Abstract
Background:
Subclinical cardiovascular changes have been associated with ambient particulate matter (PM) exposures within hours. Although the U.S. Environmental Protection Agency continues to look for additional evidence of effects associated with sub-daily PM exposure, this information is still limited because most studies of clinical events have lacked data on the onset time of symptoms to assess rapid increased risk.
Objective:
Our objective was to investigate associations between sub-daily exposures to PM and acute cardiac events using telemedicine data.
Methods:
We conducted a case-crossover study among telemedicine participants of age who called a service center for cardiac-related symptoms and were transferred to a hospital in Tel Aviv and Haifa, Israel (2002–2013). Ambient and measured by monitors located in each city during the hours before the patient called with symptoms were compared with matched control periods. We investigated the sensitivity of these associations to more accurate symptom onset time and greater certainty of diagnosis.
Results:
We captured 12,661 calls from 7,617 subscribers experiencing ischemic (19%), arrhythmic (31%), or nonspecific (49%) cardiac events. PM concentrations were associated with small increases in the odds of cardiac events. For example, odds ratios for any cardiac event in association with a increase in 6-h and 24-h average were 1.008 [95% confidence interval (CI): 0.998, 1.018] and 1.006 (95% CI: 0.995, 1.018), respectively, and for were 1.003 (95% CI: 1.001, 1.006) and 1.003 (95% CI: 1.000, 1.007), respectively. Associations were stronger when using exposures matched to the call time rather than calendar date and for events with higher certainty of the diagnosis.
Conclusions:
Our analysis of telemedicine data suggests that risks of cardiac events in telemedicine participants of age may increase within hours of PM exposures. https://doi.org/10.1289/EHP2596
Introduction
Short-term exposure to ambient particulate matter (PM) has been linked to increased cardiovascular morbidity and mortality (Brook et al. 2010; Du et al. 2016; Münzel et al. 2017; Newby et al. 2015; Zanobetti and Schwartz 2009) with large public health burdens (WHO 2016, 2018). Experimental studies indicate that subclinical cardiovascular changes may occur within minutes to hours after exposure (Ghelfi et al. 2008; Mills et al. 2007). Yet, although the U.S. Environmental Protection Agency (EPA) continues to look for additional evidence of effects associated with sub-daily exposure that may serve as a basis for establishing shorter than 24-h PM standards (U.S. EPA 2009, 2011), information about hourly associations of PM and clinical events is limited, as indicated in a review of studies examining cardiovascular outcomes and sub-daily exposures (Burgan et al. 2010). Most studies have investigated associations on a daily time scale (Ito et al. 2011; Stafoggia et al. 2013; Stieb et al. 2009; Wolf et al. 2015; Zanobetti and Schwartz 2005) because of a lack of detailed information on onset time in administrative data. In addition, the use of administrative data without additional adjudication can limit the ability of researchers to accurately identify events and classify the type of events. Error in either the onset time or outcome definition may result in an underestimation of the true magnitude of the health effects associated with air pollution (Lokken et al. 2009).
The few studies that have investigated associations between hourly exposures of PM and clinical cardiac events used patient interview (Berglind et al. 2010; Peters et al. 2001), disease register (Bhaskaran et al. 2011; Sullivan et al. 2005), or implantable cardioverter defibrillator (ICD) data (Link et al. 2013; Ljungman et al. 2008; Rich et al. 2005) to assess onset time. However, these sources are either subjective, focus on specific outcomes, or pertain to limited populations. Like emergency respondent data, telemedicine offers a novel resource for conducting environmental health research on clinical events (Leshem-Rubinow et al. 2011b). With telemedicine services, subscribers can phone a medical call center upon experiencing symptoms for a remote clinical evaluation and follow-up action, if required. Through earlier engagement with the patient, telemedicine is able to detect the onset time of confirmed clinical symptoms earlier than would be possible from a phone call for emergency respondents or hospital admissions (Audebert et al. 2005). Telemedicine also collects clinical data remotely at the time of the event, providing additional information with which to adjudicate an event with greater certainty. Although telemedicine offers a novel and clinically confirmed data source (Birati and Roth 2011; Chaudhry et al. 2010; Schwaab et al. 2003; Wennberg et al. 2010), it has been rarely used to test associations between health effects and environmental stressors (Crabbe et al. 2004).
Our study utilized unique telemedicine data to more accurately estimate associations between sub-daily PM exposure and acute clinical cardiac events among residents in two large metropolitan areas in Israel between 2002 and 2013. In secondary analyses, we further examined the impacts on our association of using more accurate information on symptom onset time and greater certainty of diagnosis from adjudicated data.
Methods
Study Subjects
SHL Telemedicine (pronounced “Shahal” an acronym for Sherut Holey Lev, a service for heart disease patients; http://www.shl-telemedicine.com/) provides medical assistance to over 300,000 subscribers worldwide (about 100,000 in Israel) (Birati et al. 2008; Roth et al. 2009; Shacham et al. 2012). Telemedicine is a popular service among the older population of Israel, with approximately 10–20% of people over 65 y of age enrolled in one of two major providers. In Shahal, 48% of the service is used by patients 60–80 y of age, and 31% by those over 80 y of age. Subscribers are often individuals with preexisting cardiovascular disease or risk factors for cardiovascular disease but are otherwise similar to the general population with respect to many characteristics such as smoking and obesity (Leshem-Rubinow et al. 2011a). The Shahal service provides its subscribers access to a 24/7 call center managed by nurses and physicians, all of whom are skilled in cardiac emergency. Subscribers carry a cardiopager (http://www.shl-telemedicine.com/solutions-products/products/), which is a hand-held 12-lead electrocardiogram (ECG) transmitter device for personal use, from which they can transmit an ECG by landline or cellular phones. The company also operates mobile intensive care units (ambulances) staffed by a physician and a paramedic.
The study subjects were Shahal subscribers living in the Tel Aviv and Haifa metropolitan areas of Israel who contacted the call center for a cardiac event between 2002 and 2013. These metropolitan areas were defined according to the Israel Central Bureau of Statistics classification (http://cbs.gov.il/reader/Milon/Milon_ByTerm_E.html?MyID=558&OnlyFinal=1). We restricted our study population to all subjects who were of age at the time of call, were examined at home by a medical team without delay (within approximately 10 min of their call), and were evacuated to a local hospital for emergency care. Available baseline data collected by a physician included demographic characteristics, cardiovascular risk factors, medical history, medications, and ECG measurements. Analysis of a de-identified dataset provided by Shahal was approved by the institutional review board at The Hebrew University of Jerusalem. No informed consent was required.
Cardiac Events
We identified clinical cardiac events based on a standard form filled out for each medical call, an ambulance medical report, and the patient’s medical background. Event-related data included the exact time of the start of each call, medications administered at home, ECG tracings, results of a physical examination, and cardiac enzyme test findings. We considered any cardiac event as well as three specific types of cardiac events: events related to ischemic heart disease (IHD), arrhythmias, and other nonspecific cardiac events (NSCEs). For IHD and arrhythmia, which have been previously linked to PM and likely reflect different biological pathways (Brook et al. 2010), events were further graded into subgroups based on our certainty level of diagnosis. A definite diagnosis of an IHD event was assigned if the hospital records included a myocardial infarction (MI) diagnosis; catheterization with a stent or bypass surgery occurring within 7 days of the event; a positive cardiac enzyme test; or ECG indications of ST-depression, ST-elevation, or T-wave inversion in both the phone-transmitted ECG and ambulance recording or if one of the ECGs had the above findings and the patient was also treated with morphine, heparin, clopidogrel bisulfate, or aspirin. Classification of probable ischemic events required that only one of the above ECG indications was present or that the patient was treated with morphine, heparin or clopidogrel bisulfate. A definite diagnosis of arrhythmia was determined by ECG findings of rhythm abnormalities of ventricular or supraventricular origin, including ventricular fibrillation, ventricular tachycardia, atrial fibrillation, atrial flutter, and supraventricular tachycardia that were not present at the baseline exam. Probable arrhythmias were defined by ECG indications of sinus tachycardia, sinus bradycardia, and non-chronic ventricular premature complexes or atrial premature complexes. Finally, an NSCE event was indicated if the patient complaints included only chest pain or shortness of breath (not related to a respiratory condition) or a physician-diagnosed pulmonary edema. In addition, this group included events in which patients were treated with resuscitation or defibrillation. In analyses by event type, calls with both IHD and arrhythmia indications were included once in each model.
