Abstract
Objective:
To use digital histology in a large autopsy cohort of Lewy Body Disorder (LBD) patients with dementia to test the hypotheses that co-occurring Alzheimer’s disease (AD) pathology impacts the anatomic distribution of α-synuclein (SYN) pathology and that co-occurring neocortical tau pathology in LBD associates with worse cognitive performance and occurs in a pattern differing from AD.
Methods:
Fifty-five autopsy-confirmed LBD (PDD: 36, DLB:19) patients and 25 AD patients were studied. LBD patients were categorized as having moderate/severe AD co-pathology (SYN+AD=20) or little/no AD co-pathology (SYN-AD=35). Digital measures of tau, Aβ, and SYN histopathology in neocortical and subcortical/limbic regions were compared between groups and related to antemortem cognitive testing.
Results:
SYN burden was higher in SYN+AD than SYN-AD in each neocortical region (F(1,54)=5.6–6.0,p<0.02) but was equivalent in entorhinal cortex and putamen (F(1,43–49)=0.7–1.7,p>0.2). SYN+AD performed worse than SYN-AD on a temporal-lobe mediated naming task (t(27)=2.1,p=0.04). Antemortem cognitive test scores inversely correlated with tau burden (r=−0.39 to −0.68,p<0.05). AD had higher tau than SYN+AD in all regions (F(1,43)=12.8–97.2,p<.001); however, SYN+AD had a greater proportion of tau in the temporal neocortex than AD, (t(41)=2.0,p<.05) whereas AD had a greater proportion of tau in the frontal neocortex than SYN+AD (t(41)=3.3,p<0.002). SYN+AD had similar severity and distribution of neocortical Aβ compared to AD (F(1,40–43)=1.6–2.0,p>.1).
Interpretation:
LBD patients with AD co-pathology harbor greater neocortical SYN pathology. Regional tau pathology relates to cognitive performance in LBD dementia, and its distribution may diverge from pure AD. Tau co-pathology contributes uniquely to the heterogeneity of cognitive impairment in LBD.
INTRODUCTION
Lewy body disorders (LBD), which include Parkinson’s disease (PD), PD with dementia (PDD), and dementia with Lewy bodies (DLB), are a clinically and pathologically heterogeneous group of neurodegenerative diseases characterized by intracellular alpha-synuclein (SYN) Lewy pathology at autopsy1. While the current clinical distinction between PDD and DLB is based on the timing of dementia onset in relation to parkinsonism, this distinction is currently under debate2, 3 due in part to the poor prediction of these clinical diagnoses for distinct pathologic substrates4, 5. The two disorders often share common motor signs, cognitive features, prodromal features such as REM sleep behavior disorder (RBD), and genetic risk factors6. Despite this overlap, there is also well-described heterogeneity in specific domains of cognitive impairment and presence or severity of distinct motor features across these conditions7, 8.
Detailed postmortem studies can provide insight into the underlying biological substrates of this variability as well as help define biologically-meaningful patient subgroups that improve upon the current clinical distinction of PDD and DLB. Previous clinicopathological studies have found that regional distribution of SYN pathology may influence certain clinical features of LBD, including the presence of hallucinations, the occurrence of dementia and survival 9–12. However, the co-occurrence of clinically-significant Alzheimer’s disease (AD) associated tau and beta-amyloid (Aβ) pathology is common and found in up to 50% of all LBD4. While we and others have demonstrated that a higher burden of AD co-pathology is associated with decreased overall survival and faster progression to dementia even when adjusting for age4, 13, 14, little is known regarding the relationship between specific, discrete cognitive features and the regional distribution of SYN and AD co-pathology in LBD. This gap is due in part to the qualitative nature of traditional neuropathological staging systems that use of limited ordinal estimates of pathological burden.
Here, we examine a large LBD dementia autopsy cohort with antemortem neuropsychological testing using digital histology to objectively measure pathologic burden in neocortical brain regions associated with cognition. Using this unique approach, we tested the hypothesis that tau and Aβ co-pathology is associated with a greater burden of neocortical SYN pathology in LBD. We previously found that tau pathology has a strong influence on the timing of onset of dementia in LBD4; therefore, we hypothesized that tau is also an important contributor to the pattern of cognitive impairment in LBD with dementia. Based on in vitro model data which suggests tau pathology may be cross-seeded by strains of pathological SYN15 and detected in human LBD brains16, we tested the hypothesis that tau in LBD has a different neocortical distribution compared to “pure AD” pathology (i.e. without neocortical SYN pathology).
METHODS
Participants
Patients and data were abstracted from the University of Pennsylvania Integrated Neurodegenerative Disease Database17. Patients selected were clinically evaluated and followed at the University of Pennsylvania’s Parkinson’s disease and Movement Disorder Clinic, Frontotemporal Dementia Center, Alzheimer’s Disease Core Center, or the Michael J. Crescenz VA Medical Center’s Parkinson’s Disease Research, Education, and Clinical Center. Cases were selected from our previously reported Penn LBD autopsy cohort4 of 133 patients who 1) met clinical criteria for LBD (PDD or DLB)18, 19 and 2) had autopsy-confirmed synucleinopathy (i.e. brainstem, limbic or neocortical stage)20. To test clinicopathological associations of dementia in LBD, we selected the subset of these patients with available antemortem neuropsychological testing data collected after the onset of dementia. Fifty-five LBD (36 PDD, 19 DLB) patients were identified who fulfilled these criteria (Table 1). An age- and sex-matched disease reference cohort of 25 patients with typical amnestic AD and a primary neuropathological diagnosis of AD with an absence of neocortical SYN was selected to examine the distribution of AD pathology in comparison to that seen in LBD with AD pathology. All autopsies were performed at the Penn Center for Neurodegenerative Disease Research using validated neuropathological criteria21 and were analyzed for the presence of co-pathologies as described22. All procedures were performed with prior informed consent in accordance with Penn Institutional Review Board guidelines.
Table 1.
