Abstract
It is widely understood that microplastics (MPs) are ubiquitous in the marine environment yet less is known about MP abundance in freshwater rivers, particularly those of the western United States. This study documents MP pollution along the Snake River (~1735 km) and from its confluence with the Columbia River to the Pacific Ocean. Grab and plankton net samples (mesh size 100 μm) were collected from the top 25 cm of surface water every 80.5 river km. MPs were identified if they met visual criteria and were verified with the hot needle test. A small representative subset of MPs from the net samples (16.7%) were selected based on appearance for micro-Raman spectroscopy in effort to provide examples of polymer types found in this study. Seventy-five percent of grab samples and 92.8% of net samples contained MPs, with concentrations ranging from 0 to 5.405 MPs L−1 and 0 to 0.014 MP L−1 (0 to 13.7 MP m−3), respectively. The majority of fragments, films and beads were between 100 μm-333 μm. This study identifies potential hotspots of MP pollution along the Snake and Lower Columbia rivers and prioritizes areas where more intensive sampling is needed. Sites with low flow or those further down river had higher numbers and the top two hotspots were located in areas with low population density but high agricultural use. Monitoring MP abundance in freshwater systems is important for establishing baseline levels of MP pollution and can direct laboratory toxicology studies in using more environmentally relevant concentrations for a better indication of how MP pollution affects ecosystems.
Keywords: Microplastics, microfibers, emerging contaminant, freshwater pollution, rivers
Graphical Abstract
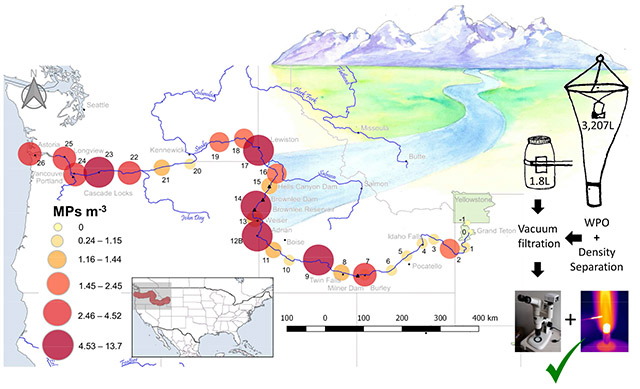
Capsule:
This study documents microplastic (MP) pollution in the Snake River using two sampling methods and identifies MP hotspots as the river flows through multi-use landscapes.
1. Introduction
As of 2015, over 5 billion tons of plastic have been produced worldwide and, based on current trends, a projected 40 billion tons will have been produced cumulatively by 2050 (Zalasiewicz et al., 2016). Less than half of plastic products are consigned to landfills or recycled leaving over half of all plastic in use or polluting the environment (Rochman et al., 2013a). Jambeck et al. (2015) estimate that 4.8 to 12.7 million metric tons of plastic enter the ocean annually. Microplastics (MPs), small plastics less than 5mm, are gaining attention in both the scientific and political realms, and are globally recognized as an emerging environmental contaminant (Hurley et al., 2018). In response there is a current boom in research addressing the prevalence of MPs and their potential negative effects within a variety of ecosystems (Ivar Do Sul and Costa, 2014).
Sources include primary MPs such as pre-production resin pellets or microbeads used as exfoliants in personal-care products, and secondary MPs resulting from the deterioration of larger plastics, including microfibers shed from textiles (Eerkes-Medrano et al., 2015; Hidalgo-Ruz et al., 2012). MPs can enter the environment via industrial spills, atmospheric deposition, littering and effluent from waste water treatment plants (WWTP) (Dris et al., 2015; Eerkes-Medrano et al., 2015). Microbeads and microfibers may not be captured by WWTPs due to their small size and buoyancy, while dense MPs are captured in sludge and made available as fertilizer on agricultural land (Browne et al. 2011; Estahbanati and Fahrenfeld, 2016; Mason et al., 2016). In 2016, researchers calculated that between 3 and 23 billion MP particles are released into U.S. waterways every day via municipal wastewater (Mason et al., 2016).
MPs are extremely mobile in aquatic environments due to their lightweight, insolubility, and durability (Holland et al., 2016). The small size of MPs allows for ingestion by aquatic organisms from different trophic levels with varying feeding strategies (De Witte et al., 2014; Graham and Thompson, 2009; Holland et al., 2016; Wright et al., 2013). Once ingested MPs can interfere with digestion and can lead to decreased feeding, impaired reproduction, oxidative stress, or death (Cole et al., 2015; Ivleva et al., 2017; Jeong et al., 2017; Sussarellu et al., 2016).
Growing evidence suggests there could also be a chemical effect of MP ingestion. Many plastic additives are considered toxic (e.g. flame retardants, phthalates, BPA), some of which are known carcinogens (Browne et al., 2007; Rochman et al., 2013a). The hydrophobic nature of plastic attracts persistent organic pollutants as well as metals and other chemicals (Brennecke et al., 2016; Llorca et al., 2018; Napper et al., 2015). Leaching of these pollutants into the tissues of organisms may cause various health issues (Nobre et al., 2015; Rochman et al., 2013b; Teuten et al., 2009). Despite growing evidence of the potential negative effects on organisms in lab studies, the ecological exposure risks of MPs are still unknown. Lenz et al. (2016) found that MP concentrations in exposure studies tended to be two to seven orders of magnitude higher than MP concentrations reported from field studies. Understanding baseline environmental concentrations of MPs in a variety of ecosystems is an important step in quantifying relevant exposure risks for future lab studies that yield realistic estimates of sub lethal effects (Lenz et al., 2016; Yu et al., 2018).
It is widely understood that MPs are ubiquitous in marine environments (Avio et al., 2017; Eriksen et al., 2014), while less on MP abundance in freshwater is known (Wagner et al., 2014). Freshwater systems are typically smaller in size and closer in proximity to sources, and therefore may present different MP distribution patterns than those of marine environments (Eerkes-Medrano et al., 2015). They are considered important MP transport pathways, including transport to marine systems (Jambeck et al., 2015; Mani et al., 2015). Although variability in sampling methods makes comparing MP concentrations across studies challenging, the few studies on U.S rivers report similar if not more MPs than have been found in marine systems. For example, McCormick et al. (2014) found MP levels (mean 1.94 MP m−3 upstream and 17.93 MP m−3 downstream from WWTP) in the North Shore Channel of Chicago, Illinois, which either match or exceed levels observed in ocean studies. Moore et al. (2002) used a similar sampling technique (333 μm mesh size net), in the coastal ocean near Long Beach, CA and found 7.25 MP m−3, while Di Mauro et al. (2017) found 11.1 ± 2.8 MP m−3 in shelf waters of the Northern Gulf of Mexico.