The time of onset of a cardiac event was defined as the time the phone call was recorded by the call center, rounded down to the nearest preceding half-hour. When an individual called the center more than once within 24 h, these calls were combined into a single event and the first call time used. Other multiple events per patient during the study period were considered as separate events.
Pollution and Weather Data
Half-hourly data for PM () and () in aerodynamic diameter, oxides of nitrogen (), ozone (), and meteorologic data were collected by the Ministry for Environmental Protection and the Israel Electric Corporation (http://www.svivaaqm.net/) at two urban background sites in Tel Aviv (Central Station and Yad Lebanim) and one in Haifa (Neve Sha’anan). We assigned the average value for the two Tel Aviv monitors to all subjects that were Tel Aviv residents and assigned the values measured at the Haifa site (for pollutants other than ) to all residents of Haifa. concentrations were not available from the Neve Sha’anan monitor in Haifa because of an instrument failure, and exposures for Haifa residents were therefore assigned based on concentrations measured at the nearby Ahuza station (also in Haifa). These stations were selected as locations that measured both and and had at least 75% of the half-hourly data available. All PM levels were measured using beta attenuation monitoring, with the results undergoing quality assurance testing by the Technion Center of Excellence in Exposure Science and Environmental Health in Haifa. Temporal correlation was high across the monitoring stations with Pearson correlations for hourly concentrations of 0.97 and 0.92 for and , respectively.
Coarse PM (2.5 to ; ) concentrations were computed as the difference between concurrent and collocated and measurements. This approach has been established as a reliable approach to estimating in urban areas (Adar et al. 2014). A heat index (HI), defined as an individual’s perceived air temperature given the humidity, was calculated for temperatures using the equation provided in the NOAA website (http://www.wpc.ncep.noaa.gov/html/heatindex_equation.shtml):
in which T is temperature in Fahrenheit (degrees) and RH is relative humidity (percentage).
Before estimating citywide average air pollution concentrations for and , we imputed missing values for (4% of all data) and (10% of all data) using regression models with autoregressive errors fitted with SAS PROC AUTOREG. These models included PM data from adjacent monitors () and were further adjusted for season, day of week, wind direction, and the heat index. This is consistent with other case-crossover studies in which individual exposure to PM was assessed using very few or even one monitoring site in a city (Peters et al. 2001; Zanobetti and Schwartz 2005) and the high correlations between monitoring stations in this region.
Statistical Analysis
Descriptive statistics for characteristics of the study population were first calculated along with distributional information for the levels of pollution and weather. We also estimated correlations between pollutants using Pearson and Spearman coefficients.
Next, we investigated the association between pollution concentrations and cardiac events using a case-crossover design (Maclure 1991; Mittleman et al. 1995) with time-stratified referent selection (Janes et al. 2005). In this design, only cases were sampled, and their exposure experience in the time period before their event was compared with control time periods relative to the same hour of the day on the same day of the week and month during the same year (resulting in three to four control time periods per case). This design eliminates confounding by subject characteristics that are stable over time and controls effectively for temporal variability in exposure (Zanobetti and Schwartz 2005).
We constructed separate conditional logistic regression models to evaluate associations between each type of cardiac event and pollutant concentration in the 1, 2, 6, 12 and 24 h before the event onset. We examined the possibly nonlinear relation between 6-h PM averages and the OR of a cardiac event nonparametrically with restricted cubic splines (Durrleman and Simon 1989) using the SAS LGTPHCURV9 Macro (https://www.hsph.harvard.edu/donna-spiegelman/software/lgtphcurv9/). Tests for nonlinearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. We used linear relations when the p-value of the nonlinearity test was . We controlled linearly for the heat index in all models because additional degrees of freedom had . Effect estimates are presented as odds ratios (ORs) and 95% confidence intervals (CIs) of a cardiac event associated with an incremental elevation of in and concentrations.
In the secondary analyses, we compared associations between models assessing exposures based on onset time as compared with calendar date of a phone call. We also compared ORs for definite versus probable IHD and arrhythmia events. Finally, we assessed modification of associations of all outcomes with 6-h and by gender; age (, 65–84, 85 y); previous history of hypertension, diabetes or morbid obesity (); and any previous history of ischemic heart disease or arrhythmia by including multiplicative interaction terms in our regression models. We note that in order to estimate an additive interaction between exposure and subject-specific characteristics in the case-crossover design, we needed information on the baseline subgroup-specific hourly rates of cardiac events (Di et al. 2017), but these data were not available in this study. All statistical analyses were conducted using SAS software (version 9.3; SAS Institute Inc.) and R (version 3.2.3; R Development Core Team).
Sensitivity Analyses
We performed sensitivity analyses to estimate associations with and averaged over the previous 2–6 days; estimated associations with 6-h PM adjusted for the average heat index during the previous 6-, 12-, and 24-h; and estimated associations for 24-h PM adjusted for 24-, 48-, and 96-h average heat index values. We also repeated models of associations with 6-h average and concentrations after adjusting for the other PM size fraction and after additional adjustment for 6-h average and concentrations (modeled as untransformed continuous covariates). In addition, we repeated models of associations with 24-h average and concentrations after restricting observations to days with of PM measurements available instead of using imputed PM values for days with incomplete PM data (as in the primary model). Finally, we repeated the models of 6-h and concentrations after excluding outcomes that occurred in the same individuals within 1 week, 1 month, or 1 y of the index event and after limiting outcomes to the first event for each individual during the study period.
Results
Study Subjects
We identified 7,617 patients who had 12,661 cardiac events and who were evacuated to the hospital for urgent care. The majority (66%) of patients called for a single cardiac event and 20, 7 and 3% called for 2–4 events, respectively (the remaining 4% had 5–20 calls). NSCEs were most common (6,258), followed by arrhythmias (3,946), and IHD-related events (1,593). In addition, 864 events had both IHD and arrhythmia indications. We were able to assign a definite classification for 52% of the IHD events, and 52% had definite arrhythmia (see Table S1).
The mean age at the first event was 77 y, 56% of the subjects were male, and most subjects had preexisting IHD (77%) or arrhythmia (62%) (Table 1).
Table 1.
