Abstract
Effective delivery still remains a major hurdle in the development of gene based therapies. While technological advances have occurred that have improved delivery in general, there is still a need for controlled delivery in order to achieve therapeutic effects. Gene electrotransfer (GET) can be utilized to accomplish this. Careful selection of parameters used for delivery such as amplitude, duration and number of pulses as well as plasmid construct can be manipulated in order to achieve appropriate levels of local expression. Previously we have shown that direct delivery of the therapeutic cytokine, interleukin 12 (IL-12), to tumors using electrotransfer can generate local and systemic anti-tumor effects in pre-clinical and clinical studies. Using this model we hypothesized that modulating local gene expression using GET can affect therapeutic outcome. To test this, we used multiple GET protocols and plasmids to achieve varying levels of local IL-12 expression. We found that high local gene expression did not give rise to a better therapeutic outcome. This suggests the level and possibly the duration of gene expression are important in mediating the host immune response against melanoma. These data also emphasize the importance of considering the desired immune outcome of the therapy when selecting parameters for GET.
Keywords: Cytokine, electrotransfer, gene therapy, immunotherapy, interleukin-12, melanoma

INTRODUCTION
Gene electrotransfer (GET), also called electroporation, is a powerful and reliable physical method of transferring DNA into tissues by applying external local electric pulses. Since electrotransfer was first described in 1982 [1], it has been shown to be an efficient and reproducible tool for gene therapy providing a safer alternative to other methods used such as viral vectors [2, 3]. When using GET, it is important to achieve the appropriate balance between transgene expression and tissue damage in order to stimulate the host immune response to reach the clinically desired outcome [4]. Parameters such as pulse duration, number, magnitude, direction, frequency and applicator are important to the overall effectiveness of gene transfer and directly impacts expression [4–6]. The selection of pulse applicator is also an important consideration. Parallel plate electrodes and needle arrays are the most commonly used applicators for GET [7, 8]. Careful selection of all parameters allows for controlled delivery of the plasmid in order to achieve the appropriate expression. This is particularly critical for immunotherapy approaches as successful outcome depends on the delicate balance between immunostimulation and immunosuppression.
Interleukin 12 (IL-12) is a potent proinflammatory cytokine, produced by antigen presenting cells, that stimulates proliferation of T cells and NK cells [9] and acts as an important mediator and regulator of innate and adaptive immunity [10, 11]. Importantly, IL-12 promotes the production of potent anti-tumor mediators including interferon gamma (IFN-γ) and has anti-angiogenic and anti-metastatic properties that have been described in the literature [12–15]. The therapeutic effects of IL-12 appear however to be limited by tumor type, delivered dose and route of injection. High levels of IL-12 induce tumor immunity by different cell populations and mediators than low levels of IL-12 [15].
Metastatic melanoma is a leading cause of death and is generally treated using systemic therapy, adoptive cell transfer or chemotherapy [16–18]. Daud et al. reported the first-in-human clinical trial using GET. In this study, a plasmid encoding IL-12 was delivered with GET to patients with metastatic melanoma [19]. Ten percent of patients in the trial that received IL-12 GET showed complete regression of all metastases (both treated and untreated lesions) with no other systemic therapy administered. An additional forty-two percent of the treated patients showed disease stabilization or partial response. These findings suggest the generation of a systemic and possibly specific immune response to melanoma antigens as only a small number (2–4) of the total number of lesions on the patient were treated. IL-12 GET is currently being examined in phase II clinical trials to treat patients with chronic T-cell lymphoma, Merkel cell carcinoma and melanoma. Preclinical studies using GET to treat established melanoma resulted in tumor regression, long term survival and resistance to challenge [20–23].
Altogether, previous clinical and preclinical studies using a murine melanoma model suggest the dose of cytokine administered is crucial to positive clinical outcome and that local delivery to the tumor results in systemic gene expression and therapeutic effects. The amount of cytokine produced can influence not only the cell types that mediate the anti-tumor immune response but their activation and function as well. These studies indicate that it is local rather than systemic expression of delivered cytokines that drives the anti-tumor and anti-metastatic effects. It remains unclear from these studies however, the appropriate “dose” of therapeutic cytokine required for optimal clinical outcome. In this study we hypothesize that modulating the level of local gene (IL-12) expression using GET can have a direct impact on therapeutic outcome.
MATERIALS AND METHODS
Study Design
In this experimental study, we sought to modulate the level of expression of the therapeutic cytokine IL-12 within the tumor in order to determine the effects on the overall therapeutic outcome. Tumor-bearing mice were randomly allocated into either treatment or control groups. The average tumor volume among groups was similar. For gene expression studies, a sample size of five animals per group was tested. Samples were collected at fixed time points after treatment. For the survival studies, a sample size of ten animals was used in a total of two replicates (of five animals each). Animals were excluded from the study if secondary untreated tumors developed. After treatment, the groups were assigned alphanumeric identifiers and the assays were conducted blinded to the intervention. The objective of this research is to determine the therapeutic effects that the level of local gene expression resulting from the modulation of pulse parameters used for gene electrotransfer have on immunotherapy of induced murine melanoma. The research subjects were 6 – 8 week old female C57BL/6J mice housed in compliance with the Guide to the care and Use of Laboratory Animals. The experiments were all designed as controlled experiments with gene expression, reduced tumor volume, overall survival and resistance to challenge as primary measures. Secondary measures would include immunological effects such as memory cell generation, cytokine production and local effects at the treatment site.
Plasmids, Cells, Mice and Tumor Generation
All plasmids used were obtained from commercial preparations by Aldevron, (Fargo, ND), had endotoxin levels <100EU/mg and were diluted in sterile physiological saline. The commercially available plasmids pUMVC3-mIL-12 and pUMVC3 were obtained from Aldevron (Fargo, ND) and the pAG250-mIL12 plasmid, which is optimized for IL-12 production was a kind gift from Dr. G. Pavlakis (NCI, Bethesda Maryland). Expression of IL-12 from the plasmids used is driven by CMV promotors. The optimized pAG250-mIL12 construct however, described by Jalah et al. [24], utilizes dual CMV promotors that drive each subunit separately. The empty vector pUMVC3 was used as a control for all experiments.
B16.F10 melanoma cells were maintained in McCoy’s 5A media (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (Life Technologies, Grand Island, NY) and 1% Gentamycin at 37°C and 5% CO2 humidified air. Female C57BL/6J mice (6 – 8 weeks old) were Purchased from Jackson Laboratories (Bar Harbour, ME) and housed in compliance with the Guide to the care and Use of Laboratory Animals. 50 μl of B16.F10 melanoma cells (1 × 106 cells) were introduced by subcutaneous injection into the shaved left flank of the animal in order to establish the tumor. Tumors were allowed to grow to a diameter of 3 to 5 mm (about 5 days) before plasmid DNA was delivered. For challenge 5 × 105 B16.F10 cells were introduced subcutaneously in 50 μl of sterile PBS into the right shaved flank. All animals were monitored closely for tumor development and tumor volume was measured using digital calipers. Tumor volume was calculated by using the formula for the volume of an ellipsoid: v = πab2/6, where a is the long diameter and b is the short diameter [25]. Mice were humanely euthanized if the tumors grew to a volume that exceeded 1000 mm3.
