Abstract
Kaoliang is a refreshing fragranced type of Chinese spirits with slight apple fragrance that comes from ethyl acetate (EA). Special aromas are produced by esterification microorganisms, which affect the taste and quality of the wine. In this study, new yeast strains were isolated from yellow water, a by-product during fermentation process. Meanwhile, the optimal culture condition was determined for its growth and EA production. Three new strains, Kazachstaniaexigua, Candida humilis and Saccharomyces cerevisiae were identified from yellow water. Among these strains, S. cerevisiae S5 was the new and dominant strain. Results from response surface methodology showed that S. cerevisiae S5 produced 161.88 ppm of EA, in the medium with 4.91% yeast extract, 9.82% peptone, and 20.91% glucose after 96 hours of cultivation at 27.53°C. GC analysis showed that aroma compounds, such as EA, isoamyl acetate and 2-phenylethanol increased from the sample of optimal condition when compared to the one from initial fermentation condition.
Introduction
Kaoliang is made from wheat-based koji, sorghum as substrate for solid-state fermentation and distillation to produce fragranced Chinese spirits [1, 2]. During fermentation, kaoliang generates various flavors such as fruits, flowers and grass aroma after blending and aging [3]. Subsequently, microorganisms conduct liquefaction, saccharification, and fermentation, resulting in the production of yellowish brown liquid, referred as yellow water, which is rich in aroma compounds and microbial flora [4]. Ethyl acetate (EA) is the major aroma compound found in kaoliang. Sensory evaluation of EA showed similarity with apple aroma that produced by microbial fermentation and metabolism [1]. Yeasts and Aspergillus spp. are the main microorganisms involved in brewing process [5]. Aspergillus produced amylase to degrade starch into smaller molecule such as carbohydrate and dextrin, yeast conducts alcohol fermentation to produce esters [6]. Ester-producing yeasts are referred as esterification microorganisms, such as Saccharomyces rouxii, Hansenula anomala and Pichia anomala [7, 8].
Cultivation optimization is a key element to optimize the production of bioactive components. The effects of initial pH, carbon source, nitrogen source, inoculation density and temperature have been investigated to optimized aroma and biomass production in wine making [9–12]. Compared to one-factor-at-a time approach, response surface methodology (RSM) is a statistical approach based on the fit of polynomial regression model, which can be applied to validate not only the value of independent variables but also the interaction among them [13, 14]. RSM has been applied for both evaluation of microorganism growth and metabolites production such as polysaccharides, proteins and organic compounds [15–17].
The purpose of this study is to isolate and identify new yeast strains with esterification capacity from sorghum yellow water. The optimal fermentation condition for the news strains to produce aroma compounds especially EA using RSM was evaluated.
Materials and methods
Isolation and purification of microorganisms
Yellow water samples used in this study was provided by the private winery in Zhongxing market (Kinmen). Sample was maintained at 4°C until use. Yeast extract peptone glucose (YEPG) agar is a selective medium used for isolation of eukaryotic microorganism [18] which is composed of 1% yeast extract, 2% peptone, 2% glucose and 2.5% agar, supplemented with 100 mg/L chloramphenicol and 50 mg/L chlortetracycline to inhibit the growth of prokaryotic microorganisms [19]. Serial dilution of yellow water samples were cultured on YEPG agar plate, at 28°C for 36 hours. Selection of strains was performed based on morphological observation. Selected strains were sub-cultured on YEPG agar by streak plate method to obtain single colony. Each strain was sub-cultured to new YEPG agar every two weeks for culture maintenance. Long-term storage of strain was done by adding 20% glycerol into the liquid culture and stored at -80°C.
Strain identification
S. cerevisiae 21447 purchased from Bioresource Collection and Research Center (BCRC, Hsinchu city, Taiwan) was used as standard strain for physiological and biochemical characteristics. Isolated yeasts were identified by comparing DNA sequences using API 20 C AUX yeast identification kit (BioMérieux, Inc., Marcy-l'Étoile, France). The strain characteristics were done by comparing with database as described previously [20]. Strain DNA extraction was conducted as described by Doyle [21]. The 5.8S rDNA amplification was performed using Internal Transcribed Spacer (ITS), ITS1 (5’ TCC GTA GGT GAA CCT GCG G 3’) and ITS4 (5’ TCC TCC GCT TAT TGA TAT GC 3’) [22]. PCR was then performed using Phusion high-fidelity PCR master mix (New England BioLabs, Inc., Massachusetts, USA). DNA sequencing was subsequently performed and the sequences comparisons were then analyzed with Basic Local Alignment Search Tool (BLAST) software (National Center for Biotechnology Information, Maryland, USA).