The objectives of this study were 1) to document MP pollution in the Snake and Lower Columbia Rivers and 2) to determine if MP hotspots identified by two different sampling techniques were the same. Volume-reduced net sampling is a commonly used method in MP field studies, with variations regarding mesh size. However, MPs smaller than the net mesh size and microfibers are susceptible to passing through a net due to their small diameter (Barrows et al., 2017). Small volume grab sampling is a simpler and more economical technique adapted from water quality studies and commonly used in citizen science programs (Barrows et al., 2018). Given that less is known about MP pollution in rivers, this study is an important step in identifying the presence of MP pollution in a large river basin in the continental United States. The Snake River stretches 1,735 km through Wyoming, Idaho, Washington, and Oregon, where it empties into the Columbia River, and eventually the Pacific Ocean. While beginning in one of the least populated and intact ecosystems worldwide, the Snake River is not immune from the impacts of human activities and flows through many different land use types: urban, rural, industrial, protected wilderness, reservoirs, and intense agriculture.
2. Methods
2.1. Study Area
The Snake River begins in the mountains of western Wyoming, flows across the arid Snake River Plain then north along the Idaho-Oregon boundary and west into Washington where it joins the Columbia River which flows to the Pacific Ocean. The Snake River Basin is approximately 240,765 km2, and is 36 percent of the Columbia River Basin (Northwest Power and Conservation Council, 2018). The headwaters (elev. 2,987 m) lie within Yellowstone National Park, a subalpine ecosystem that creates a snowmelt driven river system.
The Snake River is prized for its scenic beauty, wildlife, boating, and world class fishing. The Columbia and Snake rivers have productive populations of salmon and steelhead fish (natural and stocked). The Snake river is still the most important production area in the Columbia River Basin for these threatened anadromous fish, despite it being highly regulated and intensively used. There are 22 hydropower dams on the main stem of the Snake River, producing more than 1,100 megawatts of electricity, and simultaneously promoting the storage and pumping of water for irrigation and recreational purposes (Northwest Power and Conservation Council, 2018). Irrigation water from the Snake River transformed over three million acres of once-arid land into one of the United States most productive croplands (Collier et al., 2000). The Snake River no longer follows a natural flow regime, as over 50 percent of surface water is used primarily for irrigation and livestock, and dams manage the flow of the river to meet these irrigation needs (Clark et al., 1995). In 2016, 75% of the water reuse permits issued were for municipal water, the majority of which were used for irrigation (Idaho DEQ, 2016). In addition to irrigation needs, there are several urban and industrial areas dependent upon the Snake and Lower Columbia rivers for not only electricity, but also drinking water and industrial production.
2.2. Quality Assurance and Control
Exposure times during all stages of sample collection and processing were limited. Samples were covered at all times with clean jar lids, aluminum foil or glass eyewashes. All glassware and equipment used in this study were cleaned with Decon-90, rinsed three times with tap water followed by a final rinse using filtered water (0.45 μm cellulose nitrate, Whatman). In the field and lab, only 100% cotton clothing and white lab coats were worn. All samples were filtered inside a clean air cabinet and covered during filtration. Filters were immediately placed in clean Petri dishes until after initial visual inspection. Once all fibers/questionable particles were recorded, lids were removed to access suspect particles for verification.
Two wet peroxide oxidation controls using filtered water (0.45 μm cellulose nitrate, Whatman) were processed identical to our environmental samples to estimate procedural contamination. In addition, air contamination controls were conducted during lab processing and filtering of samples (n = 4). Filters moistened with filtered water (0.45 μm cellulose nitrate, Whatman) were exposed to the air next to where vacuum filtration occurred for 30 minutes.
2.3. Sample Collection
Surface water samples were collected once every 80.5 river km from June 25-27, July 5-7 and 15-18 and 22-23, and August 15, 2016 (Table S1), when spring runoff began to subside, but prior to the low flow, dry season. Weather during all of these time periods was hot and dry, with no significant rain events prior to and during sampling dates. Two types of samples were collected from the top 25 cm of surface water: 1) grab samples (n = 28, mean volume 1.85 L) in glass jars attached to a sampling pole approximately 3-4 m from the bank, and 2) net samples (n = 28, mean volume 3,207 L) using a 100 μm mesh circular plankton net. As a result of using a smaller mesh size, the net was submerged for only 1-2 minutes (average time 72 seconds) as any longer led to issues of clogging with organic matter (e.g. algae).
The net was submerged only halfway to a) maximize the water surface area sampled, and b) to reduce force caused by strong current. A flow meter (Sea-Gear Corp., Miami, FL, USA) was attached to the center of the net for water volume calculations. After sampling, the net was rinsed with river water from the outside to capture any remaining matter into the cod end. Organic matter and potential plastics were stored in glass jars previously cleaned with Decon 90 and rinsed three times with filtered water (0.45 μm Whatman cellulose nitrate filter). Once emptied, the net was pulled vertically through the water column and agitated without the cod end at least three times to dislodge potential particles or invasive species entangled in or on the outside of the net. All sampling gear was sprayed and immersed in Formula 409® solution to reduce the risk of spreading invasive species, and rinsed at the next sampling site with tap water. Prior to sampling, the cod end was rinsed while the net was pulled vertically at least three times through the water column and agitated prior to sampling. The purpose was to remove any particles that may have entered the net during rinsing at the previous site or during transport.
Depending on site characteristics (velocity of water, depth and access), samples were collected from a dock (n = 3), bridge (n = 2), riverbank or riprap (n = 4), boat (n = 1) or by wading (n = 18). For the boat sample, the net was held out from the bow and away from the wake using a wooden pole. For bridge samples, the net was suspended using a bright orange line. For dock samples, the net was attached to the long sampling pole and held away from the dock while being pulled upstream. For the riverbank or riprap samples, the net was held away from the bank into the current. Finally, for wading samples, one person waded approximately 5 m from shore into moving water when possible, and held the net in front and away from their body. If there was little flow (< 0.2 m/s), the sampler held the net in front while they walked parallel to the river bank upstream to simulate flow.
2.4. Lab Methods
Grab Samples
Grab samples were filtered (0.45 μm Whatman cellulose nitrate filter) using a vacuum filtration system inside a laminar flow hood. Each filter was immediately placed into a clean glass Petri dish and dried at 60°C for 2-24 hours. Plates were sealed shut until initial microscope inspection was complete.
Net Samples
All samples were sieved through a 100 μm stainless steel mesh sieve. Between samples, sieves were cleaned in an ultrasonic water bath and rinsed with filtered water (0.45 μm cellulose nitrate, Whatman). Sieved contents were rinsed into small clean glass beakers with the help of a clean metal spatula, then dried at 80°C for 24-48 hours. Potential plastics were isolated from organic material using wet peroxide oxidation (WPO) developed by National Oceanic and Atmospheric Administration (Masura et al., 2015). WPO was followed with two density separation techniques. First, NaCl was added to the digest to reach a solution density of 1.15 g cm−3 (Masura et al., 2015) and left to settle in density separation funnels for 30 to 60 minutes. The settled sediment was drained into a clean beaker. Second, a NaI saline solution (1.8 g cm−3) of equal volume was added to the drained sediment and left to settle for an additional 30-60 minutes. The supernatant collected from the density separation steps were combined and filtered through a 100 μm stainless steel mesh aided by vacuum filtration. This step helped to remove the fine salt precipitate that can clog the 0.45 μm filters. The filtered supernatant was rinsed from the mesh into a clean beaker while the mesh was added to a separate clean beaker, filled with filtered water and placed in an ultrasonic water bath for 5-7 minutes. The mesh was then removed and rinsed with filtered water into the beaker containing the filtrate, which was vacuum filtered (0.45 μm Whatman cellulose nitrate filter). The mesh was inspected under a stereoscope to ensure no particles remained behind. Each filter was immediately placed into a clean glass Petri dish and dried at 60°C for 2-24 hours. Plates were sealed shut until initial microscope inspection was complete.