Patient Demographics.
| LBD | |||
|---|---|---|---|
| SYN-AD (n=35) | SYN+AD (n=20) | AD (n=25) | |
| Clinical Characteristics | |||
| Clinical Phenotype | DLB: 7 PDD: 28 | DLB: 12 PDD: 8* | AD: 25 |
| Sex, count male (%) | 26 (74) | 15 (75) | 15 (60) |
| Age at Onseta | 61.8 (9.8) | 69.0 (6.2)* | 67.9 (5.7) |
| Age at Dementiaa | 72.5 (6.3) | 73.2 (6.7) | 67.9 (5.7)# |
| Motor Dementia Intervala | 10.8 (7.9) | 4.2 (6.2)* | NA |
| Age at Deatha | 77.7 (8.7) | 78.4 (6.1) | 79.4 (6.8) |
| Disease Durationa | 15.9 (7.2) | 9.2 (6.4)* | 11.52 (5.1) |
| Neuropathology | |||
| Brain Weightb | 1276 (260) | 1327 (131) | 1137 (157)# |
| Post Mortem Intervalc | 13.2 (11.1) | 16.0 (8.8) | 10.9(6.3)# |
| McKeith Staged | |||
| Brainstem | 2 (6) | 0 (0) | 0 (0) |
| Limbic | 9 (26) | 3 (15) | 0 (0) |
| Neocortical | 24 (66) | 17 (85) | 0 (0) |
| AD Leveld | |||
| None | 13 (37) | NA | 0 (0) |
| Low | 22 (63) | NA | 0 (0) |
| Medium | NA | 12 (60) | 2 (8) |
| High | NA | 8 (40) | 23 (92) |
| Other Co-Pathologyd | |||
| PSP | 1 (3) | 0 (0) | 0 (0) |
| HS | 2 (6) | 0 (0) | 0 (0) |
| TDP-43 | 9 (26) | 9 (45) | 17 (68) |
| ARTAG | 8/26 (31) | 13/18 (72)* | 21 (84) |
| CVD | 0 (3) | 1 (5) | 2(8) |
| AGD | 1 (3) | 0 (0) | 0 (0) |
years (SD)
Grams (SD)
hours (SD)
count (%).
Unless specified, all counts are taken from the full group
PSP: Progressive Supranuclear Palsy, AGD: Argyrophillic Grain Disease, HS: hippocampal sclerosis. CVD: Cerebrovascular disease, 10/25 AD cases had low levels of SYN in amygdala only, TDP-43 pathology present in hippocampus and or amygdala, ARTAG: age related tau astrogliopathy
p<.05 between SYN-AD and SYN+AD
p<.05 between SYN+AD and AD
Neuropathologic Diagnosis
Fresh tissue samples obtained at autopsy were fixed overnight in 70% ethanol with 150 mM sodium chloride (EtOH) or 10% neutral-buffered formalin (NBF). Tissue samples were processed as described17, 23, were embedded into paraffin blocks, and 6μm thick sections were cut for analysis. Sections were stained using immunohistochemistry (IHC) with established antibodies as described17. Expert neuropathologists (EBL, JQT) applied current diagnostic criteria to assign Thal phases24, Braak tau stages25, CERAD neuritic plaque stages26, α-synuclein Lewy body stages20, and the presence of TDP-43 and aging-related tau astrogiopathy (ARTAG) co-pathology27. Final neuropathology diagnosis for each case was rendered using standard semiquantitative assessments for each pathology in each brain region21.
Based on modern neuropathological criteria using Aβ amyloid Thal phase, Braak tau stage and CERAD plaque score (i.e. ABC scoring)21 we categorized LBD patients into those with a medium or a high-level of AD neuropathologic change (ADNPC) sufficient to contribute to dementia21 (SYN+AD) and patients with no or low-level AD pathology are referred to as those without significant AD co-pathology (SYN-AD). AD patients were similarly assessed by ABC scoring method and additionally were screened for the absence of neocortical SYN.
Digital Pathology
We selected three neocortical regions with known domain-specific contributions to cognition in neuropsychological testing for digital analysis including mid-frontal gyrus (MFC), superior temporal gyri (STC), and the angular gyrus (ANG). We also included a limbic region (entorhinal cortex, ERC) and a subcortical motor region (putamen, PUT) for comparison. Adjacent sections were immunostained for tau (AT8, Thermo Scientific), Aβ (NAB228, Santa Cruz) and SYN (SYN303, Santa Cruz) for use in digital pathology experiments. The majority of slides were fixed in NBF (750/960, 78%) and for those with missing NBF tissue, we used sections from blocks fixed in ETOH. Digital images of histology slides at 20x magnification were obtained using a Lamina slide scanning system (Perkin Elmer, Waltham MA) and Halo digital image software v1.90 (Indica Labs, Albuquerque NM) to calculate %area occupied (%AO) of reactivity for tau, Aβ, and SYN pathology as previously published, which included inter-rater validation23. Briefly, we used a vertical transect method23 to sample representative cortical grey matter in neocortical and limbic cortex and used a random sampling from this region of interest for our analyses to reduce sampling bias. Since PUT is a subcortical nucleus without laminar organization we sampled this region based on microscopic anatomical boundaries of the nucleus. Color deconvolution intensity thresholds were optimized for each stain to detect and quantify the %AO for tau and Aβ. Since SYN303 IHC can detect non-pathological monomeric SYN in axon terminal of the neuropil, as well as pathological LBs and Lewy neurites (LNs), we used an additional machine learning step (i.e. “classifier” function in HALO) to first segment LB and LN pathology from the background normal neuropil stain based on morphological features prior to applying color deconvolution algorithm to constrain our detection of pathological SYN in LBs and LNs (Figure 1). We report the average %AO in sampled regions of interest from each slide as we have done previously23.
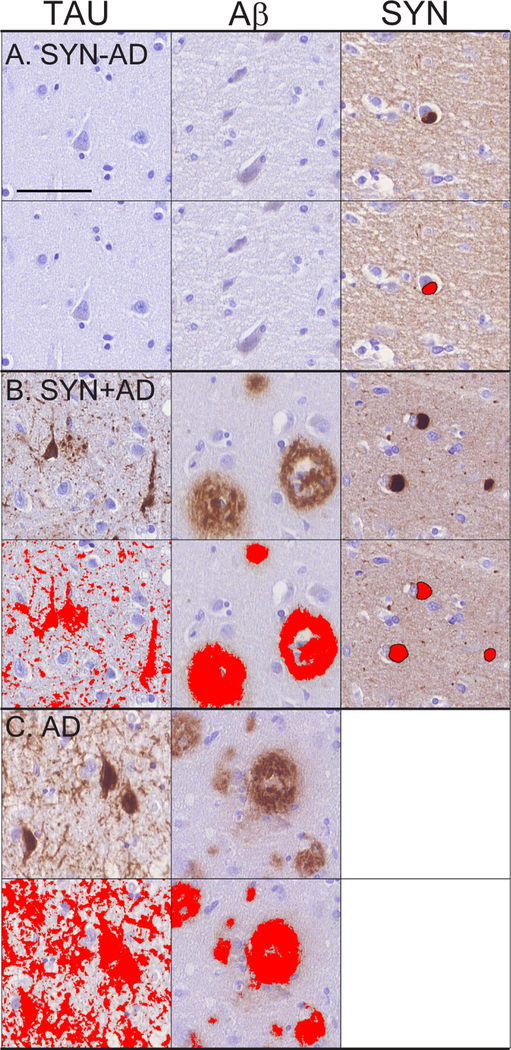
Figure 1: Microscopic Pathology of LBD and AD.
