Abstract
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines classification is based on the combination of patient risk and the severity of their symptoms.
After a busy day in the primary care clinic, having finished the day’s dictations and called a patient to discuss the results of his lipid panel, Dr. B reviews tomorrow’s schedule, and notices 2 patients with a primary diagnosis of chronic obstructive pulmonary disease (COPD). Dr. B recalls a recent publication on changes in the classification of COPD by the Global Initiative for Chronic Obstructive Lung Disease (GOLD).1 She remembers the main message being the degree of airway obstruction as measured by the forced expiratory volume in the first second (FEV1) is now considered insufficient to classify COPD severity and to make a therapeutic decision. This paradigm shift contradicts the familiar concept that FEV1 is the cornerstone piece of information in COPD, resulting in some degree of uncertainty about how to apply this in the practice. Dr. B. considers a multitude of practical questions, including: Is there a good reason to change the classification of COPD? How easy is it to use?
Will it make any therapeutic differences to my patients? In this article, the authors attempt to answer these and other questions prompted by the recent changes in the GOLD classification, with emphasis on its clinical use.
A HETEROGENEOUS CONDITION
Spirometry is central to the diagnosis of obstructive lung diseases, including COPD and asthma. The diagnosis of COPD requires demonstration of an obstructive ventilatory defect in the spirometry, usually defined as a ratio of FEV1 to forced vital capacity (FVC) below 70% (FEV1/FVC < 0.7). FEV1 is still important, not only to confirm the diagnosis of airflow obstruction, but because it predicts mortality when severely reduced. However, during the last decade severity of airflow limitation has been challenged as a descriptor of both symptom burden and consequences of COPD by data from large studies.2 For example, it has been demonstrated that 2 patients with the same degree of obstruction, measured by the FEV1 percentage predicted, can provide the physician with very different experiences about the impact of their disease in daily life.3 These differences extend to the severity of their dyspnea; their exercise capacity, as seen in the six-minute walking distance test (6MWD); or their perceived quality of life (QOL), measured by the score on the Saint George’s Respiratory Questionnaire (SGRQ). These measures of disease impact show an extremely low correlation with FEV1: a correlation of 0.36 with the severity of dyspnea, 0.34 with 6MWD, and 0.38 with the SGRQ total score.2 These newer studies imply that while spirometry is important, it captures only a small portion of the symptomatic and functional impact of COPD.
Increasing interest in understanding the differences between COPD subjects has been the main motivation in identifying distinct COPD phenotypes, subgroups of patients with similar disease experience, probable similar underlying pathogenic mechanisms, similar outcomes, and perhaps specific treatment alternatives.4,5 The severity of airflow limitation, as measured by FEV1 percent predicted, is not always related with some of the emerging COPD phenotypes (eg, chronic bronchitis predominant phenotype, frequent exacerbation phenotype). 6–8 Chronic bronchitis can be present across the whole spectrum of spirometry severity, and is always associated with poorer QOL and worse clinical outcomes. Similarly, there are patients with frequent exacerbation phenotype (defined as ≥ 2 exacerbations/year) at every level of airflow obstruction, and the phenotype tends to be stable, meaning that previous frequent exacerbations are a good predictor of future exacerbations.8
With all this information, participants in the development of the GOLD guidelines determined that although FEV1 is still a good descriptor of COPD severity and potential for poor outcomes (exacerbation frequency, mortality), a more comprehensive description of COPD needed the addition of data on the impact of symptoms (particularly dyspnea), and the future risk of poor COPD-related events (exacerbations, death, disease progression). 5 Hence, in response to Dr. B.’s question, it seems that a new approach to the way that we classify COPD was overdue, making it important to gather additional patient information, beyond FEV1.