2.5. Microplastic Identification
Filters were visually inspected under a stereoscope (Nikon SMZ800N, magnification 15x – 120x, equipped with Infinity camera) in sealed Petri plates to prevent airborne contamination and all putative MPs were recorded. If particles met visual identification criteria (Dris et al., 2015;Hidalgo-Ruz et al., 2012; Zhao et al., 2017), potential particles were photographed and measured (the longest dimension recorded as length, using Infinity Analyze software, Lumenera Corp., CAN). MPs were classified into four categories: 1) fiber, 2) fragment, 3) granule or bead, and 4) film. MPs less than 100 μm were not considered in this study because they are difficult to identify microscopically (Barrows et al., 2017; Dris et al., 2015; Lenz et al., 2015).
2.6. Microplastic Verification
Visual identification of microplastics alone is susceptible to false identification (Silva et al., 2018; Lusher et al., 2017a) and therefore MPs must be verified. It is recommended that 5-10% of MPs >100 μm identified in a study are subject to verification techniques. These techniques can be as simple as the hot needle test, or involve more expensive equipment such as FTIR or Raman spectrometry (Campbell et al., 2017; Lusher et al., 2017b). In this study, all MPs were verified using the hot needle test. Although the hot needle test cannot be used to identify MP to polymer type, it is accepted as an economical way to verify particles based on their response to a hot needle (Campbell et al., 2017; Lusher et al., 2017a; Silva et al. 2018). If putative MP identified by visual identification melted when exposed to the hot needle (Lusher et al., 2017a; Gorokhova, 2015) they were confirmed as MPs (SI Figure 1 for images of MPs found in this study). Particles that easily broke apart with forceps we excluded from this study.
2.7. Polymer Identification
A small representative subset of MPs from the net samples (16.7%, 31 of 185) were selected based on appearance for micro-Raman spectroscopy in effort to provide examples of polymer types found in this study. Only particles from the net samples were selected because they underwent cleaning during the WPO process, which reduces interference from biofouling. Raman spectrometery was chosen over FTIR due to the availability of equipment.
Raman spectra were collected using a Sierra IM-52 Portable Raman Microscope (Snowy Range Instruments, Laramie WY) equipped with a 785 nm laser. Laser power (ranging from 2.4 to 18.5 mW), integration time (average 14 seconds) and the number of multi-acquisitions varied for each individual particle to enhance spectral quality and reduce fluorescence. All spectra were analyzed using the BioRad KnowItAll Software and compared to the following spectral libraries: Horiba Raman Forensics, Horiba Raman Minerals, Bio-Rad Sadtler Raman Polymers and Monomers and Bio-Rad Sadtler Polymers and Processing Chemicals. The BioRad KnowItAll Software program compares unknown spectra to known library spectra using a correlation algorithm. Search results are ranked with a Hit Quality Index (HQI) value from 0-100%. Only values of 70% or higher were accepted as a positive match (Lusher et al., 2017a).
2.8. Statistical Analysis
All statistical analyses were conducted in RStudio (R 3.4.3, R Core Team, 2018) with p = 0.05 indicating statistical significance. A Shapiro-Wilk test indicated that the MPs per site for net and grab samples were not normally distributed, therefore only non-parametric tests (e.g. Spearman’s rank correlation, Kruskal-Wallace, Wilcoxen signed rank) were used in the analysis. Descriptive statistics, barplots and histograms were produced to display data. Maps were created using open source QGIS software (QGIS Development Team, 2018).
3. Results and Discussion
3.1. Microplastic abundance, type and size
The following results are conservative because: a) Four of the net samples (07,10, 11, and 13) contained heavy salt precipitates after filtration, interfering with microscopic identification of plastics; b) Clear, opaque, and natural colored particles are more difficult to differentiate from biological material and may be under-represented; c) All clear fibers (n = 25) were eliminated as it was impossible to determine if they were contamination from the net, which is made of clear nylon; d) When net samples were vacuum filtered through the 100 μm sieve, some particles (especially fibers) may have been forced through the sieve and lost; and e) Results from the wet peroxide oxidation controls (n = 2) yielded 1 pink fiber and therefore all similar pink fibers (a total of 2) were eliminated from the results (no particles were detected in our air contamination controls (n = 4)).
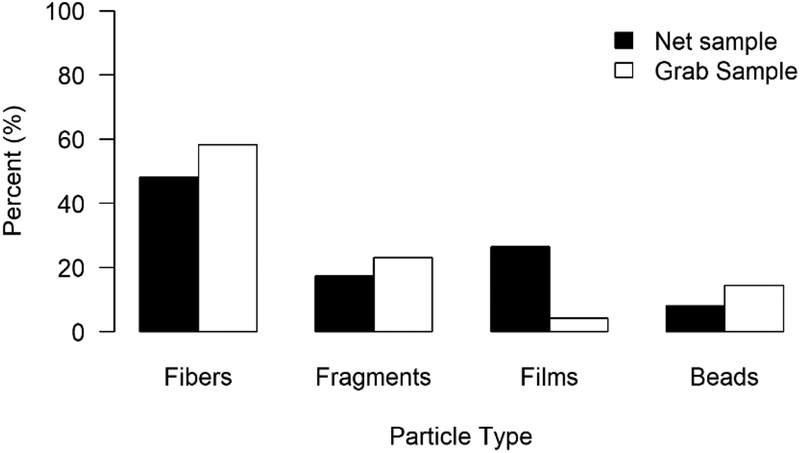
A total of 233 MPs were observed in this study. MPs were detected in 92.8% of the net samples and 75% of the grab samples. MPs were not detected at the two sites located in Grand Teton National Park, WY. Grab samples collected more MPs than net samples (Table 1). Fibers were the dominant form of MP pollution (Fig.1).
Table 1.
Results summary of MPs collected in grab and net samples.
| Grab | Net | |
|---|---|---|
| Total MPs counted | 48 | 185 |
| % containing MPs | 75% | 92.5% |
| Mean ± SD | 0.91 ± 1.14 MP L−1 | 0.00257 ± 0.003 MP L−1 |
| 2.57 ± 2.95 MP m−3 | ||
| Range | 0 to 5.405 MPs L−1 | 0 to 0.014 MP L−1 |
| 0 to 13.7 MP m−3 | ||
| Percent fibers | 58% | 48% |
| % MPs 100-500 | 90% | 76.3% |
| % MPs 100-333 | 76% | 51.3% |
Fig. 1.
Percent MPs (100μm – 5mm) by category in grab and net samples.
Length measurements were recorded for 78% of all films, fragments, and beads. The majority of those not measured were folded and twisted films from Site 14, making it difficult to obtain a reliable measurement. Of those measured, 57% were 100-333 μm and 79.4% were less than 500 μm (Fig. S1). Grab samples collected smaller MPs when compared to net samples. These results suggest that using nets with smaller mesh size (100 μm or less) or bulk sampling will collect more MPs and more accurately measure environmental concentrations.
