INTRODUCTION
What do the following three equations for predicting resting energy expenditure (REE, kcal/d) in adult women have in common?
| (1) |
| (2) |
| (3) |
The answer is that all three1–3 reveal a predictable empirical association between REE and body size. Why and how is body size related to a person’s heat production at rest? At one level the answer to this question is obvious: people who are large have more heat producing cells and tissues than do people who are small. But at a deeper level the answer is much more complex and brings to light an unforeseen connection between the fields of clinical nutrition and developmental biology.
ENERGY EXPENDITURE AND BODY SIZE
Body Mass
To delve into this topic in more detail, we know that resting mammals, including humans, produce a predictable amount of heat based on their body size as described by Kleiber’s rule4:
| (4) |
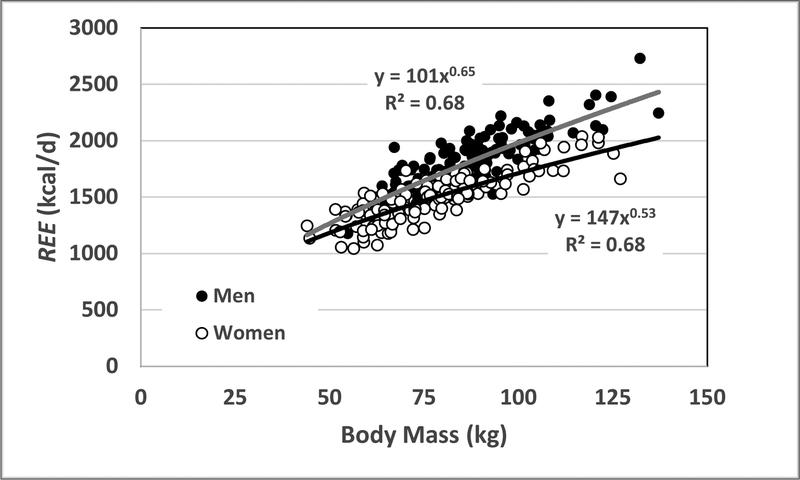
where ∝ is the symbol for proportionality and 0.75 is the “power” or scaling factor. Body mass is one measure of body size, and similar relations are observed in adult humans as shown in Figure 1. A description of this demonstration sample is presented in Supplementary Information. After controlling for adiposity and age, REE scales to body mass with respective powers (X±SE) of 0.77±0.05 (R2, 0.75) and 0.68±0.04 (R2, 0.78) in the men and women shown in the figure. Adults who are large thus expend more energy at rest than their small counterparts. But, as with mammals as a whole, there are some nuances. Since these powers are less than 1.0, animals and people who are large will expend less energy relative to their body mass compared to animals and people who are small:
| (5) |
Equation 5 describes the “quarter power” rule followed by REE and many other biological processes5, 6. According to the quarter power rule, the “mass-specific REE” (i.e., REE/Body Mass) becomes lower (i.e., the power of body mass is negative) as mammals, including humans, increase in body size. As we will see in a later section, this is the first place that developmental biology begins to shed light on longstanding observations.
Figure 1.
Resting energy expenditure (REE) versus body mass in a sample of adult healthy men (n=130) and women (n=135). The respective regression models are shown in the figure and both correlations are significant at p<0.001. The powers of body mass, after adjustment for age and %fat, are for men and women (X±SE) 0.77±0.05 (R2, 0.75) and 0.68±0.04 (R2, 0.78), respectively. The sample details are presented in Supplementary Information.
Stature
Body mass is usually the hallmark measure of body size when studying mammals as a whole4–6. However, when specifically considering humans, we know that two people can have the same body mass but differ in height. A tall person and a short person of the same body mass will differ in their relative amounts of adipose tissue, skeletal muscle, and other organs and tissues. Body mass alone is thus an inadequate phenotypic marker of an adult human’s body size and hence their REE. That’s why modern statistically-derived REE prediction equations for adults are more like equation 2 than equation 1.
Body mass and height, of course, are related to each other: people who are tall, given the same age and level of adiposity (i.e., %fat), will weigh more than people who are short. This association is mathematically described by Quetelet’s rule in adults7,
| (6) |
Quetelet’s rule is generally applicable across men and women in most of the adult race/ethnic groups evaluated so far8.