DNA Delivery by Electrotransfer
50 μg of Plasmid DNA (1mg/ml) was injected directly into the tumor using a syringe with a 25 gauge needle. The electrode was then immediately placed around the tumor and pulses were applied using an ECM 830 Square Wave Pulse Generator (BTX Harvard Apparatus, Holliston, MA). The electrodes used were; a caliper applicator (C) consisting of 2 adjustable plates that contact the tumor or an applicator with a circular array of 6 penetrating electrodes (N) as described by Gilbert et al [8]. Briefly, the needle array is applied to the perimeter of the tumor without penetrating the tumor itself. Each needle in the applicator is placed at 60° intervals around a circle 1 cm in diameter and has an independent electrical connection. A mechanical switch (BTX Harvard Apparatus, Holliston, MA) is used to direct the pulses. Four of the six needles are energized for each pulse; two anodic and two cathodic, opposing each other. For each pulse, the four needle pattern is rotated one needle counterclockwise. The field is therefore rotated 360° upon the application of six pulses. The caliper applicator is coated with ultrasound gel before contact with the tumor to ensure proper delivery of the electric field without arcing. The GET protocols that were chosen; EP1 (six rotating 1300 V/cm, 100 μs pulses delivered using a circular configuration of 6 penetrating electrodes), EP2 (ten unidirectional 600 V/cm, 5ms pulses delivered using parallel plate caliper electrodes) and EP3 (one 667 V/cm, 100 ms pulse delivered using parallel plate caliper electrodes). For the survival studies, the mice received a series of three treatments on days 0, 4 and 7. For the expression studies the mice received a single treatment.
ELISA
Tumors and serum were collected from animals at various time points after a single treatment. The tumors were mechanically homogenized in cold phosphate buffered saline (Mediatech, Manassas, VA) containing protease inhibitor (Roche, Indianapolis, IN) using a tissue homogenizer (Omni International, Kennesaw, GA). The homogenate was centrifuged and the supernatant collected and assayed for IL-12 expression using a mouse IL-12 ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Immunohistochemistry
Tumors were excised from animals 24 hours after a single treatment, placed in a cryomold with O.T.C. Compound embedding medium (Electron Microscopy Sciences, Hatfield, PA) and snap frozen. 7 μm sections were cut using a HM525 cryostat (Thermo Fisher Scientific, Waltham, MA) and mounted on a positively charged glass slide. Sections were probed for CD11b or IL-12 using a standard immunohistochemistry protocol. Briefly, sections were fixed in a cold mix of acetone and ethanol (75/25 respectively), washed and blocked in 5% bovine serum albumin then using an avidin/biotin blocking kit (Vector Labs, Burlingame, CA). Sections were incubated with the anti-CD11b (eBioscience, San Diego, CA) or anti-IL12 (R&D Systems. Minneapolis, MN) antibody at 4 °C overnight. Sections were washed and incubated with biotinylated secondary antibody (eBioscience, San Diego, CA) at room temperature followed by an FITC conjugated streptavidin (eBioscience, San Diego, CA) for anti-CD11b staining and AP conjugated streptavidin (Life Technologies, Grand Island, NY) for anti-IL12 staining. IL-12 positive sections were visualized using AP Substrate Kit I (Vector labs, Burlingame, CA).
Western Blot
Tumor homogenate from samples collected at 24 hours were pooled by group and the proteins separated by SDS-PAGE. Proteins were transferred to nitrocellulose membrane that was probed with anti-perforin (eBioscience, San Diego, CA) using a standard western blotting protocol. Levels of perforin were then normalized to the level of β-actin present in the sample.
Single Cell Suspensions and Flow Cytometry
Fresh blood was collected from the animals by cardiac punch in EDTA coated tubes. Samples were diluted 1:1 in sterile PBS and the white blood cells separated by layering the blood onto Histopaque 1083 (Life Technologies). The white cell layer was carefully removed after centrifugation at room temperature for 30 minutes and washed with PBS. Any contaminating red blood cells were lysed using ACK lysis buffer. Cells were washed twice using sterile PBS and resuspended in stain buffer (BD Biosciences). Single cell suspensions were treated with Fc Block and stained with antibodies to CD3, CD4, CD8, CD62L, and CD45RB (BD Biosciences) for 30 minutes at 4 °C. Samples were acquired (at least 100,000 events) using a FACSAria cytometer with FACSDiva software. FlowJo software was used to analyze the data generated. Samples were gated based on forward and side scatter morphology, then assessed for expression of CD3 and CD4 or CD8. The CD3+CD4+ and CD3+CD8+ populations were then assessed for expression of memory/activation markers CD45RB and CD62L.
Statistical Analysis
Data in Fig. (1) was log transformed to achieve normal distribution. Multiple groups were compared using a Kruskal-Wallis Test with Dunn’s multiple comparison post-test in GraphPad InStat statistical software. For the data in Fig. (6) pair-wise comparisons were done using a Mann-Whitney test. Critical alpha for all comparisons was 0.05.
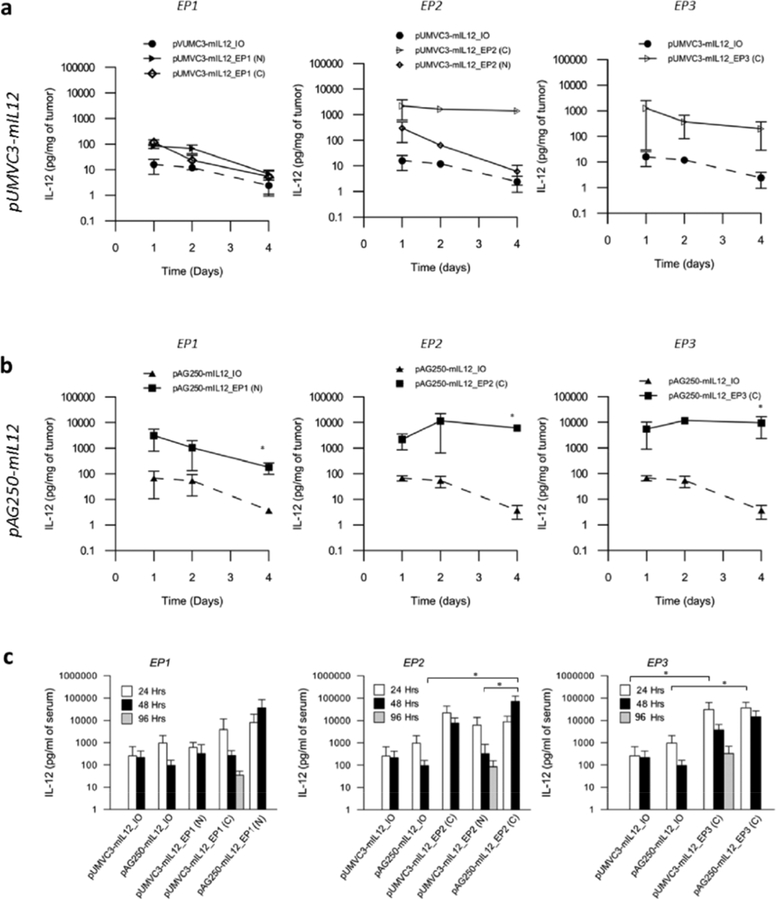
Fig. (1).
