Abstract
Although widespread hepatitis A vaccination has dramatically decreased infection rates, a large proportion of VA patients in traditionally high-risk groups remains susceptible to infection.
Hepatitis A virus (HAV) can result in acute infection characterized by fatigue, nausea, jaundice (yellowing of the skin) and, rarely, acute liver failure and death.1,2 In the US, HAV yearly incidence (per 100,000) has decreased from 11.7 cases in 1996 to 0.4 cases in 2015, largely due to the 2006 recommendations from the Centers for Disease Control and Prevention (CDC) that all infants receive HAV vaccination.3,4
In 2017, multiple HAV outbreaks occurred in Arizona, California, Colorado, Kentucky, Michigan, and Utah with infections concentrated among those who were homeless, used illicit drugs (both injection and noninjection), or had close contact with these groups (Table 1).5–7 These HAV outbreaks resulted in more than 1,000 hospitalizations and 45 reported deaths. The true scope of the outbreaks is believed to be much larger, given that HAV cases are under-reported.8
Table 1.
HAV Outbreaks Concentrated Among Those Who Are Homeless and Use Drugs
| Jurisdiction | Date Updated | Cases | Hospitalizations | Deaths |
|---|---|---|---|---|
| Arizona: Maricopa County | 10/7/17 | 17 | NR | 0 |
| California: San Diego, Santa Cruz, and Los Angeles | 1/12/18 | 688 | 449 | 21 |
| Colorado | 8/30/17 | 54 | NR | NR |
| Kentucky: Jefferson, Shelby, Bullitt, Hardin, Anderson, Mason, Christian, Madison, Fayette, McCracken, Hopkins, and Leslie counties | 11/21/17 | 31 | NR | 0 |
| Michigan: Southeast region | 1/24/18 | 715 | 582 | 24 |
| Utah: Salt Lake County, Utah County, Bear River, Southwest region, Central region, Tooele, Weber-Morgan | 1/22/18 | 154 | 81 | 0 |
Abbreviations: HAV, hepatitis A virus; NR, not reported.
In response, the CDC has recommended the administration of HAV vaccine or immune globulin (IG) as postexposure prophylaxis (PEP) to people in high-risk groups including unvaccinated individuals exposed to HAV within the prior 2 weeks.5 While the Veterans Health Administration (VHA) in the Department of Veteran’s Affairs (VA) has not noted a significant increase in the number of reported HAV infections, there have been cases of hospitalization within the VA health care system due to HAV in at least 2 of the outbreak areas. The VA facilities in outbreak areas are responding by supporting county disease-control measures that include ensuring handwashing stations and vaccinations for high-risk, in-care populations and employees in direct contact with patients at high risk for HAV.
This review provides information on HAV transmission and clinical manifestations, guidelines on the prevention of HAV infection, and baseline data on current HAV susceptibility and immunization rates in the VHA.
TRANSMISSION AND CLINICAL MANIFESTATIONS
Hepatitis A virus is primarily transmitted by ingestion of small amounts of infected stool (ie, fecal-oral route) via direct person-to-person contact or through exposure to contaminated food or water.9,10 Groups at high risk of HAV infection include those in direct contact with HAV-infected individuals, users of injection or non-injection drugs, men who have sex with men (MSM), travelers to high-risk countries, individuals with clotting disorders, and those who work with nonhuman primates.11 Individuals who are homeless are susceptible to HAV due to poor sanitary conditions, and MSM are at increased risk of HAV acquisition via exposure to infected stool during sexual activity.
Complications of acute HAV infection, including fulminant liver failure and death, are more common among patients infected with hepatitis B virus (HBV) or hepatitis C virus (HCV).12,13 While infection with HIV does not independently increase the risk of HAV acquisition, about 75% of new HIV infections in the US are among MSM or IV drug users who are at increased risk of HAV infection.14 In addition, duration of HAV viremia and resulting HAV transmissibility may be increased in HIV-infected individuals.15–17
After infection, HAV remains asymptomatic (the incubation period) for an average of 28 days with a range of 15 to 50 days.18,19 Most children younger than 6 years remain asymptomatic while older children and adults typically experience symptoms including fever, fatigue, poor appetite, abdominal pain, dark urine, clay-colored stools, and jaundice.2,20,21 Symptoms typically last less than 2 months but can persist or relapse for up to 6 months in 10% to 15% of symptomatic individuals.22,23 Those with HAV infection are capable of viral transmission from the beginning of the incubation period until about a week after jaundice appears.24 Unlike HBV and HCV, HAV does not cause chronic infection.