Since REE scales as body mass~0.75 and body mass scales as height2 in humans, we can combine these two estimates to show that REE scales to height with a power of about 1.5. When REE is plotted against height in the men and women shown in Figure 1, the observed powers of height, after controlling for adiposity and age, are 1.42±0.30 (R2, 0.38) and 1.65±0.25 (R2, 0.46), respectively. As body mass scales as height2 and REE as height~1.5, we again observe a lower mass-specific REE in people who are large, but in this case body size is characterized by height:
| (7) |
From the foregoing overview we can conclude that: resting heat production in mammals as a whole, and humans in particular, is strongly associated with body size; and that the rate at which heat is produced is smaller relative to body size in large animals and humans compared to their small counterparts.
ORGANS and THEIR SYSTEMS
How are body mass and REE linked? Body mass, and its main determinant height, reflect the summated mass of all organs of which there are more than 75 in adult humans9. Each organ has a characteristic mass-specific REE with values in adults ranging from a low of about 2 kcal/kg/d for bone and a high of about 440 kcal/kg/d for the heart and kidney10–12. Despite these large differences in organ energy production rates, REE and body mass are strongly associated with each other as described by Kleiber’s4 rule and in the men and women as depicted in Figure 1. This observation suggests that relatively stable organ mass proportions are present across mammals and humans varying widely in body mass. For example, a person with a large body size must have a large skeletal muscle, bone, liver, and heart mass that maintain proportionality similar to that observed in a small person. This observation exposes one of the great mysteries in science as reflected upon by Kennedy and Smith who queried “how do the hearts of mice and elephants each fit to the proper rib cage?”13. A critical question, one that we will return to later, is how this inter-organ mass proportionality is achieved and then maintained through molecular mechanisms across mammals differing vastly in body size.
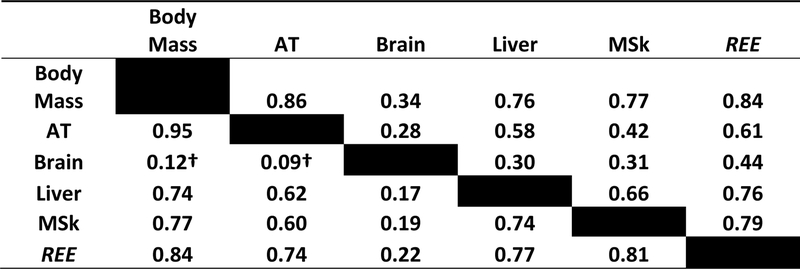
The relationships between organ and tissue masses with body size has been studied in both mammals as a whole, and humans in particular, although sample sizes by necessity tend to be small and some gaps remain5, 7, 14, 15. Two relevant observations emerge from these studies. First, on the whole, the mass of most major organs is highly correlated with animal and human body size. For example, the liver is larger in elephants than it is mice5; and liver mass is larger in people who are tall than it is in people who are short7. Body size and organ size are thus closely linked with REE through these connections. As an example, strong inter-correlations (R-values) are present between body mass, organ mass, and REE in the demonstration sample (Supplementary Information) as shown in Figure 2.
Figure 2.
Correlations (R-values) between body mass, selected body compartments, and resting energy expenditure (REE) in men (lower left) and women (upper right). The sample details are presented in Supplementary Information. AT, adipose tissue mass; MSk, musculoskeletal mass. All correlations are significant a p<0.05 except for † which are non-significant.
The second observation is that not all organs scale to body size in the same way. A long-standing observation is that the skeleton is a larger proportion of body mass in large animals (i.e., ∝body mass>1) while a smaller proportion of body mass is accounted for by brain mass (i.e., ∝body mass<1) compared to smaller animals5. The same observation applies to humans: tall adults have a larger fraction of their body mass as bone and a smaller fraction as brain7, 11, 14–16. Thus, while an organ “companionship” largely preserves organ proportionality across vast differences in body size, there also exists definable body-size related systematic variation in these relationships. Changes in organ size also follow well-established proportionality patterns in humans during periods of semi-starvation, such as with anorexia nervosa and during famines, when organs such as heart, liver, and kidney are all coordinately reduced in mass16–19. Similarly, growth restriction secondary to disturbances in the growth hormone/insulin-like growth factor axis is accompanied by a proportionally reduced size of most organs20.
Returning to the question of what the three equations at the start of this review have in common, we now see that body mass, height, or their composition surrogate fat and fat-free mass represent the mass of over 75 organs; that these organs in healthy adults each have a definable mass-specific REE; and that these organs maintain a distinct companionship pattern across large body size differences and with conditions such as semi-starvation and growth restriction.
HOW IS ORGAN SIZE REGULATED?