GOLD CATEGORY CLASSIFICATION
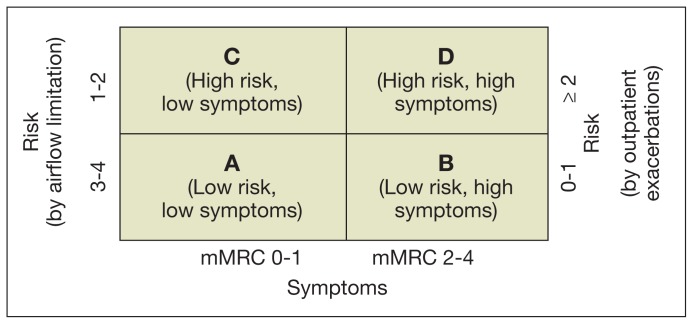
An important difference from previous classifications is that the new GOLD categories use lettered groups, from A to D, not just grades of severity; however, the severity of the ventilatory defect measured by FEV1 is still graded from 1 to 4 and is still part of the classification.9
Placing a patient in the new groups is based on 2 questions: (1) How severe are the symptoms, particularly dyspnea; and (2) Is the patient at high or low risk of poor COPD-related outcomes? The first question (symptoms severity) can be systematically approached using 1 of 2 different instruments to grade COPD symptoms: the modified Medical Research Council dyspnea score (mMRC) or the COPD Assessment Test (CAT), a more recently developed instrument to quantify COPD impact.10,11
Use of CAT score, a more comprehensive descriptor of COPD impact, is the preferred method by guideline developers. If the practitioner is more familiar with the mMRC and wishes to use it instead, the result can be simplified as low (0–1 points) or high symptoms burden (≥ 2 points). The mMRC is based on the answer to the level of effort triggering dyspnea: a score of 1 means that the patient “get[s] shorter of breath when hurrying on a level surface or walking up a slight hill”; a score of 2 means that the patient “walk[s] slower than people of the same age while walking on a level surface because of breathlessness, or I have to stop for breath when walking on my own pace on the level.” Hence, the first step to classify a patient can be as simple as asking about dyspnea, surely part of the history taking process.
The second question (risk of poor COPD-related outcomes) can be answered by using the grade of obstruction by FEV1 or asking about the frequency of exacerbations in the previous year. If FEV1 is used, those with FEV1 percentage predicted ≥ 50% are considered as “low risk”; if the airflow obstruction is more severe (previously grades 3–4), the patient is at “high risk” of future events. If the exacerbation frequency is used, ≤ 1 outpatient-treated exacerbation in the previous year qualify as “low,” and ≥ 2 as “high risk.” There is an additional alternative way to identify high risk: all patients with any (≥ 1 per year) exacerbation requiring hospital admission are considered at high risk.
The next step is combining both symptoms and risk to create 4 mutually exclusive groups, which will be relevant to select the appropriate treatment (Table 1). The groups can also be represented graphically using a 2×2 figure, with the horizontal axis being symptoms severity, and the vertical (risk) either FEV1 or exacerbation history (Figure 1). If there is a discrepancy between the risk judged by lung function and history of exacerbations, it is recommended to use the answer corresponding to the worse category.12
Table 1.
Using Clinical Information to Classify the Patient in a GOLD COPD Group
| Group | Description | Symptoms By mmMRC | Risk | |
|---|---|---|---|---|
| By FEV1 | By exacerbation frequency | |||
| A | Low symptoms, low risk | mMRC 0–1 | ≥ 50% (grades 1–2) | 0–1 outpatient or 0 hospitalized exacerbation |
| B | High symptoms, low risk | mMRC 2–4 | ≥ 50% (grades 1–2) | 0–1 outpatient or 0 hospitalized exacerbation |
| C | Low symptoms, high risk | mMRC 0–1 | < 50% (grades 3–4) | ≥ 2 outpatient or ≥ 1 hospitalized exacerbations |
| D | High symptoms, high risk | mMRC 2–4 | < 50% (grades 3–4) | ≥ 2 outpatient or ≥ 1 hospitalized exacerbations |
Abbreviations: FEV1, forced expiratory volume in the first second; mMRC, modified Medical Research Council dyspnea score.