Fulminant liver failure, characterized by encephalopathy, jaundice, and elevated international normalized ratio (INR), occurs in < 1% of HAV infections and is more common in those with underlying liver disease and older individuals.13,25–27 In one retrospective review of fulminant liver failure from HAV infection, about half of the patients required liver transplantation or died within 3 weeks of presentation.12
Other than supportive care, there are no specific treatments for acute HAV infection. However, the CDC recommends that healthy individuals aged between 1 and 40 years with known or suspected exposure to HAV within the prior 2 weeks receive 1 dose of a single-antigen HAV vaccination. The CDC also recommends that recently exposed individuals aged < 1 year or > 40 years, or patients who are immunocompromised, have chronic liver disease (CLD), or are allergic to HAV vaccine or a vaccine component should receive a single IG injection. In addition, the CDC recommends that health care providers report all cases of acute HAV to state and local health departments.28
In patients with typical symptoms of acute viral hepatitis (eg, headache, fever, malaise, anorexia, nausea, vomiting, abdominal pain, and diarrhea) and either jaundice or elevated serum aminotransferase levels, confirmation of HAV infection is required with either a positive serologic test for immunoglobulin M (IgM) anti-HAV antibody or an epidemiologic link (eg, recent household or close contact) to a person with laboratory-confirmed HAV.5 Serum IgM anti-HAV antibodies are first detectable when symptoms begin and remain detectable for about 3 to 6 months.29,30 Serum immunoglobulin G (IgG) anti-HAV antibodies, which provide lifelong protection against reinfection, appear as symptoms improve and persist indefinitely. 31,32 Therefore, the presence of anti-HAV IgG and the absence of anti-HAV IgM is indicative of immunity to HAV via past infection or vaccination.
HAV PREVENTION IN THE VHA
The mainstay of HAV prevention is vaccination with 2 doses of inactivated, single-antigen hepatitis A vaccine or 3 doses of combination (HAV and HBV) vaccine. 11 Both single antigen and combination HAV vaccines are safe in immunocompromised and pregnant patients.33–39 The HAV vaccination results in 100% anti-HAV IgG seropositivity among healthy individuals, although immunogenicity might be lower for those who are immunocompromised or with CLD.31,40–47 The VHA recommends HAV immunization, unless contraindicated, for previously unvaccinated adults who are at increased risk of contracting HAV and for any other adult who is seeking protection from HAV infection (Table 2).48 Hepatitis A virus vaccination is not specifically recommended for workers in food service, health care, sanitation, or child care.11
Table 2.
VHA HAV Immunization Guidelines
The Veterans Health Administration recommends HAV immunization (unless contraindicated) for previously unvaccinated adults who are at increased risk of contracting HAV infection and for any other adult who is seeking protection from HAV infection. High risk groups include:
|
Abbreviations: HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; VHA, Veterans Health Administration.
Since these individuals may not be immune to HAV, it is recommended that they be given the first dose in the 2-dose series, tested for HAV antibodies during the same visit, and be given a second dose of HAV vaccine if they are found to be nonimmune.