Representative photomicrographs from STC in A) SYN-AD, B) SYN+AD, and C) AD cases stained for tau (AT8,left), Aβ (NAB228,middle), and SYN (SYN303,right). Top row for each group shows raw images and lower row for each group depicts digital detection of pathology (%AO- red overlay). SYN+AD has higher burden of Tau, Aβ and SYN pathology compared to SYN-AD, while pure AD has much higher cortical Tau %AO and similar Aβ compared to SYN+AD. Images taken at 32x; scale bar is 100 micrometers.
Neuropsychological Testing
To test pathological associations with cognitive domains in our LBD dementia cohort we selected the first available research neuropsychological testing data obtained after the diagnosis of dementia as defined by the diagnostic impression of the clinician from the medical record. Neuropsychological testing was administered to participants by trained research personnel as described28. We included neuropsychological tests with sufficient data for analysis, which included two tests of global cognition (Mini-Mental State Examination (MMSE) and the Dementia Rating Scale-2 (DRS-2), one semantic category fluency task (# unique animals named in 60 seconds), and a lexical retrieval task (Boston Naming Task (BNT)).
Statistical Analysis
As %AO data were not normally distributed, a square-root transformation was used for all analyses. %AO measurements for each stain were compared to ordinal scores (i.e. 0=none, 1=mild, 2=moderate, 3=severe) as done previously23 as well as neuropathological stages using ANOVA with post-hoc t-tests. Pathology stage categories were collapsed when a category had <10 patients. Differences in pathological distribution between SYN+AD and SYN-AD groups were assessed using both independent sample t-tests and analysis of covariance (ANCOVA) models adjusted for age at death, sex, clinical diagnosis (PDD vs DLB), and fixative (NBF vs ETOH).
We performed linear mixed effects models to test the association of pathology group (SYN+AD vs SYN-AD) and neocortical regional burden (i.e. MFC, STC and reference region ANG) for each pathology (Aβ, Tau, SYN) %AO as the dependent variable. The linear mixed-effects model can account for the correlations of pathology measures across regions within each individual. Age at death and sex were included as covariates in these models.
Performance between SYN+AD and SYN-AD groups on individual neuropsychological tests were compared using independent t-tests. The LBD cohort was also dichotomized by median SYN, Tau, and Aβ AO% measurements to examine digital-pathology defined patient subgroups. Test performance was also directly compared to %AO pathology using partial correlation controlling for age at test or MMSE examining pre-hypothesized regions governing specific cognitive tasks (i.e. MFC and category fluency, STC and Boston Naming Test, average cortical pathology with MMSE and DRS).
Differences in pathological distribution of tau and Aβ between SYN+AD and the reference pure AD group were assessed using t-tests and ANCOVA adjusting for age at death and sex. We also calculated a ratio of regional tau and Aβ %AO to the average neocortical tau and Aβ %AO (e.g. region tau %AO/average neocortical tau %AO) for each region and compared ratios between groups to examine the relative neocortical distribution of pathology.
Analyses were performed using SPSS v24 or STATA v15 and were two-tailed with alpha=0.05 as we chose specific regions to test clinical-pathological correlations using pre-specified hypotheses.
RESULTS
Patients
Characteristics of the LBD and AD patients are described in Table 1. Similar to our previous observations4, patients with SYN+AD pathology were older at onset, had a shorter time interval from onset of motor to dementia, reduced survival and greater frequency of DLB phenotype than PDD (Table 1). Co-pathologies other than AD were uncommon in LBD. More cases with ARTAG were noted in the SYN+AD group than SYN-AD (χ2=7.3,p<=0.007). There were more cases with limbic TDP in the SYN+AD group as well but this did not reach significance (χ2=2.2,p=0.14). Patients with limbic TDP-43 pathology tended to have an older age at death (t(53)=2.2, p=.04), but this was not observed for patients with ARTAG (t(42)=1.0, p=.33)
Digital Measurement of Pathologic Burden in LBD
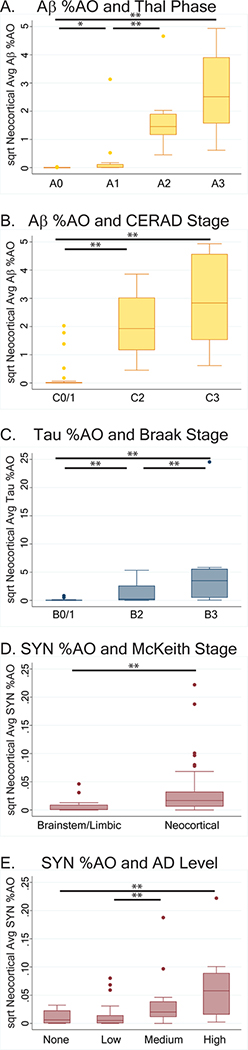
There were robust differences across 0–3 (i.e. none, mild, moderate, severe) ordinal scores for parametric %AO measures of Aβ (F(3,256)=380.0, p<0.001), tau (F(3,256)=76.1, p<0.001), and SYN (F(3,257)=152.0, p<0.001) suggesting our digital measurements accurately reflect traditional pathology rating scales. Next, we examined the relationship between traditional pathology stages (Thal24, CERAD26, Braak tau 25, and McKeith19) and average neocortical %AO measurements for each respective pathology in LBD cases. There was significant concordance of amyloid Thal phases, CERAD plaque stages, Braak tau stages and McKeith stages with neocortical average %AO for each respective pathology (Thal phase and Aβ %AO: F(3,49)=50.4, p<.001; CERAD and Aβ %AO F(2,50)=58.9 p<.001; Braak and tau %AO: F(2,52)=19.4, p<.001; McKeith Stage and SYN %AO F(1,53)=8.3 p=.006) (Figure 2). Additionally, there was greater average neocortical SYN %AO across the four-levels of ADNPC (F(3,51)=5.7, p=.002) (Figure 2).
Figure 2: Higher pathology stages are associated with higher Neocortical %Area Occupied.