Viewing these observations from another perspective, developmental biologists have as one of their great unsolved mysteries “How are the sizes of cells, organs, and bodies controlled?”21. While this area is an intense focus of research, substantial progress in answering this question has emerged over the past several decades20–22.
Organ growth is tightly regulated to achieve the “bauplan” or blueprint that characterizes the fully functional and viable adult human. But the developed systems are exposed to many functional demands across the full lifespan and therefore must retain a high level of adaptability22. Organ growth and then later maintenance of mass and function can be viewed as proceeding along two main pathways, the first coordinating growth deceleration across organs and the second establishing an organ’s adult mass.
Mechanisms Coordinating Organ Growth
Growth is maximal early in life with a gradual deceleration thereafter, with the exception of during adolescence, until cell proliferation ceases across multiple tissues when adult size organs and stature are reached20. These dynamic processes vary across organs, with brain growth in humans approaching mature levels during the first decade of life while musculoskeletal and metabolic (e.g., digestive) systems reach their peak mass towards the end of the second decade11, 23. The proportion of body mass as nervous system and brain thus gradually decline with parallel growth of the other major organ systems11. The mechanisms coordinating the parallel deceleration in growth across the major organ systems are not yet fully elucidated, but at present both systemic and local processes are recognized20–22.
Systemic mechanisms accounting for growth deceleration and cessation of cell proliferation in multiple tissues includes hormonal actions such as those by the growth hormone/insulin-like growth factor axis20, 22. At the local level, at least two factors intrinsic to organs appear to reduce cell proliferation and lead to cessation of growth. First, a genetic program in multiple organs has been identified that down regulates a large set of genes that promote growth20, 24. This mechanism appears to act as a negative feedback loop, some portions of which involve paracrine processes. The second, more recently discovered, growth coordinating factor is referred to as the Hippo pathway that when activated slows cell proliferation and increases apoptosis as a means of arriving at the appropriate organ size25.
Both systemic mechanisms and local factors thus appear involved in coordinating the rate at which organ cell proliferation slows and then ceases in multiple tissues when maturity is reached. Organ growth rates are thus synchronized across multiple systems, but what factors account for the size they reach at the time cell proliferation ceases?
Mechanisms Mediating Organ Size
At least four groups of mechanisms have been identified that provide an explanation for how organs “know” when they have reached the right size: biological clocks; cell division counters; mechanisms that monitor organ mass/cell number; and mechanisms linked with organ function20. Biological clocks monitor elapsed time as a means of establishing when an organ reaches adult mass20. Cell cycle counting may be involved in regulating the mass of some organs such as the pancreas whose growth in size is limited by the number of initial progenitor cells20.
Mechanisms monitoring an organ’s mass adjust the size of some organs, notably through negative feedback loops such as those mediated by chalones20, 22. These tissue-specific water soluble molecules reversibly inhibit cellular mitosis in the organ that secretes it, thus acting as a negative feedback signal. An example is myostatin, a protein synthesized by myocytes that acts as an autocrine signal inhibiting the growth and differentiation of muscle cells in both skeletal muscle and heart26.
The fourth factor regulating organ mass involves the metabolic and mechanical functional demands placed on organs and their systems. The classic example is the tight regulation of liver mass. Partial hepatectomy is rapidly followed by compensatory hepatocyte hyperplasia with regrowth of the liver close to its original size27, 28. Liver size adjusts upward or downward following orthotopic transplantation to a larger or smaller host, respectively20, 22, 27. While the remarkable stability of liver mass when exposed to these differing perturbations likely involves multiple mechanisms, a key factor appears to be functional stimulation of hepatocyte growth by bile acids returning to the liver via the enterohepatic circulation27.
The sizes of other organs are also adjusted by mechanisms activated by metabolic and mechanical functional demands. For example, the left ventricle decreases in mass with semi-starvation and increases in mass with high blood pressure, excess adiposity, and high exercise levels. These actions that bring about cardiomyocyte atrophy or hypertrophy include changes with these conditions in preload and afterload against a hormonal background29–31. Fine tuning through functional mechanisms is recognized for other organs and tissues such as bone, skeletal muscle, and kidney20, 32.