3.2. Comparison of sampling methods
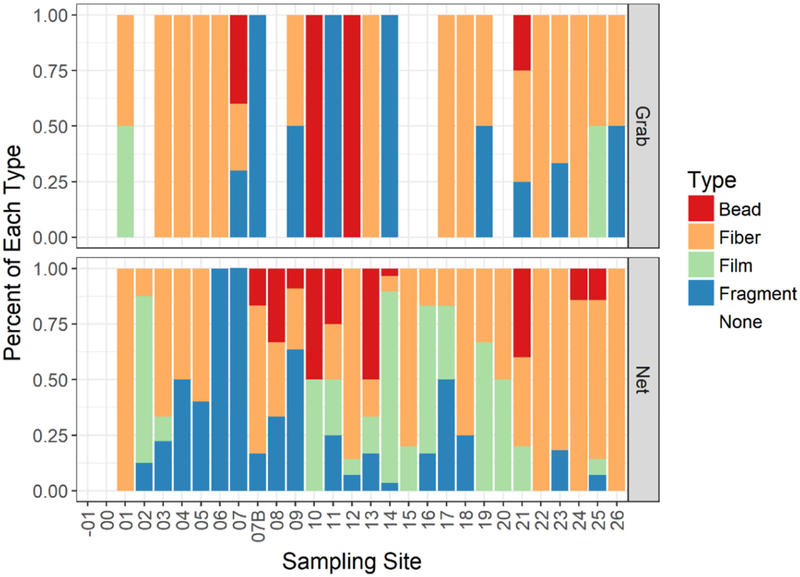
There was no significant difference in the frequencies of each MP type between net and grab samples (chi-square = 0.196, df = 3, p = 0.98); although there was a high degree of variability in the type and number between sites and sampling type (Fig. 2 and Table S1). As other studies report (Barrows et al., 2017; Lusher et al., 2017; Tamminga et al., 2018; Vermaire et al., 2017) small volume grab samples provided different MPs L−1 than net samples. A Wilcoxon Signed Ranks Test indicated that the MPs L−1 in grab samples were significantly higher than those from the net samples (Wilcoxon Signed Rank, p < 0.001).
Fig. 2.
Percent of each MP type observed in grab (top) and net (bottom) samples at each site. No MPs were found in the two sites located in Grand Teton National Park (sites −01 and 00). Site 07 was repeated one year later and is included as 07B.
Although grab samples are easier to analyze (no WPO or other digest process needed) and more economical, Tamminga et al. (2018) suggest that small volume sampling may not be representative of MP prevalence in the environment and recommend a minimum of 1000 L (1m3) for comparability among studies. According to Vermaire et al. (2017), collecting large volume samples better approximates MP prevalence in open water, however more research on the lower limit of sampling volume needs to be addressed.
3.3. Site Characteristics and Microplastic Abundance
The variation in MP prevalence among sites is not surprising (Table S1), given the number of influential environmental factors such as differing flow regimes (e.g., rapids and dams), surrounding land use (e.g., urban areas, agriculture, national park, and wilderness), population density, and proximity to wastewater treatment effluent (Baldwin et al., 2016; Mani et al., 2015). These are complex factors that require more intensive and long-term sampling than what this study provided. However, we conducted Spearman’s rank correlation tests to determine if certain sampling site characteristics showed correlations with the number of MPs found in our results. Due to the differences in the net and grab samples described above, we use only the results from net samples in the following correlation analyses. Results from the correlation tests are reported in Table 2.
Table 2.
Spearman Correlation Coefficients (rs) between certain site characteristic and MPs m−3.
| Site Characteristic | MPs m−3 |
|---|---|
| Population Density | |
| 50.3 km2 (4 km radius) | 0.25 |
| −0.02 | |
| Flow Rate (m/sec) | −0.40* |
| Distance Downriver (km) | |
| All Sites | 0.65* |
| Snake River Sites | 0.63* |
indicates significant correlations with p < 0.05
Results showed a positive correlation in MP m−3 between each site and distance in kilometers downriver (Fig. S2) A second correlation test was run solely on the Snake River sampling sites to confirm this was not a result of the Lower Columbia River sampling sites having higher MP counts. While the Lower Columbia and Snake Rivers did not differ significantly in the total number of MPs (Wilcoxon Signed Rank, W =107. p=0.13), the Columbia River did contain significantly higher numbers of MP fibers (Wilcoxon Signed Rank, W=131, p=0.006). This suggests that the increasing number of fibers could be due to their higher abundance in the Lower Columbia River.
To explore if population density was correlated with MP abundance, we calculated population density within circular plots of increasing size directly upriver from each sampling site using Population Explorer (Version 2018-01-25). Contrary to findings in other studies (Baldwin et al., 2016), Spearman’s rank correlation tests revealed no correlations between total MPs m−3 per sample site and population density. In rivers MPs can travel miles downriver and accumulate elsewhere in the water column or settle out.
Little is currently known about MP abundance in relation to flow dynamics in rivers. With 22 dams constructed along the course of the Snake River, sites exhibited variability in flow (Table S1). Zhang et al (2015) found that MPs were significantly higher in the Three Gorges Reservoir, China, and suggested that reservoirs are potential hotspots for MP accumulation. Results from this study reveal a negative correlation between MPs m−3 and velocity of water (m/sec) also suggesting that dams, or periods of low flow, may accumulate MPs.
3.4. Potential Hotspots
Hotspots are sites where the highest number of MPs were observed. Since the net and grab samples did not show similarities in identified hotspots (Fig.3 and Fig. 4), we describe only those identified in the net samples. More frequent and year-round sampling is recommended for a thorough profile of MP pollution per site and overall MP load in the river. For example, MP pollution could increase greatly during spring snowmelt and runoff events, changing the location of the hot spots identified in this study.
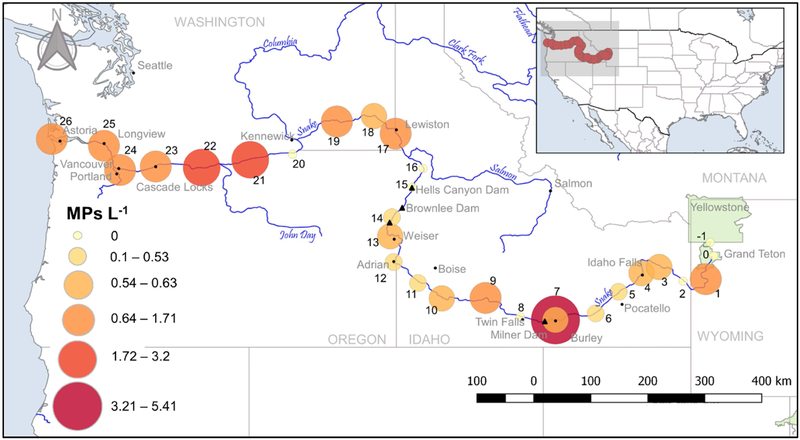
Fig. 3.
Sampling location, site number, and number of MPs L−1 for grab samples. Sites 7 and 22 contained the highest MPs. Two samples were collected at site 7, which explains the presence of two points at this site.