Box-plots depict the median, interquartile range, and range of A) Aβ %AO in each Thal Phase: F(49,3)=50.4, p<.001, B) Aβ plaque %AO in each CERAD stage: F(50,2)=58.9 p<.001, , C) tau pathology %AO in each Braak tau stage: F(52,2)=19.4, p<.001D) SYN %AO in each LBD stage: F(53,1)=8.3 p=.006 and E) Neocortical average SYN %AO for each AD level: F(51,3)=5.7, p=.002. Increasing stages of pathology are associated with higher measures of neocortical pathology, including increasing SYN pathology for each stage of coexisting AD NPC. Bars with single asterisk denote p<0.05 and bars with double asterisks denote p<0.01 between groups
Regional Distribution of Tau, Aβ, and SYN pathology in SYN+AD vs SYN-AD LBD groups
Comparison of SYN pathology between groups found higher levels in each individual neocortical region and the average of all neocortical regions in SYN+AD compared to SYN-AD. These differences persisted when in multivariate analysis controlling for sex, age at death, fixative, and clinical phenotype (DLB v PDD) (F(1,54)=5.7–12.2, p<0.001–0.02). In contrast, SYN %AO burden in the limbic (ERC) and subcortical (PUT) regions was similar levels between the two groups (ERC: t(47)=0.5, p=0.6. PUT: t(53)=1.4, p=0.2) (Table 2, Figure 3).
Table 2:
Pathology %AO Analysis between SYN-AD, SYN+AD, and pure AD
| Pathology | LBD | |||
|---|---|---|---|---|
| Region | SYN-AD N=35 | SYN+AD N=20 | AD N=25 | |
| Tau | MFC | 0.16 (.17) N=35 | 0.82 (1.02)** N=20 | 6.27 (3.3)## N=25 |
| STC | 0.22 (.18) N=35 | 1.89 (1.58)** N=20 | 7.41 (2.1)## N=25 | |
| ANG | 0.18 (.21) N=35 | 1.06 (1.09)** N=20 | 6.25 (2.2)## N=25 | |
| Neocortical Average | 0.20 (.172) N=35 | 1.43 (1.14)** N=20 | 6.90 (2.1)## N=25 | |
| ERC | 1.59 (1.76) N=35 | 4.63 (2.26)** N=20 | 7.49 (3.0)## N=25 | |
| PUT | 0.22 (.16) N=35 | 0.62 (.57)** N=20 | ||
| Aβ | MFC | 0.52 (.67) N=35 | 1.70 (.54)** N=20 | 2.09 (.67)# N=20 |
| STC | 0.29 (.50) N=34 | 1.43 (.46)** N=20 | 1.80 (.61)# N=24 | |
| ANG | 0.29 (.50) N=34 | 1.43 (.46)** N=20 | 1.80 (.61)# N=24 | |
| Neocortical Average | 0.43 (.61) N=33 | 1.57 (.42)** N=20 | 1.92 (.51)# N=20 | |
| ERC | 0.51 (.57) N=33 | 1.74 (.60)** N=19 | 2.05 (.60) N=22 | |
| PUT | 0.46 (.67) N=35 | 1.62 (1.10)** N=20 | ||
| SYN | MFC | 0.09 (.10) N=35 | 0.19 (.18)** N=20 | |
| STC | 0.09 (.09) N=35 | 0.19 (.15)** N=20 | ||
| ANG | 0.04 (.08) N=35 | 0.11 (.12)* N=20 | ||
| Neocortical Average | 0.09 (.08) N=35 | 0.19 (.12)** N=20 | ||
| ERC | 0.12 (.11) N=30 | 0.13 (.13) N=19 | ||
| PUT | 0.15 (.13) N=35 | 0.24 (.31) N=20 | ||
Square-root transformed values are reported.
p<.05
p<.01 SYN-AD v SYN+AD in univariate analysis
p<.05
p<.01 SYN+AD v AD in univariate analysis
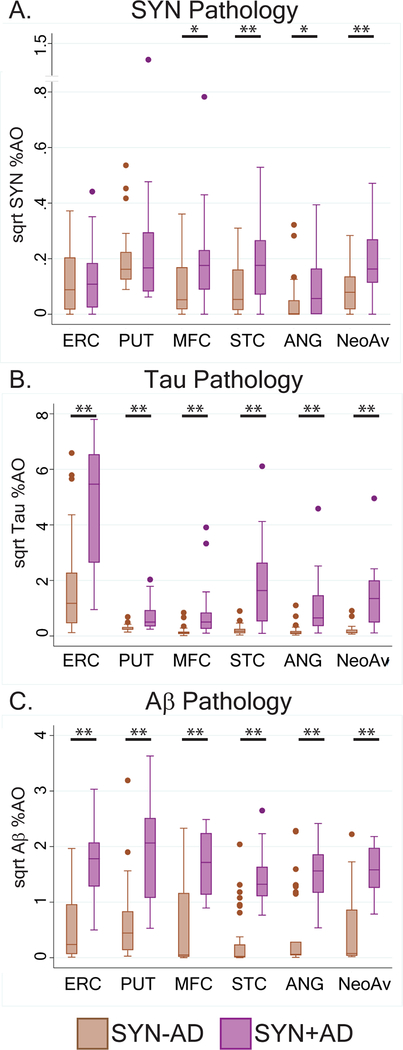
Figure 3: Regional pathology in LBD with SYN+AD compared to SYN-AD.
Box-plots depict median, interquartile range and range of %AO of SYN pathology (A), Tau pathology (B), Aβ pathology (C) in each region and in the average of the three neocortical regions. SYN-AD (brown) and SYN+AD (purple). Bars with single asterisk denote p<0.05 and bars with double asterisks denote p<0.01 in univariate analysis.
As expected, regional pathologic burden showed higher tau and Aβ throughout all brain regions in SYN+AD compared to SYN-AD which survived correction for sex, age at death, fixative, and clinical phenotype (DLB v PDD) in multivariate analysis (Tau: F(1,43–54)=6.3–23.0, p<0.02) (Aβ: F(1,43–53)=16.1–46.6, p<0.001) (Table 2, Figure 3).
Linear mixed-effect models showed a significant association of SYN+AD with greater overall neocortical Aβ (beta=1.1, SE=0.09, t=12, df=156, p<0.001) tau (beta=1.1 SE=0.13, t=8.4, df=159, p<0.001) and SYN pathology (beta=0.09, SE=0.02, t=5.3, df=159, p<0.001).
There was an independent association of region with tau (STC beta= 0.3, SE=0.2, df=159, p=0.03; in comparison to ANG) and SYN (STC beta=0.1, SE=0.02, df=159, p<0.01; MFC beta=0.1, SE=0.02, df=159, p<0.01; in comparison to ANG), indicating a preferential increase in both SYN and Tau pathology in the temporal lobe. No regional difference for Aβ was observed (F=2.4, df=(2, 156), p=0.09) (Figure 3).
