Abstract
Objectives:
Lobectomy has been compared with sublobar resection for the treatment of stage IA non-small cell lung cancer (NSCLC). Accurate long-term data are lacking on the risk of recurrence in routine clinical practice. This study utilizes a unique and representative dataset to compare recurrence, overall survival (OS) and lymph node staging between lobectomy and sublobar resection.
Methods:
The American College of Surgeons performed a Special Study of the National Cancer Data Base, reabstracting records to augment NSCLC data with enhanced information on preoperative comorbidity and cancer recurrence (2007–2012). For patients treated with lobectomy or sublobar resection (wedge/segmentectomy) for clinical stage IA NSCLC, propensity matching and competing risks models compared 5-year OS and risk of cancer recurrence. Secondary measures included lymph nodes collected, pathologic upstaging, and surgical margin status.
Results:
1687 stage IA patients were identified (1354 lobectomy and 333 sublobar resections). Propensity matching yielded 325 pairs. Lobectomy and sublobar resection groups had similar 5-year OS (61.8% vs. 55.6%, p=0.561). The sublobar group had a 39% increased risk of NSCLC recurrence (HR = 1.39, 95% CI 1.04–1.87). Median lymph node counts were higher for lobectomy patients [7 (3,10) vs. 1 (0,4) (P<0.001)].
Conclusions:
In an enhanced national dataset representative of outcomes for stage IA NSCLC, sublobar resection was associated with a 39% increased risk of cancer recurrence. The majority of patients treated with sublobar resection had an inadequate lymph node assessment. These real world results must be considered when existing clinical trial results comparing these treatments are extrapolated for clinical use.
Classifications: Database; lung cancer surgery; outcomes; statistics, survival analysis
Introduction
Anatomic lobectomy with mediastinal lymph node staging is the recommended standard of care for patients with early stage NSCLC [1]. This recommendation is from the 1995 randomized control trial performed by the Lung Cancer Study Group (LCSG), which focused on patients with early stage NSCLC receiving lobectomy or sublobar resection (segmentectomy or wedge resection). There was no significant difference in overall survival, but patients receiving lobectomy had lower rates of locoregional recurrence. Thus, lobectomy was recommended standard of care for patients with T1N0 tumors. However, sublobar resection is widely used in practice. Reviews of the National Cancer Data Base (NCDB) and Surveillance, Epidemiology, and End Results database estimate 17%−31% of patients have undergone sublobar resection [2,3]. Sublobar resection is often reserved for higher-risk patients [3–5].
Recent evidence has questioned the relevance of the LCSG study. With increased utilization of computed tomography scanning, tumors are being detected at smaller sizes [6,7]. Studies have shown similar survival outcomes between lobectomy and sublobar resection in patients whose tumors were 2.0 cm or less [8,9]. Conclusions regarding recurrence associated with resection type were mixed [10,11].
We performed a retrospective cohort study using supplemented data from the NCDB to compare lobectomy versus sublobar resection in patients with clinical stage IA NSCLC. Our primary outcomes included 5-year overall survival (OS) and disease recurrence. Our secondary outcomes included number of lymph nodes collected, frequency of pathologic upstaging, and surgical margin status. We hypothesized that disease recurrence rate and OS were similar between resection groups.
Patients and Methods
We performed a retrospective cohort study to compare OS and cancer recurrence among clinical stage IA NSCLC patients who underwent lobectomy and sublobar resection. Sublobar resection included patients who underwent wedge resection or segmentectomy.
NCDB and the Special Studies Mechanism
The NCDB is a joint program between the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The program includes 1,500 Commission on Cancer accredited US cancer programs. An estimated 70% of all newly diagnosed lung cancer cases in the US are captured by the NCDB, making it ideal for comparative effectiveness research [12,13].
To obtain the detailed level of information required for this study, we utilized a special study, which facilitated enhanced data collection of comorbidity and cancer recurrence information. The NCDB has this mechanism for the ad hoc collection of specific data to address important cancer problems. Up to 10 NSCLC patients from each accredited institution were randomly selected for further abstraction. Eligibility criteria for further data abstraction included (1) surgery for clinical stage I-III NSCLC (1/2006–12/2007) (2) alive 90 days post-surgery and (3) available medical records. Patients with unknown treatment or recurrence status were excluded without replacement. Patients were followed through 12/2012 or until first diagnosis of cancer recurrence, new primary cancer occurrence, or death.