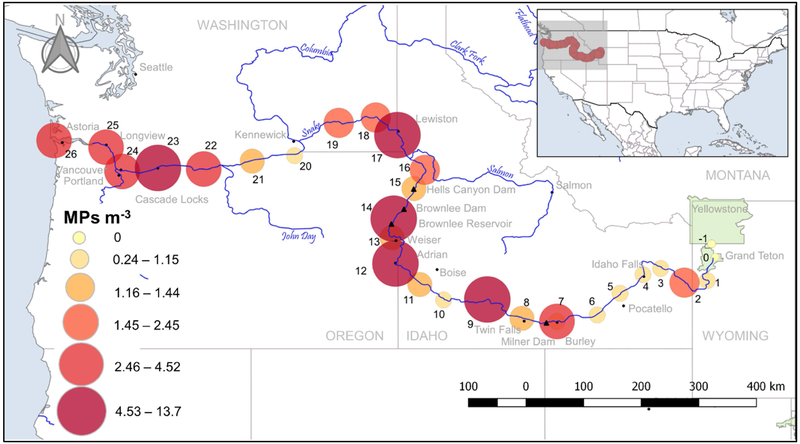
Fig. 4.
Sampling location, site number, and MPs m−3 for net samples. Sites 14 and 12 contained the highest MPs.
Site 14 had the highest number of MPs (13.7 m−3). This site is located on the western shore of Brownlee reservoir (~ 93 km-long and ~ 53 km2) along the border of Oregon and Idaho, and downriver from the confluence with the Weiser, Payette, and Boise Rivers. In 2014 it was listed as an impaired waterbody due to mercury, pesticides, reduced oxygen, total phosphorous levels, and sedimentation (EPA, 2018), most of which may be attributed to non-point source pollution (Bjorneberg et al., 2015; Etheridge, 2013). Brownlee Reservoir is the first downstream from the Snake River Plain which contains concentrated areas of agriculture. Cyanobacteria blooms have been frequent as a result of increased nutrients from agricultural runoff (Etheridge, 2013).
When river water transitions from high to low flow, buoyant MPs may accumulate in surface water until they settle due to biofouling. Thus dams can easily accumulate MPs, acting as a sink for MPs (Zhang et al., 2015). Our results suggest that Brownlee Reservoir is also a hotspot for MP pollution. It is possible that the high levels of MPs are caused by multiple effects of agriculture, recreation, and WWTP effluent from facilities serving these areas. For example, less than 100 miles upriver there are at least six WWTP that empty treated effluent into the Snake River or into one of its tributaries. Also, it cannot be overlooked that the reservoir is a popular fishing destination, and therefore the high number of films counted at this site could be the result of localized fragmentation of larger litter, such as plastic bags and food wrappers.
Site 12 is approximately 160 km upriver from Site 14 and contained the second highest number of MPs (9.5 m−3), 80% of which were fibers. The surrounding land near this site is used for agriculture (primarily alfalfa, corn, and winter wheat), as is much of the land upriver. Site 9 is also located down river (~80 river km) from an agriculturally rich region and the populated city of Twin Falls, Idaho. In agricultural areas where biosolids (wastewater treatment sludge) have been applied there is potential for MPs to enter the river during storm runoff events (Eriksen et al., 2013). Approximately 44,000-300,000 metric tons of MPs are applied to agricultural land in the US annually through land based application of biosolids (Nizzetto et al., 2016), arguing that a potential reservoir of MP in the ecosystem is farm soil. Synthetic fibers in soil may be a predictor of past biosolid application, even from 5 to 15 years prior (Zubris and Richards, 2005). The presence of MPs in biosolids from WWTP in this region has not been documented, therefore results only suggest that land based biosolids application. Other possible sources of MPs in agricultural systems are plastic mulches, irrigation tape, hay bale wrap, row covers, greenhouse plastics, and plastic foams and flakes in soil conditioners (Ng et al., 2018; Zalasiewicz et al., 2016). Further study is needed to understand if and to what extent plastics used in agriculture and biosolid application on agricultural soils contribute to MP pollution in the Snake River.
While the hotspots discussed above suggest agriculture could be a possible source of MPs, recreation may also be an important factor. Sites 17 and 23 are located at popular boating access areas with a lot of human traffic. Site 17 is located just downriver from a popular boat ramp near Asotin, Washington, while Site 23 is located at a popular sailboat racing destination near Cascade Locks, Oregon. Site 7, although not identified as a hotspot by the net samples, contained an alarming number of MPs (5.4 L−1) in the grab sample and is also located at a popular recreation area immediately downstream from a WWTP. The sample, taken from a dock frequented by fishermen, jetskiers, kayakers, and canoeists, contained globular shaped beads, colorful fragments, and fibers. Although the net samples did not reflect a high enough number of MPs for Site 7 to be identified as a hotspot, we suggest more thorough sampling at this site in addition to the other 5 identified hotspots.
3.5. Polymer Identification
Challenges using micro-Raman spectroscopy to identify polymer type in environmental plastics have been documented (Karami et al., 2017; Lenz et al., 2015; Löder, 2015; Ribeiro-Claro et al., 2017; Shim et al., 2017; Van Cauwenberghe and Janssen, 2014; Zhao et al., 2017;). Plastic additives and pigments can mask the underlying polymer spectra while environmental degradation can weaken the Raman signal (Ribeiro-Claro et al., 2017; Van Cauwenberghe and Janssen, 2014; Yu et al., 2018; Zhao et al., 2017). Furthermore, the commercial databases referenced in this study cannot cover the variety and complexity of plastic additives (Lenz et al., 2015) and the library spectra are obtained from pure materials not previously exposed to environmental conditions resulting in decreased HQI scores.
Of the MPs analyzed with micro-Raman, 67.6% were confirmed to be of synthetic nature. Of these, 35.4% had HQI scores >70 matching them to a specific type of polymer (9 immediately and 2 only after subtraction of interfering spectra). Polymers identified were polypropylene, polyethylene, polyethylene terapthalate and polyester (Fig. S4). An additional 32.3% (10 MPs) matched chemical additives or pigments commonly used in plastics (e.g. chrome-yellow, cromophtal violet B, barium yellow, copper phthalocyanine,titanium oxide and diaminobenzidine) but the underlying polymer could not be determined. It is assumed that these particles are plastic (confirmed by visual inspection, the hot needle test, and the presence of these chemicals) and the spectra of the pigment or additive interfered with the identification of polymer type. The remaining particles could not be identified due to poor quality spectra likely resulting from reasons listed above, therefore it is unlikely that the MPs reported in this study are an overestimation. Increasing the acquisition time could have allowed for more visible and identifiable peaks (Ribeiro-Claro et al., 2017) and using a different laser wavelength could reduce fluorescence (Lenz et al., 2015). Furthermore, the hot needle test used to verify MPs may have altered spectra through thermal degradation.