We also tested the relative distribution of SYN in the putamen to the neocortex. A ratio of the average neocortical SYN %AO to PUT SYN %AO showed that SYN+AD had a higher ratio than SYN-AD and therefore relatively greater neocortical burden of SYN pathology(t(42)=2.1, p=.04).
Regional Digital Histology and Cognitive Performance in LBD
Performance on neuropsychological testing in SYN-AD and SYN+AD showed similar performance on MMSE, DRS, and in category fluency. However, SYN+AD patients performed worse on confrontation naming (BNT) than SYN-AD (t(27)=2.1, df=27, p=0.04) (Table 3). There was no difference between SYN+AD and SYN-AD in age at testing, years from dementia onset to testing, years from testing to death, or education level (p>0.05). When the LBD group was divided into high- and low-tau neuropathology groups based on the median of %AO neocortical Tau alone, similar differences between high tau %AO and low tau %AO groups were seen in performance on BNT (Table 3). No differences in test scores were seen between median divisions of the cohort by %AO of Aβ or SYN. We also examined LBD patients with or without ARTAG or TDP-43 co-pathology and similarly did not find significant differences in test scores (data not shown). In all of the above comparisons there were no differences in age at test, years from dementia onset to testing, years from testing to death, or education between groups (p>.05 for each).
Table 3:
Neuropsychological Assessments in SYN+AD vs SYN-AD and Digital Pathology Defined High vs Low Neocortical Tau groups in LBD
| Neuropsychological Test | SYN-AD (n=35) | SYN+AD (n=20) | %AO Low Tau (n=28) | %AO High Tau (n=27) | %AO Low Aβ (n=27) | %AO High Aβ (n=26) | %AO Low SYN (n=28) | %AO High SYN (n=27) |
|---|---|---|---|---|---|---|---|---|
| MMSE | 21.9 (5.1) N=16 | 21.5 (7.4) N=20 | 22.6 (4.4) N=28 | 20.9 (7.2) N=27 | 22.5 (4.7) N=27 | 21.0 (7.2) N=26 | 20.9 (5.9) N=28 | 22.7 (5.9) N=27 |
| Dementia Rating Scale Total | 114.3 (17.0) N=16 | 105.8 (26.9) N=5 | 117.1 (11.6) N=14 | 102.7 (28.2) N=7 | 112.4 (12.1) N=11 | 110.3 (26.7) N=9 | 107.8 (19.0) N=12 | 118.3 (19.2) N=9 |
| Category Fluency | 9.6 (4.7) N=23 | 9.6 (6.0) N=18 | 10.2 (4.8) N=21 | 8.9 (5.5) N=20 | 10.1 (4.8) N=16 | 9.5 (5.4) N=23 | 9.3 (5.4) N=20 | 9.9 (4.9) N=21 |
| Boston Naming Test | 26.6 (3.4) N=19 | 23.1 (5.7)* N=10 | 27.0 (3.4) N=17 | 23.1 (5.0)* N=12 | 26.0 (3.5) N=14 | 24.1 (5.5) N=13 | 25.0 (5.6) N=15 | 25.8 (3.1) N=14 |
All data shown are expressed as means (SD). Abbreviations: MMSE: Mini-mental state exam,
P<.05
Comparing digital measure of pathology directly with test performance showed significant negative correlations of MMSE and DRS with average neocortical tau %AO (r=−0.45, −0.68, p<0.001 for both). For tau %AO pathology in pre-specified regions based on known anatomical associations, we found significant negative correlations for category fluency and tau %AO in MFC (r=−0.44, p=.005) and Boston Naming Test with tau %AO in the STC (r=−0.39, p=.04). There were no significant correlations of test performance with Aβ or SYN %AO pathology in their corresponding regions of interest (r= −0.32–0.12, p>0.1). When covarying for MMSE, there continues to be a significant inverse correlation between neocortical average tau %AO and performance on DRS and with STC tau %AO and Boston Naming Test (r=−.59 and −.39, p=0.006 and 0.04 respectively), however category fluency was no longer correlated significantly with MFC tau %AO (p>.1).
Regional Distribution of Tau and Aβ in SYN+AD and pure AD
We compared neocortical and hippocampal tau and Aβ %AO between SYN+AD and pure AD groups. Overall, the severity of tau pathology in the AD group was much greater (1.8–8 fold) than that seen in the SYN+AD cases for each region examined and in the neocortical average (t= 5.2–10.5, p<0.001 for all) (Figure 4). These differences persisted when controlling for demographics in multivariate analysis (F(1,44)=12.9–122.0,p<0.007). Since the pure AD group is largely high (B3) Braak tau stage (Table 1), we performed a sub-group analysis to compare SYN+AD with Braak AD tau stage B3 (n=10) to the pure AD group with Braak B3 tau stage (n=23) and still found a higher burden of tau %AO in AD in all neocortical regions and the neocortical average (t(31)=3.2–9.1, p<0.004 for all) with more similar tau %AO in the ERC (t(31)=1.8, p=.08).
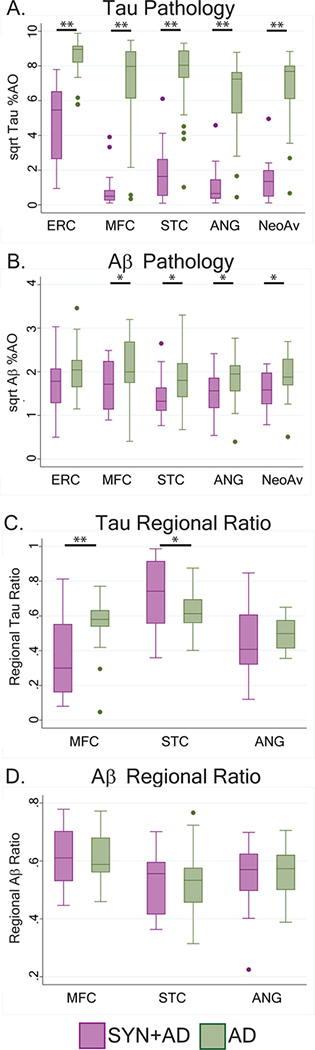
Figure 4: Regional Tau and Aβ pathology in LBD with SYN+AD compared to AD without neocortical SYN.
Box plots depict median, interquartile range and range %AO of A) Tau pathology and B) Aβ pathology in each region and average of the three neocortical regions.
Bars with single asterisk denote p<0.05 and bars with double asterisks denote p<0.01 in univariate analysis. Of note, all multivariate models were non-significant for Aβ %AO burden in SYN+AD compared to AD while all models and pathology factor variables tor Tau %AO burden were significant. Box-plots depict the proportion of total pathology in each region (e.g. MFC/neocortical average) for C) Tau D) and Aβ. SYN+AD (purple) and AD (green). Bars with single asterisk denote p<0.05 and bars with double asterisks denote p<0.01.
