Abstract
Background
This study aimed to investigate the effectiveness of perioperative parecoxib sodium combined with transversus abdominis plane (TAP) block on postoperative pain management following hepatectomy in patients with hepatocellular carcinoma (HCC).
Material/Methods
One hundred patients with HCC who underwent hepatectomy were randomized into a study group (n=51) and a control group (n=49). The study group received 40 mg of parecoxib sodium 30 minutes before anesthetic induction, and 150 mg of 0.375% ropivacaine with 5 mg dexamethasone as TAP inhibitors, before closing the abdominal incision. The control group received 40 mg of placebo 30 minutes before anesthetic induction, without TAP block. Postoperatively, all patients received patient-controlled intravenous analgesia (PCIA) and evaluation with subjective visual analog scale (VAS) pain scores. Data on adverse events, postoperative ambulation (>6 hours/day), time of flatus and defecation, and hospitalization duration were recorded.
Results
Pain scores of the study group were significantly lower compared with the control group on the first three postoperative days. No significant differences were found between the two groups in terms of adverse events. In the study group, the number of cases of postoperative ambulation was significantly more than the control group. The onset of flatus and defecation and duration of hospital stay in the study group were significantly shorter in the study group compared with the control group.
Conclusions
Parecoxib sodium combined with TAP block effectively reduced postoperative pain, improved ambulation, improved gastrointestinal function, and shortened hospitalization time following hepatectomy in patients with HCC without adverse effects.
MeSH Keywords: Analgesia; Carcinoma, Hepatocellular; Hepatectomy
Background
Hepatectomy is a surgical procedure that is associated with severe acute postoperative pain, which can result in stress leading to a series of complications and impaired clinical outcome unless there is adequate pain control [1,2]. Also, acute postoperative pain can lead to suppression of the immune function, primarily cellular immune function, which increases the risk of postoperative infection, tumor recurrence, and metastasis [3,4]. Therefore, the use of timely and effective perioperative analgesia is of great clinical and practical importance for rapid postoperative recovery and competent immune function in patients with hepatocellular carcinoma (HCC) undergoing hepatectomy.
The Enhanced Recovery After Surgery (ERAS) is a multidisciplinary approach to the peri-operative management of patients that was proposed in the 1990s, which has become increasingly implemented by medical staff including surgeons, anesthetists, nurses, and dieticians who aim to minimize postoperative complication rates and accelerate postoperative recovery. The ERAS initiative involves a series of evidence-based medical measures for reducing peri-operative patient stress to achieve the goal of rapid postoperative recovery [5,6]. Previously published studies have shown that ERAS has distinct advantages compared with traditional perioperative management methods in improving the treatment effect, shortening hospital stay, and reducing medical costs [7,8]. Effective perioperative analgesia plays a decisive role in implementing ERAS, which can reduce postoperative complications and promote early postoperative recovery. However, using the single-mode analgesic method it is difficult to achieve a satisfactory analgesic effect, and most postoperative patients still show a significant degree of pain. Therefore, the concept of multimodal and preventive analgesia was proposed to meet the analgesic needs of the postoperative patient [9,10].
Multimodal analgesia is mainly the combination of two or more different analgesic methods throughout the entire perioperative period, including preoperative, intraoperative, and postoperative analgesic management, resulting in synergistic analgesic effects [11]. Because of the cumulative or synergistic effects of different forms of analgesia with concomitant reduction of side effects, there can be a dose reduction of analgesic drugs, which reduces drug-induced adverse reactions and the incidence of postoperative complications [12]. Preventive analgesia is one of the most important ways to reduce or eliminate the incidence of allodynia and hyperalgesia by diminishing peripheral and central sensitization via intercepting the transmission of painful stimuli [13,14].
Based on the concept of ERAS, a multimodal preventive analgesia program was developed for the perioperative analgesic management of patients with HCC undergoing hepatectomy. The program includes the use of parecoxib sodium, a selective cyclooxygenase-2 (COX-2) inhibitor, which is administered intravenously 30 minutes before anesthetic induction, together with the use of a transversus abdominis plane (TAP) block technique before closing the abdomen. Although the analgesic effect of parecoxib sodium and the TAP block technique has previously been reported, the clinical value of this combined approach, as adjuvant therapy for acute perioperative pain, remains unknown.