Yeast fermentation
Yeast culture was produced by inoculating single colonyto 100 ml YEPG medium at 28°C, 150 rpm for 24 hours. The YEPG medium was sterilized medium at 121°C for 20 minutes to avoid possible microbial contamination. This culture was further centrifuged at 3,824 g for 8 minutes. The supernatant was removed and yeast was adjusted to 5% (w/w) with new YEPG medium. Fermentation culture was made by inoculating 1% (v/v) yeast into YEPG medium which supplemented with 14% glucose. This culture was fermented at 28°C, 150 rpm for 96 hours.
Response surface methodology for optimal fermentation
Determination of EA concentration of fermentation culture was conducted using three factors and three levels of Box-Behnken Design. Three factors used in RSM optimization were temperature (X1), nitrogen source (X2), glucose (X3). The ranges were 25–35°C, 6–24% and 10–30%, respectively. Optimal fermentation condition was determined based on EA production. Statistical analysis was conducted using Minitab (Minitab Inc., State College, PA, USA).
Ethyl acetate (EA) extraction and analysis
Fifty ml of fermentation culture was centrifuged at 3,824 g at 4°C for 8 minutes. Twenty ml of the supernatant with 20 ppm of pentylalcohol as internal standard were mixed with 20 ml of dichloromethane for 30 seconds. The mixture was then centrifuged at 4°C for another 8 minutes. Dichloromethane layer was taken for sampling. The procedures were conducted in triplicates. The collected extract was added with sodium sulfate and filtered through filter paper. Filtrate was vacuum concentrated and stored at -20°C. Determination of EA was performed by GC/MS (GC7890/MS5975, Agilent Technologies, Santa Clara, CA, USA). The column used in this study is HP-5MS (Agilent Technologies, Santa Clara, CA, USA).
Statistical analysis
All experiments were conducted with three independent evaluations, with three replications of each. The values were expressed as mean ± standard deviation. The RSM method followed the Box–Behnken design 3-level-3-factor with 5 center point replications. Microsoft Excel was used for the data analysis (Microsoft, Redmond, Washington, USA). Statistical Analysis System (SAS Institute Inc., Cary, North Carolina, USA) was used for T test and Duncan's new multiple range test. Minitab Statistical Software (Minitab Inc., University City, Pennsylvania, USA) was used for RSM evaluation and analysis. Statistical significant differences were all p < 0.05.
Results
Isolation and identification of yeasts
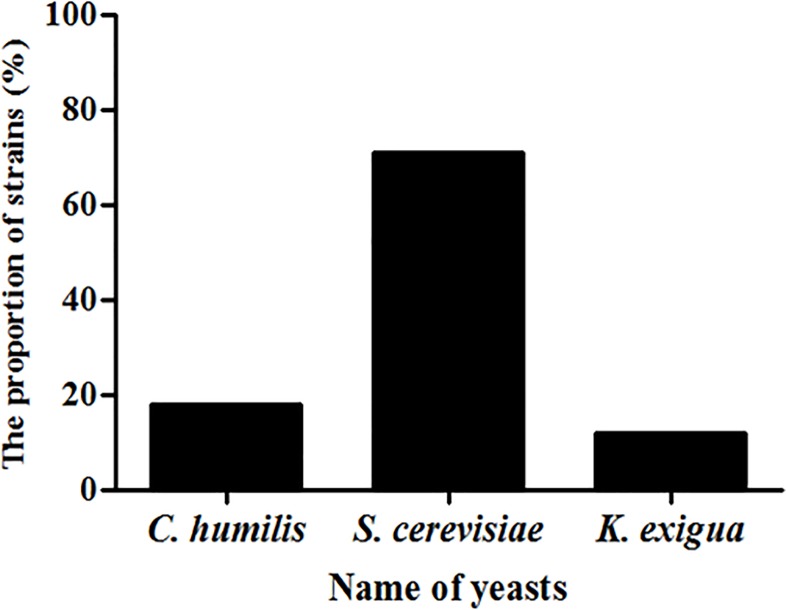
According to the morphological characteristics, biochemical reactions and DNA sequencing, three yeast strains were isolated from the yellow water, and they were identified as Candida humilis, Saccharomyces cerevisiae and Kazachstania exigua. S. cerevisiae accounted for 70.59% of the total isolates, which was the dominant strain (Fig 1).
Fig 1. The proportion of three yeast strains isolated from yellow water.