Characteristics of the study subjects experiencing a cardiac event between 2002 and 2013 ().
| Characteristic | or n (%) |
|---|---|
| Gender | |
| Female | 3,322 (43.6) |
| Male | 4,295 (56.4) |
| Age at first call (y) | |
| Age group (y) | |
| 50–64 | 1,200 (15.7) |
| 65–84 | 4,562 (59.9) |
| 1,855 (24.4) | |
| Lives alone | |
| No | 4,718 (61.9) |
| Yes | 2,899 (38.1) |
| Smoking | |
| Current | 896 (11.8) |
| Previous | 833 (10.9) |
| Never | 5,888 (77.3) |
| Comorbidities and risk factorsa | |
| Morbid obesityb | 360 (5.7) |
| Hypertension | 4,731 (74.9) |
| Hyperlipidemia | 3,912 (62.1) |
| Diabetes | 2,213 (35.1) |
| Familial cardiac history | 1,120 (17.8) |
| Chronic pulmonary disease | 1,267 (20.1) |
| Stroke | 1,166 (18.5) |
| Peripheral vascular disease | 659 (10.5) |
| Ischemic heart disease | 4,981 (77.3) |
| Congestive heart failure | 1,995 (31.0) |
| Arrhythmia | 3,967 (61.5) |
| Valvulopathy | 2,120 (33.0) |
| Coronary artery bypass grafting | 1,754 (27.3) |
| Medicationsa | |
| 4,195 (62.1) | |
| Anti-arrhythmias | 1,353 (20.0) |
| Anticoagulants | 1,529 (22.7) |
| Anti-inflammatories/platelet aggregants | 5,181 (76.7) |
| Nitrates | 2,070 (30.7) |
Note: Data for all subject characteristics were complete; study participants lived in the Tel Aviv () and Haifa () areas.
Percentages do not sum to one because multiple conditions were possible.
.
Air Pollution Exposure
Average concentrations during 6-h case and control periods were and in the Tel Aviv and Haifa areas, respectively; the respective average concentrations were and (Table 2). The interquartile ranges (IQRs) of PM for the 1-h and 24-h averaging times were similar. For example, in Tel Aviv 1-h and 24-h IQRs were 12.6 and , respectively, and the respective IQRs were 18.9 and (Table 2; see also Figure S1). Because Israel is in an arid region impacted by dust storms, approximately 1% of all measured days had very high concentrations of more than 94.2 and for and , respectively. During dust spells with , the average hourly concentrations were and for and , respectively. The Pearson correlations between the coarse and fine fractions 6-h concentrations were 0.91 (Spearman correlation ) and 0.85 () in Tel Aviv and Haifa, respectively (Table 2). The correlations between and averaged over the same time periods were similar for different time periods (see Table S2). The Pearson correlations within each PM size fraction across all averaging periods assessed ranged from between 1-h and 2-h averages to between 1-h and 6-day averages (see Table S3).
Table 2.
Distribution of and during 1-, 6-, and 24-h case and control periods in the Tel Aviv and Haifa areas, 2002–2013.
| Area | Pollutant () | Averaging Time (h) | Percentile | Correlation with a | |||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | Max | Pearson | Spearman | ||||
| Tel Aviv | 1 | 14.5 | 20.0 | 27.1 | 768.0 | 0.90 | 0.64 | ||
| 6 | 15.1 | 20.3 | 26.9 | 741.2 | 0.91 | 0.65 | |||
| 24 | 16.1 | 20.7 | 26.4 | 469.9 | 0.91 | 0.64 | |||
| 1 | 13.1 | 19.5 | 32.0 | 2455.7 | 1.00 | 1.00 | |||
| 6 | 14.1 | 19.8 | 30.9 | 2355.7 | 1.00 | 1.00 | |||
| 24 | 15.3 | 20.0 | 31.3 | 1407.3 | 1.00 | 1.00 | |||
| Haifa | 1 | 11.4 | 16.8 | 23.4 | 623.1 | 0.83 | 0.50 | ||
| 6 | 12.0 | 16.9 | 23.2 | 690.2 | 0.85 | 0.49 | |||
| 24 | 12.5 | 17.3 | 23.1 | 705.1 | 0.89 | 0.49 | |||
| 1 | 8.2 | 13.2 | 22.6 | 1005.7 | 1.00 | 1.00 | |||
| 6 | 8.8 | 13.4 | 22.2 | 1176.7 | 1.00 | 1.00 | |||
| 24 | 9.4 | 14.0 | 22.2 | 1257.2 | 1.00 | 1.00 | |||
Note: The numbers of case periods was 10,466 and 2,195 in Tel Aviv and Haifa, respectively, and the respective number of control periods was 35,431 and 7,379. Correlations between exposures during all time periods assessed and correlations within pollutants across averaging periods are provided in Tables S2 and S3. Max, maximum.
Correlations are for exposures averaged over the same 1-, 6-, or 24-h time periods.
Associations of Air Pollutants with Cardiac Events
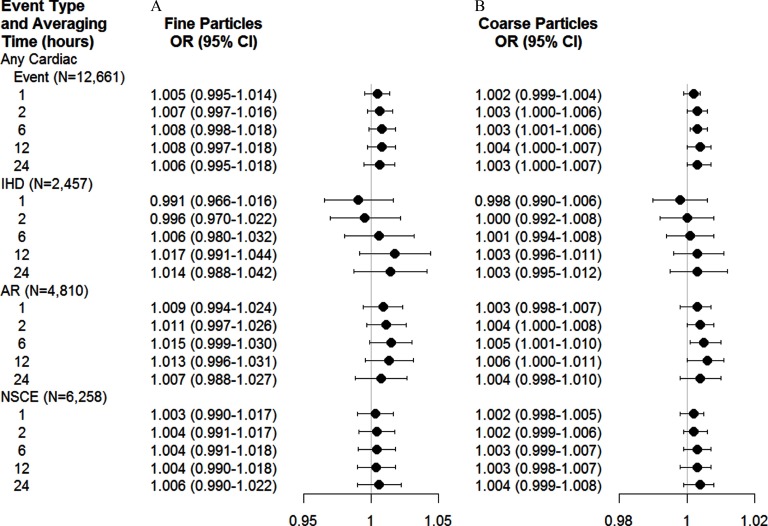
In general, and were associated with higher odds of cardiac events (Figure 1). For example, ORs for any cardiac event in association with a increase in 6-h and 24-h average were 1.008 (95% CI: 0.998, 1.018) and 1.006 (95% CI: 0.995, 1.018), respectively, and for were 1.003 (95% CI: 1.001, 1.006) and 1.003 (95% CI: 1.000, 1.007).
Figure 1.
Estimated ORs and 95% CIs for the onset of cardiac events associated with an increase of in the level of (A) fine particulate matter () and (B) coarse particulate matter () according to end points and averaging time before event onset. Estimates are from a case-crossover study and are adjusted for the heat index. The relation between PM averages and the OR of an ischemic event was nonlinear. AR, arrhythmia; IHD, ischemic heart disease; NSCE, nonspecific cardiac event.
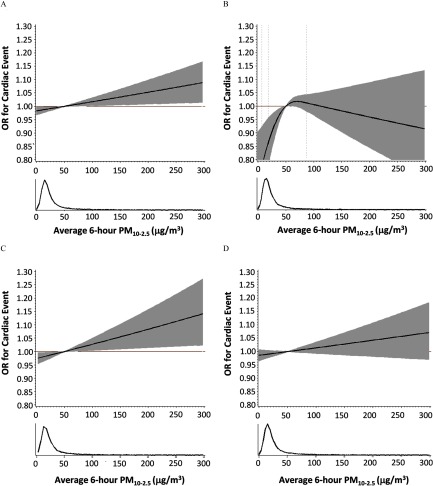
Modeling exposures using restricted cubic splines did not improve model fit relative to linear models for associations between 6-h and and all outcomes other than IHD (Figure 2; see also Figure S2). For IHD and 6-h exposures, a restricted cubic spline with 3 knots fit the data better than a linear model. Associations with increasing exposures of were inverse for concentrations lower than the reference value of and higher than (representing the 88th and 97th percentiles of the exposure distribution, respectively), whereas ORs were positive (indicating increased odds of IHD with increasing exposure) for exposures in the 88th–97th percentile range of the distribution. For , associations with increasing exposures were inverse for concentrations lower than the reference value of (50th percentile), and ORs were approximately null for higher concentrations.