Expression of interleukin 12 after gene electrotransfer. (A) Gene expression measured by ELISA from tumor homogenate (a, b) and serum (c) 1, 2 and 4 days after a single delivery of pIL-12 using various electroporation protocols compared to the plasmid DNA injection only control. EP1, six 100 μs 1300 V/cm pulses; EP2, ten 5 ms 600 V/cm pulses and EP3, one 100ms 667 V/cm pulse. Needle (N) or caliper (C) applicators used to deliver the pulses as indicated. Data are expressed as pg/mg of tumor ± standard deviation. n = 5 in each group. Difference between groups found to be significant by Kruskal-Wallis Test (P < 0.01). *P < 0.05 between groups based on Dunn’s multiple comparison post-test.
Fig. (6).
Phenotypic analysis of circulating lymphocytes in post challenge mice. PBMCs were collected and purified from the blood of all mice in Figure 3 above that were challenged with B16.F10 melanoma cells. Cells from mice that were resistant to challenge and remained tumor-free (n = 20) or non-resistant to challenge that developed a secondary tumor (n = 35) were stained with antibodies for CD3, CD4, CD8, and memory markers CD62L and CD45RB. Dot plots (a) are representative of resistant and non-resistant mice. Each dot represents a cell gated on CD4+CD3+ or CD8+CD3+ parent populations. Cumulative data for the subpopulations are presented as mean percentage of the parent T cell population from resistant (■) and non-resistant (◻) mice (b). Error bars represent standard deviation. E, effector; N, naïve; CM, central memory; EM, effector memory. * P < 0.05, ** P < 0.01, *** P < 0.001.
RESULTS
To test the hypothesis that appropriate, not necessarily higher, gene expression affects therapeutic outcome, a two-pronged approach was used in our model of IL-12 immunotherapy of murine melanoma. The first approach was the delivery of pDNA expressing murine IL-12 using three electrotransfer protocols EP1, EP2 and EP3 that have been previously characterized and extensively used in the field to deliver plasmid in vivo [7, 20, 21, 26]. Our group has demonstrated the immunotherapeutic effects of delivering pIL-12 using EP1 in both pre-clinical and clinical studies [19–21]. The second approach is to compare the effect on gene expression of using two plasmids; pUMVC3-mIL12 and pAG250-mIL12 that express different levels of IL-12 in vivo when the same amount of plasmid is used. As a control, non-expressing pUMVC3 was also delivered using these protocols. No IL-12 expression was observed (data not shown).
Selection of Electrotransfer Protocol and Applicator Impacts Gene Expression
EP1 pulses (1300 V/cm, 100 μs, 6 rotating pulses, 1 s gap) are usually delivered using a penetrating needle array while EP2 pulses (600 V/cm, 5 ms, 10 unidirectional pulses) and EP3 pulses (667 V/cm, 100ms, 1 pulse) are delivered using parallel plate caliper electrodes. These delivery parameters were selected because they are widely used in gene therapy studies. EP2 and EP3 are known to generate high levels of expression, while EP1 pulses are known to generate relatively lower expression. In order to test whether the coupling of the applicator and electrotransfer protocol plays a role in the level of gene expression in this model, the applicators used to deliver EP1 and EP2 were switched so that EP1 was delivered using a caliper electrode and EP2 delivered using the 6 needle array. The level of expression within the tumor resulting from delivery of pUMVC3-mIL12 using these parameters was measured and compared (Fig. 1a). Injection only controls were included with each condition for reference.
Of the three protocols used, EP1 showed the lowest level of IL-12 expression over the time points observed using both applicators. Using this protocol resulted in levels of intratumoral IL-12 only slightly higher, but not significantly different than the injection only control. Switching applicators used to deliver the pulses did not have a significant effect on expression levels, and in both cases expression tapered off over time.
The EP2 protocol delivered using caliper electrodes generated high levels of IL-12 that were significantly higher than the injection only control as well as the EP1 pulse protocol and were sustained over time. Interestingly, the choice of applicator used to deliver this pulse protocol appeared to affect expression. Pulses delivered using the needle array generated lower expression levels than those delivered using the caliper electrode, and expression levels were similar to those generated using either applicator to deliver the EP1 protocol.
The EP3 protocol generated relatively high levels of IL-12 expression that was higher than the injection only control. The expression levels were however, not significantly different than those generated by the other conditions tested. Due to the visibly damaging nature of the EP3 pulses and the limitations of the pulser, this protocol was not applied using the needle array.
Delivery of an Optimized IL-12 Plasmid Results in Greater Gene Expression
In order to examine whether gene expression can be modulated by plasmid selection rather than pulse conditions, we selected an optimized IL-12 plasmid pAG250, which has been shown to generate higher expression than a wild type plasmid such as pUMVC3-mIL12 [24], and delivered it using the same pulse protocol/applicator combination for EP1, EP2 and EP3 (Fig. 1b). As predicted, pAG250 generated higher levels of IL-12 expression than pUMVC3-mIL12 using similar pulses protocols and applicators. Delivery of pAG250 resulted in higher IL-12 expression compared to delivery of pUMVC3-mIL12 when using the EP1 protocol. Interestingly, using the expression conditions that generate high expression, EP2 and EP3, to deliver this plasmid, though elevated, were not significant when compared to delivery of pUMVC3-mIL12. Delivery of pAG250 using all electrotransfer protocols showed higher expression than the injection only control for each protocol tested. Electrotransfer of the control plasmid pUMVC3 resulted in IL-12 levels that were below the limit of quantitation for the ELISA kit used (data not shown). Delivery of IL-12 plasmids only with no electrotransfer resulted in detectable levels of IL-12 protein. The optimized plasmid pAG250-mIL12 induced higher levels than pUMVC3-mIL12. In both cases however, expression generated from the injection of plasmid only were lower than when plasmids were delivered using the electrotransfer protocols described. Expression of the anti-tumor mediator IFN-γ was measured in these tumor homogenate samples as well. Low levels (< 25 pg/mg of tumor) were detected that were not significantly different from each other (data not shown).
Selection of Electrotransfer Protocol Impacts the Systemic Expression of IL-12 After Treatment
The level of IL-12 present in circulation after IL-12 GET was measured using ELISA (Fig. 1c). Low levels of IL-12 were present in the serum after injection of the plasmid with no pulses applied. Gene electrotransfer of both plasmids, depending on the protocol used, resulted in higher IL-12 expression. In general, the pattern of expression over time was similar to what was noted for tumor homogenate. Mice that received treatment using EP2 and EP3 showed elevated levels compared to that of EP1 and delivery of pAG250-mIL12 generated higher serum levels of IL-12 than delivery of pUMVC3-mIL12. The level of IL-12 generated, if any, generated from electrotransfer of the control plasmid pUMVC3 was below the lower detection limit of this assay (data not shown).