Strain selection with ethyl acetate production ability
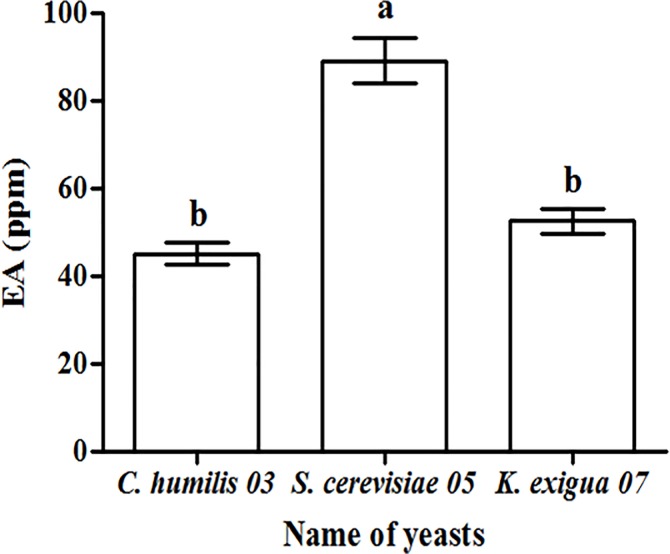
The fermented broths of three isolated strains, C. humilis 03, S. cerevisiae 05 and K. exigua 07 were analyzed for EA contentand the results were summarized in Fig 2. The EA content from C. humilis 03, S. cerevisiae 05 and K. exigua 07 samples were 45.14, 89.08 and 52.51 ppm, respectively. All strains exhibited the esterification ability, and S. cerevisiae 05 was the highest of them.
Fig 2. The EA concentrations of fermented broths from three yeast strains.
S. cerevisiae 05 was chosen for the subsequent study for the following reasons: (1) Safety: S. cerevisiae is a safe and stable food microorganism belonging to general recognized as safe (GRAS) strains as Food and Drug Administration (FDA) announced, which has long been used for baking and brewing [23]; (2) dominant species: S. cerevisiae accounted for 70.59% in yellow water microorganism population, indicating that the fermentation environment was suitable for S. cerevisiae, which may even had the ability to inhibit the growth of pathogens.
Physiological and biochemical characteristics of S. cerevisiae
Result of carbon source assimilation showed S. cerevisiae 05 and S. cerevisiae 21447 had no significant difference in carbon source preference (Table 1), and both of them were identified as S. cerevisiae in 99.9% probability (S1 Fig).
Table 1. The result of carbon source assimilation in yeast identification system API 20 C AUX tests.
| Tests | Active ingredients | S5 | BCRC#21447 |
|---|---|---|---|
| 0 | none | - | - |
| GLU | D-glucose | + | + |
| GLY | glycerol | - | - |
| 2KG | calcium 2-keto-gluconate | - | - |
| ARA | L-arabinose | - | - |
| XYL | D-xylose | - | - |
| ADO | adonitol | - | - |
| XLT | xylitol | - | - |
| GAL | D-galactose | + | + |
| INO | inositol | - | - |
| SOR | D-sorbitol | - | - |
| MDG | methyl-αD-glucopyranoside | + | + |
| NAG | N-acetyl-glucosamine | - | - |
| CEL | D-cellobiose | - | - |
| LAC | D-lactose | - | - |
| MAL | D-maltose | + | + |
| SAC | D-saccharose | + | + |
| TRE | D-trehalose | + | + |
| MLZ | D-melezitose | + | + |
| RAF | D-raffinose | + | + |
| H/PH+ | produced mycelium | - | - |
(-) means negative reaction, and (+) means positive reaction.
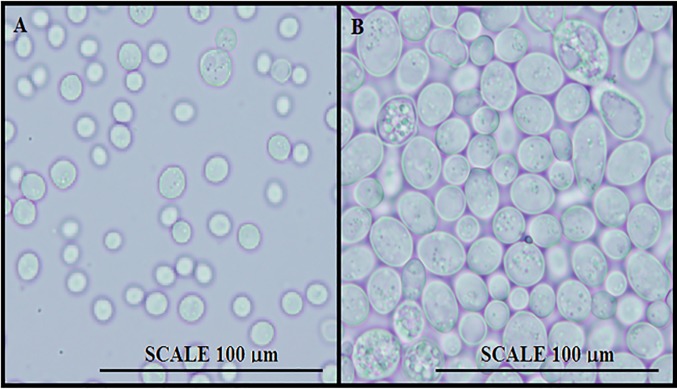
Experiments such as microscope inspection, carbon and nitrogen assimilation, carbohydrate fermentation, high glucose tolerance and cycloheximide resistance were carried out and the results were summarized in Table 2. The only difference was the appearance of the strains as shown in Fig 3. It is evident that the size of S5 strain was smaller than that of the BCRC 21447 strain. The diameters of S5 and BCRC 21447 strains were approximately for 6.3 and 10.0 μm. This phenomenon explained the rapid growth of the S5 strain, where the generation time of the S5 and BCRC 21447 strains were 73.2 and 93.7 minutes, respectively.