Figure 2.
Exposure–response curves (solid lines) and 95% confidence intervals (shaded areas) for 6 h before event onset for (A) any cardiac event, (B) ischemic events, (C) arrhythmic events, and (D) nonspecific cardiac events. (See Figure S2 for results for 6-h .) Odds ratios are depicted for concentrations relative to a reference value of . The histogram in each panel illustrates the density of exposure distribution. Estimates are from a case-crossover study and are adjusted for the heat index. For any event, arrhythmia events, and nonspecific events, PM is modeled linearly in the conditional logistic regression models. For ischemic events, PM is modeled with restricted cubic splines with 3 knots. Levels of exposure are truncated at (99th percentile) for clearer presentation.
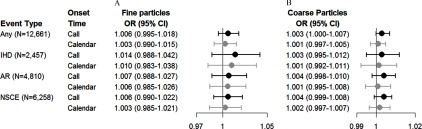
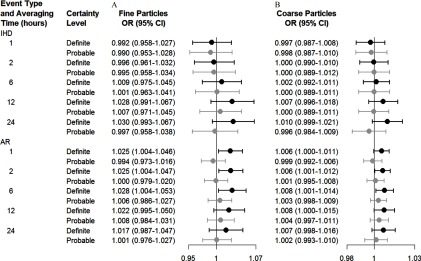
In the secondary analyses, we found that associations based on exposures linked to the call time were slightly stronger than ORs for exposures linked to the calendar day of the call (Figure 3). For example, the OR for an arrhythmic event in association with a increase in 24-h was 1.004 (95% CI: 0.998, 1.010) when based on the call time compared with 1.001 (95% CI: 0.995, 1.008) based on the calendar day of the event. Associations with both and were consistently positive for definite arrhythmia events, whereas ORs for probable arrhythmia events were close to the null (Figure 4). For example, an increase of of and over the previous 6 h was associated with 1.028 (95% CI: 1.004, 1.053) and 1.008 (95% CI: 1.001, 1.014) increased odds with definite arrhythmia as compared with 1.006 (95% CI: 0.986, 1.027) and 1.003 (95% CI: 0.998, 1.009) for probable certainty. For ischemic events, ORs for exposures averaged over 12-h and 24-h were positive for definite events but close to the null for probable events. However, ORs for definite and probable ischemic events were similar for exposures averaged over shorter time periods.
Figure 3.
Estimated ORs and 95% CIs for an increase of in the level of (A) fine particulate matter () and (B) coarse particulate matter () using the actual time of call (black) and the calendar date (gray) for 24-h exposures. Estimates are from a case-crossover study and are adjusted for the heat index. The relation between PM averages and the OR of an ischemic event was nonlinear. AR, arrhythmia; IHD, ischemic heart disease; NSCE, nonspecific cardiac event.
Figure 4.
Estimated ORs and 95% CIs for the onset of cardiac events associated with an increase of in the level of (A) fine particulate matter () and (B) coarse particulate matter () for definite (black) versus probable (gray) diagnoses of ischemic and arrhythmic events by averaging time before event onset. Estimates are from a case-crossover study and are adjusted for the heat index. The relation between PM averages and the OR of an ischemic event was nonlinear. AR, arrhythmia; IHD, ischemic heart disease.
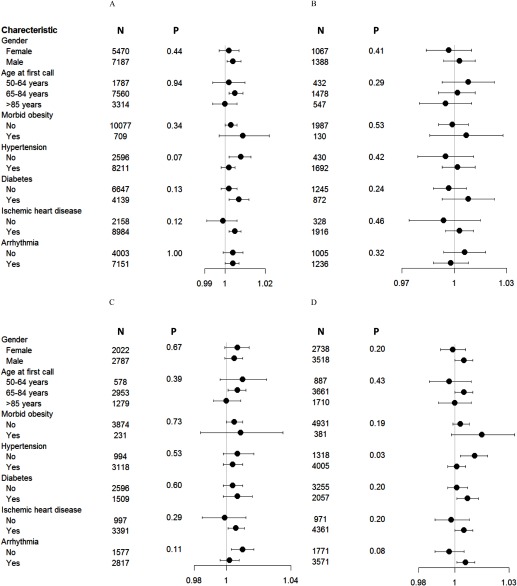
Associations between 6-h PM and cardiac events were positive across all end points for subjects who had diabetes, were morbidly obese, or had a history of ischemic heart disease; whereas corresponding ORs were closer to the null or inverse for people who did not have these conditions (p for interaction = 0.06–0.80 and 0.13–0.73 for and , respectively) (Figure 5; see also Figure S3). Associations with 6-h and were consistently null or negative among those of age, but patterns of associations by age varied among the outcomes (p for interaction = 0.32–0.92 and 0.29–0.94 for and , respectively) (Figure 5; see also Figure S3). Associations of 6-h with any cardiac event and nonspecific cardiac events were significantly stronger for subjects without a history of hypertension than for those with a history of hypertension (p for interaction = 0.07 and 0.03, respectively), but ORs for ischemic events and arrhythmias were not significantly modified by hypertension. Patterns of associations by hypertension were similar for 6-h , although the OR for nonspecific cardiac events was not significantly modified by a history of hypertension (see Figure S3).
Figure 5.
ORs and 95% CIs for an increase of in the level of 6 h before event onset according to selected characteristics for (A) any cardiac event, (B) ischemic events, (C) arrhythmias, and (D) nonspecific cardiac events. (See Figure S3 for results for 6-h .) p-Values are for multiplicative interaction terms between and individual characteristics. Morbid obesity was defined as . Hypertension, ischemic heart disease, and arrhythmia were indicated for people with a history of these conditions. Estimates are from a case-crossover study and are adjusted for the heat index. The relation between averages and the OR of an ischemic event was nonlinear. BMI, body mass index.
Sensitivity Analyses
In general, ORs for PM exposures averaged over were slightly stronger for exposures averaged over 6-h or 12-h versus exposures averaged over 24-h, 2 days, or 3 days for any cardiac event, arrhythmias, and ischemic events (see Table S4). However, for arrhythmias and ischemic events, the strongest associations were estimated for PM exposures averaged over 6 days. ORs for any cardiac event, arrhythmias, and nonspecific events were slightly stronger based on models that included 6-h , , , and compared with corresponding ORs from single-pollutant models for ; whereas ORs for from the multi-pollutant models changed from positive to inverse (see Table S5). ORs for ischemic events were similar between single- and multi-pollutant models for both PM exposures. Increasing the heat index averaging times had little influence on ORs for 6-h or 24-h PM exposures (see Table S6). ORs for 24-h PM exposures were similar to ORs from primary models when restricted to days with of missing PM data (vs. using imputed PM values for days with missing data) (see Table S7). ORs for 6-h PM exposures were similar to primary model estimates when restricted to subjects who did not experience additional cardiac events during the week or month after the index event, but ORs were closer to the null when restricted to subjects without any cardiac events within a year of the index event (see Table S8). Finally, when models with 6-h exposures were based only on the first event experienced by subjects during the study period, ORs were attenuated for any cardiac events and arrhythmias and were approximately null for ischemic and nonspecific events (see Table S8).
Discussion
In this study, we leveraged telemedicine data from two large populations to examine associations between hourly fluctuations in air pollution levels and acute cardiac events. We found that increases in and concentrations were associated with small increases in the odds of a clinical cardiac event within a matter of hours. Our findings also suggest that using more accurate event onset times and outcome information available through telemedicine records may strengthen estimated associations between acute outcomes and short-term exposures to air pollutants. Broadly, this research illustrates the potential benefits of using telemedicine data as a novel population-level data source for use in epidemiological studies of clinical events with sub-daily exposures.