4. Conclusion
This study aimed to investigate MP pollution along the Snake River as it flows through Wyoming, Idaho, Oregon and Washington, and is the first to confirm MP presence in the river. We determined if MP pollution existed at the sample sites and, if so, identified areas of increased MP concentration (hotspots) using two sampling techniques. The grab and net sampling techniques did not identify similar hotspots, which could be a result of the different sampling volumes. We placed more emphasis on the hotspots identified with the net samples due to the larger volume sampled, however further study is needed to identify ideal sampling volumes in MP research. Due to the size of the study area, a comprehensive study measuring the MP load would require extensive resources and collaboration efforts to increase the number of samples collected at each sampling site multiple times during the year. The hotspots identified in this study can be used to prioritize areas for further research designed to identify and quantify the sources and dynamics driving MP pollution in this large river basin.
Although variations in sampling protocols abound, MP abundance in this study is comparable to those reported in other rivers. Vermaire et al. (2017) also used a 100 μm mesh net and reported a mean of 1.35 MPs m−3 in the Ottawa River, Canada. This is less than the 2.54 m−3 MPs that we report in this study. Baldwin et al. (2016), although they used a 333 μm mesh net, reported a mean of 4.2 MPs m−3 in tributaries of the Great Lakes, United States. Miller et al. (2017) reported a mean of 0.98 anthropogenic microfibers L−1 in the Hudson River, New York using 1 L grab samples. In this study, although we considered only microplastic fibers, we report a mean of 0.53 microfibers L−1. Variety in freshwater and marine ecosystems (e.g., current, depth, wind) has prevented the adoption of a standardized sampling protocol for identifying MP pollution (Browne et al., 2015). To improve comparability among MP studies, such protocols must be established.
With minimal MP pollution data available on rivers in the U.S. and worldwide, these results are valuable in providing information on the abundance of MPs along a river that flows through multiple land uses (e.g., National Park, wilderness, urban, agriculture, and recreation areas). Further research is needed to address MP prevalence in agricultural areas, particularly those irrigated with recycled WWTP water and where biosolids have been applied. By comparing hotspots to sites with less MP pollution, scientists can begin to understand the ecological effects of MPs. Monitoring freshwater systems is important in establishing baseline levels of MP pollution and can direct laboratory toxicology studies in using more environmentally relevant concentrations for a better indication of how MP pollution affects ecosystems.
Supplementary Material
Fig. S1. Histogram comparing size distributions of MPs between sampling methods.
Fig. S2. Spearman’s rank correlation reveals a positive correlation between MPs m−3 and location in kilometers downriver from river source.
Fig. S3. Examples of MP types identified in this study.
Fig.S4. Example Raman spectra for identified MPs
Table S1. Location, flow rate, volume of water sampled, total and types of MPs abundance (in both net and grab samples) for each sampling site.
Highlights:
First surface water sampling for microplastics along the Snake and Lower Columbia Rivers
MPs were detected in 75% of grab samples and 92.8% of net samples
57% of the plastic beads, fragments and films identified were less than 333 microns in length
Fibers were the dominant type of MPs, comprising 48% (net) and 58% (grab) of particles
Highest MPs were observed at sites with low population density but high agricultural use
Acknowledgements
We thank Dr. Rene Rodriguez at Idaho State University and Kathryn McHenry of Metrohm and Snowy Range Instruments for their guidance and help with micro-Raman spectroscopy and Nic Bluoin with University of Wyoming INBRE Bioinformatics Core for his technical help using Program R. We are grateful for the financial support provided by the Teton Conservation District (TCD) and through an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant # 2P20GM103432. The contents in this article are solely the responsibility of the authors and do not necessarily represent the official views of TCD or NIH.
Footnotes
Declarations of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Avio CG, Gorbi S, Regoli F, 2017. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 128, 2–11. 10.1016/j.marenvres.2016.05.012 [DOI] [PubMed] [Google Scholar]
- Baldwin AK, Corsi SR, Mason SA, 2016. Plastic Debris in 29 Great Lakes Tributaries: Relations to Watershed Attributes and Hydrology. Environ. Sci. Technol. 50, 10377–10385. 10.1021/acs.est.6b02917 [DOI] [PubMed] [Google Scholar]
- Barrows APW, Neumann CA, Berger ML, Shaw SD, 2017. Grab vs. neuston tow net: a microplastic sampling performance comparison and possible advances in the field. Anal. Methods 9, 1446–1453. 10.1039/C6AY02387H [DOI] [Google Scholar]
- Barrows APW, Cathey SE, & Petersen CW 2018. Marine environment microfiber contamination: Global patterns and the diversity of microparticle origins. Environ Pollut 237, 275–284. 10.1016/j.envpol.2018.02.062 [DOI] [PubMed] [Google Scholar]
- Beer S, Garm A, Huwer B, Dierking J, Nielsen TG, 2017. No increase in marine microplastic concentration over the last three decades - A case study from the Baltic Sea. Sci. Total Environ. 10.1016/j.scitotenv.2017.10.101 [DOI] [PubMed] [Google Scholar]
- Bjorneberg DL, Leytem AB, Ippolito JA, Koehn AC, 2015. Phosphorus Losses from an Irrigated Watershed in the Northwestern United States: Case Study of the Upper Snake Rock Watershed. J. Environ. Qual. 44, 552 10.2134/ieq2014.04.0166 [DOI] [PubMed] [Google Scholar]
- Brennecke D, Duarte B, Paiva F, Caçador I, Canning-Clode J, 2016. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 178, 189–195. 10.1016/j.ecss.2015.12.003 [DOI] [Google Scholar]
- Browne M, Galloway T, Thompson R, 2007. Microplastic: An emerging contaminant of potential concern? Integr. Environ. Assess. Manag 3, 1. 10.1002/ieam.563003Q412 [DOI] [PubMed] [Google Scholar]
- Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R 2011. Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ. Sci. Technol, 45, 9175–9179. 10.1021/es201811s [DOI] [PubMed] [Google Scholar]
- Browne MA, Chapman MG, Thompson RC, Amaral Zettler LA, Jambeck J, Mallos NJ 2015. Spatial and temporal patterns of stranded intertidal marine debris: is there a picture of global change? Environ. Sci. Technol, 49, 7082–7094. https://pubs.acs.org/doi/10.1021/es5060572 [DOI] [PubMed] [Google Scholar]