INTEGRATION
Why Strong Associations Are Present Between REE and Body Size
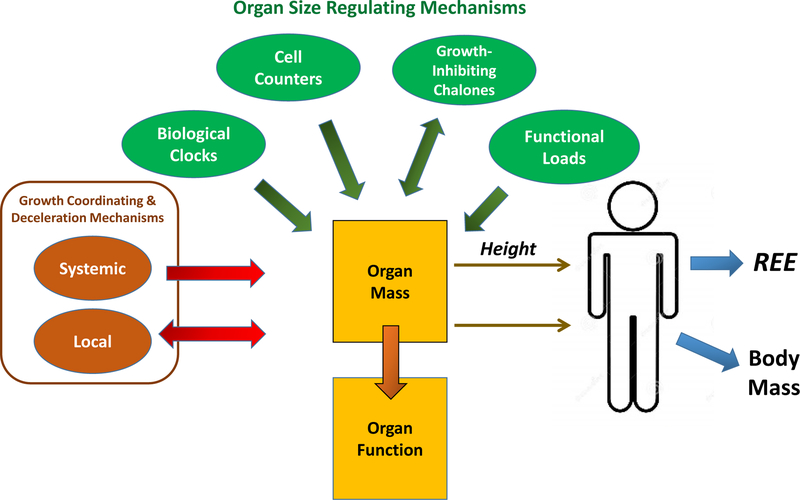
A vast complex array of mechanisms thus intricately controls the synchronized growth and ultimately the size of body organs and their related systems (Figure 3). Once adulthood is reached, these mechanisms must continue to maintain sufficient plasticity to adjust the structure and function of organs to meet the variable metabolic and mechanical demands placed on them.
Figure 3.
The connections between factors regulating organ growth, organ mass, REE, and body mass. Organ mass and function are tightly coupled and conceptually are “designed” to accommodate maximal metabolic and mechanical functional demands33. Growth deceleration appears coordinated by systemic mechanisms and a large set of locally-acting growth-promoting genes that serve as part of a negative feedback loop and the Hippo pathway24. The four groups of mechanisms recognized as contributing to the size reached and maintained by adult organs20 are shown in the figure.
These developmental biology and organ mass regulatory concepts can be viewed in the context of a widely discussed evolutionary biology theory referred to as symmorphosis33. According to the symmorphosis hypothesis, organs and their systems are highly adaptable, closely inter-related, and are “designed” to accommodate maximal functional demands33. The sizes of organs in the symmorphosis model are optimized to accommodate functional loads so as to be maximally efficient while avoiding energy wastage by carrying an excess mass. This useful conceptual model creates a framework by which we can link bodily functions with the size of organs and through those pathways to REE and body size as a whole.
Adults at stable body weight and in good health maintain a relatively constant daily food intake and activity level over long time periods. Functional demands across organs and organ systems are thus relatively stable when integrated over long time periods. Increased functional demands brought about by high activity levels, weight gain, and other individual variations over time are accommodated by adaptations spread across multiple organ systems. For example, increasing adipose tissue mass with development of obesity is followed by structure-function adaptations mediated by increased mechanical and metabolic loads in heart, liver, bone, skeletal muscle, and other organs11. These functional demands are similar across most healthy adults and thus we can surmise that organ inter-relations and their proportionality are reasonably stable in most people. In a sense the functionally synchronized body components collectively act as a single unit whose size relates to a person’s body size as a whole, hence the strong associations observed across individuals between REE and body mass. The association between REE and body mass in our demonstration sample accounted for almost three-fourths of the variance after controlling for between-individual differences in age and adiposity.
In sum, these complex structural and functional physiological connections are manifest by the relatively simple observation that REE is strongly associated with adult body size. While we can now see the biological complexity involved in creating the human body plan as it relates to heat production at rest, the evolutionary explanations for this universal design still are uncertain and controversial (4–6).
Why a Large Body Size in Adults is Accompanied by a Relatively Low REE
As in mammals as a whole, human brain growth plateaus relatively early in life23 and remains stable in mass thereafter. By contrast, other organs and their systems continue to grow into the second decade and the result is that only weak correlations are present between brain mass and other body compartments (Figure 2)11. Moreover, structural components such as the skeleton form a larger proportion of body mass in people who are tall compared to people who are short7. The result is that REE/Body Mass is inversely correlated with height (equation 7) since brain has a high mass-specific REE (440 kcal/kg/d) compared to bone (~2 kcal/kg/d) and other structural components10, 11. People with a large body size, notably who are tall, thus have a different organ proportionality than people who are short7 and this variation in the human bauplan accounts for the lower relative rate of resting heat production in people who are tall.