Table 2. The result of “Yeasts: Characteristics and identification tests”.
| Appearance of yeasts | Paper result | S5 | BCRC#21447 |
|---|---|---|---|
| Mycelium | - | - | - |
| Ascospore | + | + | + |
| Ballistospore | - | - | - |
| Carbon source assimilation tests | Paper result | S5 | BCRC#21447 |
| D-galactose | + | + | + |
| D-glucosamine | - | - | - |
| D-xylose | - | - | - |
| L-arabinose | - | - | - |
| D-saccharose | + | + | + |
| D-maltose | + | + | + |
| D-cellobiose | - | - | - |
| Salicin | - | - | - |
| D-lactose | - | - | - |
| starch | - | - | - |
| glycerol | - | - | - |
| D-mannitol | + | + | + |
| Lactic acid | - | - | - |
| succinic acid | - | - | - |
| citric acid | - | - | - |
| methanol | - | - | - |
| ethanol | + | + | + |
| L-fructose | + | + | + |
| D-raffinose | + | + | + |
| Nitrogen source assimilation tests | Paper result | S5 | BCRC#21447 |
| Sodium nitrate | - | - | - |
| L-lysine | - | - | - |
| Ammonium sulfate | + | + | + |
| Sugar fermentation tests | Paper result | S5 | BCRC#21447 |
| D-glucose | + | + | + |
| D-galactose | + | + | + |
| D-maltose | + | + | + |
| D-saccharose | + | + | + |
| D-lactose | - | - | - |
| D-cellobiose | - | - | - |
| starch | - | - | - |
| L-fructose | + | + | + |
| D-raffinose | + | + | + |
| Glucose solution osmolaritytests | Paper result | S5 | BCRC#21447 |
| 40% glucose solution | + | + | + |
| 50% glucose solution | + | + | + |
| 60% glucose solution | - | - | - |
| cycloheximide test | Paper result | S5 | BCRC#21447 |
| 100 ppm | - | - | - |
| 1000 ppm | - | - | - |
Fig 3.
The appearance of S5 (A) and BCRC21447 (B) under microscopy.
Response model fitting and adequacy checking
The medium composition and the fermented parameters certainly affect the EA yield of yeasts [24]. Different yeasts prefer specific cultivation system. In this study, three factors namely fermented temperature (X1), nitrogen sources (X2), and glucose (X3) on EA production were investigated using BBD-RSM design (Table 3). The nitrogen sources were yeast extract and peptone, and the ratio of them was kept at 1:2. The results of BBD were summarized in Table 4 and analyzed by multiple regression analysis. Following second-order polynomial equation was obtained.
Table 3. Levels of factors chosen for the Box-Behnken design.
| Factors | Symbols | Coded levels | ||
|---|---|---|---|---|
| -1 | 0 | +1 | ||
| Temperature (oC) | X1 | 25 | 30 | 35 |
| Nitrogen sources (%)* | X2 | 6 | 15 | 24 |
| Glucose (%) | X3 | 10 | 20 | 30 |
* The nitrogen source was composed by peptone and yeast extract. (Peptone: Yeast extract = 2:1)
Table 4. Box-Behnken design matrix and experimental results of EA production and the theoretical EA production of equation.
| Run Order | Factors | EA(ppm) | Theoretical EA | ||
|---|---|---|---|---|---|
| X1 | X2 | X3 | |||
| 1 | 35 | 15 | 30 | 63.00 | 55.10 |
| 2 | 25 | 6 | 20 | 127.15 | 123.79 |
| 3 | 30 | 15 | 20 | 150.03 | 153.53 |
| 4 | 35 | 15 | 10 | 72.64 | 71.52 |
| 5 | 25 | 24 | 20 | 124.34 | 118.44 |
| 6 | 30 | 24 | 30 | 93.60 | 98.07 |
| 7 | 30 | 15 | 20 | 152.15 | 153.53 |
| 8 | 30 | 15 | 20 | 161.47 | 153.53 |
| 9 | 25 | 15 | 10 | 112.06 | 119.60 |
| 10 | 35 | 24 | 20 | 50.89 | 53.89 |
| 11 | 30 | 6 | 30 | 96.94 | 98.88 |
| 12 | 30 | 15 | 20 | 153.72 | 153.53 |
| 13 | 30 | 15 | 20 | 151.83 | 153.53 |
| 14 | 30 | 6 | 10 | 107.66 | 102.77 |
| 15 | 30 | 24 | 10 | 95.82 | 93.49 |
| 16 | 25 | 15 | 30 | 136.01 | 136.71 |
| 17 | 35 | 6 | 20 | 53.11 | 58.64 |
X1 = Temperature; X2 = Nitrogen source; X3 = Glucose
| (Eq 1) |
The analysis of variance for the model is shown in Table 5, and the fitness of it was examined using the determination coefficient (R2 = 0.964), which suggests that the sample variation of 96.4% for EA production was associated with the variable factors. In addition, the lack of fit for the model was insignificant (p> 0.05), verifying the accuracy fit of the second-order model (Eq 1) to the true response of EA production. Moreover, the F value of 49.19 and p value < 0.05 for the regression, supporting the second-order model, adequately approximated the response surface. As a result, canonical analysis demonstrated that the predicted maximum of EA production was 161.88 ppm at fermented temperature 27.5°C, 14.73% nitrogen sources, and 20.91% glucose. The results clearly indicated that all these variables influenced EA yield.
