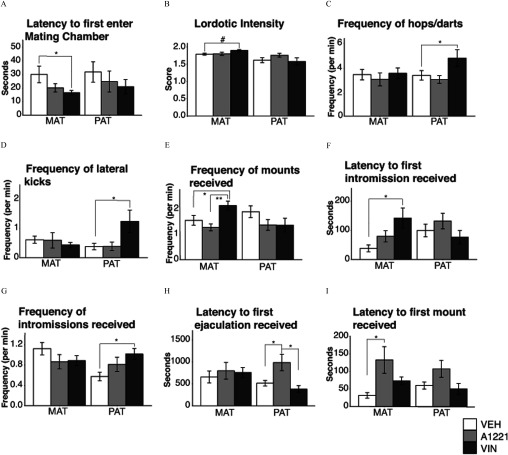
Several aspects of paced mating behaviors differed significantly between treatment groups in a lineage-specific manner (Figure 9). Maternal VIN females had a shorter latency to enter the mating chamber (; ; Figure 9A), and tended to have greater lordotic intensity than VEH females (; ; Figure 9B). The frequency of hops/darts (Figure 9C) and lateral kicks (Figure 9D) were unaffected in the maternal line. Maternal VIN females also received mounts more frequently (Figure 9E) than both VEH () and A1221 () females (). The latency to first intromission from a stimulus male was longer for VIN than VEH females (; ; Figure 9F). Paternal-lineage VIN females hopped/darted more frequently (; ; Figure 9C) and delivered lateral kicks more frequently to the stimulus male (; ; Figure 9D). F2 paternal VIN females received intromissions more frequently than VEH (; ; Figure 9G), and paternal VIN females had a shorter latency to receive their first ejaculation than A1221 females (; ; Figure 9H).
Figure 9.
Females were observed for sexual behavior in a paced mating chamber. Latency to first enter the (A) mating chamber, (B) lordotic intensity, (C) frequency of hops/darts, (D) frequency of lateral kicks to males, (E) frequency of mounts received, (F) latency to receive a first intromission, (G) frequency of intromissions, (H) latency to first ejaculation, and (I) latency to first mount are shown as . Note: A1221, Aroclor 1,221; MAT, maternal; PAT, paternal; VEH, vehicle; VIN, vinclozolin. #; *; ** as determined by analysis of variance (ANOVA) followed by Tukey’s HSD posthoc analysis.
For A1221, in the maternal lineage, females had significantly longer latencies to receive their first mount than VEH females (; ; Figure 9I). They also had a longer latency to receive the first ejaculation from a male compared to VEH () and VIN (described above and Figure 9H).
A PCA on female sexual behavior revealed that the first two PCs explained less than 50% of the variability in the data, and a one-way ANOVA investigating the main effect of treatment revealed no differences in PC1 or PC2.
F2 Functional Landscape Results
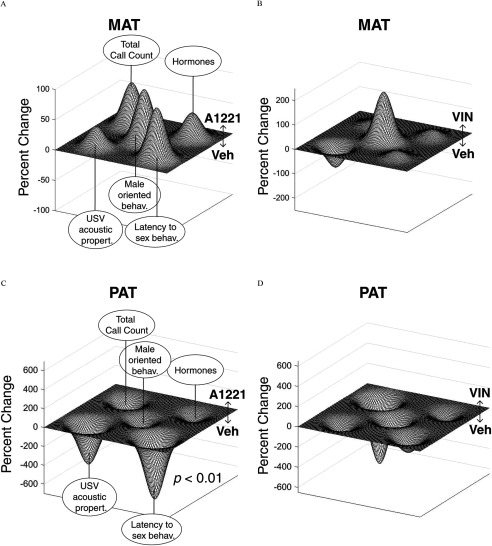
Because most of the effects of treatment were found in males, functional landscapes were used to depict certain male behaviors and physiology in the maternal lineage (Figure 10A, B) and the paternal lineages (Figure 10C, D). Selected traits were the principal components of the USV properties, latency to engage in sexual behavior, frequency of male-oriented sexual behavior and serum hormone levels, as well as z-scaled total USV call counts. Using an LDA, we determined that only the VEH and A1221 landscape profiles differed significantly () and that this was limited to the males of the paternal lineage.