Examination of sub-daily associations between PM and clinical cardiac events has important implications because the U.S. EPA still seeks evidence on effects occurring from exposures at sub-daily averaging times that may serve as a basis for establishing shorter than 24 h PM standards (U.S. EPA 2009, 2011). Although associations with some outcomes were slightly stronger for exposures averaged over shorter time periods, the differences from estimates averaged over 24 h are too small to rule out random variation, and strong correlations between exposures limit our ability to identify critical exposure time windows during the 24 h before a cardiac event.
Previous case-crossover studies of stroke in association with (Ljungman et al. 2008; Lokken et al. 2009) and of ventricular arrhythmias in association with nitrogen dioxide (), , and (in a subset of patients) (Ljungman et al. 2008) reported stronger associations when exposures were estimated relative to the specific time of event onset instead of the day on which the event occurred. Consistent with the previous studies, we hypothesize that this is due to reduced exposure misclassification. Associations with arrhythmias (and some associations with ischemic events) were stronger when clinical information from the telemedicine examinations was used to subclassify events as definite versus probable, consistent with a reduction in outcome misclassification. These results support the value of telemedicine data as a source of information for epidemiologic research. Rapid developments in e-health technology and a growing adoption of these services around the world (Dorsey and Topol 2016; European Commission 2018; Kakria et al. 2015; Müller et al. 2010; Singh et al. 2014) are increasing the potential availability of telemedicine data for research. For example, in the United States there are now nearly 200 telemedicine networks with 3,500 service sites, through which nearly 1 million individuals are equipped with remote cardiac monitors (http://www.americantelemed.org/main/about/about-telemedicine/telemedicine-faqs).
Previous studies of short-term (daily and sub-daily) exposures to PM pollution and clinically relevant arrhythmia were mostly carried out in patients with ICDs (Anderson et al. 2010; Dockery et al. 2005; Link et al. 2013; Ljungman et al. 2008; Metzger et al. 2007; Rich et al. 2005, 2006). Although some of the results were consistent with our findings of an association, the literature on arrhythmia and short-term exposures is largely mixed, as reviewed by Link and Dockery (2010). For example, Link et al. (2013) conducted a prospective follow-up study that evaluated the association of particle number count, , black carbon (BC), sulfate (), , , and with the onset of atrial fibrillation in 176 patients with ICDs. They reported associations with 2-, 6-, and 12-h but not with 24-h or 48-h averages. Another case-crossover study of ventricular arrhythmias in association with , , and (for one site only) in 211 ICD patients found an association with 2-h but not with 24-h averages or with (Ljungman et al. 2008). In contrast, a single case-crossover study of ventricular arrhythmias in 203 ICD patients that evaluated , BC, , CO, , and in the 3, 7, 24, and 48 h before the arrhythmia found clear association only for 24-h and averages (Rich et al. 2005). A subsequent analysis of paroxysmal atrial fibrillation episodes in the same cohort found a clear association only with during the concurrent hour (Rich et al. 2006). In a study of 105 individuals wearing ECG monitors, He et al. (2011) reported an association of within the same hour with the total number of premature ventricular contractions but not with premature atrial contractions. In a series of crossover studies of a variety of controlled exposures (including exposure to concentrated ambient particles for 2 h), Langrish et al. (2014) concluded that based on continuous ECG monitoring there was “no evidence to suggest an increased tendency to arrhythmia after brief controlled exposures…”. Two studies investigating associations with emergency room visits for clinical arrhythmia events reported positive associations with same-day PM averages. In a case-crossover study in Taipei, Taiwan, Chiu et al. (2013) found associations with on warm [] and cool days; and in a time-series study in São Paolo, Brazil, Santos et al. (2008) reported a clear association with . Finally, a meta-analysis of studies assessing associations with hospital admissions indicated a percentage increase in admissions of 0.8% per increase in daily (95% CI: 0.1%, 1.4%) (COMEAP 2006).
Regarding ischemic events, the existing literature on hourly exposures focused primarily on MIs and reported varied results. In a case-crossover study of 79,288 MI diagnoses in 15 conurbations in England and Wales, Bhaskaran et al. (2011) found increased risk of MI 1–6 h after exposure to , but ORs for longer lags were inverse. Similarly, another case-crossover study among 772 patients in the greater Boston area indicated that the strongest association between the onset of MI and were observed within 1–2 h before the onset of MI (Peters et al. 2001). In contrast, case-crossover studies in Augsburg, Germany (691 cases) (Peters et al. 2005), and in King County, Washington (5,793 cases) (Sullivan et al. 2005), found no associations between MI and concentrations in the previous hours or days; and a case-crossover study in Stockholm, Sweden (660 MI cases) (Berglind et al. 2010), found no associations with hourly or daily . Pooling findings across different studies by recent meta-analyses, however, indicated positive overall associations between MI and PM (Luo et al. 2015; Mustafić et al. 2012). For example, Mustafić et al. (2012) found ORs of 1.006 (95% CI: 1.002, 1.009) and 1.025 (95% CI: 1.015, 1.036) per increase in daily and , respectively. Finally, diverse results were also reported for out-of-hospital cardiac arrests using reports by emergency medical services. For example, Rosenthal et al. (2008) found in a case-crossover study of 551 witnessed cardiac arrests in Indianapolis clear association only with exposure during the hour of the arrest, whereas in another case–control study, Ensor et al. (2013) found clear associations with 1-day or 2-day averages but not with hourly exposures for 11,667 cases in Houston. Our analysis of the shape of the concentration–response relationship with 6-h showed evidence of linearity for all outcomes except ischemic events. For this outcome, we found that the slope of the curve was negative at higher exposures. With only about 10% of our data at these high concentration levels, however, our estimates were imprecise and the observed inverse associations could not be distinguished from no or weaker positive associations.
In addition to the many strengths from using telemedicine data noted above, another contribution of this work was our assessment of associations between hourly concentrations and cardiac events in a location with high levels of , which is relevant to large populations around the world (Brook et al. 2014; De Longueville et al. 2013) that are not commonly studied in the literature. Although there is still a lack of knowledge regarding the components of (Adar et al. 2014), a few studies indicated possible toxicity of , including metals and biological materials from bacteria, viruses, and fungi that may be local or collected during long-range transport of dust. In a human controlled-exposure study, Behbod et al. (2013) demonstrated that exposure to coarse PM induced acute systemic inflammatory responses in healthy adults. Perez et al. (2008) found metals that trigger oxidative stress, such as iron, copper, lead and zinc, in on Saharan and non-Saharan dust days. A study in North Carolina (Alexis et al. 2006) found that 13% of the mass of ambient (25% by number) was composed of pollens, fungal spores, and bacteria. PM pollution in Israel comprises mineralogical dust from long-range transport and immediate sources as well as fine-mode aerosols emitted from source combustions and traffic. Previous studies indicated substantial contamination of dust particles by toxic metals such as Pb, Cu, Zn, and Ni, as well as by semi-volatile organic compounds such as the pesticide malathion, during dust storms affecting Israel (Erel et al. 2006, 2013; Kalderon-Asael et al. 2009).
A review of the health implications of pointed to the inconclusive nature of the literature (Adar et al. 2014). Yet, a meta-analysis by the same authors of six hospital admissions studies around the world (London, Helsinki, 6 French cities, 108 counties in the United States, Hong Kong, and 8 cities in Southern Europe) indicated that the pooled rate ratio for a cardiovascular admission in association with a increase in daily was 1.005 (95% CI: 1.003, 1.008). More recently, a study in the United States among adults of age living in 110 large urban counties found evidence for associations between cardiovascular admissions and same-day exposure (Powell et al. 2015). More research is needed to investigate the cardiac health impacts of , especially in locations where there is a large variability in such as Israel because this likely improved our statistical power to detect associations with this pollutant in this study.