In addition to vaccination, addressing risk factors for HAV infection and its complications could reduce the burden of disease. For instance, recent outbreaks highlight that homeless individuals and users of injection and noninjection drugs are particularly vulnerable to infections transmitted via fecal-oral contamination. Broad strategies to address homelessness and related sanitation concerns are needed to help reduce the likelihood of future HAV outbreaks.49 Specific measures to combat HAV include providing access to clean water, adequate hygiene, and clean needles for people who inject drugs.11 Hepatitis A virus can be destroyed by heating food to ≥ 185 °F for at least 1 minute, chlorinating contaminated water, or cleaning contaminated surfaces with a solution of household bleach and water.50 Moreover, it is important to identify and treat risk factors for complications of HAV infection. This includes identifying individuals with HCV and ensuring that they are immune to HAV, given data that HCV-infected individuals are at increased risk of fulminant hepatic failure from HAV.12,13
Active-duty service members have long been considered at higher risk of HAV infections due to deployments in endemic areas and exposure to contaminated food and water.51,52 Shortly after the FDA approved HAV vaccination in 1995, the Department of Defense (DoD) mandated screening and HAV immunization for all incoming active-duty service members and those deployed to areas of high endemicity.53 However, US veterans who were discharged before the adoption of universal HAV vaccination remain at increased risk for HAV infection, particularly given the high prevalence of CLD, homelessness, and substance use disorder (SUD) in this cohort. 54–56 Given the importance of HAV prevention for high-risk individuals, an analysis was performed to determine rates of HAV vaccination and testing within VA-enrolled individuals with selected risk factors for HAV acquisition or complications.
METHODS
A cross-sectional analysis of veterans in VA care from June 1, 2016 to June 1, 2017 was performed to determine national rates of HAV susceptibility among patients with HCV exposure, homelessness, SUD, or HIV infection. The definitions of homelessness, SUD (alcohol, cannabis, opioid, sedatives, hallucinogens, inhalants, stimulants, or tobacco), and HIV infection were based on the presence of appropriate ICD-9 or ICD-10 codes. History of HCV exposure was based on a positive HCV antibody test. Presence of HAV vaccination was determined based on CPT codes for administration of the single-antigen HAV vaccination or combination HAV/HBV vaccination.
While HIV infection is not independently considered an indication for HAV vaccination, the authors included this group given its high proportion of patients with other risk factors, including MSM and IV drug use. All data were obtained from the VA Corporate Data Warehouse (CDW), a comprehensive national repository of all laboratory, diagnosis, and prescription results (including vaccines) within the VHA since 1999.
Hepatitis A virus nonsusceptibility was defined as (1) documented receipt of HAV vaccination within the VHA; (2) anti-HAV IgG antibody testing within the VHA; or (3) active-duty service after October 1997. It was considered likely that patients who received HAV testing either showed evidence of HAV immunity (eg, positive anti-HAV IgG) or were anti-HAV IgG negative and subsequently immunized. Therefore, patients with anti-HAV IgG antibody testing were counted presumptively as nonsusceptible. The DoD implemented a universal HAV vaccination policy in 1995, therefore, 1997 was chosen as a time at which the military’s universal HAV vaccination campaign was likely to have achieved near 100% vaccination coverage of active-duty military.
RESULTS
The cohort included 5,896,451 patients in VA care, including 381,628 (6.5%) who were homeless, 455,344 (7.7%) with SUD, 225,889 (3.8%) with a lifetime history of positive HCV antibody (indicating past HCV exposure), and 29,166 (0.5%) with HIV infection. National rates of HAV susceptibility were lowest among patients with HIV (mean 21.8%, facility range 0%–56.5%) followed by SUD (mean 47.4%, facility range 3.8%–70.4%), homelessness (mean 48.4%, facility range 5.9%–69.3%), and HCV exposure (mean 48.9%, facility range 30.5%–71.6%) (Table 3).
Table 3.
Hepatitis A Vaccination and Testing Among US Veterans Within High-Risk Subgroupsa
| Vaccinated, % | Tested, % | Active Duty After 9/1997, % | HAV Tested/Vaccinated, % | HAV Susceptible, mean, % | |
|---|---|---|---|---|---|
| Homeless | 24.5 | 18.5 | 22.3 | 51.6 | 48.4 |
| SUD | 24.8 | 19.7 | 22.1 | 52.6 | 47.4 |
| HCV Ab+ | 28.2 | 32.2 | 13.2 | 55.7 | 44.3 |
| HIV | 48.8 | 48.9 | 18.6 | 78.2 | 21.8 |
Abbreviations: HAV, hepatitis A virus; HCV Ab+, hepatitis C virus antibody positive; SUD, substance use disorder.