Figure 10.
Functional landscapes for male behavior and serum hormones are shown for maternal and paternal lineages for A1221 (A and C, respectively) and VIN (B and D, respectively) compared to VEH. Each landscape displays the relative difference of each endocrine-disrupting chemical (EDC) treatment from VEH within each lineage. A relative increase for one group over the other is shown by directionality and height of a peak of valley. Nodes around the outer corners (beginning upper left and going clockwise) are: total call counts, serum hormones module, latency to engage in sex behavior module, ultrasonic vocalization (USV) call properties module, and male-oriented sex behaviors in the middle. Note that the y-axis scale differs between each landscape. Note: A1221, Aroclor 1,221; MAT, maternal; PAT, paternal; VEH, vehicle; VIN, vinclozolin.
F1 Serum Hormones
To ascertain whether effects of treatment in the F2 generation were due to physiological alterations in their F1 parents, we measured serum hormones (estradiol, testosterone, corticosterone) in a subset of the F1 sires and dams. Estradiol concentrations (, ) were (VEH), (A1221), and (VIN) in females, and (VEH), (A1221), and (VIN) in males. Serum concentrations of testosterone (, ) in males were (VEH), (A1221), and (VIN). Serum corticosterone concentrations (, ) in females were (VEH), (A1221), and (VIN); in males, they were (VEH), (A1221), and (VIN). No treatment effects were observed in either sex for any hormone in these F1 rats.
Discussion
Prenatal exposures to EDCs affect the individual and also have intergenerational (F2) and transgenerational (F3 and beyond) actions (Anway et al., 2005; Brieño-Enriquez et al., 2015; Manikkam et al. 2013; Elshenawy and Simmons 2016). Several classes of EDCs, particularly VIN (Anway et al., 2005), phthalates (Brehm et al. 2018; Doyle et al. 2013), bisphenol A (BPA) (Susiarjo et al. 2015; Wolstenholme et al. 2012), tributyltin (Chamorro-García et al. 2017), and recently A1221 (Mennigen et al. 2018) have effects in rodent models, for which metabolic, hormonal, reproductive and behavioral changes have been reported. Because the F3 generation is the first to be entirely unexposed in the traditional transgenerational EDC model of prenatal exposure, it has been the most heavily investigated, with surprisingly little known about the F2 generation. Yet, the F2s provide a unique window into the programming effects of EDCs caused by germline exposure. Our focus on physiological outcomes, developmental milestones, and affective and sexual behaviors in adulthood revealed a number of phenotypic effects that varied by sex and were dependent on the type of EDC and the lineage of the individual, especially males from the paternal lineage.
Endocrine-Disrupting Chemicals Increase F2 Body Weight over the Lifespan
Researchers have recently uncovered an epigenetic basis of developmental programming and multigenerational inheritance of obesity (Elshenawy and Simmons 2016). In rats, EDCs such as tributyltin (TBT) (Chamorro-García et al. 2017), diethylstilbestrol (Newbold et al. 2005), triflumizole (Li et al. 2012), tolylfluanid (Regnier et al. 2015), and PCBs (Mennigen et al., 2018) are associated with increased BW and/or obesity. In the F3 generation, a transgenerational impact of EDCs such as BPA, dichlorodiphenyltrichloroethane, VIN, and dibutyl phthalates, albeit at relatively high doses, has been reported on obesity and/or BW (Crews et al. 2012; Manikkam et al. 2013; Skinner et al. 2013). For TBT, low-dose exposures increased white adipose tissue and reprogrammed mesenchyme stem cells away from bone and towards the adipocyte lineage in pregnant F0 exposed animals, with effects persisting through the F4 generation (Chamorro-García et al. 2017; 2013).