Registry staff obtained complete information on patient comorbidity, tumor characteristics, treatment, and loco-regional recurrence information for five years following lung cancer resection. The data obtained were combined with existing NCDB data, de-identified, and transferred to our study team. This study was exempted form IRB review.
We evaluated individuals with clinical stage IA NSCLC, defined as tumors less than or equal to 3 cm in diameter with no evidence of nodal or distant metastases [9].
Descriptive Statistics
Descriptive statistics were expressed as mean ± standard deviation for variables that were approximately normally distributed, median (Q1, Q3) for highly skewed variables, and count (percent) for categorical variables. Continuous variables were compared using a 2-sample t-test or Wilcoxon rank sum test, as appropriate, while categorical data were assessed using chi square tests. All p-values were two-tailed, and values <0.05 were considered significant. Survival risks for both cohorts were modeled using multivariable Cox proportional hazards regression analysis, and covariate effects are presented as hazard ratios with 95% confidence ratios. Kaplan-Meier curves were constructed to provide OS survival estimates.
Propensity-score Matching and Competing Risks Analyses
We initially examined OS between lobectomy and sublobar resection groups in an unmatched comparison. Overall survival was defined as percentage of patients alive in each subgroup five years after resection, and was determined by examining last known vital status and time between resection and the last known follow-up date. To adjust for treatment selection bias, we matched patients in both groups for comparison using a propensity score matching technique [14,15]. Propensity scores were generated using a logistic regression model accounting for age, sex, race, zip-code, income quartile, urban/rural, academic/non-academic hospital, tumor histology and grade, and all comorbidities present in more than 5% of the cohort. Matched cohorts were carefully assessed for, and exhibited, good balance across all factors. Missing covariate data were handled with separate categorical variables.
Cumulative incidence of locoregional recurrence was compared across resection groups by time using a Fine and Gray competing risk analysis, which accounted for the competing risks of new primary cancer development or death without cancer recurrence [16]. Patients with positive surgical margins were excluded from competing risk analysis. All analyses were performed using R version 3.4 [17].
Results
We identified 1687 patients with clinical stage IA NSCLC from the Special Study that met inclusion criteria. A comparison between patients selected from the Special Study versus patients who were not included in the Special Study is provided in Supplementary Table 1. Of included patients, 1354 underwent lobectomy, 48 underwent segmentectomy, and 285 underwent wedge resection. Segmentectomy and wedge resection patients were combined to create the sublobar resection group. Approximately 91.3% of patients were followed for a minimum of 5 years. The remainder was censored at loss to follow-up.
Patients undergoing sublobar resection were older and more likely to have pre-existing comorbidities (Table 1). Patients who underwent lobectomy were more likely to have a greater number of lymph nodes sampled (6 vs. 1, p<0.001). This corresponded to more patients in the lobectomy group undergoing pathologic upstaging (p=0.002). Surgical margin positivity was significantly different between lobectomy and sublobar resection cohorts (2.5% vs. 6.6%, p=0.003). Information on new primary lung cancer development is shown in Supplemental Table 2.