Modulation of GET has an Impact on the Tumor Environment
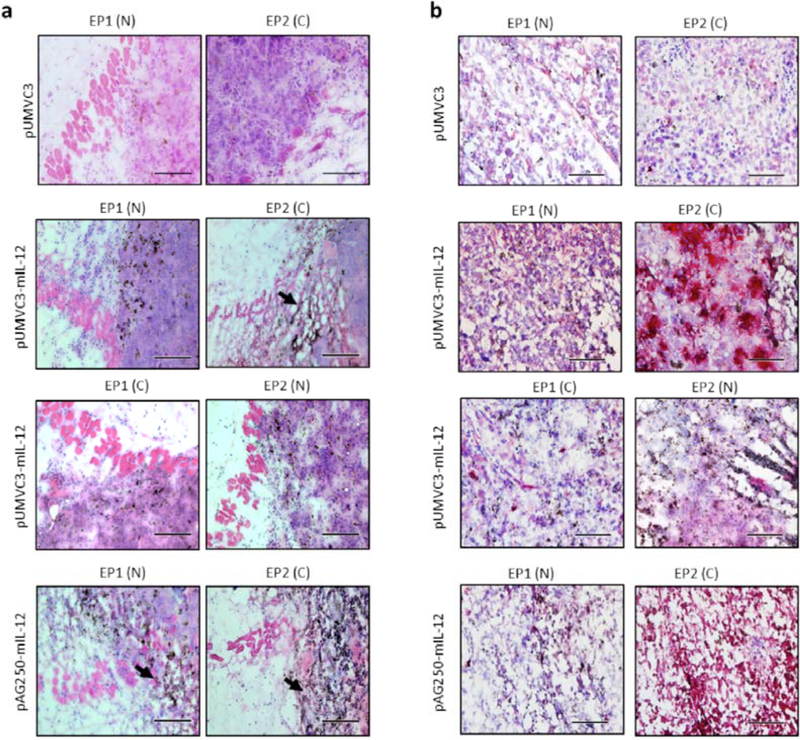
In order to examine the effects delivery of these plasmids using various protocols have on the tumor microenvironment, Tumors were collected 24 hours after a single treatment, and sectioned for histological analysis (Fig. 2). Histological examination of tumor sections collected after electrotransfer using the specified protocol and plasmid show different levels of lymphocytic infiltrate and tissue damage as well as pattern of IL-12 expression within the tumor. Hematoxylin and eosin staining (Fig. 2a) reveals tumors treated with pAG250 −mIL12 using EP2 (C) had the most damage evidenced by decreased nuclear staining (blue) and increased presence of pigment (brown/black). Similar damage was observed, but to a lesser extent when EP1 (N) was used to deliver pAG250-mIL12 and EP2 (C) was used to deliver pUMVC3-mIL12. Delivering pUMVC3-mIL-12 using any combination of electrotransfer protocol and applicator appears to induce less tissue damage than delivering pAG250-mIL12. Delivery of the vector control pUMVC3 using the described GET protocols did not result in visible damage.
Fig. (2).
Histology of tumors 24 hrs after IL-12 GET. Hematoxylin and eosin staining (a) reveal tumor morphology and damage as a result of GET. Images were taken towards the edge of the tumor. Immunohistological staining (b) of IL-12 (red) expressed in central region of the tumor after electrotransfer. Sections are counterstained with haematoxylin (blue). Images are best representative sections from n = 2 tumors. Scale bar represents (a) 100 μm and (b) 200 μm.
The sections were probed with anti-IL12 antibodies to examine the distribution of IL-12 within the tumor due to GET (Fig. 2b). There appears to be a distinct difference in the pattern of gene expression that is protocol dependent. This is most notable in tumors that received GET of pUMVC3-mIL-12 as these tumors showed less damage at this timepoint. GET of pUMVC3-mIL12 using EP2 resulted in punctate regions of IL-12 expression (red) within the tumor, but delivery of the same plasmid using EP1 appeared to give a more even distribution. Interestingly, when the electrode applicators were switched, that is EP2 delivered using needle applicator and EP1 delivered using the caliper applicator, this effect seemed to be lost. GET of pAG250-mIL12 with EP2 resulted in higher IL-12 expression than EP1, but due to the extensive amount of damage a pattern could not be discerned. Delivery of vector control pUMVC3 using the described get protocols did not show distinctive regions of IL-12 positive staining.
There appears to be more immune cell infiltrate in the tumors treated with pUMVC3-mIL12 using EP1 (N) than the other conditions (Fig. 2a). In order to more closely examine this, the sections were probed with anti-CD11b antibodies (Fig. 3a.). CD11b is a cell surface marker that is present on antigen presenting cells as well as a subset of NK cells and T lymphocytes. Fig. (3a) shows the best representative sections of the center of the treated tumors compared with injection of the IL-12 expressing plasmids only. While there was infiltrate in all sections, delivery of both plasmids using the conventional protocol-applicator combination showed an increased presence of CD11b positive cells within the tumor when compared to the mismatched protocol-applicator combinations and the injection only controls.
Fig. (3).
Effects of IL-12 GET on the tumor microenvironment. A functional consequence of intratumoral IL-12 expression is cytotoxic and natural killer cell-mediated tumor destruction mediated by perforin. Anti-CD11b staining (green) of tumor sections taken 24 hours after a single treatment (a) reveal the presence of immune cell infiltrate within the tumor. Cell nuclei are identified by DAPI (blue) staining. Images are the best representative sections from n = 2 tumors. Images captured with a 10 X objective lens. Scale bar represents 100 μm (b) Levels of perforin present in the pooled tumor samples at 24 hours after a single treatment were determined by western blot. Data represents the ratio of perforin relative to β-actin for each treatment group. n = 5 samples were pooled prior to loading on SDS-PAGE for each group.
Perforin, a pore-forming protein released by cytotoxic T lymphocytes and natural killer cells, is an important mediator of the anti-tumor response generated by IL-12 GET as it induces lysis of tumor cells. The level of perforin present in tumors was measured by western blot of tissue homogenate collected 24 hours after a single treatment and then normalized to the level of β-actin present (Fig. 3b). The levels of perforin present in the treated tumors correlate with the findings from the histology with regard to the presence of lymphocytic infiltrate into the tumor and the amount of tissue damage observed. Electrotransfer of pUMVC3-mIL-12 using the mismatched pairing of protocol and applicator which resulted in little lymphocytic infiltrate and no tissue damage showed low levels of perforin. Delivery of both plasmids using the conventional protocol/applicator pairing showed increased levels of perforin present. The highest levels observed were in the group treated with pAG250-mIL12 using EP2 which also showed the most tissue damage as a result of IL-12 GET. The amount of perforin present decreased when electrotransfer protocols were used to deliver pUMVC3-mIL12 with switched applicators, EP1 (C) and EP2 (N), compared to pUMVC3-mIL12 delivery with EP1 (N) and EP2 (C). Delivery of the empty vector pUMVC3 using the prescribed protocols and delivery of IL-12 plasmids without pulses showed low levels of perforin in the tumor 24 hours after treatment.