Table 5. Analysis of variance (ANOVA) for response surface quadratic model.
| Source | Coefficient | Degree of freedom | Sum of squares | F value | P value |
|---|---|---|---|---|---|
| Constant | 153.84 | ||||
| X1 | -32.49 | 1 | 0.000 | ||
| X2 | -2.53 | 1 | 0.339 | ||
| X3 | 0.17 | 1 | 0.946 | ||
| X12 | -33.77 | 1 | 0.000 | ||
| X22 | -31.20 | 1 | 0.000 | ||
| X32 | -24.14 | 1 | 0.000 | ||
| X1X2 | 0.15 | 1 | 0.967 | ||
| X1X3 | -8.40 | 1 | 0.047 | ||
| X2X3 | 2.12 | 1 | 0.561 | ||
| Regression | 9 | 21,449.5 | 49.19 | 0.000 | |
| Linear | 3 | 8,495.9 | 58.45 | 0.000 | |
| Square | 3 | 12,653.3 | 87.05 | 0.000 | |
| Interaction | 3 | 300.2 | 2.07 | 0.193 | |
| Residual | 7 | 339.2 | |||
| Lack-of-Fit | 3 | 259.5 | 4.34 | 0.095 | |
| Pure Error | 4 | 79.7 | |||
| Total | 16 | 21,788.6 |
Effects of factors on EA production
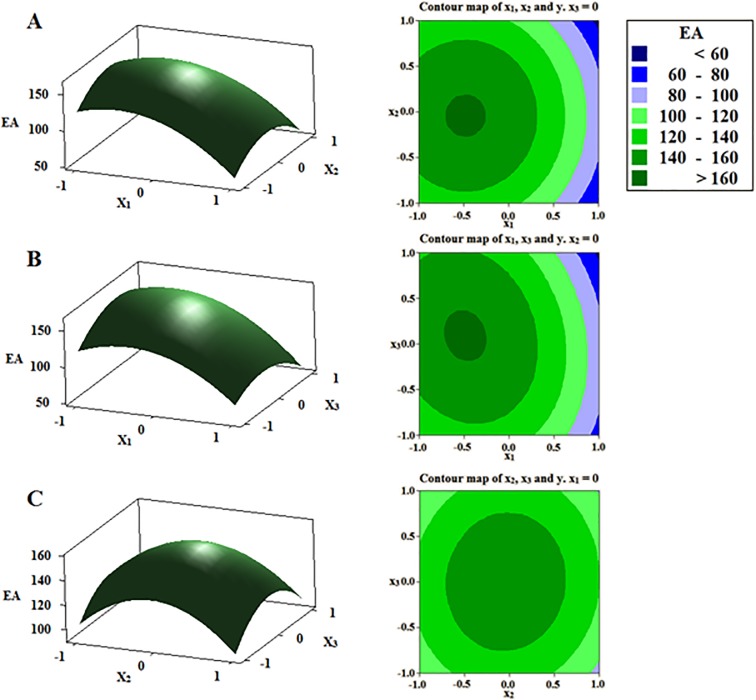
According to the mathematical model, three-dimensional surface and contour plots were generated to reveal the interaction among the three independent variables studied and to depict the combined effects of these variables on EA production. As the influence of two variables on response surface was plotted, the other variable was kept at its zero level. As shown in Fig 4, EA production gradually increased with the increase of fermented temperature, nitrogen sources and glucose. However, it was found that the yield of EA will decrease while these three factors continuously increased (Fig 4). These results demonstrated that our response surface generated from a quadratic model was defined as maximum surface.