In our study, we found significantly increased BW in F2 VIN males. We acknowledge that BW is not a measure of adiposity or obesity; in fact, it is possible that our rats are simply larger, not fatter, as we did not measure length or investigate metabolism or fat. The BW difference in our rats (5–10%) was also smaller than that reported for obesogenic chemicals. Because the only significant result was limited to F2 VIN males exposed to EDCs as developing germ cells, it is possible that exposure to the antiandrogenic effects of VIN acted upon the germline and, together with the high levels of circulating testosterone concentrations in male (but not female) fetuses at this life stage, contributed to this outcome. By contrast, A1221 males did not show this BW phenotype, suggesting the mechanism of action (weakly estrogenic) was not involved. In F2 females, it is interesting that maternal-lineage A1221 but not VIN females tended to have higher BWs than VEH rats, indicative of the sensitivity of females to estrogenic but not antiandrogenic disruption. Based on our pattern of results, it is evident that the sex and lineage of the F2 offspring, as well as the mechanism of action of the EDC, determine the impact on an individual’s BW over their lifespan. Future work should include comprehensive metabolic profiling and measures of adiposity and energy balance.
Effects of Endocrine-Disrupting Chemicals on Adult Sociosexual Ultrasonic Vocalizations
USVs are a mechanism by which rats communicate information on their affective state to other conspecifics (Burgdorf et al., 2011; Willadsen et al. 2014; Wöhr et al. 2008; Barker et al. 2010; Brudzynski 2013). The class of USVs is considered as appetitive or social, due to their prevalence in situations involving reward or the anticipation of reward. For example, these USVs are emitted at greater rates in anticipation of drugs such as cocaine, sexual contact, and play (Bialy et al. 2000; Knutson et al. 1998; Ma et al. 2010; Maier et al. 2010; Wright et al. 2010) as well as in response to entering a familiar location and meeting familiar members of a group (Garcia et al. 2017). Effects of USVs are also strongly modulated by steroid hormones in adult male and female rats (Garcia et al. 2017).
In our paradigm, all of the significant effects of EDCs were in males of the paternal lineage. Paternal VIN males emitted fewer flat and FM USV calls during the 5-min test interval immediately after brief exposure to a sexually receptive stimulus female. These males also had lower estradiol than their untreated counterparts, suggesting a link between circulating serum estradiol and number of vocalizations. In fact, a previous study in male gerbils showed that estradiol benzoate was sufficient to restore high levels of vocalizations after castration (Holman et al. 1991). By contrast, USV acoustic characteristics of power and mean frequency were significantly altered in the A1221 paternal-lineage males. In our study, the A1221 males with altered acoustic properties also had significantly lower circulating testosterone than controls, consistent with the importance of this hormone as shown in rat models of castration and testosterone replacement in males (Harding and McGinnis 2003).
Mating Behaviors in F2 Males and Females
Germ cell exposure to EDCs altered adult male sexual behavior only in the paternal lineage. Surprisingly, A1221 and VIN increased the frequency of intromissions and A1221 decreased the latency to ejaculate, a finding that is associated with greater mating efficiency (de Medeiros et al. 2010). However, this result should not be interpreted as beneficial or improved for reproductive success. In fact, female rodents have the best pregnancy outcomes when they receive a particular number and timing of intromissions before ejaculation (Adler 1969; Erskine 1985). In general, stimulus females paired with our paternal-lineage A1221 and VIN males also showed increases in proceptive behaviors. This is interesting given that both A1221 and VIN males had lower concentrations of testosterone and estradiol respectively; thus, the females may have altered their proceptive and receptive behaviors in response to the males’ behavior or chemosensory cues. Previous work from our lab showed that stimulus females, when given a choice, preferred to spend time in proximity to F3 VEH males than F3 descendants of high-dose VIN males (Crews et al. 2007), indicative of altered phenotypic signal(s) in males of the VIN lineage. A follow-up study observing both the female’s choice behavior along with mating behavior would further delineate the effects of exposure to EDCs.