Limitations
Limitations of this study should be considered. First, our sample consisted of subjects who chose to subscribe to a commercial service, which is very popular in Israel among older adults. Although subscribers are similar to the general population with respect to smoking habits and prevalence of obesity (ICBS 2012), they are more likely to have cardiovascular disease, high blood pressure, hyperlipidemia, and diabetes (ICDC 2012). If any of these characteristics enhance susceptibility to the impacts of air pollution, then our results would not be generalizable to a random sample of the general population. Our analysis of effect modification by age seemed to go in the inverse direction; for example, the ORs for those are smaller than the ORs for those in the 65- to 84-y-old group. There may be a selection issue in the study in that the older individuals who have survived a previous heart attack may be a healthier subset of the population than others and the younger individuals who enroll in telemedicine insurance are likely to be less healthy than the average young individual. Similarly, we found stronger associations for participants without a history of hypertension than for those with a history of hypertension for all outcomes other than IHD. This result may be related to treatment for hypertension that often slows the heart rate and thus may decrease the risk of arrhythmia but not the risk of an ischemic event. Another limitation of this work is that we did not have the exact time of onset of symptoms. Although the use of telemedicine data provides earlier detection of symptoms than emergency room time, for example, not all individuals will call when they first experience an event. Examination of the hourly distribution of call time indicated that it was approximately uniform over the 24 h, suggesting that there was not a systematic delay during the night hours. Matching on person, time of day, and day of week should also minimize systematic bias from confounding, but bias could still exist due to exposure misclassification. Because we expect any exposure misclassification to be non-differential, this would likely attenuate the estimates toward the null.
We cannot rule out some effect attenuation for the shorter averaging times because it is likely that instrumentation errors in PM are minimized with longer averaging periods in which overestimates are compensated by underestimates. Similarly, the indirect estimation of concentrations by subtraction of from may add uncertainty due to errors in both filters and possibly reduce the estimates toward the null. We used a exposure contrast for all effect estimates to facilitate the presentation of results and facilitate comparisons with other studies rather than using IQRs to generate effect estimates for the same relative increase in exposure across all pollutants and averaging times within our study. Finally, this presentation may not have been appropriate for evaluating IHD given evidence of nonlinearity of the relation between PM and the OR of an ischemic event.
Conclusions
Telemedicine offers a novel and clinically validated data source for epidemiologic studies of associations between short-acting exposures and clinical outcomes. Our findings for Isreali telemedicine service subscribers of age suggest that sub-daily increases in fine and coarse PM may increase the risks of any cardiac events and the risks of acute arrhythmic events specifically. Although some associations were slightly stronger for exposures averaged over shorter time periods, they were generally consistent with findings for 24-h average and exposures.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Environment and Health Fund (RG1201). This paper is dedicated to the memory of our colleague Prof. Arie Roth.
References
- Adar SD, Filigrana PA, Clements N, Peel JL. 2014. Ambient coarse particulate matter and human health: a systematic review and meta-analysis. Curr Environ Health Rep 1(3):258–274, PMID: 25152864, 10.1007/s40572-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, et al. 2006. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol 117(6):1396–1403, PMID: 16751003, 10.1016/j.jaci.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Anderson HR, Armstrong B, Hajat S, Harrison R, Monk V, Poloniecki J, et al. 2010. Air pollution and activation of implantable cardioverter defibrillators in London. Epidemiology 21(3):405–413, PMID: 20386173, 10.1097/EDE.0b013e3181d61600. [DOI] [PubMed] [Google Scholar]
- Audebert HJ, Kukla C, Clarmann von Claranau S, Kühn J, Vatankhah B, Schenkel J, et al. 2005. Telemedicine for safe and extended use of thrombolysis in stroke: the Telemedic Pilot Project for Integrative Stroke Care (TEMPiS) in Bavaria. Stroke 36(2):287–291, PMID: 15625294, 10.1161/01.STR.0000153015.57892.66. [DOI] [PubMed] [Google Scholar]
- Behbod B, Urch B, Speck M, Scott JA, Liu L, Poon R, et al. 2013. Endotoxin in concentrated coarse and fine ambient particles induces acute systemic inflammation in controlled human exposures. Occup Environ Med 70(11):761–767, PMID: 24143017, 10.1136/oemed-2013-101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind N, Ljungman P, Möller J, Hallqvist J, Nyberg F, Rosenqvist M, et al. 2010. Air pollution exposure—a trigger for myocardial infarction? Int J Environ Res Public Health 7(4):1486–1499, PMID: 20617041, 10.3390/ijerph7041486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran K, Hajat S, Armstrong B, Haines A, Herrett E, Wilkinson P, et al. 2011. The effects of hourly differences in air pollution on the risk of myocardial infarction: case crossover analysis of the MINAP database. BMJ 343:d5531, PMID: 21933824, 10.1136/bmj.d5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birati EY, Malov N, Kogan Y, Yanay Y, Tamari M, Elizur M, et al. 2008. Vigilance, awareness and a phone line: 20 years of expediting CPR for enhancing survival after out-of-hospital cardiac arrest. The 'SHL'-Telemedicine experience in Israel. Resuscitation 79(3):438–443, PMID: 18952353, 10.1016/j.resuscitation.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Birati EY, Roth A. 2011. Telecardiology. Isr Med Assoc J 13(8):498–503, PMID: 21910377. [PubMed] [Google Scholar]
- Brook RD, Bard RL, Morishita M, Dvonch JT, Wang L, Yang HY, et al. 2014. Hemodynamic, autonomic, and vascular effects of exposure to coarse particulate matter air pollution from a rural location. Environ Health Perspect 122(6):624–630, PMID: 24618231, 10.1289/ehp.1306595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. 2010. Particulate matter air pollution and cardiovascular disease: an update to the Scientific Statement from the American Heart Association. Circulation 121(21):2331–2378, PMID: 20458016, 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Burgan O, Smargiassi A, Perron S, Kosatsky T. 2010. Cardiovascular effects of sub-daily levels of ambient fine particles: a systematic review. Environ Health 9:26, PMID: 20550697, 10.1186/1476-069X-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, et al. 2010. Telemonitoring in patients with heart failure. N Engl J Med 363(24):2301–2309, PMID: 21080835, 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu HF, Tsai SS, Weng HH, Yang CY. 2013. Short-term effects of fine particulate air pollution on emergency room visits for cardiac arrhythmias: a case-crossover study in Taipei. J Toxicol Environ Health A 76(10):614–623, PMID: 23859081, 10.1080/15287394.2013.801763. [DOI] [PubMed] [Google Scholar]
- COMEAP (Committee on the Medical Effects of Air Pollutants). 2006. “Cardiovascular Disease and Air Pollution: A Report by the Committee on the Medical Effects of Air Pollutants Cardiovascular Sub-Group.” London, UK:Department of Health, National Health Service. [Google Scholar]