Therefore, the aim of this prospective study was to investigate the effectiveness of perioperative parecoxib sodium combined with the TAP block on postoperative pain management following hepatectomy in patients with HCC.
Material and Methods
Study design
A single center, randomized, controlled, prospective clinical study of multimodal preventive analgesia for hepatic surgery was undertaken. The study was conducted following the Ethical Principles of the Declaration of Helsinki and was approved by the local Ethics Committee of the First Affiliated Hospital of University of Science and Technology of China. Written informed consent was obtained from all patients.
Study inclusion criteria were patients with hepatocellular carcinoma (HCC) who underwent hepatectomy, aged 18–75 years, and with American Society of Anesthesia (ASA) class I or II physical status. Exclusion criteria were cardiac insufficiency, respiratory insufficiency, uncontrolled hypertension, diabetes mellitus, peptic ulcer disease or a history of peptic bleeding, hematologic disorders, renal insufficiency, allergic to non-steroidal anti-inflammatory drugs (NSAIDs) or ropivacaine, bronchial asthma and long-term treatment with analgesics (NSAIDs, opioids) or steroids, and paresthesia or peripheral neuropathy.
Following patient selection using the inclusion and exclusion criteria, the patients with HCC who underwent hepatectomy from January 2015 to December 2016 in the Department of General Surgery, Anhui Provincial Hospital (Hefei, China) were included in this study. A randomized, single-blind controlled method was used by giving the study participants random envelopes containing their group assignment (study, or control) using a random number table. Envelopes were drawn from the patients and randomized into two groups with a 1: 1 allocation ratio: the study group (group S, n=60) and the control group (group C, n=60). In this study group, open hepatectomy was performed for all patients with HCC with the surgical incision below the right costal margin.
Analgesic strategy
All patients were routinely given intravenous anesthesia, underwent endotracheal intubation, and their vital signs were monitored. The patients in the study group received intravenous doses of 40 mg of parecoxib sodium (diluted to 4 ml with normal saline) 30 min before anesthetic induction, and 150 mg of 0.375% ropivacaine combined with 5 mg of dexamethasone were infused into the fascia between the muscles of the obliquus internus abdominis and the transversus abdominis by the transversus abdominis plane (TAP) block technique before closing of the abdomen. The patients in the control group, received 40 mg of placebo intravenously (normal saline) 30 minutes before anesthetic induction, without TAP block, before the closing of the abdomen. Then, 40 mg of parecoxib sodium was injected intravenously every 12 hours for 72 hours for all patients after surgery. Patient-controlled intravenous analgesia (PCIA) was then commenced, consisting of sufentanil, tropisetron, and dezocine for all patients after surgery. The infusion dose for sufentanil was 0.2 μg/kg/h, the controlled volume was 2 mL each time, with an interval time of 15 minutes, and ceased after 48 h. If the pain score was >3 points, patients could increase the amount of analgesic dose by self-control. A summary of analgesia schedules is shown in Table 1.
Table 1.
Schemes and schedule of the analgesia for the study group and the control group.
| Perioperation | Group S | Group C |
|---|---|---|
| Preoperative | The patients were administered intravenously 40 mg of parecoxib sodium (diluted to 4 ml with saline) 30 min before anesthetic induction | The patients were administered intravenously 40 mg of placebo (normal saline) 30 min before anesthetic induction |
| Intraoperative | TAP block was performed with 150 mg of 0.375% ropivacaine and 5 mg dexamethasone before the closing of the abdomen | Without TAP block management before closing abdomen |
| Postoperative | The 40 mg of parecoxib sodium was then injected intravenously every 12 hours for 72 hours for all patients after surgery | |
| Postoperative | PCIA was started with the constituent of sufentanil, tropisetron and dezocine for all patients after surgery | |
Group S – study group; Group C – control group; TAP – transversus abdominis plane; PCIA – patient controlled intravenous analgesia.