Fig 4. Three-dimensional surface and contour plots of three factors on EA production.
The effect of temperature (X1) and nitrogen sources (X2) (A), temperature (X1) and glucose (X3) (B), and nitrogen sources (X2) and glucose (X3) (C) on EA production.
Verification of optimization
To validate the predicted S. cerevisiae S5 EA production, verification fermentation with the predicted optimal value of variables was carried out.As shown in Table 6, a high correlation between predicted (161.88 ppm) and experimental yield of EA was observed. The EA production was 163.22 ppm after the 96-hours fermentation, which was approximately 1.83 times higher than the one from basic medium (Table 6).
Table 6. Comparison of basic and optimum medium on EA production.
| Factors | Basic medium | Optimum medium |
|---|---|---|
| Temperature (oC) | 28.0 | 27.5 |
| Nitrogen source (%) | 3.00 | 14.73 |
| Glucose (%) | 14.00 | 20.91 |
| EA yield (ppm)a | 89.08 ± 9.09 | 163.22 ± 19.15 |
EA = ethyl acetate.
aThe results are presented as the means of five replicated.
Content of aroma compounds in fermented broth
We compared the aroma compounds between the fermented broths from basic and optimum medium (Table 7). Results showed that the content of major aroma compounds, such as EA, acetoin, isoamyl alcohol, isoamyl acetate, 2-methylbutyl acetate, 2-phenylethanol, 2-phenethyl acetate and tryptophol, increased after optimization, and the increase ratio were between 1.28–2.01.
Table 7. Comparison of basic and optimum fermented broth content of aroma compounds (ppm).
| Aroma compounds | Basic medium | Optimum medium | Increase fold |
|---|---|---|---|
| Ethyl acetate | 90.29 ± 9.32 | 168.43 ± 16.64 | 1.87 |
| Acetoin | 116.47 ± 4.22 | 219.92 ± 12.95 | 1.89 |
| Isoamyl alcohol | 113.17 ± 11.24 | 159.25 ± 13.84 | 1.41 |
| Isoamyl acetate | 58.98 ± 2.57 | 118.84 ± 4.36 | 2.01 |
| 2-Methylbutyl acetate | N/A | 15.15 ± 1.67 | - |
| 2-Phenylethanol | 401.86 ± 35.40 | 515.42 ± 54.12 | 1.28 |
| 2-Phenethyl acetate | 29.91 ± 1.09 | 48.17 ± 3.04 | 1.61 |
| Tryptophol | 53.09 ± 4.39 | 68.88 ± 5.69 | 1.29 |
EA = ethyl acetate.
The results are presented as the means of three replicated.
Discussion
S. cerevisiae also known as brewing yeast and baking yeast is profoundly used in food industry [25]. Since the whole genome sequence of S. cerevisiae has been elucidated, it has been used as model for disease prevention and molecule biology [26]; more than that, S. cerevisiae can produce many organic compounds such as ethanol, lactic acid, glycerol and EA [27]. C. humilis was often used for fermenting traditional Italian rye sour dough with Lactobacillus spp. [28], both C. humilis and L. spp. exhibits acid-fastness and salt-tolerance properties. C. humilis can inhibit growth of other bacteria and eventually became the dominant species during fermentation [29]. C. humilis can also produce aroma compounds such as ethanol, acetaldehyde and EA [30]. K. spp. are common in pineapple, star fruits etc. [31], which can be used for making Turkish kefir [32]. K. spp. can also be used as feed additive which will reduce the poultry infection of Salmonella [33]. Etienne-Mesmin et al. [34] found a new probiotic yeast strain, S. cerevisiae CNCM I-3856, which can inhibit the growth of Escherichia coli O157:H7 in digestive system. Fadel et al. [35] isolated a new thermotolerant strain S. cerevisiae F-514 from Egyptian distillery factory, which could improve ethanol yield by fermenting sugarcane molasses. Parapouli et al. [36] successful induced several new enzymes from new yeast strain S. cerevisiae Z622 which could contribute to a better understanding of how S. cerevisiae cells adapt to wine fermentation.
Esterification involves many enzyme reactions such as alcohol acetyltransferase (AAT) [37] and is also regulated by many genes such as alcohol acetyltransferase gene (ATF1) [38]. EA is the major aroma compound in kaoliang [39], and it’s also a crucial esterification component of yeasts [40]. Yeasts with higher EA production efficiency means that the higher amount of ester aroma compounds were produced during the winemaking process.