Selection of GET Protocol Impacts Tumor Regression and Long-Term Survival
Previous studies by our lab have shown the efficacy of GET using IL-12 for tumor regression and long term survival. To further test our hypothesis that the amount of local gene expression can influence therapeutic outcome we used the previously described delivery protocols. GET was administered as a series of three i.t. deliveries of pIL-12 (pUMVC3-mIL12 or pAG250-mIL12) on days 0, 4 and 7 using either EP1 or EP2. Tumor volume was monitored over the course of nine weeks after which the mice were characterized as either complete responders (CRs), partial responders (PRs) or non-responders (NRs) (Fig. 4). The CRs showed steady tumor regression and were tumor free at around week 3 or 4 and remained tumor free for the duration of the experiment. The PRs had tumors that started to regress after treatment but then progressed, and the NRs showed no tumor regression. The percentages of surviving mice over time are shown in Fig. (4b). At the end of the observation period the surviving mice were all tumor free.
Fig. (4).
Long term Survival of mice that received gene electrotransfer. Tumors (3 – 6 mm diameter) on mice received three deliveries of pDNA on days 0, 4 and 7. Tumor volume was monitored over the course of 9 weeks. (A) Response to treatment is depicted as percentage on the bar graph. Animals were characterized as either complete responders (CR), partial responders (PR), or non-responders (NR) based on tumor regression or progression. Mice with tumors that completely regressed and remained tumor free for the experiment duration are described as complete responders. Mice with tumors that showed initial regression but eventual progression were described as partial responders. Mice with tumors that showed no regression, only tumor volume progression were described as non-responders. n ≥ 9 for each group. (B) Percentage survival of these mice over time is depicted by step plots.
All animals receiving IL-12 GET had a higher percentage of animals that responded to treatment compared to controls. IL-12 plasmid only controls and empty vector GET controls showed partial and complete responses to treatment. The data was analyzed from two perspectives; the protocol/applicator selection and the resulting level of gene expression. From the response data the selection of protocol/applicator has some effect on tumor regression. When the caliper applicator was used to deliver pUMVC3-mIL12 using EP1 pulses complete tumor regression was only observed in 77% of animals compared to 100% of animals when the conventional 6 needle applicator was used to deliver the same plasmid using the same pulse protocol. The remaining animals did not respond to treatment. When using the EP2 protocol, the choice of applicator did not seem to matter as all animals in both groups were responsive to treatment and showed tumor regression.
From the perspective of IL-12 expression levels, there is an inverse relationship between increased level of gene expression and complete response in the animals. Delivery of pAG250-mIL12 using EP1 (N) resulted in higher expression of IL-12 than deliver of pUMVC3-mIL12, but did not induce complete regression in 100% of animals. All in the group had some response to the therapy but only 10% showed partial response. Similarly, using EP2 (C) to deliver pAG250 resulted in higher expression than delivery of pUMVC3-mIl12, and though all animals in the group were responsive to treatment, only 40% showed a complete response compared to 90% complete response using EP2 (C) to deliver pUMVC3-mIL12. Overall, the groups that expressed elevated levels of IL-12 showed a lower percentage of complete responders while groups that expressed low levels of IL-12 showed a higher percentage of complete responders.
High Transgene Expression Does not Protect Animals From Challenge
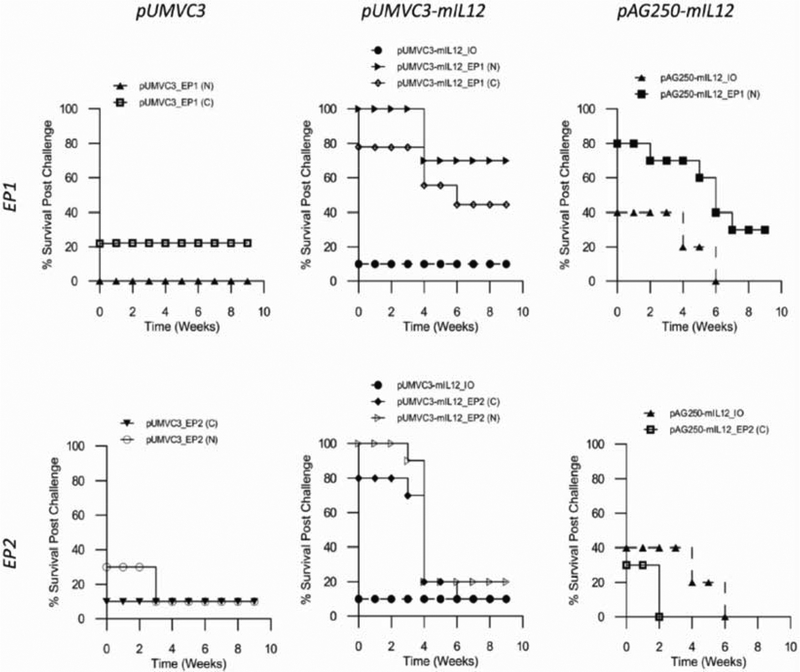
In order to test whether the tumor-free CRs were protected from new tumor growth, the mice were inoculated with fresh B16.F10 melanoma cells on the opposite flank. If the mice developed tumors they were considered not to be resistant to challenge. Mice that did not develop tumors were monitored for 50 days, after which if no tumors developed they were considered resistant to challenge.
High levels of IL-12 generated within the tumor by GET did not offer protection from challenge (Fig. 5). Groups expressing elevated levels of local IL-12 did not confer protection to more than 30% of the animals. Groups expressing low levels of IL-12 conferred protection in over 40% of the animals treated. pAG250 delivered using EP2 (C) showed the highest expression but did not confer resistance to any animals. pUMVC3-mIL12 delivered using EP1 (N) showed the lowest level of expression, just above background, and conferred resistance to 70% of the treated animals. Overall the protocol/applicator choice did not impact the outcome. When delivering pUMVC3-mIL12 however, the mismatch proved to be less effective than the conventional combination. GET of the vector control pUMVC3 and injection of plasmid IL-12 without pulses protected a small percentage of animals from challenge.
Fig. (5).
Survival of tumor-free mice after challenge with B16.F10 melanoma cells. Survival plots of mice that showed tumor regression and remained tumor free after electrotransfer and were challenged on the opposite flank with B16.F10 melanoma cells. Mice resistant to challenge remained tumor free for the 10 week period post challenge. Mice that developed tumors were termed non-resistant and developed tumors shortly after challenge. Tumor bearing mice either succumbed to disease or were euthanized after weeks 3 and 5 and samples collected for further analysis.