Evaluation of analgesic efficacy and safety
A visual analog scale (VAS) pain score was used to assess the degree of postoperative pain at 24, 48, and 72 hours, both at rest and during coughing. The VAS scores were as follows: 0, no pain; 1–3, mild pain; 4–6, moderate pain; 7–9, severe pain, 10, extremely severe pain [15]. Pain history was assessed in all patients, and all patients included in the study reported no preoperative pain.
Safety was evaluated based on the occurrence of postoperative adverse events, including nausea, vomiting, pruritus, urinary retention, hypotension, respiratory depression, and other adverse reactions. Postoperative gastrointestinal function was evaluated by time of onset of passing flatus, and defecation. Postoperative ambulation (>6 hours a day), and hospital stay were also recorded in the two groups. The standard requirements for hospital discharge for patients was defined as the presence of flatus, defecation, daily ambulation time >6 h, a surgical incision that healed well without infection, a VAS score <2 points without analgesics, and normothermia.
Statistical analysis
Data were analyzed using the Microsoft Excel 2003, and statistical analysis was performed using SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA). Categorical variables were presented as the number of cases. Continuous (numerical) data were expressed as the mean ± standard deviation (SD) with the normally distributed data, or as the median and quartiles for skewed data distributions. The Pearson chi-squared (χ2) test and Fisher’s exact test were used to analyze categorical variables. Student’s t-test, analysis of variance (ANOVA) for repeated measures, and the Mann-Whitney test were used to analyze continuous data. A two-tailed t-test determined was used and a P-value <0.05 was considered to be statistically significant.
Results
Demographic and clinical patient data
One hundred and forty patients with hepatocellular carcinoma (HCC) were enrolled in the study before surgery, and 120 patients were randomized into two groups, the study group, and the control group, according to the inclusion and exclusion criteria (1: 1 ratio), and 100 participants completed this study (group S=51; group C=49) (Figure 1). The demographic and clinical data were acquired from the medical records and included age, sex, body weight, body height, body mass index (BMI), American Society of Anesthesia (ASA) physical status class, intraoperative blood loss, and operative time.
Figure 1.
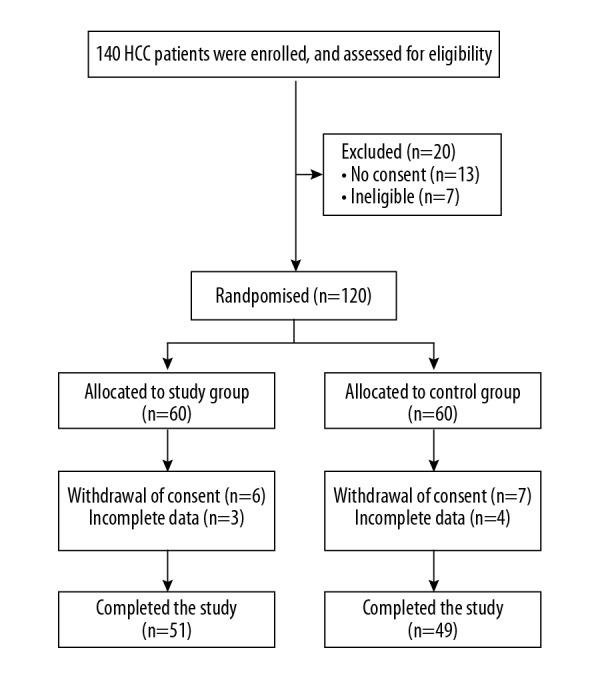
Flow diagram of the study design. One hundred and forty patients with hepatocellular carcinoma (HCC) were enrolled in the study, before hepatectomy, and 100 participants completed this study.
The patients included in the study consisted of 68 men and 32 women, with a mean age of 57.9±7.2 years (range, 45.0–68.0 years), a mean body weight of 65.8±7.6 kg (range, 50.0–78.0 kg), a mean height of 171.9±7.4 cm (range, 157.0–182.0 cm), and a mean BMI of 22.2±1.1 kg/m2 (range, 20.0–25.3 kg/m2). Following the ASA grading standards, 64 patients were grade I, and 36 patients were grade II. The mean intraoperative blood loss was 196.0±83.1 mL (range, 50.0–350.0 mL). The mean operative time was 160.8 ±46.0 min (range, 100.0–400.0 min). There was no significant difference in age, sex, body weight, body height, BMI, ASA grade, intraoperative blood loss, and operation time between the two groups (P>0.05) (Table 2).