Fermentation time is an important parameter in fermentation engineering since the microorganisms affect the composition of the fermentation broth. In this study, we performed an eight-day-fermentation and monitored EA production every 24 hours. The results showed that EA production reached the highest on the fourth day and then declined (S2(A) Fig). Glucose also played an important role on EA synthesis. In alcoholic fermentation, glucose could be converted to pyruvate by glycolysis pathway, and produce ethanol in an anaerobic environment [41]. In this process, glucose not only provides the energy for growth, but also produces the ethanol for EA synthesis. There were three metabolic pathways to synthesize EA: esterification [40], hemiacetal reaction [42] and alcoholysis reaction [43], and these metabolic pathways require ethanol to participate the action. However, excess glucose had an inhibitory effect in the yield of EA that might be attributable to unfavorable osmotic pressure (S2(B) Fig). The sluggish growth of microorganisms was often observed at high osmotic pressures, while initial sugar concentration exceeded a certain level [41].
Organic nitrogen source was widely used in the cultivation of yeasts, suggesting that essential amino acid could be synthesized from organic nitrogen sources instead of inorganic ones [44]. The biochemical characteristics results of this study also supported that an organic nitrogen source (yeast extract and peptone) was more favorable for biomass production in S. cerevisiae S5 than an inorganic nitrogen source (ammonium sulfate). Yeast extract and peptone are rich in vitamins, essential amino acids and trace elements such as magnesium cation. Magnesium cation acts as a mainly co-factor of several enzymes of fermentation metabolism and protecting yeast cells from stressful conditions [45]. The yield of EA increased with the elevation of nitrogen source, but excess nitrogen source may also suppress EA production (S2(C) Fig). It was supposed that high concentrations of cations would decrease the amount of live cells during the fermentation process, which results the decrease in alcoholic content and fermentative efficiency [46]. In addition, certain by-products existing at high concentrations of yeast extract and peptone would lead to abatement in EA yield, such as L-pyroglutamate [47].
Fermented temperature was associated with the yield of EA (S2(D) Fig). Previous study suggested that, in fermentation process, temperature would influence the yeasts’ gene regulation [48], target product yield [49] and enzyme activity [50]. Each yeast strain had distinct cultivation temperature. It was generally believed that the EA-synthesis enzymes had higher activity at 30°C [51], which was in agreement with our results.
Many studies discussed about winemaking concerning S. cerevisiae and the concentration of EA. Mateos et al. [52] used nine S. cerevisiae strains in winemaking and found that the concentration of EA in wine was 44.1–56.9 ppm. Roza et al. [53] explored the biomass, sugars and ethanol influence the aroma during the cider industrial fermentation, and found that the concentration of EA was about 44 ppm.The previous study indicated that the concentration of EA in wines is generally lower than 150 ppm. When EA went above 200 ppm will be considered negative for the wine aroma [54]. Therefore, S. cerevisiae S5 was regarded as a winemaking strain with acceptable EA production.
The EA assists S. cerevisiae to disseminate to the environment by attracting insects [55]. These aroma compounds also play important roles in wine. In winemaking, for example, carbonic maceration process could induce cell wall hydrolysis, which generated esters, such as isoamyl acetate [56], which is one of the esters remarkably contributes to the aroma profile of white wines [57]. Therefore, medium optimization provides a reinforced approach to produce aroma compounds.
Conclusion
The current study screened a new yeast strain, S. cerevisiae S5, with winemaking-potentiality from Kinmen kaoliang yellow water. Furthermore, the optimum value of independent variables (fermented temperature, nitrogen sources and glucose) for EA production was evaluated and predicted by BBD-RSM. As a result, fermentation conditions of 27.5°C, 14.73%, and 20.91% were suggested for EA production. EA production was 163.22 ppm at RSM-optimized medium, which was 100.83% of the software-predicted value. It is noteworthy that the content of many aroma compounds were increased in the optimum medium compared with those obtained from basic medium.
In conclusion, this study reported a new yeast strain with enhanced aroma production ability, which provides a new insight into the aroma compounds production or winemaking industrial application of S. cerevisiae.
Supporting information
Both S. cerevisiae 05 and S. cerevisiae 21447 were identified as S. cerevisiae in 99.9% probability.
(TIF)
The highest EA concentration at 100.75 ppm in fermented broth was obtained on the 4th day (A).The correlation of carbon source concentration and EA concentration. The treatment of glucose 20% yielded the highest EA concentration 120.20 ppm in fermented broth (B). The correlation of nitrogen source concentration and EA concentration. The treatment nitrogen 12% yielded the highest EA concentration 125.96 ppm in fermented broth (C). The correlation of fermented temperature and EA concentration. The temperature 30°C yielded the highest EA concentration 90.80 ppm in fermented broth (D).