We assessed the relative neocortical regional burden of tau %AO in SYN+AD compared to pure AD by comparing ratios of tau %AO in each region to the average neocortical tau %AO (e.g. (MFC tau %AO)/(neocortical average tau %AO)). We found pure AD had relatively greater proportion of tau %AO in MFC compared to SYN+AD (t(41)=3.3, p<0.01) while SYN+AD had relatively greater proportion of tau %AO in STC compared pure AD (t(41)=2.0, p<.05) (Figure 4). SYN+AD and pure AD had similar amounts of Aβ in the ERC and higher amounts of Aβ in AD in the MFC, STC, ANG, and neocortical average in univariate analysis, however these comparisons were not significant in multivariate analysis (for model F(3,39–43)=1.6–2.0, p>.10)) (Table 2. There were no differences in the relative distribution of Aβ between SYN+AD and AD (p>.05).
DISCUSSION
Previous postmortem studies using traditional pathologic methods suggest clinically-significant AD co-pathology (i.e. medium-high ADNPC) is present in ~50% of all LBD1, 4, is associated with greater overall SYN pathology, and corresponds with decreased survival and faster progression to dementia4, 9, 10, 13, 14. This literature suggests that AD co-pathology plays an integral role in the pathophysiological process of LBD. Here, using a digital histology approach, we found that SYN+AD patients have greater neocortical SYN than SYN-AD while having equivalent SYN burden in the entorhinal cortex and putamen (Figure 3, Table 2). We also found the topology of neocortical tau pathology in LBD appears to map more closely to the distribution of SYN pathology (Figure 3) and diverges from the neocortical pattern of tau pathology in AD (Figure 4), while the diffuse pattern of Aβ amyloidosis is similar in LBD and AD (Table 2, Figure 4). Finally, the severity of tau pathology in LBD correlates with cognitive performance on both global cognitive measures and two cortical region-dependent cognitive tasks (Table 3).
Digital pathology is a novel approach for fine-grained, parametric assessment of disease severity and facilitates improved detection of clinicopathological associations23, 29, 30. Neuropathological staging systems for LBD20, 31 and AD24–26 are useful measures of overall disease severity that are reproducible across centers32; however, they are largely based on the topology of pathology with less emphasis on severity. We found our digitized measurements of increasing overall neocortical averages of tau, Aβ, and SYN reflected ordinal stages of pathology for AD and LBD (Figure 2). These results suggest that digital methods may provide complimentary data to traditional staging schemes for future clinicopathological studies.
When we dichotomized the LBD cohort based on presence or absence of sufficient ADNPC to contribute to dementia21, an increase in neocortical SYN pathology in SYN+AD was noted with particular increases in the STC and MFC. In contrast, there were equivalent levels of SYN pathology in the putamen and entorhinal cortex (Figure 3). The entorhinal cortex and the putamen are thought to be affected earlier in the spread of SYN in LBD20, 31, 33. Thus, one potential interpretation of these results is that spread of SYN pathology to the neocortex in SYN+AD may be facilitated by the pathophysiological processes of tau and Aβ.
While tau, Aβ and SYN pathology are all correlated in LBD4, 9, 10, 34, our digitized pathology analysis revealed differences in the regional patterns of pathology. While Aβ has a relatively diffuse neocortical pattern, tau has a higher concentration in the STC in SYN+AD in a manner that more closely resembles the pattern of neocortical SYN in SYN+AD (Table 2, Figure 3) Thus, it appears that SYN pathology appears in a distinct distribution in the neocortex when accompanied by tau co-pathology. While we cannot rule out that these patterns of tau and SYN pathology in SYN+AD represent in part greater overall severity of pathology, as traditional Braak staging of both SYN20, 31 and tau25 suggest STC may be affected earlier in the disease compared to other neocortical regions sampled, we do not find a difference in subcortical pathological burden of SYN between SYN+AD and SYN-AD groups.
Our cohort included both PDD and DLB clinical phenotypes and we found a greater proportion of clinical DLB patients in the SYN+AD group (Table 1), consistent with previous studies finding higher neocortical burdens of SYN, Tau and Aβ in DLB compared to PDD4, 9; however, we still found a robust difference in the distribution and severity of SYN pathology between SYN+AD and SYN-AD after adjusting for PDD/DLB clinical phenotype (Table 2), which was not a significant predictor in our analyses (data not shown). Further, while cognitive impairment35 and dementia36 is near universal long-term in LBD, previous studies suggest a clinicopathological spectrum4–6 with no clear biological substrate to substantiate the “1-year rule” of dementia onset to distinguish DLB from PDD2, 19. As the clinical definitions of LBD continue to evolve, future efforts should consider clinical and biomarker features predictive of biologically-based (i.e. pathologic and/or genetic) subgroups of LBD which may require different treatment strategies.
Our observations suggest that SYN+AD is an important biological subgroup of LBD with unique clinical features that are largely driven by tau pathology. AD co-pathology has been shown to influence gross clinical outcomes4, 10, 14, 22, 34, clinical features37–39, and MRI atrophy patterns40. Here we examined region-specific associations of pathological burden with cognitive testing in an LBD cohort, and found that regional burden of tau is a robust correlate of domain-specific cognitive tasks in LBD (Table 3). Our direct comparisons found preliminary evidence that SYN+AD performs worse on a naming task reliant on temporal lobe function than SYN-AD, despite a similar level of overall cognitive impairment. Previous work also suggested worse performance in naming tasks38, 41 in LBD with AD co-pathology, and mixed temporal lobe and prefrontal cortex pathology both strongly associated with cognitive decline in LBD42.
Previous work has highlighted the importance of SYN in the occurrence of dementia and clinical features in LBD10–12. Here we focused on LBD with dementia and did not include non-demented PD patients which could explain the lack of association of SYN with cognitive measures in this study. Further, it is possible SYN pathology is more influential to early stages of cognitive impairment in LBD, prior to onset of dementia. Longitudinal cognitive assessments in autopsy-confirmed cohorts will help resolve these issues. ARTAG was associated with higher ADNPC and TDP-43 was associated with advanced age and higher ADNPC in LBD, similar to previous reports of these co-pathologies in AD and aging27. While we did not detect an association of these with cognitive scores in this study, we acknowledge that other age-related pathological co-morbidities, including cerebrovascular disease, that were less common in our cohort may influence cognition LBD.