Phenotype of Circulating Lymphocytes Post Challenge is Unaffected by Level of Gene Expression
In order to further examine the effects of the selected EP protocols post challenge, the phenotype of circulating lymphocytes were analyzed using flow cytometry. Peripheral blood mononuclear cells (PMBCs) were collected from all resistant mice 50 days post challenge and from all non-resistant mice after a palpable tumor was detected. Fast growing tumors were collected about 3 weeks after challenge and the slower growing tumors were collected after 5 weeks (Fig. 6). Though not ideal, these data collection points provide insight into the cellular composition of the secondary immune response to tumor antigen. If the animals are able to mount an effective immune response to the presence of B16.F10 cells post treatment and regression of the primary tumor, it is most likely due to the presence and action of memory T cells.
CD4+ and CD8+ T cells are characterized by their expression of either marker in conjunction with CD3 expression. These double positive populations are further evaluated for memory T cell subsets using activation and memory markers CD45RB and CD62L. Cells that express low CD45RB are characterized as memory cells and those that express high CD45RB are thought to be non-memory or naive cells [27]. Naïve cells also express high levels of CD62L which are lost on encounter with antigen [9]. Ericsson et al suggest that CD8+CD45RB+ cells are responsible for anti-tumor cytotoxicity [28]. The sub-populations that were observed using this technique are delineated as follows: Effector memory cells (EM) are CD45RB−CD62L−, central memory cells (CM) are CD45RB−CD62L+, naïve cells (N) are CD45RB+CD62L+, and the activated effector cells (E) are CD45RB+CD62L−.
There was no significant difference in the percentages of the T cell populations present in animals that were treated using the various protocols, plasmids and applicators tested. The pattern that emerged from the data is that the animals can be grouped based on clinical outcome rather than on parameter selection. If the mice were resistant to challenge and remained tumor free for the experiment duration, their phenotypic profile was similar regardless of treatment condition or plasmid and significantly different from their non-resistant counterparts (Fig. 6). Of the circulating CD4+CD3+ cell population, there were higher pro portion of memory cells (both central and effector) than naïve and activated effector cells. The converse is true of the CD8+CD3+ cells which showed low memory cell and high effector and naïve cell populations. There were significantly higher circulating CD4+ effector cells (activated and memory) in the mice that were resistant to challenge compared with those that were nonresistant. These resistant mice also showed significantly lower proportion of naïve and central memory cells.
DISCUSSION
These experiments show that in this mouse model of induced melanoma, we are able to modulate the expression of IL-12 by changing the parameters used to deliver the electro-transfer pulses as well as by changing the plasmid used. Typically, the objective of GET and other gene transfer approaches has been to achieve a high level of expression in the tissue. The rationale was that the high expression in the target tissue should lead to a more successful outcome of the gene therapy. This report shows that this is not always the case and in some instances low short-term expression is needed for effective therapeutic outcome.
Of the electrotransfer protocols tested in this study, EP1 resulted in lower gene expression than EP2 and EP3. Though the EP3 protocol generated high levels of IL-12 expression comparable with that of EP2, application of these pulses resulted in visible damage to the animal and its use was discontinued for the remainder of the studies.
Parameter selection and resulting IL-12 expression does not appear to have a tremendous impact on the regression of primary tumors, as most treated groups achieved complete regression of treated tumors in at least 70% of the animals in the group. However, the group that expressed the highest level of IL-12 which received pAG250-mIL12 using EP2 (C), showed only a 40% survival rate after treatment and none of those animals were protected from developing secondary tumors after challenge. The other groups with high expression after treatment were only able to protect at most 33% from developing secondary tumors after challenge. From the data, we see that high local expression does not correspond with lasting immunity and a successful long-term clinical outcome, possibly due to inadequate generation of a memory response. There appears to be a limit to the level of gene expression that will result in both primary tumor regression and protection from challenge. This demonstrates the importance of being able to modulate gene expression in the target tissue and makes a case for the use of “appropriate” levels of expression based on desired clinical outcome and protein kinetics. This can be achieved by selection of the right combination of plasmid, electrotransfer protocol and applicator [19–21, 23].
The pattern of expression over time in the serum was similar to what was observed in the tumor homogenate. The EP conditions that resulted in higher levels of IL-12 expression caused larger amounts of the protein to enter circulation (Fig. 1c). This may only serve to enhance the immune response to the local treatment of the tumor. This may also play a role in the enhanced and most likely non-specific response mediated by the cellular infiltrate observed in Fig. (3).
We found that pulses delivered using EP1 were in general more successful than EP2 at generating lasting immunity and conferring protection from challenge in a larger number of animals (Fig. 5). Delivery using EP2 resulted in higher gene expression than EP1 (Fig. 1) but caused more tissue damage and elevated levels of perforin (Fig. 2, 3). Cantanella et al. reported that pulses delivered using lower voltage, longer pulse width and delivering a larger number of pulses results in greater plasmid uptake [4]. However, they suggest the uptake of a large amount of molecules is associated with a large loss of cell viability. A train of unidirectional pulses of the same amplitude and duration have been shown to cause more cell death than when similar bidirectional pulses are applied [29]. Conversely, changing the orientation of pulse delivery, as is the case with the needle applicator, has been shown to improve the efficacy of electrically-mediated therapy [8, 30, 31].
It is noteworthy to mention that animals in the control groups that received IL-12 plasmid but no pulses or those that received pulses and were treated with an empty vector expressing no IL-12 (pUMVC3), had a small percentage of animals that not only showed tumor regression after treatment but were protected from challenge. The levels of IL-12 generated in tumors injected with plasmid alone, with no pulses applied, were statistically similar to the levels generated when pUMVC3-mIL12 was delivered using EP1 and the needle electrode. The group that was treated with pAG250-mIL12 injection only showed long term survival in 40 % of the animals compared to only 10% of those treated with pUMVC3-mIL12 only. Upon challenge however, the pAG250-mIL12 only mice were not protected from secondary tumor formation. These mice expressed similar amounts of IL-12 as the group treated with pUMVC3-mIL12 using EP1 with needle electrodes. This observation indicates elevated levels of intratumoral IL-12 alone, while sufficient to induce tumor regression in almost half of the treated animals, is not sufficient to stimulate a robust enough systemic/memory response to confer protection.
In this study, delivery of the non-coding plasmid pUMVC3 using the electrotransfer protocols described can induce tumor regression, although at a much lower rate than with pUMVC3-IL-12 delivered with EP1, in some treated animals and protect them from challenge. Previous studies have shown that electrotransfer of empty vector plasmids that do not encode therapeutic genes can induce tumor regression and confer protection from challenge [23, 32–36]. The authors suggest that this anti-tumor effect could be due to the presence of CpG motifs on the plasmid DNA or to alteration of endogenous mRNA and proteins, or activation of stress genes within the tumor due to the pulses themselves.