Table 2.
Demographic and clinical characteristics of all patients.
| Demographic and clinical data | Group S (n=51) (%) | Group C (n=49) (%) | P-value |
|---|---|---|---|
| Age (years) | 57.6±6.9 | 58.1±7.6 | 0.744 |
| Sex (Males/Female) | 34/17 (66.7%/33.3%) | 34/15 (69.4%/30.6%) | 0.771 |
| Body weight (kg) | 65.8±7.6 | 65.9±7.6 | 0.946 |
| Body height (cm) | 171.7±7.2 | 172.2±7.8 | 0.720 |
| BMI (kg/m2) | 22.2±1.2 | 22.1±1.0 | 0.649 |
| ASA grade (I/II) | 34/17 (66.7%/33.3%) | 30/19 (61.2%/38.8%) | 0.571 |
| Intraoperative blood loss (mL) | 186.3±86.6 | 206.1±78.8 | 0.234 |
| Operative time (min) | 157.1±33.4 | 164.7±56.2 | 0.409 |
Group S – study group; Group C – control group; ASA – American Society of Anesthesiologists; BMI – body mass index; Continuous data are expressed as means ±SD; Categorical data were presented as number of cases and constituent ratio.
Postoperative visual analog scale (VAS) pain scores
The VAS pain scores in the study group were significantly lower compared with the control group for the 1st, 2nd and 3rd postoperative days (P<0.001). The mean VAS score in the study group was 3.0±0.8 (range, 1.0–5.0) for the 1st day, 2.2±1.0 (range, 1.0–4.0) for the 2nd day, and 1.6±0.8 (range, 0–3.0) for the 3rd day. The mean values of VAS in the control group were 4.6±1.1 (range 3.0–8.0) for the 1st day, 3.6±1.2 (range, 1.0–7.0) for the 2nd day, and 2.4±1.2 (range, 1.0–6.0) for the 3rd day (Table 3).
Table 3.
Postoperative VAS pain scores in the two groups.
| Postoperative VAS scores | Groups | P-value | |
|---|---|---|---|
| Group S (n=51) | Group C (n=49) | ||
| 1st day | 3.0±0.8 | 4.6±1.1 | <0.001 |
| 2nd day | 2.2±1.0 | 3.6±1.2 | <0.001 |
| 3rd day | 1.6±0.8 | 2.4±1.2 | <0.001 |
Group S – study group; Group C – control group; VAS – visual analogue scales.
Postoperative adverse events
Postoperative nausea and vomiting occurred in 9 cases in the study group (9/51, 17.6%), and in 13 cases in the control group (13/49, 26.5%). Pruritus occurred in 4 cases in the study group (4/51, 7.8%), and in 2 cases in the control group (2/49, 4.1%). Urinary retention was found in 3 cases in the study group (3/51, 5.9%), and in 4 cases in the control group (4/49, 8.2%). The difference in the incidence of nausea, vomiting, pruritus and urinary retention between the two groups was not statistically significant (P>0.05) (Table 4). None of the patients had respiratory depression, hypotension, and other adverse reactions.
Table 4.
Postoperative adverse events in the two groups.
| Adverse events | Groups | P-value | |
|---|---|---|---|
| Group S (n=51) (%) | Group C (n=49) (%) | ||
| Nausea and vomiting | |||
| Absent | 42 (82.4%) | 36 (73.5%) | 0.284 |
| Present | 9 (17.6%) | 13 (26.5%) | |
| Pruritus | |||
| Absent | 47 (92.2%) | 47 (95.9%) | 0.428 |
| Present | 4 (7.8%) | 2 (4.1%) | |
| Urinary retention | |||
| Absent | 48 (94.1%) | 45 (91.8%) | 0.655 |
| Present | 3 (5.9%) | 4 (8.2%) | |
Group S – study group; Group C – control group.