Several strands of evidence suggest a link between tau and SYN pathology in LBD. Genetic variation in the H1 haplotype of the tau gene MAPT has been linked to increased risk for PD43 and DLB44, and the Contursi kindred of hereditary LBD with Ala53Thr mutations in SNCA was found to have high levels of tau pathology in addition to SYN45. A subset of tau inclusions in these cases were surrounded by pathological SYN within the same cell45. Moreover, a subset of SYN Lewy bodies in sporadic LBD have peripheral deposits of tau pathology 46 or tau tangles within the same cell47. There is a large proportion of SYN pathology located at the synapse in LBD which are visualized only with non-traditional tissue preparations 48, 49 and thus, co-polymerization of tau an SYN at the presynaptic compartment may also influence regional spread of tau and SYN pathology. Further, in vitro studies suggest the co-incubation of recombinant tau and SYN can accelerate polymerization of both proteins into fibrils consisting largely of homopolymers of either tau or SYN50. A significant proportion of transgenic mice harboring the human Ala53Thr mutation in SNCA show tau co-pathology50 and when these mice are bred to also contain transgenes for human mutations in APP and MAPT there is acceleration of cognitive decline and deposition of all three pathologies, further suggesting a synergistic interaction of these proteins. Finally, recent data suggests a distinct strain of recombinant SYN that can induce both tau and SYN pathology in cell models15 which is detected in human LBD brain tissue16.
Our direct comparisons of SYN+AD and AD suggest that the pattern of tau deposition is different in these two conditions. Tau %AO is several-fold higher in AD than SYN+AD, even when comparing patients with similar Braak tau stages. Nevertheless, we found a greater relative pathologic burden of neocortical tau pathology in STC in SYN+AD compared to greater relative pathologic burden of tau in MFC in pure AD (Figure 4). It is tempting to hypothesize that the aforementioned strains of pathogenic SYN that cross-seed tau pathology in model systems could contribute to the altered pattern of tau pathology in LBD observed here15, 16. Recently published studies using the PET ligand flortaucipir similarly found overall lower levels of flortaucipir uptake in LBD compared to AD patients and increased uptake in the posterior temporal-parietal lobes compared to controls 51, 52. Although the limited sampling in pathological studies cannot completely recapitulate whole-brain PET imaging analyses, these studies provide converging evidence for a distinct pattern of tau in LBD. A recent digital pathology study found higher tau pathology in AD compared to SYN+AD in the hippocampus alone29. This discrepancy may be due in part because the previous study largely focused on clinical AD with neocortical SYN pathology while our AD reference cohort was free of neocortical SYN pathology and was age- and sex-matched to our SYN+AD group. Nonetheless, these studies provide complementary views of the clinicopathological spectrum of AD and LBD, and with the emerging in vivo molecular imaging data, highlight intriguing distinctions of tau pathology in LBD compared to AD.
There are limitations to the current study. Despite the large-scale digital histology effort (>900 slides digitally analyzed), we sampled limited brain regions in a focused cohort to facilitate correlation with cognitive performance. Future digital pathology studies in larger multicenter cohorts using the full spectrum of LBD with extensive sampling will be needed to fully elucidate the staging of tau, Aβ and SYN in LBD and compared to AD. Autopsy cohorts from tertiary academic centers may not be completely generalizable to the clinical LBD population; results would benefit from confirmation in population-based cohorts. Finally, this study details the results of harmonized neuropsychological testing across cognitive and movement disorder clinics28, but despite this significant effort we had limited clinical data across cognitive domains, and were lacking a test for episodic memory. Future efforts with harmonized cognitive and motor assessments are needed to fully resolve the clinical phenotype of LBD with AD co-pathology.
With these caveats in mind, we conclude that concurrent AD co-pathology is associated with an altered pattern of SYN deposition in LBD. Nevertheless, it is the anatomic distribution of tau pathology that appears to be associated with specific antemortem cognitive features in dementia and may contribute to observed clinical heterogeneity of LBD. Furthermore, the distribution of tau pathology in LBD may be distinct from that in AD, possibly related to the strain of SYN that also elicits tau co-pathology only in LBD. Thus, we contend that SYN+AD is a clinically meaningful subtype of LBD that may be more informative than current clinical distinctions between PDD and DLB; antemortem detection of SYN+AD in LBD could improve prognostication and may aid in clinical trial stratification for more homogenous patient populations for both symptomatic and protein-targeted therapies.
Acknowledgements
We would like to thank Cecilia Zhou, John Robinson, and Terry Shuck for their technical assistance. We thank the patients and families for their participation in research. Research reported in this publication was supported by funding from TL1TR001880, NIA AG010124, and NINDS NS088341, NS053488.
Footnotes
Potential Conflicts of Interest:
Nothing to report.
REFERENCES
- 1.Irwin DJ, Lee VM-Y, Trojanowski JQ. Parkinson’s disease dementia: convergence of [alpha]-synuclein, tau and amyloid-[beta] pathologies. Nature Reviews Neuroscience 2013;14(9):626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 2015. October;30(12):1591–601. [DOI] [PubMed] [Google Scholar]
- 3.Boeve BF, Dickson DW, Duda JE, et al. Arguing against the proposed definition changes of PD. Movement disorders : official journal of the Movement Disorder Society 2016. November;31(11):1619–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irwin DJ, Grossman M, Weintraub D, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol 2017. January;16(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballard C, Ziabreva I, Perry R, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006. December 12;67(11):1931–4. [DOI] [PubMed] [Google Scholar]
- 6.Lippa C, Duda J, Grossman M, et al. DLB and PDD boundary issues diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68(11):812–9. [DOI] [PubMed] [Google Scholar]
- 7.Goldman JG, Williams‐Gray C, Barker RA, Duda JE, Galvin JE. The spectrum of cognitive impairment in Lewy body diseases. Movement Disorders. 2014;29(5):608–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990. October;40(10):1529–34. [DOI] [PubMed] [Google Scholar]
- 9.Halliday GM, Holton JL, Revesz T, Dickson DW. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta neuropathologica. 2011;122(2):187–204. [DOI] [PubMed] [Google Scholar]
- 10.Irwin DJ, White MT, Toledo JB, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012. October;72(4):587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurtig H, Trojanowski J, Galvin J, et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology. 2000;54(10):1916–21. [DOI] [PubMed] [Google Scholar]
- 12.Graff-Radford J, Aakre J, Savica R, et al. Duration and Pathologic Correlates of Lewy Body Disease. JAMA Neurol. 2017. March 1;74(3):310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jellinger K, Seppi K, Wenning G, Poewe W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson’s disease. Journal of neural transmission. 2002;109(3):329–39. [DOI] [PubMed] [Google Scholar]