- Campbell SH, Williamson PR, and Hall BD, 2017. Microplastics in the gastrointestinal tracts of fish and the water from an urban prairie creek. FACETS 2: 395–409. 10.1139/facets-2017-0008 [DOI] [Google Scholar]
- Clark GM, Maret TR, Rupert MG, Maupin MA, Low WH, Ott DS, 1998. Water quality in the Upper Snake River Basin, Idaho and Wyoming, 1992–95: US Geological Survey Circular 1160. Dep. of Int. 40pp. [Google Scholar]
- Clark GM, Naymik J, Krabbenhoft DP, Eagles-Smith CA, Aiken GR, Marvin-DiPasquale MC, Harris RC Myers R, 2016. Mercury Cycling in the Hells Canyon Complex of the Snake River, Idaho and Oregon. Tacoma: 10.3133/fs20163051 [DOI] [Google Scholar]
- Cole M, Lindeque P, Fileman E, Halsband C, Galloway TS, 2015. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 49, 1130–1137. 10.1021/es504525u [DOI] [PubMed] [Google Scholar]
- Collier M, Webb RH, and Schmidt JC, 2000. Dams and rivers: a primer on the downstream effects of dams. DIANE Publishing. [Google Scholar]
- De Witte B, Devriese L, Bekaert K, Hoffman S, Vandermeersch G, Cooreman K, Robbens J, 2014. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 85, 146–155. 10.1016/j.marpolbul.2014.06.006 [DOI] [PubMed] [Google Scholar]
- Di Mauro R, Kupchik MJ, & Benfield MC (2017). Abundant plankton-sized microplastic particles in shelf waters of the northern Gulf of Mexico. Environ. Pollut, 230, 798–809. 10.1016/i.envpol.2017.07.030. [DOI] [PubMed] [Google Scholar]
- Dris R, Gasperi J, Rocher V, Saad M, Renault N, Tassin B, 2015. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 12, 592–599. 10.1071/EN14167 [DOI] [Google Scholar]
- Eerkes-Medrano D, Thompson RC, Aldridge DC, 2015. Microplastics in freshwater systems : A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 75, 63–82. 10.1016/j.watres.2015.02.012 [DOI] [PubMed] [Google Scholar]
- Eriksen M, Lebreton LCM, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, 2014. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS One 9, el11913 10.1371/journal.pone.0111913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen M, Mason S, Wilson S, Box C, Zellers A, Edwards W, Farley H, Amato S, 2013. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 77, 177–182. 10.1016/j.marpolbul.2013.10.007 [DOI] [PubMed] [Google Scholar]
- Estahbanati S, Fahrenfeld NL, 2016. Influence of wastewater treatment plant discharges on microplastic concentrations in surface water. Chemosphere 162, 277–284. 10.1016/i.chemosphere [DOI] [PubMed] [Google Scholar]
- Etheridge AB, 2013. Evaluation of Total Phosphorus Mass Balance in the Lower Boise River, Southwestern Idaho. U.S. Geol. Surv. Sci. Investig. Rep. 2013 5220, 82 [Google Scholar]
- Gorokhova E 2015. Screening for microplastic particles in plankton samples: How to integrate marine litter assessment into existing monitoring programs?. Mar. Pollut. Bull, 99, 271–275. 10.1016/j.marpolbul.2015.07.056 [DOI] [PubMed] [Google Scholar]
- Graham ER, Thompson JT, 2009. Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J. Exp. Mar. Bio. Ecol. 368, 22–29. 10.1016/j.jembe.2008.09.007 [DOI] [Google Scholar]
- Hidalgo-Ruz V, Gutow L, Thompson RC, and Thiel M, V., 2012. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 46, 3060–3075. [DOI] [PubMed] [Google Scholar]
- Holland ER, Mallory ML, Shutler D, 2016. Plastics and other anthropogenic debris in freshwater birds from Canada. Sci. Total Environ. 571, 251–258. 10.1016/j.scitotenv.2016.07.158 [DOI] [PubMed] [Google Scholar]
- Hurley R, Woodward J, Rothwell JJ, 2018. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 11, 1–7. 10.1038/s41561-018-0080-1 [DOI] [Google Scholar]
- Idaho Department of Environmental Quality, 2016. Water Reuse in Idaho Newsletter. Retrieved from http://www.deq.idaho.gov/permitting/water-quality-permitting/wastewater-reuse.aspx [Google Scholar]
- Ivar Do Sul JA, Costa MF, 2014. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 185, 352–364. 10.1016/j.envpol.2013.10.036 [DOI] [PubMed] [Google Scholar]
- Ivleva NP, Wiesheu AC, Niessner R, 2017. Microplastic in Aquatic Ecosystems. Angew. Chemie - Int. Ed. 56, 1720–1739. 10.1002/anie.201606957 [DOI] [PubMed] [Google Scholar]
- Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, and Law KL, 2015. Plastic waste inputs from land into the ocean. Science, 347, 768–771. 10.1126/science.1260352 [DOI] [PubMed] [Google Scholar]
- Jeong CB, Kang HM, Lee MC, Kim DH, Han J, Hwang DS, Souissi S, Lee SJ, Shin KH, Park HG, Lee JS, 2017. Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana. Sci. Rep. 7, 1–11. 10.1038/srep41323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami A, Golieskardi A, Ho YB, Larat V, Salamatinia B 2017. Microplastics in eviscerated flesh and excised organs of dried fish. Scientific reports, 7, 5473 10.1038/s41598-017-05828-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson TM, Vethaak AD, Almroth BC, Ariese F, van Velzen M, Hassellöv M, Leslie HA, 2017. Screening for microplastics in sediment, water, marine invertebrates and fish: Method development and microplastic accumulation. Mar. Pollut. Bull. 122, 403–408. 10.1016/j.marpolbul.2017.2017.06.081 [DOI] [PubMed] [Google Scholar]
- Lenz R, Enders K, Nielsen TG, 2016. Microplastic exposure studies should be environmentally realistic. Proc Natl Acad Sci. 113, E4121–E4122. https://doi.org/10.1073.pnas.1606615113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz R, Enders K, Stedmon CA, MacKenzie DMA, Nielsen TG, 2015. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 100, 82–91. 10.1016/j.marpolbul.2015.09.026 [DOI] [PubMed] [Google Scholar]
- Llorca M, Schirinzi G, Martínez M, Barceló D, Farré M, 2018. Adsorption of perfluoroalkyl substances on microplastics under environmental conditions. Environ. Pollut. 235, 680–691. 10.1016/j.envpol.2017.12.075 [DOI] [PubMed] [Google Scholar]
- Löder MGJ, Gerdts G, 2015. Methodology Used for the Detection and Identification of Microplastics—A Critical Appraisal, In: Bergmann M, Gutow L, K M (Ed.), Marine Anthropogenic Litter (pp 201–227) Springer, Cham; 10.1007/978-3-319-16510-3_8 [DOI] [Google Scholar]
- Lusher AL, Tirelli V, O’Connor I, Officer R 2015. Microplastics in Arctic polar waters: the first reported values of particles in surface and sub-surface samples. Sci. Rep. 5, 14947 10.1038/srep14947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusher AL, Welden NA, Sobral P, Cole M, 2017a. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods 9, 1346–1360. 10.1039/C6AY02415G [DOI] [Google Scholar]
- Lusher AL, Hurley R, Vogelsang C, Nizzetto L, and Olsen M 2017b. Mapping microplastics in sludge. Norwegian Institute for Water Research Report No. 7215–2017, Norwegian Environment Agency Report No. M-907/2017, 55 pages. [Google Scholar]
- Mani T, Hauk A, Walter U, Burkhardt-Holm P, 2015. Microplastics profile along the Rhine River. Sci. Rep. 5, 1–7. 10.1038/srep17988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason SA, Garneau D, Sutton R, Chu Y, Ehmann K, Barnes J, Fink P, Papazissimos D, Rogers DL, 2016. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 218, 1045–1054. 10.1016/i.envpol.2016.08.056 [DOI] [PubMed] [Google Scholar]