Table 1:
Patient and tumor-related factors, all patients
| All Patients (n=1687) |
Lobectomy (n=1354) |
Sublobar Resection (n=333) |
P value | |
|---|---|---|---|---|
|
Patient-Related
Factors | ||||
| Age (year) | 66.9± 10.0 | 66.3± 10.0 | 69.6±9.6 | <0.001 |
| Male gender | 737 (43.7%) | 579 (42.8%) | 158 (47.4%) | 0.138 |
| Insured | 1622 (97.9%) | 1300 (97.8%) | 322 (98.5%) | 0.597 |
| Race | ||||
| Caucasian | 1527 (90.5%) | 1221 (90.2%) | 306 (91.9%) | 0.553 |
| African American | 108 (6.4%) | 91 (6.7%) | 17 (5.1%) | |
| Other | 52 (3.1%) | 42 (3.1%) | 10 (3.0%) | |
| Comorbidity | ||||
| COPD | 731 (43.7%) | 538 (39.7%) | 193 (58.0%) | <0.001 |
| Congestive Heart Failure (CHF) | 731 (43.3%) | 89 (6.6%) | 28 (8.4%) | 0.289 |
| Coronary Artery Disease (CAD) | 117 (6.9%) | 279 (20.6%) | 89 (26.7%) | 0.019 |
| Diabetes | 368 (21.8%) | 213 (15.7%) | 59 (17.7%) | 0.424 |
| Peripheral Vascular Disease | 155 (9.2%) | 122 (9.0%) | 33 (9.9%) | 0.687 |
| Psychiatric History | 127 (7.5%) | 105 (7.8%) | 22 (6.6%) | 0.552 |
| Substance Abuse | 92 (5.5%) | 74 (5.5%) | 18 (5.4%) | 1.000 |
| Chemotherapy | 241 (14.7%) | 206 (15.6%) | 35 (10.9%) | 0.038 |
| Radiation | 92 (5.6%) | 62 (4.7%) | 30 (9.3%) | 0.002 |
|
Tumor-related
Factors | ||||
| Tumor Size, mm (IQR) | 20 (15, 25) | 20 (15,25) | 17 (14,22) | 0.001 |
| Number of lymph nodes sampled (IQR) | 5 (2, 9) | 6 (4,10) | 1 (0,4) | <0.001 |
| Pathologic Stage | ||||
| Stage 1 | 1516 (89.9%) | 1199 (88.6%) | 317 (95.2%) | 0.002 |
| Stage 2 | 105 (6.2%) | 96 (7.1%) | 9 (2.7%) | |
| Stage 3 | 66 (3.9%) | 59 (4.4%) | 7 (2.1%) | |
| Histology | ||||
| Adenocarcinoma | 1047 (62.1%) | 844 (62.3%) | 203 (61.0%) | 0.706 |
| Squamous | 452 (26.8%) | 357 (26.4%) | 95 (28.5%) | |
| Other | 188 (11.1%) | 153 (11.3%) | 35 (10.5%) | |
| Positive Surgical Margins | 56 (3.3%) | 34 (2.5%) | 22 (6.6%) | 0.003 |
Propensity Score Matching and Survival Analysis
Propensity score matching yielded 325 pairs. Differences in age and comorbidity in unmatched patients were no longer observed (Table 2). However, lobectomy patients still had a greater number of lymph nodes sampled (7 nodes vs. 1 node, p<0.001) and a lower rate of positive margins (2.5% vs. 6.8%, p=0.013). Of those with positive margins, 2 lobectomy (25%) and 11 sublobar resection (55%) patients underwent post-operative chemotherapy or radiation (Supplemental Table 3).
Table 2:
Patient and Tumor-related Factors, propensity matched patients
| Lobectomy (n=325) |
Sublobar Resection (n=325) |
P value | |
|---|---|---|---|
|
Patient-Related
Factors | |||
| Age (year) | 69.4± 9.6 | 69.3±9.6 | 0.877 |
| Male gender | 154 (47.4%) | 153 (47.1%) | 1.000 |
| Insured | 315 (98.1%) | 314 (98.4%) | 1.000 |
| Race | |||
| Caucasian | 297 (91.4%) | 298 (91.7%) | 0.985 |
| African American | 18 (5.5%) | 17 (5.2%) | |
| Other | 10 (3.1%) | 101 (3.1%) | |
| Comorbidity | |||
| COPD | 176 (54.2%) | 185 (56.9%) | 0.528 |
| Congestive Heart Failure (CHF) | 36 (11.1%) | 28 (8.6%) | 0.357 |
| Coronary Artery Disease (CAD) | 93 (28.6%) | 84 (25.8%) | 0.481 |
| Diabetes | 51 (15.7%) | 55 (16.9%) | 0.750 |
| Peripheral Vascular Disease | 34 (10.5%) | 30 (9.2%) | 0.693 |
| Psychiatric History | 24 (7.4%) | 21 (6.5%) | 0.757 |
| Substance Abuse | 16 (4.9%) | 17 (5.2%) | 1.000 |
| Chemotherapy | 35 (11.2%) | 35 (11.1%) | 1.000 |
| Radiation | 10 (3.2%) | 30 (9.6%) | 0.002 |
|
Tumor-Related
Factors | |||
| Tumor Size, mm (IQR) | 18 (13, 22) | 17 (14, 22) | 0.970 |
| Number of lymph nodes sampled (IQR) | 7 (3,10) | 1 (0,4) | <0.001 |
| Pathologic Stage | |||
| Stage 1 | 298 (91.7%) | 309 (95.1%) | 0.203 |
| Stage 2 | 17 (5.2%) | 9 (2.8%) | |
| Stage 3 | 10 (3.1%) | 7 (2.2%) | 0.203 |
| Histology | |||
| Adenocarcinoma | 199 (61.2%) | 198 (60.9%) | 0.861 |
| Squamous | 96 (29.5%) | 93 (28.6%) | |
| Other | 30 (9.2%) | 34 (10.5%) | |
| Positive Surgical Margins | 8 (2.5%) | 22 (6.8%) | 0.013 |
Five-year survival for the overall cohort was 64.6% (95% CI: 62.3%−66.9%). Only age, race, histologic grade, radiation therapy, and Diabetes Mellitus were found to be independent risk factors for OS (Table 3). Sublobar resection was not associated with OS (OR 1.18, 0.95–1.47, p=0.147). Kaplan-Meier survival curves modeling OS were performed for both unmatched and propensity-matched patients (Figures 1 and 2). On propensity-matched analysis, lobectomy and sublobar resection cohorts demonstrated similar OS probability (61.7% vs. 55.6%, p= 0.561).