Derived from analysis of VA Corporate Data Warehouse data; rates of HAV susceptibility based on rates of HAV vaccination, testing, and recent active-duty service among patients with homelessness, SUD, HCV antibody positivity, and HIV infection in the national VHA.
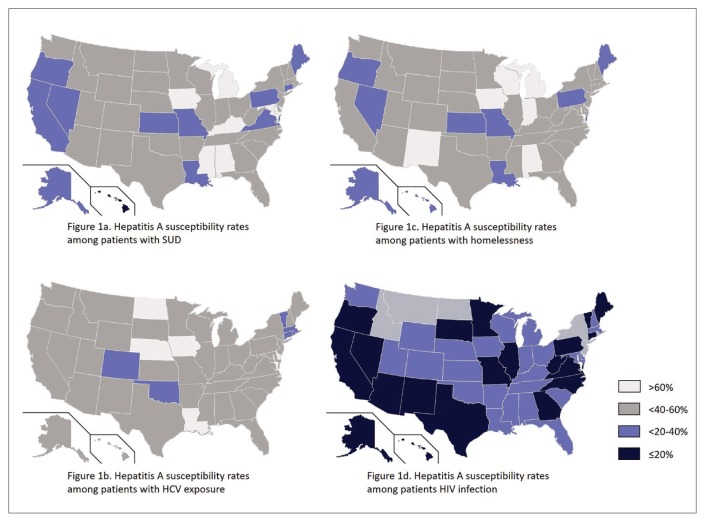
There was wide geographic variability in rates of HAV susceptibility (Figure 1). When limiting the analysis to patients with confirmed vaccination within the VHA or active duty military service after October 1997, VA facilities in states with active outbreaks had a mean HAV vaccination rate of 38.1% (range 31.5%–44.3%) among patients who were homeless and 42.0% (range 33.8%–49.0%) among patients with SUD.
Figure 1.
HAV Average Statewide Rates of Selected VA Subgroups
Abbreviations: HAV, hepatitis A virus; HCV, hepatitis C virus; SUD, substance use disorder.
Overall HAV susceptibility rates were lowest among patients with HIV followed by homelessness, SUD, and HCV exposure. There was wide variability in statewide HAV susceptibility rates in all subgroups.
DISCUSSION
Widespread HAV vaccination has decreased the incidence of HAV infection in the US dramatically. Nevertheless, recent outbreaks demonstrate that substantial population susceptibility and associated risk for HAV-related morbidity and mortality remains, particularly in high-risk populations. Although the VHA has not experienced a significant increase in acute HAV infections to date, this cross-sectional analysis highlights that a large proportion of VA patients in traditionally high-risk groups remain susceptible to HAV infection.
Strengths
Strengths of this analysis include a current reflection of HAV susceptibility within the national VHA, thus informing HAV testing and vaccination strategies. This study also involves a very large cohort, which is possible because the VHA is the largest integrated healthcare system in the US. Lastly, because the VHA uses electronic medical records, there was nearly complete capture of HAV vaccinations and testing obtained through the VHA.
Limitations
This cross-sectional analysis has several potential limitations. First, findings may not be generalizable outside the VHA. In addition, determination of homelessness, substance abuse, and HIV infection were based on ICD-9 and ICD-10 codes, which have been used in previous studies but may be subject to misclassification. The authors deliberately included all patients with positive HCV antibody testing to include those with current or prior risk factors for HAV acquisition. This population does not reflect patients with HCV viremia who received HAV testing or vaccination. Lastly, misattribution of HAV susceptibility could have occurred if patients with negative HAV IgG results were not vaccinated or if patients previously received HAV vaccination outside the VHA.