To further illustrate the importance of selecting the appropriate parameters for IL-12 GET immunotherapy, when pUMVC3-mIL12 is delivered using switched applicators; EP1 (C) instead of EP1 (N) and EP2 (N) instead of EP2 (C), similar amounts of IL-12 are produced. The outcome however, is markedly different. Using EP1 (C) was less effective at protection from challenge, and EP2 (N) was not effective at conferring protection compared to EP2 (C). This indicates that the level of IL-12 generated within the tumor as a result of GET is only one part of the story, and suggests a role for the GET protocol selected.
Proinflammatory cytokines such as IL-12 either directly or indirectly mediate wide-ranging effects on the tumor microenvironment that results in tumor regression. The presence of IL-12 within the tumor environment has been shown to increase antigen presentation and generation of a tumor-specific immune response [37]. It also promotes upregulation of mediators within the tumor cells that slow their growth, increase expression of lymphocyte adhesion molecules that increase the infiltration of lymphocytic effector cells into the tumor and leads to the increased presence of cytotoxic T and NK cells within the tumor [38–40]. The application of strong electric fields has been associated with reduced cell viability [4]. Delivery of the non-coding control pUMVC3 using the specified protocols did not appear to induce tissue damage. We observed increased tissue damage when EP2 pulses are used to deliver plasmid IL-12 compared to EP1 pulses. The resulting gene expression and increased presence of immune cells, could result in rapid tumor cell destruction that does not allow adequate time for the generation of a specific immune response and recruitment of memory cells which are essential in establishing lasting immunity. The levels of the pore-forming protein perforin detected in the tumor correspond with elevated levels of IL-12 expressed in the tumor which indicates that the tumor destruction is cell-mediated.
This study reveals that low to moderate expression levels of a therapeutic gene in tumors are best for generating local and systemic anti-tumor responses that correspond with a more successful outcome. It is also clear that IL-12 alone is not sufficient to generate this response, it requires selection and application of the appropriate electrotransfer pulse protocol. This finding is important in improving GET-based therapies for melanoma patients. From these data, it remains unclear which subset of immune cells are mediating the local anti-tumor response and what role the GET parameter selection plays, in the composition of the cellular infiltrate. Previous studies using this tumor model and IL-12 GET showed tumor regression was most likely mediated by the induced local expression of IFN-γ, increased presence of infiltrating lymphocytes and inhibition of tumor angiogenesis [20]. The reduction of formation of distant lesions in treated mice implies the generation of a memory response [21]. This led us to examine the circulating T cell populations in challenged mice.
We found the proportion of memory, naïve and effector T cell sub-populations differ in mice that were resistant to challenge from those mice that developed secondary tumors. The EP conditions and plasmid selection did not appear to impact the disparity between the two groups. The most noteworthy finding, in our opinion, is the CD8+CD3+ T cell subset appeared to have more CD45+CD62L− cells circulating in mice that were resistant to challenge than those that were not. We consider this sub-population “active effectors” because the loss of expression of CD62L indicates that it has encountered antigen and the expression of CD45RB indicates it is not a memory cell though some studies suggest that antigen-experienced T memory cells can regain CD45RB expression [41]. In addition, CD45RB+ CD8+ cells are thought to mediate anti-tumor cytotoxic activity [28]. It is likely that this sub-population may be responsible for the protection from formation of new tumors seen in these animals. It is important to note that our conclusions are limited by the reliability of the expression of these cell surface phenotypic markers, as their expression can be affected by activation state of the cell and the presence of cytokines. The markers used in this study were intended to identify the memory sub-populations that may be present in the circulation. In order to make more definitive statements about the populations present, a more extensive study of surface markers needs to be conducted along with functional studies.
GET of therapeutic cytokines is an effective way to induce tumor regression and generate systemic immunity as shown by our lab [19–23]. The mechanism by which this occurs has yet to be elucidated and may involve many pathways and cell types. A few candidate genes have been suggested to play a role in tumor regression. Elevated levels of Stat1, IRF7 and Mig (CXCL9) have been generated locally in response to intratumoral IL-12 GET [42]. We have demonstrated that we can modulate gene expression at the delivery site by selecting pulse parameters and applicators. Furthermore we demonstrated that while high levels of the therapeutic gene locally are effective in inducing regression of the treated primary tumor, it is not effective in protecting the animal from developing secondary tumors. This finding warrants further investigation with other tumor models and therapeutic genes. Moderating gene expression and selecting the appropriate parameters for GET is essential to achieving the desired clinical outcome for this form of immunotherapy.
ACKNOWLEDGEMENTS
We would like to thank Yi Jing for her assistance with the flow cytometry experiments and Dr. G. Pavlakis (National Cancer Institute) for the gift of the plasmids pAG250-mIL12.
FUNDING
Partial funding was provided by the National Cancer Institute R01 CA122518 Grant and by the Frank Reidy Research Center for Bioelectrics.
Footnotes
CONFLICT OF INTEREST
Dr. R. Heller is an inventor on patents which cover the technology that was used in the work reported in this manuscript. In addition, Dr. R. Heller owns stock in Inovio Pharmaceuticals, Inc. and stock and stock options in OncoSec, Inc.
PATIENT CONSENT
Declared none.