EDCs also had effects on F2 female rats in the paced mating paradigm. Pacing is important because it allows the female to control the timing of mating events, which in turn optimizes reproductive outcomes (Erskine et al. 2004). The results were interesting, as they revealed that the interaction of treatment with lineage was associated with seemingly contradictory phenotypic outcomes. For example, paternal-lineage VIN females showed more proceptive behaviors and received more frequent intromissions than VEH, but they also gave more lateral kicks, considered a rejection behavior (Spiteri et al. 2010; Steinberg et al. 2007). VIN maternal-lineage females had a different phenotype, with shorter latencies to enter the mating chamber, higher lordotic intensity, and elicited mounts from males more frequently, indicating greater receptivity to the male. However, stimulus males paired with these females had delayed latencies to their first intromission, possibly reflecting an alteration in male preference or the experimental female’s chemosensory cues. The effects of A1221 on paced mating in the F2 females were few, limited to increased latency for the male to mount in the maternal lineage, and increased latency to receive an ejaculation in the paternal lineage. Again, this may indicate a decrease in attractiveness. Taken together, these results indicate that EDC exposure during germ cell development can alter nuanced aspects of mating behavior. To understand the reproductive consequences of these behavioral changes, future studies would need to comprehensively investigate both the EDC-treated and stimulus animals’ mate choice, sexual behavior, and offspring outcomes.
Conclusions
Our study is among the first to demonstrate the impact of an individual’s exposure to EDCs during the germ cell stage of development. Each sex and lineage had unique phenotypic profiles due to treatment, but the paternal-lineage males showed particular vulnerability. This sex difference may be attributable to the timing of exposure to EDCs (E8–18 of gestation of the F1 generation). In the developing fetus, DNA methylation marks in germ cells—the F2 generation—undergo erasure and subsequent reestablishment of DNA methylation marks in a sexually dimorphic manner (Smallwood and Kelsey 2012). In male rats, remethylation is completed in the fetus, whereas in female rats, remethylation does not happen until ova exit meiotic arrest during ovulatory processes that begin at puberty (Reik et al. 2001). Furthermore, the critical period of brain sexual differentiation in response to gonadal hormones in rats begins at about E18 due to the occurrence of a testosterone surge in males but not females (Ward and Weisz 1980). The overlap of these key developmental periods in germ cells and on the brain likely led to the increased sensitivity of paternal-lineage males. Recent research demonstrated the sensitivity of undifferentiated primordial germ cells to a similar dose of VIN, with male mice exhibiting alterations to microRNA in these germ cells as well as reduced fertility (Brieño-Enriquez et al. 2015). It is important to note that our selected exposure window, while informative, needs to be expanded to represent the real-world situation for humans and wildlife, namely, lifelong exposure to a mixture of EDCs overlapping across the generations.
Other factors could contribute to the transmission of EDC effects from the F1 to the F2 generation. For example, maternal care can serve as a nongenomic method by which physiological and behavioral traits are transmitted to and manifest in the offspring (Champagne and Meaney 2001; Champagne et al. 2003). The intrauterine environment, intrauterine position, and circulating steroid hormones during pregnancy could potentially serve as nongenomic modulators. Future studies should attempt to delineate between these context-dependent EDC effect (i.e., maternal care, hormonal exposures in gestation) vs. a germline-dependent EDC effect mediated through molecular reprogramming (Crews 2011; Crews and Gore 2011).
Acknowledgments
Funding was provided by NIH grant (RO1 ES023254) to A.C.G. and NIAAA INIA project grant (013517) to C.L.D. We thank A. Hasbum, D. Morales, and C. Weeks for their assistance with behavioral scoring and analysis, and Dr. R. Gillette and Dr. A. Holley for partnership on colony maintenance.