Table 3:
Cox Proportional Hazard Model of 5-year overall survival, propensity- matched patients
| Covariate | Hazard Ratio | P Value |
|---|---|---|
| Age (decile) | 1.34 (1.17–1.57) | <0.001 |
| Gender | ||
| Male | -- | 0.122 |
| Female | 0.84 (0.67–1.05) | |
| Race | ||
| Caucasian | -- | 0.027 |
| African American | 0.44 (0.22–0.84) | |
| Other | 1.29 (0.78–2.13) | |
| Comorbidity | ||
| Cerebrovascular Disease | 1.26 (0.68–2.34) | 0.485 |
| COPD | 1.22 (0.97–1.53) | 0.090 |
| Congestive Heart Failure | 1.26 (0.89–1.80) | 0.206 |
| Coronary Artery Disease | 0.89 (0.68–1.17) | 0.355 |
| Diabetes | 1.60 (1.18–2.15) | 0.003 |
| Peripheral Vascular Disease | 1.00 (0.68–1.46) | 0.996 |
| Psychiatric History | 1.48 (1.01–2.17) | 0.073 |
| Substance Abuse | 0.92 (0.58–1.45) | 0.727 |
| Tumor size (mm) | 1.01 (0.99–1.02) | 0.544 |
| Positive margin | 1.04 (0.60–1.81) | 0.884 |
| Chemotherapy | 1.06 (0.73–1.53) | 0.782 |
| Radiation | 1.62 (0.96–2.65) | 0.037 |
| Pathologic Stage | ||
| Stage 1 | -- | 0.379 |
| Stage 2 | 1.44 (0.84–2.48) | |
| Stage 3 | 1.07 (0.47–2.41) | |
| Histology | ||
| Adenocarcinoma | -- | 0.175 |
| Squamous | 0.83 (0.63–1.09) | |
| Other | 0.72 (0.46–1.14) | |
| Histologic Grade | ||
| Well differentiated | -- | 0.025 |
| Moderately differentiated | 1.56 (1.13–2.16) | |
| Poorly Differentiated | 1.67 (1.19–2.34) | |
| Unknown | 1.26 (0.67–2.38) | |
| Resection type | ||
| Lobectomy | -- | 0.134 |
| Sublobar resection | 1.18 (0.95–1.47) | |
Figure 1.