Figure 1.
Classification in GOLD COPD Groups1
Abbreviation: mMRC, modified Medical Research Council dyspnea score.
GOLD GUIDELINE-BASED TREATMENT
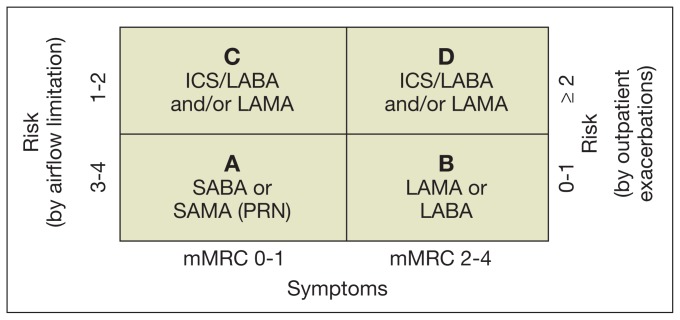
The new classification should also help to identify the patient’s main needs: controlling symptoms, reducing future risks, or both. Based on the results of available randomized clinical trials, GOLD guideline developers suggest group-tailored strategies of management (Table 2, Figure 2).
Table 2.
| Group | Description | First choice | Alternative choice |
|---|---|---|---|
| A | Low symptoms, low risk | SABA or SAMA (as needed) |
LAMA or LABA or SABA/SAMA |
| B | High symptoms, low risk | LAMA or LABA |
LAMA and LABA |
| C | Low symptoms, high risk | ICS/LABA or LAMA |
LAMA and LABA |
| D | High symptoms, high risk | ICS/LABA or LAMA |
ICS/LABA and LAMA or ICS/LABA and PD4-inh or LAMA and LABA or LAMA and PD4-inh |
This table is presented for educational purposes. Any treatment decision should be weighted at the individual level. Only most common options are displayed, and the order does not indicate any preference.
Abbreviations: ICS, inhaled corticosteroids; LABA, long-acting beta agonist; LAMA, long-acting antimuscarinic; PD4-inh, phosphodiesterase-4 inhibitor; SABA, short-acting beta agonists; SAMA, short-acting antimuscarinic.
Figure 2.
First-line Therapies Based on the Classification in GOLD COPD Groups1
Abbreviations: ICS, inhaled corticosteroids; LABA, long-acting beta agonist; LAMA, long-acting antimuscarinic; mMRC, modified Medical Research Council dyspnea score; PRN, pro re nata (as needed); SABA, short-acting beta agonists; SAMA, short-acting antimuscarinic.
Group A: Low risk and low symptoms
The goal is to treat only as needed, using short-acting medications. No preference was given to the type of short-acting medication and the practitioner could select between short-acting beta agonists (SABAs) or short-acting anticholinergic (also known as short-acting antimuscarinic [SAMA]) medication as first-line therapy. Second-line therapy includes either the combination of both families of short-acting medications in 1 inhaler, or the use of 1 long-acting inhaler. As a rule of thumb, no patient in this group should be on more than 1 inhaler, and the combination of short and long-acting medications is not part of the recommendations. Patients in group A, and indeed everyone with COPD, benefit from respiratory immunizations and tobacco cessation.