CONCLUSION
To mitigate the risk of future HAV outbreaks, continued efforts should be made to increase vaccination among high-risk groups, improve awareness of additional prevention measures, and address risk factors for HAV acquisition, particularly in areas with active outbreaks. Further study is suggested to identify geographic areas with large caseloads of at-risk patients and to highlight best practices utilized by VHA facilities that achieved high vaccine coverage rates. Recommended approaches likely will need to include efforts to improve hygiene and reduce risks for HAV exposure associated with SUD and homelessness.
Footnotes
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
REFERENCES
- 1.Kemmer NM, Miskovsky EP, Hepatitis A. Infect Dis Clin North Am. 2000;14(3):605–615. doi: 10.1016/s0891-5520(05)70123-9. [DOI] [PubMed] [Google Scholar]
- 2.Tong MJ, el-Farra NS, Grew MI. Clinical manifestations of hepatitis A: recent experience in a community teaching hospital. J Infect Dis. 1995;171(suppl 1):S15–S18. doi: 10.1093/infdis/171.supplement_1.s15. [DOI] [PubMed] [Google Scholar]
- 3.Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis a disease in the United States. MMWR Suppl. 2016;65(1):29–41. doi: 10.15585/mmwr.su6501a6. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Viral hepatitis surveillance, United States. 2015. [Accessed February 12, 2018]. https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Published 2015.
- 5.Centers for Disease Control and Prevention. 2017– Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. [Accessed February 12, 2018]. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Updated February 7, 2018.
- 6.Hepatitis A cases more than double in 2017 if you’re at risk, get vaccinated [press release] [Accessed February 12, 2018]. https://www.colorado.gov/pacific/cdphe/news/hep-a-cases-doubled. Published August 30, 2017.
- 7.Alltucker K. Hepatitis A outbreak spread to Maricopa County homeless from San Diego, officials say. Azcentral website. Oct 7, 2017. [Accessed February 12, 2018]. https://www.azcentral.com/story/news/local/arizona-health/2017/10/07/hepatitis-outbreak-spread-maricopa-county-homeless-san-diego-officials-say/740185001/
- 8.Savage RD, Rosella LC, Brown KA, Khan K, Crowcroft NS. Underreporting of hepatitis A in non-endemic countries: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:281. doi: 10.1186/s12879-016-1636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purcell RH, Wong DC, Shapiro M. Relative infectivity of hepatitis A virus by the oral and intravenous routes in 2 species of nonhuman primates. J Infect Dis. 2002;185(11):1668–1671. doi: 10.1086/340520. [DOI] [PubMed] [Google Scholar]
- 10.Tassopoulos NC, Papaevangelou GJ, Ticehurst JR, Purcell RH. Fecal excretion of Greek strains of hepatitis A virus in patients with hepatitis A and in experimentally infected chimpanzees. J Infect Dis. 1986;154(2):231–237. doi: 10.1093/infdis/154.2.231. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Hepatitis A questions and answers for health professionals. [Accessed February 12, 2018]. https://www.cdc.gov/hepatitis/hav/havfaq.htm. Updated November 8, 2017.