- Crabbe H, Barber A, Bayford R, Hamilton R, Jarrett D, Machin N. 2004. The use of a European telemedicine system to examine the effects of pollutants and allergens on asthmatic respiratory health. Sci Total Environ 334–335:417–426, PMID: 15504527, 10.1016/j.scitotenv.2004.04.045. [DOI] [PubMed] [Google Scholar]
- De Longueville F, Ozer P, Doumbia S, Henry S. 2013. Desert dust impacts on human health: an alarming worldwide reality and a need for studies in West Africa. Int J Biometeorol 57(1):1–19, PMID: 22552909, 10.1007/s00484-012-0541-y. [DOI] [PubMed] [Google Scholar]
- Di Q, Dai L, Wang Y, Zanobetti A, Choirat C, Schwartz JD, et al. 2017. Association of short-term exposure to air pollution with mortality in older adults. JAMA 318(24):2446–2456, PMID: 29279932, 10.1001/jama.2017.17923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery DW, Luttmann-Gibson H, Rich DQ, Link MS, Mittleman MA, Gold DR, et al. 2005. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implanted cardioverter defibrillators. Environ Health Perspect 113(6):670–674, PMID: 15929887, 10.1289/ehp.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey ER, Topol EJ. 2016. State of telehealth. N Engl J Med 375(2):154–161, PMID: 27410924, 10.1056/NEJMra1601705. [DOI] [PubMed] [Google Scholar]
- Du Y, Xu X, Chu M, Guo Y, Wang J. 2016. Air particulate matter and cardiovascular disease: the epidemiological, biomedical and clinical evidence. J Thorac Dis 8(1):E8–E19, PMID: 26904258, 10.3978/j.issn.2072-1439.2015.11.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrleman S, Simon R. 1989. Flexible regression models with cubic splines. Stat Med 8(5):551–561, PMID: 2657958, 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- Ensor KB, Raun LH, Persse D. 2013. A case-crossover analysis of out-of-hospital cardiac arrest and air pollution. Circulation 127(11):1192–1199, PMID: 23406673, 10.1161/CIRCULATIONAHA.113.000027. [DOI] [PubMed] [Google Scholar]
- Erel Y, Dayan U, Rabi R, Rudich Y, Stein M. 2006. Trans boundary transport of pollutants by atmospheric mineral dust. Environ Sci Technol 40(9):2996–3005, PMID: 16719103, 10.1021/es051502l. [DOI] [PubMed] [Google Scholar]
- Erel Y, Tirosh O, Kessler N, Dayan U, Belkin S, Stein M, et al. 2013. Atmospheric particulate matter (PM) in the Middle East: toxicity, trans-boundary transport, and influence of synoptic conditions. In: Medical Geochemistry: Geological Materials and Health. Censi P, Darrah T, Erel Y, eds. Dordrecht, Netherlands:Springer, 31–46. [Google Scholar]
- European Commission. 2018. EU-funded Research & Innovation in the Field of ICT for Health, Wellbeing & Ageing: An Overview. Luxembourg, Europe: European Union. http://ec.europa.eu/newsroom/document.cfm?doc_id=2852 [accessed 22 July 2018].
- Ghelfi E, Rhoden CR, Wellenius GA, Lawrence J, Gonzalez-Flecha B. 2008. Cardiac oxidative stress and electrophysiological changes in rats exposed to concentrated ambient particles are mediated by TRP-dependent pulmonary reflexes. Toxicol Sci 102(2):328–336, PMID: 18184637, 10.1093/toxsci/kfn005. [DOI] [PubMed] [Google Scholar]
- He F, Shaffer ML, Rodriguez-Colon S, Yanosky JD, Bixler E, Cascio WE, et al. 2011. Acute effects of fine particulate air pollution on cardiac arrhythmia: the APACR study. Environ Health Perspect 119(7):927–932, PMID: 21398201, 10.1289/ehp.1002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICBS (Israel Central Bureau of Statistics). 2012. Social Survey 2010 [in Hebrew]. Jerusalem, Israel: Israel Central Bureau of Statistics; http://cbs.gov.il/publications12/seker_hevrati10/pdf/h_print.pdf [accessed 22 July 2018]. [Google Scholar]
- ICDC (Israel Center for Disease Control). 2012. Israel National Health Interview Survey INHIS-2, 2007-2010—Selected Findings [in Hebrew]. Jerusalem, Israel:Israel Center for Disease Control, Ministry of Health; http://www.health.gov.il/publicationsfiles/inhis_2.pdf [accessed 22 July 2018]. [Google Scholar]
- Ito K, Mathes R., Ross Z., Nádas A., Thurston G., Matte T. 2011. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ Health Perspect 119(4):467–473, PMID: 21463978, 10.1289/ehp.1002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes H, Sheppard L, Lumley T. 2005. Case–crossover analyses of air pollution exposure data: referent selection strategies and their implication for bias. Epidemiology 16(6):717–726, 10.1097/01.ede.0000181315.18836.9d. [DOI] [PubMed] [Google Scholar]
- Kakria P, Tripathi NK, Kitipawang P. 2015. A real-time health monitoring system for remote cardiac patients using smartphone and wearable sensors. Int J Telemed Appl 2015:373474, PMID: 26788055, 10.1155/2015/373474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon-Asael B, Erel Y, Sandler A, Dayan U. 2009. Mineralogical and chemical characterization of suspended atmospheric particles over the east Mediterranean based on synoptic-scale circulation patterns. Atmos Environ 43(25):3963–3970, 10.1016/j.atmosenv.2009.03.057. [DOI] [Google Scholar]
- Langrish JP, Watts SJ, Hunter AJ, Shah AS, Bosson JA, Unosson J, et al. 2014. Controlled exposures to air pollutants and risk of cardiac arrhythmia. Environ Health Perspect 122(7):747–753, PMID: 24667535, 10.1289/ehp.1307337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem-Rubinow E, Abramowitz Y, Malov N, Hadad M, Tamari M, Golovner M, et al. 2011a. Prehospital cardiac markers in defining ambiguous chest pain. Arch Intern Med 171(22):2056–2057, PMID: 22158578, 10.1001/archinternmed.2011.477. [DOI] [PubMed] [Google Scholar]
- Leshem-Rubinow E, Berger M, Shacham J, Birati EY, Malov N, Tamari M, et al. 2011b. New real-time loop recorder diagnosis of symptomatic arrhythmia via telemedicine. Clin Cardiol 34(7):420–425, PMID: 21618252, 10.1002/clc.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link MS, Dockery DW. 2010. Air pollution and the triggering of cardiac arrhythmias. Curr Opin Cardiol 25(1):16–22, PMID: 19881339, 10.1097/HCO.0b013e32833358cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link MS, Luttmann-Gibson H, Schwartz J, Mittleman MA, Wessler B, Gold DR, et al. 2013. Acute exposure to air pollution triggers atrial fibrillation. J Am Coll Cardiol 62(9):816–825, PMID: 23770178, 10.1016/j.jacc.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman PL, Berglind N, Holmgren C, Gadler F, Edvardsson N, Pershagen G, et al. 2008. Rapid effects of air pollution on ventricular arrhythmias. Eur Heart J 29(23):2894–2901, PMID: 19004842, 10.1093/eurheartj/ehn463. [DOI] [PubMed] [Google Scholar]
- Lokken RP, Wellenius GA, Coull BA, Burger MR, Schlaug G, Suh HH, et al. 2009. Air pollution and risk of stroke: underestimation of effect due to misclassification of time of event onset. Epidemiology 20(1):137–142, PMID: 19244659, 10.1097/ede.0b013e31818ef34a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Zhu X, Yao C, Hou L, Zhang J, Cao J, et al. 2015. Short-term exposure to particulate air pollution and risk of myocardial infarction: a systematic review and meta-analysis. Environ Sci Pollut Res Int 22(19):14651–14662, PMID: 26298338, 10.1007/s11356-015-5188-x. [DOI] [PubMed] [Google Scholar]
- Maclure M. 1991. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 133(2):144–153, PMID: 1985444, 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- Metzger KB, Klein M, Flanders WD, Peel JL, Mulholland JA, Langberg JJ, Tolbert PE. 2007. Ambient air pollution and cardiac arrhythmias in patients with implantable defibrillators. Epidemiology 18(5):585–592, PMID: 17700247, 10.1097/EDE.0b013e318124ff0e. [DOI] [PubMed] [Google Scholar]