Footnotes
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Adler NT. 1969. Effects of the male's copulatory behavior on successful pregnancy of the female rat. J Comp Physiol Psychol 69(4):613–622, PMID: 5391014, 10.1037/h0028244. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. 2000. Toxicological Profile For Polychlorinated Biphenyls (PCBs). 1–948. [PubMed] [Google Scholar]
- Alyea RA, Gollapudi BB, Rasoulpour RJ. 2014. Are we ready to consider transgenerational epigenetic effects in human health risk assessment? Environ Mol Mutagen 55(3):292–298, PMID: 24259352, 10.1002/em.21831. [DOI] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. 2005. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308(5727):1466–1469, PMID: 15933200, 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Root DH, Ma S, Jha S, Megehee L, Pawlak AP, et al. 2010. Dose-dependent differences in short ultrasonic vocalizations emitted by rats during cocaine self-administration. Psychopharmacology (Berl) 211(4):435–442, PMID: 20571780, 10.1007/s00213-010-1913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, Thompson LM, Rodriguez K, Gore AC. 2016. Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 1. Sexually dimorphic effects on social and anxiety-like behaviors. Horm Behav 78:168–177, PMID: 26592453, 10.1016/j.yhbeh.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict GS, Williams CL. 1993. Hormonal modulation of the cutaneous initiation of lordosis in infant and adult rats. Horm Behav 27(4):449–469, PMID: 8294116, 10.1006/hbeh.1993.1033. [DOI] [PubMed] [Google Scholar]
- Bialy M, Rydz M, Kaczmarek L. 2000. Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behav Neurosci 114(5):983–990, PMID: 11085613, 10.1037/0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- Brehm E, Rattan S, Gao L, Flaws JA. 2018. Prenatal exposure to di(2-ethylhexyl) phthalate causes long-term transgenerational effects on female reproduction in mice. Endocrinology 159(2):795–809, PMID: 29228129, 10.1210/en.2017-03004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieño-Enriquez MA, García-López J, Cárdenas DB, Guibert S, Cleroux E, Ded L, et al. 2015. Exposure to endocrine disruptor induces transgenerational epigenetic deregulation of microRNAs in primordial germ cells. PLoS One 10(4):e0124296–e0124219, PMID: 25897752, 10.1371/journal.pone.0124296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzynski SM. 2013. Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr Opin Neurobiol 23(3):310–317, PMID: 23375168, 10.1016/j.conb.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Moskal JR. 2011. Frequency-modulated 50 kHz ultrasonic vocalizations: a tool for uncovering the molecular substrates of positive affect. Neurosci Biobehav Rev 35(9):1831–1836, PMID: 21144859, 10.1016/j.neubiorev.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Chamorro-García R, Diaz-Castillo C, Shoucri BM, Käch H, Leavitt R, Shioda T, et al. 2017. Ancestral perinatal obesogen exposure results in a transgenerational thrifty phenotype in mice. Nat Commun 8(1):2012, PMID: 29222412, 10.1038/s41467-017-01944-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro-García R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. 2013. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ Health Perspect 121:359–366, PMID: 23322813, 10.1289/ehp.1205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. 2003. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav 79(3):359–371, PMID: 12954431, 10.1016/S0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. 2001. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res 133:287–302, PMID: 11589138, 10.1016/S0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Colbert NKW, Pelletier NC, Cote JM, Concannon JB, Jurdak NA, Minott SB, et al. 2005. Perinatal exposure to low levels of the environmental antiandrogen vinclozolin alters sex-differentiated social play and sexual behaviors in the rat. Environ Health Perspect 113(6):700–707, PMID: 15929892, 10.1289/ehp.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colciago A, Casati L, Mornati O, Vergoni AV, Santagostino A, Celotti F, et al. 2009. Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat Part 2: effects on reproductive parameters, on sex behavior, on memory retention and on hypothalamic expression of aromatase and 5alpha-reductases in the offspring. Toxicol Appl Pharmacol 239(1):46–54, PMID: 19464308, 10.1016/j.taap.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Coopersmith C, Erskine MS. 1994. Influence of paced mating and number of intromissions on fertility in the laboratory rat. J Reprod Fertil 102(2):451–458, PMID: 7861400, 10.1530/jrf.0.1020451. [DOI] [PubMed] [Google Scholar]