REFERENCES
- [1].Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J 1982; 1(7): 841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Heller LC, Heller R. In vivo electroporation for gene therapy. Hum Gene Ther 2006; 17(9): 890–7. [DOI] [PubMed] [Google Scholar]
- [3].Favard C, Dean DS, Rols MP. Electrotransfer as a non viral method of gene delivery. Curr Gene Ther 2007; 7(1): 67–77. [DOI] [PubMed] [Google Scholar]
- [4].Canatella PJ, Karr JF, Petros JA, Prausnitz MR. Quantitative study of electroporation-mediated molecular uptake and cell viability. Biophys J 2001; 80(2): 755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gehl J, Mir LM. Determination of optimal parameters for in vivo gene transfer by electroporation, using a rapid in vivo test for cell permeabilization. Biochem Biophys Res Commun 1999; 261(2): 377–80. [DOI] [PubMed] [Google Scholar]
- [6].Wolf H, Rols MP, Boldt E, Neumann E, Teissie J. Control by pulse parameters of electric field-mediated gene transfer in mammalian cells. Biophys J 1994; 66(2 Pt 1): 524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cemazar M, Sersa G, Wilson J, et al. Effective gene transfer to solid tumors using different nonviral gene delivery techniques: electroporation, liposomes, and integrin-targeted vector. Cancer Gene Ther 2002; 9(4): 399–406. [DOI] [PubMed] [Google Scholar]
- [8].Gilbert RA, Jaroszeski MJ, Heller R. Novel electrode designs for electrochemotherapy. Biochim Biophys Acta 1997; 1334(1): 9–14. [DOI] [PubMed] [Google Scholar]
- [9].Berard M, Tough DF. Qualitative differences between naive and memory T cells. Immunology 2002; 106(2): 127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Trinchieri G Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol 1995; 13: 251–76. [DOI] [PubMed] [Google Scholar]
- [11].Trinchieri G Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 2003; 3(2): 133–46. [DOI] [PubMed] [Google Scholar]
- [12].Brunda MJ, Luistro L, Warrier RR, et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med 1993; 178(4): 1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Del Vecchio M, Bajetta E, Canova S, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res 2007; 13(16): 4677–85. [DOI] [PubMed] [Google Scholar]
- [14].Voest EE, Kenyon BM, O’Reilly MS, Truitt G, D’Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst 1995; 87(8): 581–6. [DOI] [PubMed] [Google Scholar]
- [15].Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev 2002; 13(2): 155–68. [DOI] [PubMed] [Google Scholar]
- [16].Gogas HJ, Kirkwood JM, Sondak VK. Chemotherapy for metastatic melanoma: time for a change? Cancer 2007; 109(3): 455–64. [DOI] [PubMed] [Google Scholar]
- [17].Riker AI, Jove R, Daud AI. Immunotherapy as part of a multidisciplinary approach to melanoma treatment. Front Biosci 2006; 11: 1–14. [DOI] [PubMed] [Google Scholar]
- [18].Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 2005; 23(10): 2346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Daud AI, DeConti RC, Andrews S, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol 2008; 26(36): 5896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lucas ML, Heller L, Coppola D, Heller R. IL-12 plasmid delivery by in vivo electroporation for the successful treatment of established subcutaneous B16.F10 melanoma. Mol Ther 2002; 5(6): 668–75. [DOI] [PubMed] [Google Scholar]
- [21].Lucas ML, Heller R. IL-12 gene therapy using an electrically mediated nonviral approach reduces metastatic growth of melanoma. DNA Cell Biol 2003; 22(12): 755–63. [DOI] [PubMed] [Google Scholar]
- [22].Ugen KE, Kutzler MA, Marrero B, et al. Regression of subcutaneous B16 melanoma tumors after intratumoral delivery of an IL-15-expressing plasmid followed by in vivo electroporation. Cancer Gene Ther 2006; 13(10): 969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Marrero B, Shirley S, Heller R. Delivery of Interleukin-15 to B16 Melanoma by Electroporation Leads to Tumor Regression and Long-term Survival. Technology in cancer research & treatment 2014; 13(6): 551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jalah R, Rosati M, Ganneru B, et al. The p40 subunit of interleukin (IL)-12 promotes stabilization and export of the p35 subunit: implications for improved IL-12 cytokine production. The Journal of biological chemistry 2013; 288(9): 6763–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol 1989; 24(3): 148–54. [DOI] [PubMed] [Google Scholar]
- [26].Cemazar M, Golzio M, Sersa G, et al. Control by pulse parameters of DNA electrotransfer into solid tumors in mice. Gene Ther 2009; 16(5): 635–44. [DOI] [PubMed] [Google Scholar]
- [27].Swain SL, Croft M, Dubey C, et al. From naive to memory T cells. Immunological reviews 1996; 150: 143–67. [DOI] [PubMed] [Google Scholar]
- [28].Ericsson PO, Hedlund G, Hansson J, Dohlsten M, Sjogren HO. Tumor-selective cytolysis is executed exclusively by CD45R+ CTL whereas allo-specific cytotoxicity can be executed also by CD45RCTL. Cellular immunology 1990; 126(1): 69–79. [DOI] [PubMed] [Google Scholar]
- [29].Kotnik T, Mir LM, Flisar K, Puc M, Miklavcic D. Cell membrane electropermeabilization by symmetrical bipolar rectangular pulses. Part I. Increased efficiency of permeabilization. Bioelectrochemistry 2001; 54(1): 83–90. [DOI] [PubMed] [Google Scholar]
- [30].Sersa G, Cemazar M, Semrov D, Miklavcic D. Changing electrode orientation improves the efficacy of electrochemotherapy of solid tumors in mice. Bioelectroch Bioener 1996; 39(1): 61–6. [Google Scholar]
- [31].Todorovic V, Kamensek U, Sersa G, Cemazar M. Changing electrode orientation, but not pulse polarity, increases the efficacy of gene electrotransfer to tumors in vivo. Bioelectrochemistry 2014; 100:119–27. [DOI] [PubMed] [Google Scholar]
- [32].Heller LC, Cruz YL, Ferraro B, Yang H, Heller R. Plasmid injection and application of electric pulses alter endogenous mRNA and protein expression in B16.F10 mouse melanomas. Cancer Gene Ther 2010; 17(12): 864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Heller LC, Coppola D. Electrically mediated delivery of vector plasmid DNA elicits an antitumor effect. Gene Ther 2002; 9(19): 1321–5. [DOI] [PubMed] [Google Scholar]
- [34].Peng B, Zhao Y, Xu L, Xu Y. Electric pulses applied prior to intramuscular DNA vaccination greatly improve the vaccine immunogenicity. Vaccine 2007; 25(11): 2064–73. [DOI] [PubMed] [Google Scholar]
- [35].Liu H, Huang G, Tang Q, et al. The immunogenicity and safety of vaccination with purified Vero cell rabies vaccine (PVRV) in China under a 2-1-1 regimen. Hum Vaccin 2011; 7(2): 220–4. [DOI] [PubMed] [Google Scholar]
- [36].Shen W, Waldschmidt M, Zhao X, Ratliff T, Krieg AM. Antitumor mechanisms of oligodeoxynucleotides with CpG and polyG motifs in murine prostate cancer cells: decrease of NF-kappaB and AP-1 binding activities and induction of apoptosis. Antisense Nucleic Acid Drug Dev 2002; 12(3): 155–64. [DOI] [PubMed] [Google Scholar]
- [37].Cavallo F, Di Carlo E, Butera M, et al. Immune events associated with the cure of established tumors and spontaneous metastases by local and systemic interleukin 12. Cancer Res 1999; 59(2): 414–21. [PubMed] [Google Scholar]
- [38].Yu WG, Ogawa M, Mu J, et al. IL-12-induced tumor regression correlates with in situ activity of IFN-gamma produced by tumor-infiltrating cells and its secondary induction of anti-tumor pathways. J Leukoc Biol 1997; 62(4): 450–7. [DOI] [PubMed] [Google Scholar]
- [39].Yu WG, Yamamoto N, Takenaka H, et al. Molecular mechanisms underlying IFN-gamma-mediated tumor growth inhibition induced during tumor immunotherapy with rIL-12. Int Immunol 1996; 8(6): 855–65. [DOI] [PubMed] [Google Scholar]
- [40].Watkins SK, Li B, Richardson KS, et al. Rapid release of cytoplasmic IL-15 from tumor-associated macrophages is an initial and critical event in IL-12-initiated tumor regression. Eur J Immunol 2009; 39(8): 2126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Taylor HR, West SK, Mmbaga BB, et al. Hygiene factors and increased risk of trachoma in central Tanzania. Arch Ophthalmol 1989; 107(12): 1821–5. [DOI] [PubMed] [Google Scholar]
- [42].Li S, Xia X, Mellieon FM, Liu J, Steele S. Candidate genes associated with tumor regression mediated by intratumoral IL-12 electroporation gene therapy. Molecular Therapy 2004; 9(3): 347–54. [DOI] [PubMed] [Google Scholar]