- McCormick A, Hoellein TJ, Mason SA, Schluep J, Kelly JJ, 2014. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 48, 11863–11871. 10.1021/es503610r [DOI] [PubMed] [Google Scholar]
- Moore CJ, Moore SL, Weisberg SB, Lattin GL, Zellers AF 2002. A comparison of neustonic plastic and zooplankton abundance in southern California’s coastal waters. Marine Pollut Bull 44, 1035–1038. 10.1016/S0025-326X(02)00150-9 [DOI] [PubMed] [Google Scholar]
- Miller RZ, Watts AJR, Winslow BO, Galloway TS, Barrows APW, 2017. Mountains to the sea: River study of plastic and non-plastic microfiber pollution in the northeast USA. Mar. Pollut. Bull. 124, 245–251. 10.1016/j.marpolbul.2017.07.028 [DOI] [PubMed] [Google Scholar]
- Napper IE, Bakir A, Rowland SJ, Thompson RC, 2015. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 99, 178–185. 10.1016/j.marpolbul.2015.07.029.2015.07.029 [DOI] [PubMed] [Google Scholar]
- Ng E, Huerta E, Eldridge SM, Johnston P, Hu H, Geissen V, Chen D, 2018. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 627, 1377–1388. 10.1016/j.scitotenv.2018.01.341 [DOI] [PubMed] [Google Scholar]
- Nizzetto L, Futter M, Langaas S, 2016. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 50, 10777–10779. 10.1021/acs.est.6b04140 [DOI] [PubMed] [Google Scholar]
- Nobre CR, Santana MFM, Maluf A, Cortez FS, Cesar A, Pereira CDS, Turra A, 2015. Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Mar. Pollut. Bull. 92, 99–104. 10.1016/j.marpolbul.2014.12.050 [DOI] [PubMed] [Google Scholar]
- Northwest Power and Conservation Council. 2018, June 1 Snake River. Retrieved from https://www.nwcouncil.org/reports/columbia-river-history/snakeriver [Google Scholar]
- QGIS Development Team. 2018. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org [Google Scholar]
- Ribeiro-Claro P, Nolasco MM, Araujo C, 2017. Characterization of microplastics by Raman spectroscopy. Compr Anal Chem, 75, 119–151. 10.1016/bs.coac.2016.10.001 [DOI] [Google Scholar]
- Rochman CM, Browne MA, Halpern BS, Hentschel BT, Hoh E, Karapanagiati HK, Rios-Mendoza LM, Takada H, Teh S, Thompson RC, 2013a. Classify plastic waste as hazardous. Nature 494, 169–171. 10.1038/494169a [DOI] [PubMed] [Google Scholar]
- Rochman CM, Hoh E, Kurobe T, Teh SJ, 2013b. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 3, 1–7. 10.1038/srep03263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setälä O, Magnusson K, Lehtiniemi M, Norén F 2016. Distribution and abundance of surface water microliter in the Baltic Sea: a comparison of two sampling methods. Mar. Pollut. Bull. 110, 177–183. 10.1016/j.marpolbul.2016.06.065 [DOI] [PubMed] [Google Scholar]
- Shim WJ, Hong SH, Eo SE, 2017. Identification methods in microplastic analysis: a review. Anal. Methods 9, 1384–1391. 10.1039/C6AY02558G [DOI] [Google Scholar]
- Silva AB, Bastos AS, Justino CI, da Costa JP, Duarte AC, Rocha-Santos TA 2018. Microplastics in the environment: Challenges in analytical chemistry- A review. Analytica chimica acta. 1017, 1–19. 10.1016/j.aca.2018.02.043 [DOI] [PubMed] [Google Scholar]
- Sussarellu R, Suquet M, Thomas Y, Lambert C, Fabioux C, Pernet MEJ, Le Goiïc N, Quillien V, Mingant C, Epelboin Y, Corporeau C, Guyomarch J, Robbens J, Paul-Pont I, Soudant P, Huvet A, 2016. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. 113, 2430–2435. 10.1073/pnas.1519019113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga M, Hengstmann E, Fischer EK, 2018. Microplastic analysis in the South Funen Archipelago, Baltic Sea, implementing manta trawling and bulk sampling. Mar. Pollut. Bull. 128, 601–608. 10.1016/j.marpolbul.2018.01.066 [DOI] [PubMed] [Google Scholar]
- Team RC, 2018. R: A language and environment for statistical computing [Google Scholar]
- Teuten EL, Saquing JM, Knappe DRU, Barlaz MA, Jonsson S, Bjorn A, Rowland SJ, Thompson RC, Galloway TS, Yamashita R, Ochi D, Watanuki Y, Moore C, Viet PH, Tana TS, Prudente M, Boonyatumanond R, Zakaria MP, Akkhavong K, Ogata Y, Hirai H, Iwasa S, Mizukawa K, Hagino Y, Imamura A, Saha M, Takada H, 2009. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 364, 2027–2045. 10.1098/rstb.2008.0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauwenberghe L, Janssen CR, 2014. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 193, 65–70. 10.1016/j.envpol.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Vermaire JC, Pomeroy C, Herczegh SM, Haggart O, 2017. Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries. Facets 2, 301–314. 10.1139/facets-2016-0070 [DOI] [Google Scholar]
- Wagner M, Scherer C, Alvarez-Munoz D, Brennholt N, Bourrain X, Buchinger S, 2014. Microplastics in freshwater ecosystems: what we know and what we need to know. Env. Sci Eur. 26, 1–9. 10.1186/s12302-014-0012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SL, Thompson RC, Galloway TS, 2013. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 178, 483–492. 10.1016/i.envpol.2013.02.031 [DOI] [PubMed] [Google Scholar]
- Yonkos LT, Friedel EA, Perez-Reyes AC, Ghosal S, Arthur CD, 2014. Microplastics in four estuarine rivers in the chesapeake bay, U.S.A. Environ. Sci. Technol. 48, 14195–14202. 10.1021/es5036317 [DOI] [PubMed] [Google Scholar]
- Yu Y, Zhou D, Li Z, Zhu C, 2018. Advancement and Challenges of Microplastic Pollution in the Aquatic Environment: a Review. Water Air Soil Pollut. 229,140 10.1007/s11270-018-3788-z [DOI] [Google Scholar]
- Zalasiewicz J, Waters CN, Ivar do Sul JA, Corcoran PL, Barnosky AD, Cearreta A, Edgeworth M, Gałuszka A, Jeandel C, Leinfelder R, McNeill JR, Steffen W, Summerhayes C, Wagreich M, Williams M, Wolfe AP, Yonan Y, 2016. The geological cycle of plastics and their use as a stratigraphic indicator of the Anthropocene. Anthropocene 13, 4–17. 10.1016/j.ancene.2016.01.002 [DOI] [Google Scholar]
- Zhao S, Danley M, Ward JE, Li D, Mincer TJ, 2017. An approach for extraction, characterization and quantitation of microplastic in natural marine snow using Raman microscopy. Anal. Methods 9, 1470–1478. 10.1039/C6AY02302A [DOI] [Google Scholar]
- Zubris KAV, Richards BK, 2005. Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 138, 201–211. 10.1016/j.envpol.2005.04.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Histogram comparing size distributions of MPs between sampling methods.
Fig. S2. Spearman’s rank correlation reveals a positive correlation between MPs m−3 and location in kilometers downriver from river source.
Fig. S3. Examples of MP types identified in this study.
Fig.S4. Example Raman spectra for identified MPs
Table S1. Location, flow rate, volume of water sampled, total and types of MPs abundance (in both net and grab samples) for each sampling site.