Postoperative ambulation time
The number of cases with postoperative ambulation (>6 hours a day) in the study group was significantly higher compared with the control group (P=0.026 for the 1st day; P=0.002 for the 2nd day; P=0.028 for the 3rd day). The number of cases with postoperative ambulation (>6 hours a day) in the study group was 11 (21.6%) for the 1st day, 23 (45.1%) for the 2nd day, and 32 (62.7%) for the 3rd day. The number of cases with postoperative ambulation in the control group (>6 hours a day) was 3 (6.1%) for the 1st day, 8 (16.3%) for the 2nd day, and 20 (40.8%) for the 3rd day (Table 5).
Table 5.
The number of cases of postoperative ambulation (>6 h for every day) in the two groups.
| Postoperative ambulation time | Groups | P-value | |
|---|---|---|---|
| Group S (n=51) (%) | Group C (n=49) (%) | ||
| 1st day | |||
| ≤6 h | 40 (78.4%) | 46 (93.9%) | 0.026 |
| >6 h | 11 (21.6%) | 3 (6.1%) | |
| 2nd day | |||
| ≤6 h | 28 (54.9%) | 41 (83.7%) | 0.002 |
| >6 h | 23 (45.1%) | 8 (16.3%) | |
| 3rd day | |||
| ≤6 h | 19 (37.3%) | 29 (59.2%) | 0.028 |
| >6 h | 32 (62.7%) | 20 (40.8%) | |
Group S – study group; Group C – control group.
Time of flatus, defecation, and hospitalization after surgery
The mean value of flatus time was 30.4±7.2 h in the study group and 36.6±7.1 h in the control group. The mean postoperative time to commencing defecation was 50.9±5.3 h in the study group and 61.3±7.3 h in the control group. The mean postoperative hospital stay was 6.2±0.8 days in the study group and 9.6±1.1 days in the control group. The time of flatus, defecation, and postoperative hospitalization time were significantly shorter in the study group compared with the control group (all, P<0.001) (Table 6).
Table 6.
Time of anal exhaust, defecation and hospitalization after surgery in the two groups.
| Time | Groups | P-value | |
|---|---|---|---|
| Group S (n=51) | Group C (n=49) | ||
| Anal exhaust (h) | 30.4±7.2 | 36.6±7.1 | <0.001 |
| Defecation (h) | 50.9±5.3 | 61.3±7.3 | <0.001 |
| Hospitalization (d) | 6.2±0.8 | 9.6±1.1 | <0.001 |
Group S – study group; Group C – control group.
Discussion
This study aimed to investigate the clinical value of perioperative multimodal preventive analgesia by using the combination of intravenous parecoxib sodium and the transversus abdominis plane (TAP) block method following hepatectomy in patients with hepatocellular carcinoma (HCC). The results showed that the postoperative visual analog scale (VAS) pain scores in the study group were significantly lower than in the control group. There was no difference between two groups in the incidence of adverse events such as nausea, vomiting, pruritus, and urinary retention, which indicates the safety of parecoxib combined with the TAP block in the management of acute postoperative pain. The number of cases with postoperative ambulation (>6 hours a day) in the study group was significantly greater than in the control group. The time of flatus, defecation, and hospitalization in the study group was significantly shorter than in the control group.
Currently, opioids are frequently used to relieve pain, which act by binding to the opioid receptors in the central and peripheral nervous system [16]. The side effects of opioids may include nausea, vomiting, dizziness, itching, sedation, respiratory depression, uroschesis, constipation, and euphoria [17]. Excessive or concurrent use of other strong analgesics can lead to respiratory depression [17]. Non-steroidal anti-inflammatory drugs (NSAIDs) prevent the synthesis of prostaglandins from arachidonic acid by inhibiting cyclooxygenase (COX) and are considered to be a better choice as an adjuvant during the perioperative period [18]. The major advantage of COX inhibitors over opiates is that they produce effective analgesia without respiratory depression.