- 12.Taylor RM, Davern T, Munoz S, et al. US Acute Liver Failure Study Group. Fulminant hepatitis A virus infection in the United States: Incidence, prognosis, and outcomes. Hepatology. 2006;44(6):1589–1597. doi: 10.1002/hep.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vento S, Garofano T, Renzini C, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338(5):286–290. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Johnson AS, McCray E, Hall HI. Conference on Retroviruses and Opportunistic Infections (CROI) Seattle, WA: Feb 13–16, 2017. [Accessed February 12, 2018]. CDC - HIV incidence, prevalence and undiagnosed infections in men who have sex with men - HIV incidence decreased among all transmission categories except MSM. http://www.natap.org/2017/CROI/croi_116.htm. [Google Scholar]
- 15.Fonquernie L, Meynard JL, Charrois A, Delamare C, Meyohas MC, Frottier J. Occurrence of acute hepatitis A in patients infected with human immunodeficiency virus. Clin Infect Dis. 2001;32(2):297–299. doi: 10.1086/318478. [DOI] [PubMed] [Google Scholar]
- 16.Ida S, Tachikawa N, Nakajima A, et al. Influence of human immunodeficiency virus type 1 infection on acute hepatitis A virus infection. Clin Infect Dis. 2002;34(3):379–385. doi: 10.1086/338152. [DOI] [PubMed] [Google Scholar]
- 17.Costa-Mattioli M, Allavena C, Poirier AS, Billaudel S, Raffi F, Ferré V. Prolonged hepatitis A infection in an HIV-1 seropositive patient. J Med Virol. 2002;68(1):7–11. doi: 10.1002/jmv.10163. [DOI] [PubMed] [Google Scholar]
- 18.Neefe JR, Gellis SS, Stokes J., Jr Homologous serum hepatitis and infectious (epidemic) hepatitis; studies in volunteers bearing on immunological and other characteristics of the etiological agents. Am J Med. 1946;1:3–22. doi: 10.1016/0002-9343(46)90017-4. [DOI] [PubMed] [Google Scholar]
- 19.Krugman S, Giles JP, Hammond J. Infectious hepatitis. Evidence for two distinctive clinical, epidemiological, and immunological types of infection. JAMA. 1967;200(5):365–373. doi: 10.1001/jama.200.5.365. [DOI] [PubMed] [Google Scholar]
- 20.Hadler SC, Webster HM, Erben JJ, Swanson JE, Maynard JE. Hepatitis A in day-care centers. A community-wide assessment. N Engl J Med. 1980;302(22):1222–1227. doi: 10.1056/NEJM198005293022203. [DOI] [PubMed] [Google Scholar]
- 21.Lednar WM, Lemon SM, Kirkpatrick JW, Redfield RR, Fields ML, Kelley PW. Frequency of illness associated with epidemic hepatitis A virus infections in adults. Am J Epidemiol. 1985;122(2):226–233. doi: 10.1093/oxfordjournals.aje.a114093. [DOI] [PubMed] [Google Scholar]
- 22.Gordon SC, Reddy KR, Schiff L, Schiff ER. Prolonged intrahepatic cholestasis secondary to acute hepatitis A. Ann Intern Med. 1984;101(5):635–637. doi: 10.7326/0003-4819-101-5-635. [DOI] [PubMed] [Google Scholar]
- 23.Schiff ER. Atypical clinical manifestations of hepatitis A. Vaccine. 1992;10(suppl 1):S18–S20. doi: 10.1016/0264-410x(92)90534-q. [DOI] [PubMed] [Google Scholar]
- 24.Richardson M, Elliman D, Maguire H, Simpson J, Nicoll A. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20(4):380–391. doi: 10.1097/00006454-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Willner IR, Uhl MD, Howard SC, Williams EQ, Riely CA, Waters B. Serious hepatitis A: an analysis of patients hospitalized during an urban epidemic in the United States. Ann Intern Med. 1998;128(2):111–114. doi: 10.7326/0003-4819-128-2-199801150-00006. [DOI] [PubMed] [Google Scholar]
- 26.Rezende G, Roque-Afonso AM, Samuel D, et al. Viral and clinical factors associated with the fulminant course of hepatitis A infection. Hepatology. 2003;38(3):613–618. doi: 10.1053/jhep.2003.50366. [DOI] [PubMed] [Google Scholar]
- 27.Lemon SM. Type A viral hepatitis. New developments in an old disease. N Engl J Med. 1985;313(17):1059–1067. doi: 10.1056/NEJM198510243131706. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Guidelines for viral hepatitis surveillance and case management. [Accessed February 8, 2018]. https://www.cdc.gov/hepatitis/statistics/surveillanceguidelines.htm. Updated May 31, 2015.