- 14.Compta Y, Parkkinen L, O’sullivan SS, et al. Lewy-and Alzheimer-type pathologies in Parkinson’s disease dementia: which is more important? Brain. 2011;134(5):1493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo JL, Covell DJ, Daniels JP, et al. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell. 2013;154(1):103–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Covell DJ, Robinson JL, Akhtar RS, et al. Novel conformation-selective alpha-synuclein antibodies raised against different in vitro fibril forms show distinct patterns of Lewy pathology in Parkinson’s disease. Neuropathol Appl Neurobiol. 2017. December;43(7):604–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toledo JB, Van Deerlin VM, Lee EB, et al. A platform for discovery: The University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimers Dement. 2014. July;10(4):477–84 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2007;22(12):1689–707. [DOI] [PubMed] [Google Scholar]
- 19.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005. December 27;65(12):1863–72. [DOI] [PubMed] [Google Scholar]
- 21.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta neuropathologica. 2012. January;123(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018. July 1;141(7):2181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin DJ, Byrne MD, McMillan CT, et al. Semi-Automated Digital Image Analysis of Pick’s Disease and TDP-43 Proteinopathy. J Histochem Cytochem. 2016. January;64(1):54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002. June 25;58(12):1791–800. [DOI] [PubMed] [Google Scholar]
- 25.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. [DOI] [PubMed] [Google Scholar]
- 26.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991. April;41(4):479–86. [DOI] [PubMed] [Google Scholar]
- 27.Kovacs GG, Robinson JL, Xie SX, et al. Evaluating the patterns of aging-related tau astrogliopathy unravels novel insights into brain aging and neurodegenerative diseases. Journal of Neuropatholgy & Experimental Neurology. 2017;76(4):270–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson GS, Cholerton BA, Gross RG, et al. Neuropsychologic assessment in collaborative Parkinson’s disease research: A proposal from the National Institute of Neurological Disorders and Stroke Morris K. Udall Centers of Excellence for Parkinson’s Disease Research at the University of Pennsylvania and the University of Washington. Alzheimer’s & Dementia. 2013;9(5):609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker L, McAleese KE, Thomas AJ, et al. Neuropathologically mixed Alzheimer’s and Lewy body disease: burden of pathological protein aggregates differs between clinical phenotypes. Acta Neuropathol. 2015. May;129(5):729–48. [DOI] [PubMed] [Google Scholar]
- 30.Neltner JH, Abner EL, Schmitt FA, et al. Digital pathology and image analysis for robust high-throughput quantitative assessment of Alzheimer disease neuropathologic changes. Journal of Neuropathology & Experimental Neurology. 2012;71(12):1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003. Mar-Apr;24(2):197–211. [DOI] [PubMed] [Google Scholar]
- 32.Montine TJ, Monsell SE, Beach TG, et al. Multisite assessment of NIA-AA guidelines for the neuropathologic evaluation of Alzheimer’s disease. Alzheimer’s & Dementia. 2016;12(2):164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duda JE, Giasson BI, Mabon ME, Lee VM, Trojanowski JQ. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol. 2002. August;52(2):205–10. [DOI] [PubMed] [Google Scholar]
- 34.Halliday G, Hely M, Reid W, Morris J. The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta neuropathologica. 2008;115(4):409–15. [DOI] [PubMed] [Google Scholar]
- 35.Pigott K, Rick J, Xie SX, et al. Longitudinal study of normal cognition in Parkinson disease. Neurology. 2015: 10.1212/WNL.0000000000002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kempster PA, O’Sullivan SS, Holton JL, Revesz T, Lees AJ. Relationships between age and late progression of Parkinson’s disease: a clinico-pathological study. Brain. 2010;133(6):1755–62. [DOI] [PubMed] [Google Scholar]
- 37.Lopez OL, Becker JT, Kaufer DI, et al. Research evaluation and prospective diagnosis of dementia with Lewy bodies. Archives of neurology. 2002;59(1):43–6. [DOI] [PubMed] [Google Scholar]
- 38.Peavy GM, Edland SD, Toole BM, Hansen LA, Galasko DR, Mayo AM. Phenotypic differences based on staging of Alzheimer’s neuropathology in autopsy-confirmed dementia with Lewy bodies. Parkinsonism Relat Disord. 2016. October;31:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merdes A, Hansen L, Jeste D, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003;60(10):1586–90. [DOI] [PubMed] [Google Scholar]
- 40.McMillan CT, Wolk DA. Presence of cerebral amyloid modulates phenotype and pattern of neurodegeneration in early Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016. October;87(10):1112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraybill ML, Larson EB, Tsuang D, et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology. 2005;64(12):2069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howlett DR, Whitfield D, Johnson M, et al. Regional Multiple Pathology Scores Are Associated with Cognitive Decline in Lewy Body Dementias. Brain pathology. 2015. July;25(4):401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zabetian CP, Hutter CM, Factor SA, et al. Association analysis of MAPT H1 haplotype and subhaplotypes in Parkinson’s disease. Annals of neurology. 2007. August;62(2):137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Labbe C, Heckman MG, Lorenzo-Betancor O, et al. MAPT haplotype H1G is associated with increased risk of dementia with Lewy bodies. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2016. December;12(12):1297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duda JE, Giasson BI, Mabon ME, et al. Concurrence of alpha-synuclein and tau brain pathology in the Contursi kindred. Acta neuropathologica. 2002. July;104(1):7–11. [DOI] [PubMed] [Google Scholar]
- 46.Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of Tau and Alpha-Synuclein Epitopes in Lewy Bodies. Journal of Neuropathology & Experimental Neurology. 2003;62(4):389–97. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt ML, Martin JA, Lee VM-Y, Trojanowski JQ. Convergence of Lewy bodies and neurofibrillary tangles in amygdala neurons of Alzheimer’s disease and Lewy body disorders. Acta neuropathologica. 1996;91(5):475–81. [DOI] [PubMed] [Google Scholar]
- 48.Colom-Cadena M, Pegueroles J, Herrmann AG, et al. Synaptic phosphorylated α-synuclein in dementia with Lewy bodies. Brain. 2017;140(12):3204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kramer ML, Schulz-Schaeffer WJ. Presynaptic α-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. Journal of Neuroscience. 2007;27(6):1405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giasson BI, Forman MS, Higuchi M, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300(5619):636–40. [DOI] [PubMed] [Google Scholar]
- 51.Kantarci K, Lowe VJ, Boeve BF, et al. AV‐1451 tau and β‐amyloid positron emission tomography imaging in dementia with Lewy bodies. Annals of neurology. 2017;81(1):58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomperts SN, Locascio JJ, Makaretz SJ, et al. Tau positron emission tomographic imaging in the Lewy body diseases. JAMA neurology. 2016;73(11):1334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]