Parecoxib sodium is a highly selective COX-2 inhibitor that selectively inhibits the activity of COX-2 but does not affect COX-1 activity, which significantly reduces the risk of developing peptic ulcer or hemorrhage. Therefore, parecoxib sodium is favored as an adjunct in acute postoperative pain management [19]. Previously published studies have shown that parecoxib sodium, alone or in combination with other analgesics, was safe and feasible for the management of postoperative pain in the patients who underwent surgery, including hepatectomy [20–25]. Also, postoperative pain, the consumption of opioids, and the incidence of drug-related adverse reactions were significantly lower with parecoxib, which increased the postoperative recovery of patients [20–25]. The results of the present study also showed that in patients with HCC, following hepatectomy, the use of a multimodal preventive analgesia program that included parecoxib significantly reduced the degree of the postoperative pain without any significant drug-related adverse reactions.
Dexamethasone is a long-acting glucocorticoid that inhibits the accumulation of inflammatory cells and the release of inflammatory mediators, which can improve the nonspecific response to surgical stress and reduce surgery-induced harm. Therefore, dexamethasone can achieve a good analgesic effect as an effective drug in multimodal analgesic management. Ropivacaine is a long-acting local anesthetic drug belonging to the aminoamide group, and adverse drug reactions are rare when it is used as a nerve block. Low concentrations (0.6%) of ropivacaine can result in a satisfactory independent sensory and motor nerve block without affecting gastrointestinal function.
The TAP block is a peripheral nerve block technique designed to anesthetize the nerves supplying the anterior abdominal wall (T6 to L1) [26]. The local anesthetic is infused into the space between the obliquus internus abdominis and the transversus abdominis muscles by using the TAP block method, only in the plane through which the sensory nerves pass [27]. The TAP block is a practical technique that can facilitate postoperative pain management following abdominal, gynecologic, obstetric, and urologic surgery that usually affect the T6 to L1 sensory nerves [28–32]. Some studies have shown that the TAP block is an effective form of analgesia in colorectal surgery, bariatric surgery, cholecystectomy, open appendectomy, and laparoscopic ventral hernia repair [32–35]. The skin, muscle, and parietal peritoneum of the operation field are innervated by the thoracic and lumbar nerves (T6–L1). After leaving the intervertebral foramen, these sensory nerves cross the transverse process and penetrate the lateral abdominal wall muscle into the fascia between the obliquus internus abdominis and the transversus abdominis muscles. Therefore, injection of ropivacaine, using the TAP block technique, can effectively block the abdominal T6–L1 sensory nerves distribution and achieve a local analgesic effect. In this study, the patients in the study group had an intravenous injection of parecoxib sodium before surgery, combined with the TAP block method. In the control group, only intravenous parecoxib sodium was used after surgery. The results showed that the analgesic effect of the combination program was significantly better when compared with postoperative intravenous parecoxib alone.
This study had several limitations. Firstly, this was a single-center prospective study with small patient study size. Second, the effect of intravenous parecoxib sodium and the TAP block method on postoperative pain were not compared separately. Third, the difference in the consumption of opioid pain medication was not evaluated. Also, differences in serum inflammatory mediators were not studied. Therefore, additional, multi-center prospective studies with larger cohorts are required to assess the safety and effectiveness of perioperative parecoxib sodium administration combined with the TAP block method, as well as their long-term effect on prognosis after hepatectomy in patients with HCC.
Conclusions
The findings of this study showed that combined preventive analgesia with parecoxib sodium plus the transversus abdominis plane (TAP) block technique significantly relieved postoperative pain without significant adverse effects, and also resulted in early ambulation, recovery of gastrointestinal function, and reduced the length of hospitalization after hepatectomy in patients with hepatocellular carcinoma (HCC). Therefore, the application of preventive analgesia with parecoxib sodium and the TAP block technique is safe and effective and can help patients with HCC recover more rapidly after hepatectomy.
Footnotes
Source of support: This study was supported by the Science and Technology Research Foundation of Anhui Province (No. 1704a0802150)
Conflict of interest
None.