Group B: Low risk, high symptoms
Again, the goal of treatment is symptom control. Based on the available evidence, this can be achieved using long-acting bronchodilators, without the need of inhaled corticosteroids (ICS). The first line of treatment should be just 1 bronchodilator, either a long-acting antimuscarinic (LAMA) or long-actingbeta agonist (LABA). These could be used together as second-line treatment (LAMA plus LABA), still without indication for ICS. It is important to remember that dyspnea, or other symptoms, could also be a manifestation of comorbid conditions, such as cardiovascular disease, obesity, deconditioning, and musculoskeletal diseases.13 When spirometry is not used to confirm the diagnosis of COPD, patients may receive incremental types of inhalers instead of being evaluated for other causes of dyspnea, which might have led to more appropriate specific therapy.14 As a result, judicious evaluation of the patient’s symptomsis recommended. The guidelines also recommend programs that increase physical activity for this group of patients, as well to those in groups C and D, as this can improve symptoms and decrease risk of exacerbations.15
Group C: High risk, low symptoms
The combination of ICS/LABA is the first-line therapy for this group, based on data showing the superiority of the ICS/LABA combination over monotherapy to reduce exacerbations and symptoms, as well as to improve QOL.16,17 Monotherapy LABA is also a first-line GOLD recommendation. Selecting between ICS/LABA vs LABA should be individualized based on the reason that the patient was judged as high risk. In the authors’ practice, if the risk is based only in spirometry values, using LABA as monotherapy is a good choice, while if the definition of high risk was based on the frequency of exacerbations, ICS/LABA is the first choice. The GOLD guidelines list the combination of LABA/LAMA as second-line therapy.
Group D: High risk and high symptoms
First-line therapy for this group is essentially the same that for group C, with similar considerations. The combination of LABA/LAMA is also recommended as second-line therapy, as well as the use of ICS/LABA and LAMA (all 3 major classes of controller medications together). It is worth noting that phosphodiesterase-4 inhibitors (PDE4-inh, roflumilast being the best known) can be considered as a third-line of therapy (in group C) or as part of secondline combinations (in group D).
BENEFITS AND LIMITATIONS OF GOLD
There is no doubt that the new classification system and treatment guidelines are a significant step forward, intended to foster the development of more personalized decisions for COPD patients. The guidelines are the first attempt to incorporate the concepts of phenotypes (frequent exacerbation phenotype), disease heterogeneity (the variation in outcomes for the same degree of airflow obstruction), and the differences between the burden of symptoms and the risk of outcomes. The guidelines incorporate the need to weigh the benefits and risks of medications at the individual level (eg, ICS without an accompanying long-acting agent are not recommended in any group, and ICS use is reserved for those with high risk, especially if the designation is based on exacerbation frequency). The guidelines also stress the importance of examining comorbidities, emphysizing that their management should in no way be altered just because the patient also has COPD. Relative to the previous staging based only on FEV1 values, this new classification system has been shown to have appropriate predictive ability and association with the risk of exacerbations, and better correlation with measures of quality of life and costs of care.18,19 The guidelines, initially released in 2011 and slightly updated recently, are in continuous development and have been subject to intense evaluation.
Some limitations have been found (eg, the classification is still not the best predictor of mortality, but has the same ability to predict hospital admission as the previous spirometry-based system).18,20,21 Hence, it should be no surprise that modifications will likely be released in the near future.