- Crews D. 2011. Epigenetic modifications of brain and behavior: theory and practice. Horm Behav 59(3):393–398, PMID: 20633562, 10.1016/j.yhbeh.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK. 2012. Epigenetic transgenerational inheritance of altered stress responses. Proc Natl Acad Sci USA 109(23):9143–9148, PMID: 22615374, 10.1073/pnas.1118514109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gore AC. 2011. Life imprints: living in a contaminated world. Environ Health Perspect 119(9):1208–1210, PMID: 21571618, 10.1289/ehp.1103451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, et al. 2007. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci USA 104(14):5942–5946, PMID: 17389367, 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Medeiros CB, Rees SL, Llinas M, Fleming AS, Crews D. 2010. Deconstructing early life experiences: distinguishing the contributions of prenatal and postnatal factors to adult male sexual behavior in the rat. Psychol Sci 21(10):1494–1501, PMID: 20807895, 10.1177/0956797610382122. [DOI] [PubMed] [Google Scholar]
- Dombret C, Capela D, Poissenot K, Parmentier C, Bergsten E, Pionneau C, et al. 2017. Neural mechanisms underlying the disruption of male courtship behavior by adult exposure to di(2-ethylhexyl) phthalate in mice. Environ Health Perspect 125(9):097001, PMID: 28934723, 10.1289/EHP1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH. 2013. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod 88(5):112, PMID: 23536373, 10.1095/biolreprod.112.106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshenawy S, Simmons R. 2016. Maternal obesity and prenatal programming. Mol Cell Endocrinol 435:2–6, PMID: 27392495, 10.1016/j.mce.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Erskine MS. 1985. Effects of paced coital stimulation on estrus duration in intact cycling rats and ovariectomized and ovariectomized-adrenalectomized hormone-primed rats. Behav Neurosci 99(1):151–161, PMID: 4041227, 10.1037/0735-7044.99.1.151. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Lehmann ML, Cameron NM, Polston EK. 2004. Co-regulation of female sexual behavior and pregnancy induction: an exploratory synthesis. Behav Brain Res 153(2):295–315, PMID: 15265625, 10.1016/j.bbr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EF, Belanger EE, Gomez MI, Cayo M, McCaffrey RJ, Seegal RF, et al. 2008. Polychlorinated biphenyl exposure and neuropsychological status among older residents of upper Hudson River communities. Environ Health Perspect 116(2):209–215, PMID: 18288320, 10.1289/ehp.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame G. 1997. A collaborative study of 209 PCB congeners and 6 Aroclors on 20 diffrent HRGC column, 2. Semi-quantitative Aroclor congener distributions. Fresenius J Anal Chem 357(6):714–722, 10.1007/s002160050238. [DOI] [Google Scholar]
- Garcia AN, Bezner K, Depena C, Yin W, Gore AC. 2017. The effects of long-term estradiol treatment on social behavior and gene expression in adult female rats. Horm Behav 87:145–154, PMID: 27871902, 10.1016/j.yhbeh.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R, Miller-Crews I, Nilsson EE, Skinner MK, Gore AC, Crews D. 2014. Sexually dimorphic effects of ancestral exposure to vinclozolin on stress reactivity in rats. Endocrinology 155(10):3853–3866, PMID: 25051444, 10.1210/en.2014-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R, Reilly MP, Topper VY, Thompson LM, Crews D, Gore AC. 2017. Anxiety-like behaviors in adulthood are altered in male but not female rats exposed to low dosages of polychlorinated biphenyls in utero. Horm Behav 87:8–15, PMID: 27794483, 10.1016/j.yhbeh.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. 2015. Executive summary to EDC-2: The Endocrine Society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev 36(6):593–602, PMID: 26414233, 10.1210/er.2015-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, et al. 2001. Effects of environmental antiandrogens on reproductive development in experimental animals. Human Reprod Update 7(3):248–264, PMID: 11392371, 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- Harding SM, McGinnis MY. 2003. Effects of testosterone in the VMN on copulation, partner preference, and vocalizations in male rats. Horm Behav 43(2):327–335, PMID: 12694643, 10.1016/s0018-506x(02)00049-1. [DOI] [PubMed] [Google Scholar]
- Hellwig J, van Ravenzwaay B, Mayer M, Gembardt C. 2000. Pre- and postnatal oral toxicity of vinclozolin in Wistar and Long-Evans rats. Regul Toxicol Pharmacol 32(1):42–50, PMID: 11029267, 10.1006/rtph.2000.1400. [DOI] [PubMed] [Google Scholar]