References
- 1.Argoff CE. Recent management advances in acute postoperative pain. Pain Pract. 2014;14:477–87. doi: 10.1111/papr.12108. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 3.Conrick-Martin I, Buggy DJ. The effects of anesthetic and analgesic techniques on immune function. J Clin Anesth. 2013;25:253–54. doi: 10.1016/j.jclinane.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Cata JP, Bauer M, Sokari T, et al. Effects of surgery, general anesthesia, and perioperative epidural analgesia on the immune function of patients with non-small cell lung cancer. J Clin Anesth. 2013;25:255–62. doi: 10.1016/j.jclinane.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson A, Lowe MC, Parker J, et al. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg. 2014;101:172–88. doi: 10.1002/bjs.9394. [DOI] [PubMed] [Google Scholar]
- 6.Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: A review. JAMA Surg. 2017;152:292–98. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 7.Ni TG, Yang HT, Zhang H, et al. Enhanced recovery after surgery programs in patients undergoing hepatectomy: A meta-analysis. World J Gastroenterol. 2015;21:9209–16. doi: 10.3748/wjg.v21.i30.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant MC, Yang D, Wu CL, et al. Impact of enhanced recovery after surgery and fast track surgery pathways on healthcare-associated infections: Results from a systematic review and meta-analysis. Ann Surg. 2017;265:68–79. doi: 10.1097/SLA.0000000000001703. [DOI] [PubMed] [Google Scholar]
- 9.Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77:1048–56. doi: 10.1213/00000539-199311000-00030. [DOI] [PubMed] [Google Scholar]
- 10.Dionne R. Preemptive vs. preventive analgesia: Which approach improves clinical outcomes? Compend Contin Educ Dent. 2000;21:48, 51–54, 56. [Google Scholar]
- 11.Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: What do they really mean? Plast Reconstr Surg. 2014;134:85S–93S. doi: 10.1097/PRS.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 12.Luo J, Min S. Postoperative pain management in the postanesthesia care unit: An update. J Pain Res. 2017;10:2687–98. doi: 10.2147/JPR.S142889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vadivelu N, Mitra S, Schermer E, et al. Preventive analgesia for postoperative pain control: A broader concept. Local Reg Anesth. 2014;7:17–22. doi: 10.2147/LRA.S62160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–88. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 15.McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: A critical review. Psychol Med. 1988;18:1007–19. doi: 10.1017/s0033291700009934. [DOI] [PubMed] [Google Scholar]
- 16.Barletta JF, Asgeirsson T, Senagore AJ. Influence of intravenous opioid dose on postoperative ileus. Ann Pharmacother. 2011;45:916–23. doi: 10.1345/aph.1Q041. [DOI] [PubMed] [Google Scholar]
- 17.Goettsch WG, Sukel MP, van der Peet DL, et al. In-hospital use of opioids increases rate of coded postoperative paralytic ileus. Pharmacoepidemiol Drug Saf. 2007;16:668–74. doi: 10.1002/pds.1338. [DOI] [PubMed] [Google Scholar]
- 18.Kaye AD, Baluch A, Kaye AJ, et al. Pharmacology of cyclooxygenase-2 inhibitors and preemptive analgesia in acute pain management. Curr Opin Anaesthesiol. 2008;21:439–45. doi: 10.1097/ACO.0b013e3283007e8d. [DOI] [PubMed] [Google Scholar]
- 19.Gajraj NM. COX-2 inhibitors celecoxib and parecoxib: Valuable options for postoperative pain management. Curr Top Med Chem. 2007;7:235–49. doi: 10.2174/156802607779941323. [DOI] [PubMed] [Google Scholar]
- 20.Nong L, Sun Y, Tian Y, et al. Effects of parecoxib on morphine analgesia after gynecology tumor operation: A randomized trial of parecoxib used in postsurgical pain management. J Surg Res. 2013;183:821–26. doi: 10.1016/j.jss.2013.02.059. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Wang S, Wu H, Wu Y. Effect of perioperative parecoxib on postoperative pain and local inflammation factors PGE2 and IL-6 for total knee arthroplasty: A randomized, double-blind, placebo-controlled study. Eur J Orthop Surg Traumatol. 2014;24:395–401. doi: 10.1007/s00590-013-1203-4. [DOI] [PubMed] [Google Scholar]