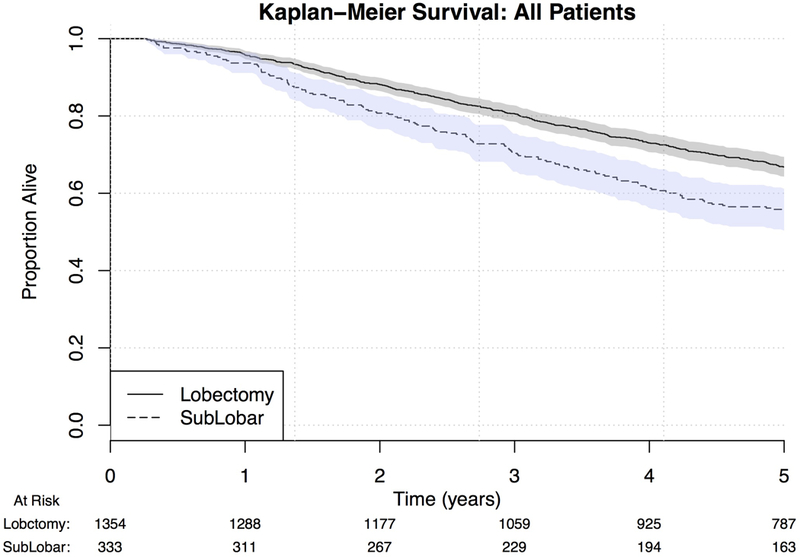
Kaplan-Meier curve for OS by extent of surgical resection, unmatched patients
Figure 2.
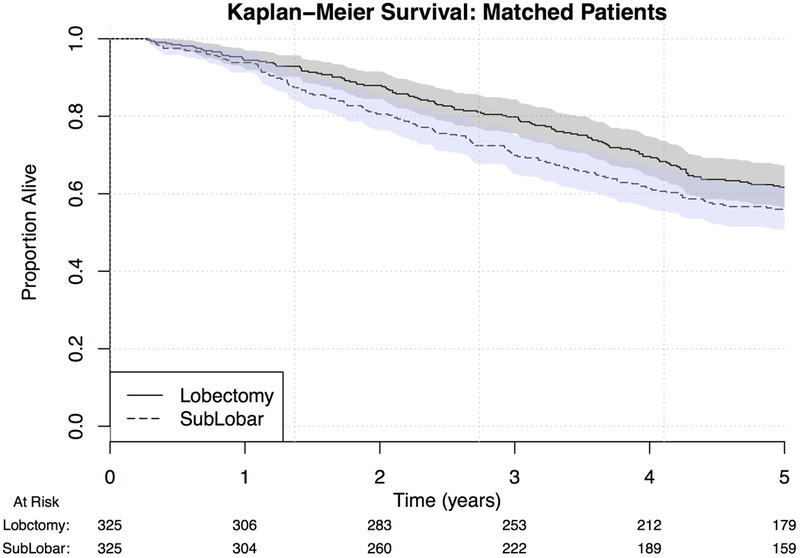
Kaplan-Meier curve for OS by extent of surgical resection, propensity score matched patients
Competing Risks Analysis and Disease Recurrence
Sublobar resection was associated with a significantly increased risk of recurrence (1.39, 1.04–1.87, p=0.026). The sublobar group had a shorter median time to recurrence (17.7 months, IQR 8.6–29.1) compared to lobectomy (21.0 months, IQR 12.1–24.7). Cumulative predicted recurrence over five years from surgical resection is demonstrated for a theoretical patient in Figure 3.
Figure 3.
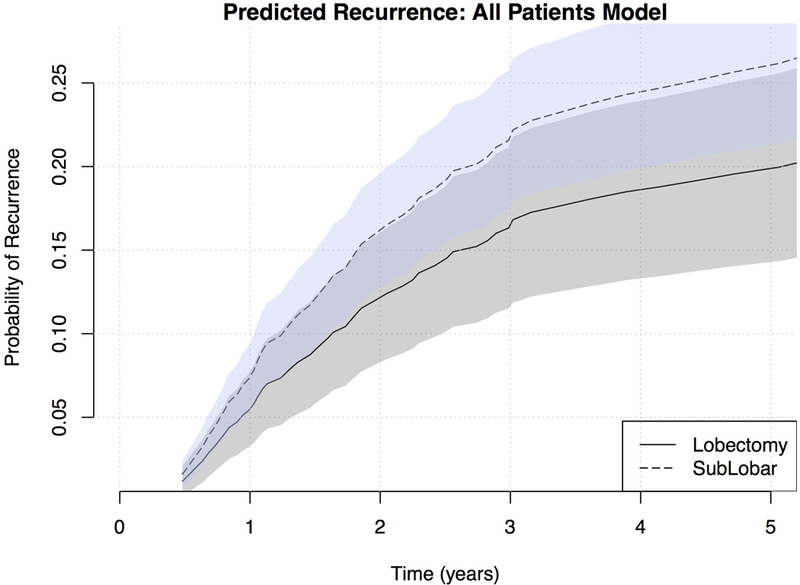
Risk of recurrence estimated for a 67 year old female patient with pathologic stage 1A NSCLC adenocarcinoma, by resection type
Comments
The 1995 LCSG trial represents the only randomized trial to date to examine outcomes of lobectomy and sublobar resection for T1N0 NSCLC [1]. There were criticisms that challenged its applicability to current practice. The LCSG enrolled patients with tumors ≤3 cm. Subsequent smaller studies have demonstrated higher survival rates in patients focused on patients with tumors ≤ 2 cm [9,10].