- Holman SD, Hutchison RE, Hutchison JB. 1991. Microimplants of estradiol in the sexually dimorphic area of the hypothalamus activate ultrasonic vocal behavior in male Mongolian gerbils. Horm Behav 25(4):531–548, PMID: 1813379, 10.1016/0018-506x(91)90019-e. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. 1998. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol 112(1):65–73, PMID: 9528115, 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. 2002. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull 128(6):961–977, PMID: 12405139, 10.1037//0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, McCarthy MM. 2012. Sexual differentiation of the rodent brain: dogma and beyond. Front Neurosci 6:26, PMID: 22363256, 10.3389/fnins.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Pham HT, Janesick AS, Blumberg B. 2012. Triflumizole is an obesogen in mice that acts through peroxisome proliferator activated receptor gamma (PPARγ). Environ Health Perspect 120(12):1720–1726, PMID: 23086663, 10.1289/ehp.1205383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL. 2010. Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res 212(1):109–114, PMID: 20382187, 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EY, Ma ST, Ahrens A, Schallert TJ, Duvauchelle CL. 2010. Assessment of ultrasonic vocalizations during drug self-administration in rats. J Vis Exp 22(41):e2041–e2041, PMID: 20689507, 10.3791/2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. 2013. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One 8(1):e55387, PMID: 23359474, 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez I, Paredes RG. 2001. Only self-paced mating is rewarding in rats of both sexes. Horm Behav 40(4):510–517, PMID: 11716580, 10.1006/hbeh.2001.1712. [DOI] [PubMed] [Google Scholar]
- McBirney M, King SE, Pappalardo M, Houser E, Unkefer M, Nilsson E, et al. 2017. Atrazine induced epigenetic transgenerational inheritance of disease, lean phenotype and sperm epimutation pathology biomarkers. PLoS One 12(9):e0184306, PMID: 28931070, 10.1371/journal.pone.0184306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennigen JA, Thompson LM, Bell M, Tellez Santos M, Gore AC. 2018. Transgenerational effects of polychlorinated biphenyls: 1. Development and physiology across 3 generations of rats. Environ Health 17(1):18, PMID: 29458364, 10.1186/s12940-018-0362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN. 2005. Developmental exposure to estrogenic compounds and obesity. Birth Defects Res Part A Clin Mol Teratol 73(7):478–480, PMID: 15959888, 10.1002/bdra.20147. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Vazquez B. 1999. What do female rats like about sex? Paced mating. Behav Brain Res 105(1):117–127, PMID: 10553695, 10.1016/S0166-4328(99)00087-X. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. 1979. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J. Physiol. 288:189–202, PMID: 469715, 10.1111/(ISSN)1469-7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Kippin TE, Coria-Avila GA, Gelez H, Afonso VM, Ismail N, et al. 2012. Who, what, where, when (and maybe even why)? How the experience of sexual reward connects sexual desire, preference, and performance. Arch Sex Behav 41(1):31–62, PMID: 22402996, 10.1007/s10508-012-9935-5. [DOI] [PubMed] [Google Scholar]
- Piedrafita B, Erceg S, Cauli O, Monfort P, Felipo V. 2008. Developmental exposure to polychlorinated biphenyls PCB153 or PCB126 impairs learning ability in young but not in adult rats. Eur J Neurosci 27(1):177–182, PMID: 18093177, 10.1111/j.1460-9568.2007.5988.x. [DOI] [PubMed] [Google Scholar]
- Regnier SM, Kirkley AG, Ye H, El-Hashani E, Zhang X, Neel BA, et al. 2015. Dietary exposure to the endocrine disruptor tolylfluanid promotes global metabolic dysfunction in male mice. Endocrinology 156(3):896–910, PMID: 25535829, 10.1210/en.2014-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Dean W, Walter J. 2001. Epigenetic reprogramming in mammalian development. Science 293(5532):1089–1093, PMID: 11498579, 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- Reilly MP, Weeks CD, Topper VY, Thompson LM, Crews D, Gore AC. 2015. The effects of prenatal PCBs on adult social behavior in rats. Horm Behav 73:47–55, PMID: 26093262, 10.1016/j.yhbeh.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reno JM, Marker B, Cormack LK, Schallert T, Duvauchelle CL. 2013. Automating ultrasonic vocalization analyses: the WAAVES program. J Neurosci Methods 219(1):155–161, PMID: 23832016, 10.1016/j.jneumeth.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpino SV, Gillette R, Crews D. 2014. multiDimBio: An R Package for the Design, Analysis, and Visualization of Systems Biology Experiments. 1–12, https://arxiv.org/abs/1404.0594.