- 22.Essex MN, Cheung R, Li C, Xie L. Safety of parecoxib when used for more than 3 days for the management of postoperative pain. Pain Manag. 2017;7:383–89. doi: 10.2217/pmt-2017-0017. [DOI] [PubMed] [Google Scholar]
- 23.Essex MN, Xu H, Parsons B, et al. Parecoxib relieves pain and has an opioid-sparing effect following major gastrointestinal surgery. Int J Gen Med. 2017;10:319–27. doi: 10.2147/IJGM.S143837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz-Borjon E, Torres-Gomez A, Essex MN, et al. Parecoxib provides analgesic and opioid-sparing effects following major orthopedic surgery: A subset analysis of a randomized, placebo-controlled clinical trial. Pain Ther. 2017;6:61–72. doi: 10.1007/s40122-017-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim KI, Chiu YC, Chen CL, et al. Effects of pre-existing liver disease on acute pain management using patient-controlled analgesia fentanyl with parecoxib after major liver resection: A retrospective, pragmatic study. Transplant Proc. 2016;48:1080–82. doi: 10.1016/j.transproceed.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 26.Petersen PL, Mathiesen O, Torup H, Dahl JB. The transversus abdominis plane block: A valuable option for postoperative analgesia? A topical review. Acta Anaesthesiol Scand. 2010;54:529–35. doi: 10.1111/j.1399-6576.2010.02215.x. [DOI] [PubMed] [Google Scholar]
- 27.Lissauer J, Mancuso K, Merritt C, et al. Evolution of the transversus abdominis plane block and its role in postoperative analgesia. Best Pract Res Clin Anaesthesiol. 2014;28:117–26. doi: 10.1016/j.bpa.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Rao Kadam V, Van Wijk RM, Moran JL, et al. Continuous transversus abdominis plane block vs. intermittent bolus for analgesia after abdominal surgery: A randomized trial. J Pain Res. 2017;10:1705–12. doi: 10.2147/JPR.S132891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawahara R, Tamai Y, Yamasaki K, et al. The analgesic efficacy of ultrasound-guided transversus abdominis plane block with a mid-axillary approach after gynecologic laparoscopic surgery: A randomized controlled trial. J Anaesthesiol Clin Pharmacol. 2015;31:67–71. doi: 10.4103/0970-9185.150547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Champaneria R, Shah L, Wilson MJ, Daniels JP. Clinical effectiveness of transversus abdominis plane (TAP) blocks for pain relief after caesarean section: A meta-analysis. Int J Obstet Anesth. 2016;28:45–60. doi: 10.1016/j.ijoa.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Güner Can M, Göz R, Berber İ, et al. Ultrasound/laparoscopic camera-guided transversus abdominis plane block for renal transplant donors: A randomized controlled trial. Ann Transplant. 2015;20:418–23. doi: 10.12659/AOT.893926. [DOI] [PubMed] [Google Scholar]
- 32.Ripollés J, Mezquita SM, Abad A, Calvo J. Analgesic efficacy of the ultrasound-guided blockade of the transversus abdominis plane – a systematic review. Braz J Anesthesiol. 2015;65:255–80. doi: 10.1016/j.bjane.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Qazi N, Bhat WM, Iqbal MZ, et al. Postoperative analgesic efficacy of bilateral transversus abdominis plane block in patients undergoing midline colorectal surgeries using ropivacaine: A randomized, double-blind, placebo-controlled trial. Anesth Essays Res. 2017;11:767–72. doi: 10.4103/0259-1162.194577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batko I, Kościelniak BK, Al-Mutari I, Kobylarz K. Benefits of ultrasound-guided transversus abdominis plane block for open appendectomy in children. Anaesthesiol Intensive Ther. 2017;49:198–203. doi: 10.5603/AIT.a2017.0039. [DOI] [PubMed] [Google Scholar]
- 35.Fields AC, Gonzalez DO, Chin EH, et al. Laparoscopic-assisted transversus abdominis plane block for postoperative pain control in laparoscopic ventral hernia repair: A randomized controlled trial. J Am Coll Surg. 2015;221:462–69. doi: 10.1016/j.jamcollsurg.2015.04.007. [DOI] [PubMed] [Google Scholar]