- 29.Kao HW, Ashcavai M, Redeker AG. The persistence of hepatitis A IgM antibody after acute clinical hepatitis A. Hepatology. 1984;4(5):933–936. doi: 10.1002/hep.1840040525. [DOI] [PubMed] [Google Scholar]
- 30.Liaw YF, Yang CY, Chu CM, Huang MJ. Appearance and persistence of hepatitis A IgM antibody in acute clinical hepatitis A observed in an outbreak. Infection. 1986;14(4):156–158. doi: 10.1007/BF01645253. [DOI] [PubMed] [Google Scholar]
- 31.Plumb ID, Bulkow LR, Bruce MG, et al. Persistence of antibody to Hepatitis A virus 20 years after receipt of Hepatitis A vaccine in Alaska. J Viral Hepat. 2017;24(7):608–612. doi: 10.1111/jvh.12676. [DOI] [PubMed] [Google Scholar]
- 32.Koff RS. Clinical manifestations and diagnosis of hepatitis A virus infection. Vaccine. 1992;10(suppl 1):S15–S17. doi: 10.1016/0264-410x(92)90533-p. [DOI] [PubMed] [Google Scholar]
- 33.Clemens R, Safary A, Hepburn A, Roche C, Stanbury WJ, André FE. Clinical experience with an inactivated hepatitis A vaccine. J Infect Dis. 1995;171(suppl 1):S44–S49. doi: 10.1093/infdis/171.supplement_1.s44. [DOI] [PubMed] [Google Scholar]
- 34.Ambrosch F, André FE, Delem A, et al. Simultaneous vaccination against hepatitis A and B: results of a controlled study. Vaccine. 1992;10(suppl 1):S142–S145. doi: 10.1016/0264-410x(92)90570-a. [DOI] [PubMed] [Google Scholar]
- 35.Gil A, González A, Dal-Ré R, Calero JR. Interference assessment of yellow fever vaccine with the immune response to a single-dose inactivated hepatitis A vaccine (1440 EL.U.). A controlled study in adults. Vaccine. 1996;14(11):1028–1030. doi: 10.1016/0264-410x(96)00059-x. [DOI] [PubMed] [Google Scholar]
- 36.Jong EC, Kaplan KM, Eves KA, Taddeo CA, Lakkis HD, Kuter BJ. An open randomized study of inactivated hepatitis A vaccine administered concomitantly with typhoid fever and yellow fever vaccines. J Travel Med. 2002;9(2):66–70. doi: 10.2310/7060.2002.21955. [DOI] [PubMed] [Google Scholar]
- 37.Nolan T, Bernstein H, Blatter MM, et al. Immunogenicity and safety of an inactivated hepatitis A vaccine administered concomitantly with diphtheria-tetanus-acellular pertussis and haemophilus influenzae type B vaccines to children less than 2 years of age. Pediatrics. 2006;118(3):e602–e609. doi: 10.1542/peds.2005-2755. [DOI] [PubMed] [Google Scholar]
- 38.Usonis V, Meriste S, Bakasenas V, et al. Immunogenicity and safety of a combined hepatitis A and B vaccine administered concomitantly with either a measles-mumps-rubella or a diphtheria-tetanus-acellular pertussis-inactivated poliomyelitis vaccine mixed with a Haemophilus influenzae type b conjugate vaccine in infants aged 12–18 months. Vaccine. 2005;23(20):2602–2606. doi: 10.1016/j.vaccine.2004.11.062. [DOI] [PubMed] [Google Scholar]
- 39.Moro PL, Museru OI, Niu M, Lewis P, Broder K. Reports to the Vaccine Adverse Event Reporting System after hepatitis A and hepatitis AB vaccines in pregnant women. Am J Obstet Gynecol. 2014;210(6):561.e1–561.e–6. doi: 10.1016/j.ajog.2013.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.André FE, D’Hondt E, Delem A, Safary A. Clinical assessment of the safety and efficacy of an inactivated hepatitis A vaccine: rationale and summary of findings. Vaccine. 1992;10(suppl 1):S160–S168. doi: 10.1016/0264-410x(92)90576-6. [DOI] [PubMed] [Google Scholar]
- 41.Just M, Berger R. Reactogenicity and immunogenicity of inactivated hepatitis A vaccines. Vaccine. 1992;10(suppl 1):S110–S113. doi: 10.1016/0264-410x(92)90560-7. [DOI] [PubMed] [Google Scholar]