We hypothesized that survival and recurrence were similar among clinical stage IA patients who underwent either resection type. Similar to the LCSG trial, we observed similar OS but differing recurrence rates between cohorts. Propensity-matched analysis showed similar OS probability in patients undergoing lobectomy and sublobar resection (61.8% vs. 55.6%, p=0.561). However, when examining disease recurrence, our findings fail to support our original hypothesis. Sublobar resection was associated with a 39% increased risk of recurrence (p=0.026).
Our findings parallel the results of the LCSG, with specific applicability to the clinical stage IA population. The LCSG recommended that sublobar resection should be reserved for high risk stage I NSCLC patients. Defining “high-risk” is still a subject for debate. Preoperative forced expiratory volume in one second (FEV1) and diffusing capacity of the lung for carbon monoxide (DLCO) have often been combined with other variables (i.e. age, frailty, comorbidities) to predict postoperative morbidity and mortality [18]. The American College of Surgeons Oncology Group (ACOSOG) performed multiple large prospective trials focusing on outcomes of “high-risk” NSCLC patients exposed to various treatments [19,20]. Classification as “high risk” was contingent on FEV1 and DLCO ≤50% predicted. Puri et al. assessed whether there were differences in short-term morbidity and mortality in patients characterized as “high-risk” and “normal-risk” by ACOSOG trial enrollment criteria [18]. On retrospective review of ACOSOG trials z4032 (sublobar resection with or without brachytherapy for cT1N0M0 NSCLC) and z4099 (sublobar resection vs. stereotactic body radiation therapy for cT1N0M0 NSCLC), they found that there was not strict adherence to treatment allocation based on risk categorization. Approximately 60% of “high-risk” patients underwent lobectomy. In patients who received lobectomy, major morbidity and hospital mortality were identical between “high-risk” and “normal-risk” patients. ACOSOG “high-risk” status was not associated with major perioperative morbidity in patients undergoing sublobar resection or lobectomy. Puri et al. demonstrated that many patients deemed “high-risk” can safely undergo lobectomy, and challenged the traditional notion of allocation of sublobar resection based on pre-operative pulmonary function tests. It may be too simplistic to recommend sublobar resection’s use based on pre-operative risk. Treatment allocation to sublobar resection is much more nuanced.
Examination of our secondary outcomes (margin status, lymph node collection, and pathologic upstaging) provides insight into how surgical resection strategies are being performed in routine practice. There are currently no guidelines on adequate surgical margin distance in sublobar resection. El-Sherif et al. performed a single institution study examining margin status in wedge resection [21]. They found that wedge resection was frequently associated with margins less than 1 cm, and this distance was associated with increased local recurrence. Mohiuddin et al. examined 479 patients with cT1AN0M0 NSCLC to see the effect of varying margin distance on local recurrence risk [22]. The authors demonstrated a decreasing risk of local recurrence (p=0.033) with increasing margin distance, with a maximal benefit achieved at 15 mm. Our study did not have access to data on margin distance. However, we found that rates of positive margins were almost tripled in patients receiving sublobar resection (6.8% vs. 2.5%, p=0.013). A sizable amount of the clinical stage IA NSCLC patient population may be receiving incomplete resection, which is a major risk factor for locoregional disease [23].
We also observed notable differences in the number of lymph nodes examined by lobectomy and sublobar resection (7 vs. 1, p<0.001). This suggests that patients undergoing sublobar resection are not receiving adequate pathologic staging. Previous studies have examined lymph node sampling by resection type and its association with survival. Khullar et al. performed an analysis of the NCDB examining patients with clinical stage IA NSCLC who underwent lobectomy, wedge resection, and segmentectomy [24]. They found a significant difference in lymph nodes sampled. Dividing lymph nodes examined into two groups (0–3 nodes and >3 nodes), Khullar et al. observed that 78.5% of lobectomy patients had > 3 lymph nodes sampled compared to only 23.6% of wedge resection patients and 46% of segmentectomy patients. Khullar et al. further compared overall survival by resection type stratified by number of lymph nodes examined (0–7 vs. 8+). Even when adequate numbers of lymph nodes were examined in both cohorts, lobectomy was still associated with improved survival.