- Mills NL, Törnqvist H, Gonzalez MC, Vink E, Robinson SD, Soderberg S, et al. 2007. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med 357(11):1075–1082, PMID: 17855668, 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Maclure M, Robins JM. 1995. Control sampling strategies for case-crossover studies: an assessment of relative efficiency. Am J Epidemiol 142(1):91–98, PMID: 7785679, 10.1093/oxfordjournals.aje.a117550. [DOI] [PubMed] [Google Scholar]
- Müller A, Schweizer J, Helms TM, Oeff M, Sprenger C, Zugck C. 2010. Telemedical support in patients with chronic heart failure: experience from different projects in Germany. Int J Telemed Appl 2010:1–11, 10.1155/2010/181806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzel T, Sørensen M, Gori T, Schmidt FP, Rao X, Brook J, et al. 2017. Environmental stressors and cardio-metabolic disease: part I–epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J 38(8):550–556, PMID: 27460892, 10.1093/eurheartj/ehw269. [DOI] [PubMed] [Google Scholar]
- Mustafić H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, et al. 2012. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA 307(7):713–721, PMID: 22337682, 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, et al. 2015. Expert position paper on air pollution and cardiovascular disease. Eur Heart J 36(2):83–93b, PMID: 25492627, 10.1093/eurheartj/ehu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez L, Tobias A, Querol X, Künzli N, Pey J, Alastuey A, et al. 2008. Coarse particles from Saharan dust and daily mortality. Epidemiology 19(6):800–807, PMID: 18938653, 10.1097/ede.0b013e31818131cf. [DOI] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Muller JE, Mittleman MA. 2001. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 103(23):2810–2815, PMID: 11401937, 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Peters A, von Klot S, Heier M, Trentinaglia I, Cyrys J, Hörmann A, et al. 2005. Air pollution, personal activities, and onset of myocardial infarction in a case-crossover study, part I. In: Particulate Air Pollution and Nonfatal Cardiac Events, Research Report 124. Boston, MAHealth Effects Institute, 1–66. [PubMed] [Google Scholar]
- Powell H, Krall JR, Wang Y, Bell ML, Peng RD. 2015. Ambient coarse particulate matter and hospital admissions in the Medicare Cohort Air Pollution Study, 1999–2010. Environ Health Perspect 123(11):1152–1158, PMID: 25872223, 10.1289/ehp.1408720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Mittleman MA, Link MS, Schwartz J, Luttmann-Gibson H, Catalano PJ, et al. 2006. Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environ Health Perspect 114(1):120–123, PMID: 16393668, 10.1289/ehp.8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Schwartz J, Mittleman MA, Link M, Luttmann-Gibson H, Catalano PJ, et al. 2005. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am J Epidemiology 161(12):1123–1132, PMID: 15937021, 10.1093/aje/kwi143. [DOI] [PubMed] [Google Scholar]
- Rosenthal FS, Carney JP, Olinger ML. 2008. Out-of-hospital cardiac arrest and airborne fine particulate matter: a case-crossover analysis of emergency medical services data in Indianapolis, Indiana. Environ Health Perspect 116(5):631–636, PMID: 18470283, 10.1289/ehp.10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A, Malov N, Steinberg DM, Yanay Y, Elizur M, Tamari M, et al. 2009. Telemedicine for post-myocardial infarction patients: an observational study. Telemed J E Health 15(1):24–30, PMID: 19199844, 10.1089/tmj.2008.0068. [DOI] [PubMed] [Google Scholar]
- Santos UP, Terra-Filho M, Lin CA, Pereira LA, Vieira TC, Saldiva PH, et al. 2008. Cardiac arrhythmia emergency room visits and environmental air pollution in São Paulo, Brazil. J Epidemiol Community Health 62(3):267–272, PMID: 18272743, 10.1136/jech.2006.058123. [DOI] [PubMed] [Google Scholar]
- Schwaab B, Katalinic A, Riedel J, Kiepe W, Huhmann W, Sheikhzadeh A. 2003. Feasibility and reliability of a transtelephonic 12 leads ECG [in German]. Z Kardiol 92(1):31–38, PMID: 12545299, 10.1007/s00392-003-0876-9. [DOI] [PubMed] [Google Scholar]
- Shacham J, Birati EY, Malov N, Yanay Y, Steinberg DM, Tamari M, et al. 2012. Telemedicine for diagnosing and managing paroxysmal atrial fibrillation in outpatients. The phone in the pocket. Int J Cardiol 157(1):91–95, PMID: 21195490, 10.1016/j.ijcard.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Singh M, Agarwal A, Sinha V, Manoj Kumar R, Jaiswal N, Jindal I, et al. 2014. Application of handheld tele-ECG for health care delivery in rural India. Int J Telemed Appl 2014:981806, PMID: 25368654, 10.1155/2014/981806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafoggia M, Samoli E, Alessandrini E, Cadum E, Ostro B, Berti G, et al. 2013. Short-term associations between fine and coarse particulate matter and hospitalizations in Southern Europe: results from the MED-PARTICLES project. Environ Health Perspect 121(9):1026–1033, PMID: 23777832, 10.1289/ehp.1206151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieb DM, Szyszkowicz M, Rowe BH, Leech JA. 2009. Air pollution and emergency department visits for cardiac and respiratory conditions: a multi-city time-series analysis. Environ Health 8:25, PMID: 19515235, 10.1186/1476-069X-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J, Sheppard L, Schreuder A, Ishikawa N, Siscovick D, Kaufman J. 2005. Relation between short-term fine-particulate matter exposure and onset of myocardial infarction. Epidemiology 16(1):41–48, PMID: 15613944, 10.1097/01.ede.0000147116.34813.56. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2009. “Integrated Science Assessment (ISA) for Particulate Matter (Final Report, Dec 2009).” Research Triangle Park, NC:U.S. EPA, National Center for Environmental Assessment, Office of Research and Development; EPA/600/R-08/139F http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=216546 [accessed 22 July 2018]. [Google Scholar]
- U.S. EPA. 2011. “Policy Assessment for the Review of the Particulate Matter National Ambient Air Quality Standards.” Research Triangle Park, NC:U.S. EPA, Office of Air Quality Planning and Standards; EPA 452/R-11-003. http://www.epa.gov/ttn/naaqs/standards/pm/data/20110419pmpafinal.pdf [accessed 22 July 2018]. [Google Scholar]
- Wennberg DE, Marr A, Lang L, O’Malley S, Bennett G. 2010. A randomized trial of a telephone care-management strategy. N Engl J Med 363(13):1245–1255, PMID: 20860506, 10.1056/NEJMsa0902321. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2016. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease. Geneva, Switzerland:WHO; http://apps.who.int/iris/bitstream/10665/250141/1/9789241511353_eng.pdf [accessed 22 July 2018]. [Google Scholar]
- WHO. 2018. 9 out of 10 people worldwide breathe polluted air, but more countries are taking action. http://www.who.int/news-room/detail/02-05-2018-9-out-of-10-people-worldwide-breathe-polluted-air-but-more-countries-are-taking-action [accessed 22 July 2018].
- Wolf K, Schneider A, Breitner S, Meisinger C, Heier M, Cyrys J, et al. 2015. Associations between short-term exposure to particulate matter and ultrafine particles and myocardial infarction in Augsburg, Germany. Int J Hyg Environ Health 218(6):535–542, PMID: 26013401, 10.1016/j.ijheh.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. 2005. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environ Health Perspect 113(8):978–982, PMID: 16079066, 10.1289/ehp.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. 2009. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect 117(6):898–903, PMID: 19590680, 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.