The treatment recommendations associated with the current classification are based on the best evidence available and expert opinion, as no published clinical trials have compared the group-based therapy system to standard therapies. Evaluations of their effectiveness in real-life practice are still to be released. Previous, less complex guidelines, based on spirometry stages, were followed < 60% of the time in actual practice, thus it will be surprising to find high adherence to the current recommendations, but evaluations are still in progress.22
CONCLUSION
The best way for primary care providers to incorporate the GOLD guidelines into daily practice is to remember that COPD is very heterogeneous. Although spirometry is important, it is also essential to inquire about exacerbation frequency and symptoms severity. It is encouraging that for each of the relevant questions needed to classify the patient, there is a clear, easy to remember cut point. First, look at symptoms (low or high burden, based on the presence of dyspnea), then to judge risk look at FEV1 percentage predicted (using 50% as a cut-point) and at exacerbation frequency (using 2 per year as the cut point). With those simple questions, build the groups, based on the combination of answers, and select the appropriate therapy. The general assumptions are that short-acting medications are appropriate for infrequent symptoms, long-acting medications are used to control symptoms and prevent exacerbations in more severe disease, and that ICS (always in combination with LABA) are reserved for those in the high-risk groups, especially if high risk is defined by frequent exacerbations. This summary should be supplemented with the judicious use of the tables and figures provided in this review, and available with detailed description and discussion in the original sources.1
Footnotes
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner; Frontline Medical Communications Inc.; the Department of Defense, or its Components; and the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
REFERENCES
- 1.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Agusti A, Calverley PM, Celli B, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. [Google Scholar]
- 3.Jones PW. Health status and the spiral of decline. COPD. 2009;6(1):59–63. doi: 10.1080/15412550802587943. [DOI] [PubMed] [Google Scholar]
- 4.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han MK, Kazerooni EA, Lynch DA, et al. COPDGene Investigators. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim V, Han MK, Vance GB, et al. COPDGene Investigators. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140(3):626–633. doi: 10.1378/chest.10-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181. doi: 10.1186/1741-7015-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurst JR, Vestbo J, Anzueto A, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 9.Qaseem A, Wilt TJ, Weinberger SE, et al. American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434–1440. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- 11.Lee SD, Huang MS, Kang J, et al. Investigators of the Predictive Ability of CAT in Acute Exacerbations of COPD (PACE) Study. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. Respir Med. 2014;108(4):600–608. doi: 10.1016/j.rmed.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Haughney J, Gruffydd-Jones K, Roberts J, Lee AJ, Hardwell A, McGarvey L. The distribution of COPD in UK general practice using the new GOLD classification. Eur Respir J. 2014;43(4):993–1002. doi: 10.1183/09031936.00065013. [DOI] [PubMed] [Google Scholar]
- 13.Martinez CH, Han MK. Contribution of the environment and comorbidities to chronic obstructive pulmonary disease phenotypes. Med Clin North Am. 2012;96(4):713–727. doi: 10.1016/j.mcna.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins BF, Feemster LC, Rinne ST, Au DH. Factors predictive of airflow obstruction among veterans with presumed empiric diagnosis and treatment of COPD. Chest. 2015;147(2):369–376. doi: 10.1378/chest.14-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;10:CD005305. doi: 10.1002/14651858.CD005305.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Nannini LJ, Poole P, Milan SJ, Kesterton A. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;8:CD006826. doi: 10.1002/14651858.CD006826.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829. doi: 10.1002/14651858.CD006829.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goossens LM, Leimer I, Metzdorf N, Becker K, Rutten-van Mölken MP. Does the 2013 GOLD classification improve the ability to predict lung function decline, exacerbations and mortality: a post-hoc analysis of the 4-year UPLIFT trial. BMC Pulm Med. 2014;14:163. doi: 10.1186/1471-2466-14-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boland MR, Tsiachristas A, Kruis AL, Chavannes NH, Rutten-van Mölken MP. Are GOLD ABCD groups better associated with health status and costs than GOLD 1234 grades? A cross-sectional study. Prim Care Respir J. 2014;23(1):30–37. doi: 10.4104/pcrj.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han MK, Muellerova H, Curran-Everett D, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med. 2013;1:43–50. doi: 10.1016/S2213-2600(12)70044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johannessen A, Nilsen RM, Storebø M, Gulsvik A, Eagan T, Bakke P. Comparison of 2011 and 2007 Global Initiative for Chronic Obstructive Lung Disease guidelines for predicting mortality and hospitalization. Am J Respir Crit Care Med. 2013;188(1):51–59. doi: 10.1164/rccm.201212-2276OC. [DOI] [PubMed] [Google Scholar]
- 22.Sharif R, Cuevas CR, Wang Y, Arora M, Sharma G. Guideline adherence in management of stable chronic obstructive pulmonary disease. Respir Med. 2013;107(7):1046–1052. doi: 10.1016/j.rmed.2013.04.001. [DOI] [PubMed] [Google Scholar]