This study has some important limitations. First are the inherent biases associated with retrospective studies, especially treatment allocation bias. We utilized propensity matching to adjust for this treatment bias. Our propensity-matched cohorts were relatively small, yielding only 325 well-matched pairs. Still, there was a higher rate of positive margins in sublobar resection patients. We adjusted for this in our survival analysis and excluded positive margin patients from recurrence analysis. Additionally, the majority of sublobar resection patients were treated with wedge resection. Due to the small sample size of segmentectomies in the data (n=48), we did not analyze this procedure separately. Finally, our analysis is limited to the data captured by the NCDB. The NCDB does not include data on surgeon specialty, patient functional status, or pre-operative staging procedures or imaging. We could not provide information on surgical approach given that this data was only available after 2010.
This study also had several strengths to be noted. The special studies mechanism provided complete five year follow up information, which allowed us to accurately describe OS and recurrence patterns. As the NCDB accounts for nearly 70% of new cases of NSCLC, our findings are likely to be representative of the general population of early stage NSCLC [13]. Additionally, we captured ongoing trends in real clinical practice that should be considered when choosing resection strategies. The LCSG trial required that all patients undergo thorough intra-operative mediastinal lymph node sampling prior to randomization. Our study found that patients undergoing sublobar resection frequently did not undergo adequate lymphadenectomy. There is an ongoing phase III randomized non-inferiority trial (Cancer and Leukemia Group B 140504) that examines efficacy associated with lobectomy and sublobar resection types in patients with NSCLC ≤ 2 cm [25]. This trial will also implement the same strict inclusion criteria as the LCSG, and thus may not be generalizable to what happens in routine clinical practice. This point will be of critical importance when disseminating and implementing practice changes based on their results. The present study, using retrospective but broadly generalizable data, found similar 5-year survival between the resection groups for stage IA NSCLC but an increased risk of recurrence with sublobar resection.
Supplementary Material
Table 4:
Competing risks model for time to lung cancer recurrence
| HR (95% CI) | P-value | |
|---|---|---|
| Age (per decade) | 1.01 (1.00, 1.02) | 0.230 |
| Sex: Male | (reference) | |
| Female | 0.86 (0.68, 1.10) | 0.231 |
| Surgery: Lobectomy | (reference) | |
| Sublobar Resection | 1.39 (1.04, 1.87) | 0.026 |
| Pathologic Stage 1 | (reference) | |
| Stage 2 | 1.54 (1.03, 2.30) | 0.035 |
| Stage 3 | 1.70 (1.07, 2.72) | 0.026 |
| Histology: Adenocarcinoma | (reference) | |
| Squamous | 0.66 (0.49, 0.89) | 0.007 |
| Other histology | 0.97 (0.67, 1.41) | 0.876 |
Patients with positive surgical margins were excluded for competing risks analysis
Acknowledgments and Disclosures
Funding support was provided by the Patient Centered Outcomes Research Institute (R-APD-1306–00727). Dr. Benjamin Kozower is the grant recipient.