Why REE in some Individuals and Groups May Differ from that Predicted for Their Body Size
The focus so far has been on the strong associations observed between REE and body size, one that is pervasive across mammals as a whole and humans in particular. The thesis presented so far is that most humans are designed according to a distinct organ bauplan that develops according to molecular mechanisms and that ultimately is reflected in a group’s resting heat production and body size. There are, however, anatomic variations between individuals and groups in body proportions that do or can lead to differences in REE. Considering a few examples, compared to non-Hispanic whites, African Americans as a group tend to have a relatively larger proportion of body mass as muscle and bone and a smaller proportion as visceral organs34, 35. These anatomic differences are reflected in a lower REE for people who are African American relative to people who are non-Hispanic white. Mutations in the myostatin gene are recognized that lead to a muscular human phenotype26, an anatomic change that likely would reduce a person’s REE/Body Mass since muscle has a low mass-specific REE (i.e., 13 kcal/kg)10. The recent discovery of an adaptive mutation leading to an enlarged spleen mass in people with a long tradition of deep sea diving36 is yet another example of between-person or group variation in organ proportions that may influence REE-body size relations.
CONCLUSIONS
One of the oldest and most studied relationships in clinical nutrition is the association between REE and body size. Here I show how advances in developmental, molecular, and evolutionary biology mechanistically connect to the ideas surrounding human heat production according to the clinical nutrition paradigm. Some of these connections are well defined while others are largely conjecture or hypotheses. These ideas reflect the efforts of those of us working in the field to move classic empirical associations such as those described by equations 1–3 to a more mechanistic and physiological level11, 12. Advancing these observations and hypotheses provide many opportunities for future clinical nutrition research.
Supplementary Material
ACKNOWLEDGEMENTS
The author acknowledges Melanie Peterson for her support in preparing this manuscript and Drs. Anja Bosy-Westphal and Manfred J. Muller for their generous data support used in this review.
FUNDING
This work was partially supported by two National Institutes of Health NORC Center Grants P30DK072476, Pennington/Louisiana; and P30DK040561, Harvard; and R01DK109008, Shape UP! Adults.
Abbreviations:
- REE
resting energy expenditure
Footnotes
CONFLICTS OF INTEREST
The author is on the Medical Advisory Boards of Tanita and Medifast Corporations.
REFERENCES
- 1.Food and Agriculture Organization (FAO), Committee on Calorie Requirements. Calorie Requirements: Report of the Second Committee on Calorie Requirements. Nutritional Studies No. 15. In. Rome: Food and Agriculture Organization of the United Nations, 1957. [Google Scholar]
- 2.Harris JA, Benedict FG. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci U S A. 1918; 4: 370–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielsen S, Hensrud DD, Romanski S, Levine JA, Burguera B, Jensen MD. Body composition and resting energy expenditure in humans: role of fat, fat-free mass and extracellular fluid. Int J Obes Relat Metab Disord. 2000; 24: 1153–1157. [DOI] [PubMed] [Google Scholar]
- 4.Kleiber M Body size and metabolism. Hilgardia. 1932; 6: 315–353. [Google Scholar]
- 5.Calder WA. Size, function, and life history, Harvard University Press: Cambridge, Mass, 1984. [Google Scholar]
- 6.Glazier DS. Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol Rev Camb Philos Soc. 2005; 80: 611–662. [DOI] [PubMed] [Google Scholar]
- 7.Heymsfield SB, Gallagher D, Mayer L, Beetsch J, Pietrobelli A. Scaling of human body composition to stature: new insights into body mass index. Am J Clin Nutr. 2007; 86: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heymsfield SB, Peterson CM, Thomas DM, Heo M, Schuna JM Jr., Hong S et al. Scaling of adult body weight to height across sex and race/ethnic groups: relevance to BMI. Am J Clin Nutr. 2014; 100: 1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Commission on Radiological Protection, Task Group on Reference Man. Report of the Task Group on Reference Man: A Report. In. New York: Pergamon Press, 1975. [Google Scholar]
- 10.Elia M. Organ and tissue contribution to metabolic rate. In: Kinney JM, Tucker HN (eds). Energy metabolism: Tissue determinants and cellular corollaries. Raven Press: New York, 1992, pp 61–79. [Google Scholar]