- Sierra-Santoyo A, Castañeda-Hernández G, Harrison RA, Barton HA, Hughes MF. 2008. Pharmacokinetics and dosimetry of the antiandrogen vinclozolin after oral administration in the rat. Toxicol Sci 106(1):55–63, PMID: 18703562, 10.1093/toxsci/kfn167. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Manikkam M, Tracey R, Guerrero-Bosagna C, Haque M, Nilsson EE. 2013. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med 11:228, PMID: 24228800, 10.1186/1741-7015-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood SBA, Kelsey G. 2012. De novo DNA methylation: a germ cell perspective. Trends Genet 28(1):33–42, PMID: 22019337, 10.1016/j.tig.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, Ågmo A. 2010. Estrogen-induced sexual incentive motivation, proceptivity and receptivity depend on a functional estrogen receptor alpha in the ventromedial nucleus of the hypothalamus but not in the amygdala. Neuroendocrinology 91(2):142–154, PMID: 19887773, 10.1159/000255766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RM, Juenger TE, Gore AC. 2007. The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm Behav 51(3):364–372, PMID: 17274994, 10.1016/j.yhbeh.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RM, Walker DM, Juenger TE, Woller MJ, Gore AC. 2008. Effects of perinatal polychlorinated biphenyls on adult female rat reproduction: development, reproductive physiology, and second generational effects. Biol Reprod 78(6):1091–1101, PMID: 18305224, 10.1095/biolreprod.107.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström G, Hutzinger O, Safe S. 1976. The metabolism of chlorobiphenyls — a review. Chemosphere 5(5):267–298, 10.1016/0045-6535(76)90002-3. [DOI] [Google Scholar]
- Susiarjo M, Xin F, Bansal A, Stefaniak M, Li C, Simmons RA, et al. 2015. Bisphenol A exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology 156(6):2049–2058, PMID: 25807043, 10.1210/en.2014-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Aburada S, Hashimoto K, Kitaura T. 1986. Transfer and distribution of accumulated (14C)polychlorinated biphenyls from maternal to fetal and suckling rats. Arch. Arch Environ Contam Toxicol 15(6):709–715, PMID: 3098190, 10.1007/BF01054917. [DOI] [PubMed] [Google Scholar]
- Ward IL, Weisz J. 1980. Maternal stress alters plasma testosterone in fetal males. Science 207(4428):328–329, PMID: 7188648, 10.1126/science.7188648. [DOI] [PubMed] [Google Scholar]
- Willadsen M, Seffer D, Schwarting RKW, Wöhr M. 2014. Rodent ultrasonic communication: male prosocial 50-kHz ultrasonic vocalizations elicit social approach behavior in female rats (Rattus norvegicus). J Comp Psychol 128(1):56–64, PMID: 24188619, 10.1037/a0034778. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Houx B, Schwarting RKW, Spruijt B. 2008. Effects of experience and context on 50-kHz vocalizations in rats. Physiol Behav 93(4-5):766–776, PMID: 18191963, 10.1016/j.physbeh.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Edwards M, Shetty SRJ, Gatewood JD, Taylor JA, Rissman EF, et al. 2012. Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology 153(8):3828–3838, PMID: 22707478, 10.1210/en.2012-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Kelce WR, Sar M, Wilson EM. 1995. Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. J Biol Chem 270(34):19998–20003, PMID: 7650017, 10.1074/jbc.270.34.19998. [DOI] [PubMed] [Google Scholar]
- Wright JM, Gourdon JC, Clarke PB. 2010. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacology (Berl) 211(1):1–13, PMID: 20443111, 10.1007/s00213-010-1859-y. [DOI] [PubMed] [Google Scholar]