- 42.McMahon BJ, Williams J, Bulkow L, et al. Immunogenicity of an inactivated hepatitis A vaccine in Alaska Native children and Native and non-Native adults. J Infect Dis. 1995;171(3):676–679. doi: 10.1093/infdis/171.3.676. [DOI] [PubMed] [Google Scholar]
- 43.Balcarek KB, Bagley MR, Pass RF, Schiff ER, Krause DS. Safety and immunogenicity of an inactivated hepatitis A vaccine in preschool children. J Infect Dis. 1995;171(suppl 1):S70–S72. doi: 10.1093/infdis/171.supplement_1.s70. [DOI] [PubMed] [Google Scholar]
- 44.Bell BP, Negus S, Fiore AE, et al. Immunogenicity of an inactivated hepatitis A vaccine in infants and young children. Pediatr Infect Dis J. 2007;26(2):116–122. doi: 10.1097/01.inf.0000253253.85640.cc. [DOI] [PubMed] [Google Scholar]
- 45.Arguedas MR, Johnson A, Eloubeidi MA, Fallon MB. Immunogenicity of hepatitis A vaccination in decompensated cirrhotic patients. Hepatology. 2001;34(1):28–31. doi: 10.1053/jhep.2001.25883. [DOI] [PubMed] [Google Scholar]
- 46.Overton ET, Nurutdinova D, Sungkanuparph S, Seyfried W, Groger RK, Powderly WG. Predictors of immunity after hepatitis A vaccination in HIV-infected persons. J Viral Hepat. 2007;14(3):189–193. doi: 10.1111/j.1365-2893.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- 47.Askling HH, Rombo L, van Vollenhoven R, et al. Hepatitis A vaccine for immunosuppressed patients with rheumatoid arthritis: a prospective, open-label, multicentre study. Travel Med Infect Dis. 2014;12(2):134–142. doi: 10.1016/j.tmaid.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 48.US Department of Veterans Affairs. VHA national hepatitis A immunization guidelines. http://vaww.prevention.va.gov/CPS/Hepatitis_A_Immunization.aspNonpublic document. Source not verified.
- 49.Kushel M. Hepatitis A outbreak in California - addressing the root cause. N Engl J Med. 2018;378(3):211–213. doi: 10.1056/NEJMp1714134. [DOI] [PubMed] [Google Scholar]
- 50.Millard J, Appleton H, Parry JV. Studies on heat inactivation of hepatitis A virus with special reference to shellfish. Part 1. Procedures for infection and recovery of virus from laboratory-maintained cockles. Epidemiol Infect. 1987;98(3):397–414. doi: 10.1017/s0950268800062166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoke CH, Jr, Binn LN, Egan JE, et al. Hepatitis A in the US Army: epidemiology and vaccine development. Vaccine. 1992;10(suppl 1):S75–S79. doi: 10.1016/0264-410x(92)90550-4. [DOI] [PubMed] [Google Scholar]
- 52.Dooley DP. History of U.S. military contributions to the study of viral hepatitis. Mil Med. 2005;170(suppl 4):71–76. doi: 10.7205/milmed.170.4s.71. [DOI] [PubMed] [Google Scholar]
- 53.Grabenstein JD, Pittman PR, Greenwood JT, Engler RJ. Immunization to protect the US Armed Forces: heritage, current practice, and prospects. Epidemiol Rev. 2006;28:3–26. doi: 10.1093/epirev/mxj003. [DOI] [PubMed] [Google Scholar]
- 54.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology. 2015;149(6):1471–1482.e1475. doi: 10.1053/j.gastro.2015.07.056. quiz e17–e18. [DOI] [PubMed] [Google Scholar]
- 55.Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45. [PMC free article] [PubMed] [Google Scholar]
- 56.Teeters JB, Lancaster CL, Brown DG, Back SE. Substance use disorders in military veterans: prevalence and treatment challenges. Subst Abuse Rehabil. 2017;8:69–77. doi: 10.2147/SAR.S116720. [DOI] [PMC free article] [PubMed] [Google Scholar]