- 11.Heymsfield SB, Bourgeois B, Peterson CM. Body Composition-Energy Expenditure Organ System Model: Initial Evaluation of Predictions. Obesity Reviews. (Under review). [Google Scholar]
- 12.Muller MJ, Bosy-Westphal A, Kutzner D, Heller M. Metabolically active components of fat-free mass and resting energy expenditure in humans: recent lessons from imaging technologies. Obes Rev. 2002; 3: 113–122. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy D, Norman C. What don’t we know? Science. 2005; 309: 75. [DOI] [PubMed] [Google Scholar]
- 14.Heymsfield SB, Chirachariyavej T, Rhyu IJ, Roongpisuthipong C, Heo M, Pietrobelli A. Differences between brain mass and body weight scaling to height: potential mechanism of reduced mass-specific resting energy expenditure of taller adults. J Appl Physiol (1985). 2009; 106: 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heymsfield SB, Muller MJ, Bosy-Westphal A, Thomas D, Shen W. Human brain mass: similar body composition associations as observed across mammals. Am J Hum Biol. 2012; 24: 479–485. [DOI] [PubMed] [Google Scholar]
- 16.Bosy-Westphal A, Reinecke U, Schlorke T, Illner K, Kutzner D, Heller M et al. Effect of organ and tissue masses on resting energy expenditure in underweight, normal weight and obese adults. Int J Obes Relat Metab Disord. 2004; 28: 72–79. [DOI] [PubMed] [Google Scholar]
- 17.Webb JG, Kiess MC, Chan-Yan CC. Malnutrition and the heart. CMAJ. 1986; 135: 753–758. [PMC free article] [PubMed] [Google Scholar]
- 18.Keys AB, Brožek J, Henschel A, Mickelsen O, Taylor HL. The biology of human starvation, University of Minnesota Press: Minneapolis, 1950. [Google Scholar]
- 19.Fohlin L, Freyschuss U, Bjarke B, Davies CT, Thoren C. Function and dimensions of the circulatory system in anorexia nervosa. Acta Paediatr Scand. 1978; 67: 11–16. [DOI] [PubMed] [Google Scholar]
- 20.Lui JC, Baron J. Mechanisms limiting body growth in mammals. Endocr Rev. 2011; 32: 422–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hafen E, Stocker H. How are the sizes of cells, organs, and bodies controlled? PLoS Biol. 2003; 1: E86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penzo-Mendez AI, Stanger BZ. Organ-Size Regulation in Mammals. Cold Spring Harb Perspect Biol. 2015; 7: a019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dekaban AS. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978; 4: 345–356. [DOI] [PubMed] [Google Scholar]
- 24.Lui JC, Garrison P, Baron J. Regulation of body growth. Curr Opin Pediatr. 2015; 27: 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watt KI, Harvey KF, Gregorevic P. Regulation of Tissue Growth by the Mammalian Hippo Signaling Pathway. Front Physiol. 2017; 8: 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carnac G, Ricaud S, Vernus B, Bonnieu A. Myostatin: biology and clinical relevance. Mini Rev Med Chem. 2006; 6: 765–770. [DOI] [PubMed] [Google Scholar]
- 27.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006; 43: S45–53. [DOI] [PubMed] [Google Scholar]
- 28.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. J Hepatol. 2012; 57: 692–694. [DOI] [PubMed] [Google Scholar]
- 29.Alpert MA, Omran J, Bostick BP. Effects of Obesity on Cardiovascular Hemodynamics, Cardiac Morphology, and Ventricular Function. Curr Obes Rep. 2016; 5: 424–434. [DOI] [PubMed] [Google Scholar]
- 30.Ford LE. Heart size. Circ Res. 1976; 39: 297–303. [DOI] [PubMed] [Google Scholar]
- 31.Manetti P, Toncelli L, Vono MC, Capalbo A, Boddi V, Rostagno C et al. [The effects of training on skeletal and cardiac muscle mass in professional soccer players]. Ann Ital Med Int. 1999; 14: 166–171. [PubMed] [Google Scholar]
- 32.Frost HM. From Wolff’s law to the Utah paradigm: insights about bone physiology and its clinical applications. Anat Rec. 2001; 262: 398–419. [DOI] [PubMed] [Google Scholar]
- 33.Weibel ER. Symmorphosis : on form and function in shaping life, Harvard University Press: Cambridge, Mass, 2000. [Google Scholar]
- 34.Gallagher D, Albu J, He Q, Heshka S, Boxt L, Krasnow N et al. Small organs with a high metabolic rate explain lower resting energy expenditure in African American than in white adults. Am J Clin Nutr. 2006; 83: 1062–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heymsfield SB, Peterson CM, Thomas DM, Heo M, Schuna JM Jr. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes Rev. 2016; 17: 262–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ilardo MA, Moltke I, Korneliussen TS, Cheng J, Stern AJ, Racimo F et al. Physiological and Genetic Adaptations to Diving in Sea Nomads. Cell. 2018; 173: 569–580 e515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.