Footnotes
Meeting Presentation: Oral presentation at the 64th Annual Meeting of the Southern Thoracic Surgical Association; San Antonio, Texas; November 8–11, 2017
References
- [1].Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 No non-small cell lung cancer. Lung Cancer Study group. Ann Thorac Surg 1995; 60:615–622.. [DOI] [PubMed] [Google Scholar]
- [2].Speicher PJ, Gu L, Gulack BC, et al. Sublobar Resection for clinical stage IA non-small cell lung cancer in the United States. Clin Lung Cancer 2016; 17:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McMurry TL, Shah PJ, Samson P, Robinson CG, Kozower BD. Treatment of stage I non-small cell lung cancer: What’s trending? J Thorac Cardiovasc Surg 2017; 154:1080–1087.. [DOI] [PubMed] [Google Scholar]
- [4].Harada H, Okada M, Sakamoto T, Matsuoka H, Tsubota N. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005; 80:2041–5. [DOI] [PubMed] [Google Scholar]
- [5].Fernandez FG, Kosinski AS, Burfeind W, Park B, DeCamp MM, et al. STS lung cancer resection risk model: higher quality data and superior outcomes. Ann Thorac Surg 2016; 102: 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumors. J Thorac Oncol 2007; 2:706–714.. [DOI] [PubMed] [Google Scholar]
- [7].Okada M, Nishio W Sakamoto T, et al. Evolution of surgical outcomes for nonsmall cell lung cancer: time trends in 1465 consecutive patients undergoing complete resection. Ann Thorac Surg 2004; 77: 1926–1930. [DOI] [PubMed] [Google Scholar]
- [8].MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology 2017; 284:228–243. [DOI] [PubMed] [Google Scholar]
- [9].Gajra A, Newman N, Gamble GP, Abraham NZ, Kohman LJ, Graziano SL. Impact of tumor size on survival in stage IA non-small cell lung cancer: a case for subdividing stage IA disease. Lung Cancer 2003; 42: 51–57. [DOI] [PubMed] [Google Scholar]
- [10].Port JL, Kent MS, Korst RJ, Libby D, Pasmantier M, Altorki NK. Tumor size predicts survival within stage IA non-small cell lung cancer. Chest 2003; 124:1828–1833. [DOI] [PubMed] [Google Scholar]
- [11].Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity matched analysis. J Clin Oncol 2014; 32:2449–2455.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Koike T, Yoshiya K, Tsuchida M, Toyabe S. Risk factor analysis of locoregional recurrence after sublobar resection in patients with clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2013; 146:372–378. [DOI] [PubMed] [Google Scholar]
- [13].National Cancer Data Base. 2017. (Accessed August 2, 2017, at https://www.facs.org/quality-programs/cancer/ncdb).
- [14].Mcmurry TL, Hut Y, Blackstone EH, Kozower BD. Propensity scores: Methods, considerations, and applications in the Journal of Thoracic and Cardiovascular Surgery. J Thorac Cardiovasc Surg 2015; 150:14.19. [DOI] [PubMed] [Google Scholar]
- [15].Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm 2011; 10:150–161.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fine Jason P., and Gray Robert J.. “A proportional hazards model for the subdistribution of a competing risk.” Journal of the American statistical association 94.446 (1999): 496–509. [Google Scholar]
- [17].R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- [18].Puri V, Crabtree TD, Bell JM, Kreisel D, Krupnick A, et al. National Cooperative Group Trials of “High-Risk” patients with lung cancer: are they truly “high-risk”? Ann Thorac Surg 2014; 97:1678–1685.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fernando HC, Landreneau RJ, Mandrekar SJ, Nichols FC, Hillman SL, et al. Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small cell lung cancer. J Clin Oncol 2014; 32:2456–2462.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fernando HC, Timmerman R. American College of Surgeons Oncology Group Z4099/Radiation Therapy Oncology Group 1021: a randomized study of sublobar resection compared with stereotactic body radiotherapy for high-risk stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2012; 144:S35–S38.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].El-Sherif A, Fernando HC, Santos R, Pettiford B, Luketich JD, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol 2007; 14:2400–2405.. [DOI] [PubMed] [Google Scholar]
- [22].Mohiuddin K, Haneuse S, Sofer T, Gill R, Jaklitsch MT, et al. Relationship between margin distance and local recurrence among patients undergoing wedge resection for small (≤2 cm) non-small cell lung cancer. J Thorac Cardiovasc Surg 2014; 147:1169–1177.. [DOI] [PubMed] [Google Scholar]
- [23].Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the Lung Cancer Study Group. J Thorac Oncol 2010; 5:1583–1593.. [DOI] [PubMed] [Google Scholar]
- [24].Khullar OV, Liu Y, Gillespie T,, Higgins KA, Ramalingam S, Lipscomb, Fernandez FG. Survival after sublobar resection versus lobectomy for clinical stage IA lung cancer: an analysis from the National Cancer Data Base. J Thorac Onc 2015; 10:1625–1633.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fox N, Bauer T. CALGB 140503: A randomized phase III trial of lobectomy versus sublobar resection for small (< 2 cm) peripheral non-small cell lung cancer. Oncology Issues 2017;23: 20–21.. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