(TIF)
Acknowledgments
The authors greatly acknowledge the article proofreading by Dr. Shella Permatasari Santoso and Mr. Francisco German Blanco Parte who are native speakers.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This article was supported by Kinmen County Government, 104230230250 to Y-JL. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Callejon RM, Clavijo A, Ortigueira P, Troncoso AM, Paneque P, Morales ML.Volatile and sensory profile of organic red wines produced by different selected autochthonous and commercial Saccharomyces cerevisiae strains. Anal Chim Acta. 2010; 660(1–2):68–75. 10.1016/j.aca.2009.09.040 [DOI] [PubMed] [Google Scholar]
- 2.Peng Q, Tian R, Chen F, Li B, Gao H. Discrimination of producing area of Chinese Tongshan kaoliang spirit using electronic nose sensing characteristics combined with the chemometrics methods. Food Chem. 2015; 178:301–305. 10.1016/j.foodchem.2015.01.023 [DOI] [PubMed] [Google Scholar]
- 3.Li LY. Quality Control Measures of “Erguotou” Fen-flavor Liquor. Liquor-Making Science & Technology 2 2007. [Google Scholar]
- 4.Liu Y. Application of Yellow Water in the Blending & Fermentation of New-type Liquor and Xiaoqu Fen-flavor Liquor. Liquor-Making Science & Technology. 2009; 12:030 [Google Scholar]
- 5.Kuo CP. Production of kaoliang liquor using submerged culture and flavor analysis. 2008.
- 6.Liao YH, Yang CX, Hu JY, Hu JH, Xie JC. Comparison of aroma compounds in mild aromatic Niulanshan, erguotou liquor and strong aromatic Niulanshan liquor by GC-MS. Food Sci.2012; 33:181–185 [Google Scholar]
- 7.Yang G, Qiu S, Huang Y. Optimization of protease-producing conditions of aroma-forming bacteria in Maotai sauce-flavor Daqu. China Brew. 2011; 12:015 [Google Scholar]
- 8.Yu W, Zeng Z, Wu S, Zhang Z. Influence of Monascus on the style and flavor components of special-flavor liquor. China Brew.2012; 31:87–91 [Google Scholar]
- 9.Hai J, Liu Y, Qiu S. Optimization of Fermentation Process of'Sanhua'Plum Wine and Analysis of Its Aroma Components. Food Science. 2016; 23:037 [Google Scholar]
- 10.Kim EJ, Kim YH, Kim JW, Lee HH, Ko YJ, Park MH, et al. Optimization of fermentation process and quality properties of wild grape wine. Journal of the Korean Society of Food Science and Nutrition.2007b; 36(3):366–370. 10.3746/jkfn.2007.36.3.366 [DOI] [Google Scholar]
- 11.Matias-Guiu P, Rodríguez-Bencomo JJ, Pérez-Correa JR, López F. Aroma profile design of wine spirits: Multi-objective optimization using response surface methodology. Food chem. 2018; 245:1087–1097. 10.1016/j.foodchem.2017.11.062 [DOI] [PubMed] [Google Scholar]
- 12.Sevda SB, Rodrigues L. Fermentative behavior of Saccharomyces strains during guava (Psidium guajava L) must fermentation and optimization of guava wine production. J Food Process Technol. 2011; 2.118:2 10.4172/2157-7110.1000118 [DOI] [Google Scholar]
- 13.Box GEP, Wilson KB. On the experimental attainment of optimum conditions. Journal of the Royal Statistical Society Series B (Methodological).1951; 13(1):1–45 [Google Scholar]
- 14.Vadde KK, Syrotiuk VR, Montgomery DC. Optimizing protocol interaction using response surface methodology. Mobile Computing, IEEE Transactions on 2006; 5(6):627–639 [Google Scholar]
- 15.Cheng KC, Ren M, Ogden KL. Statistical optimization of culture media for growth and lipid production of Chlorella protothecoides UTEX 250. Bioresource technol.2013; 128:44–48. 10.1016/j.biortech.2012.09.085 [DOI] [PubMed] [Google Scholar]
- 16.Hsieh SC, Liu JM, Pua XH, Ting Y, Hsu RJ, Cheng KC. Optimization of Lactobacillus acidophilus cultivation using taro waste and evaluation of its biological activity. Appl microbiol biotechnol.2016; 100(6):2629–2639. 10.1007/s00253-015-7149-1 [DOI] [PubMed] [Google Scholar]
- 17.Hsu KD, Wu SP, Lin SP, Lum CC, Cheng KC. Enhanced active extracellular polysaccharide production from Ganoderma formosanum using computational modeling. JFood Drug Anal. 2017; 25(4):804–811. 10.1016/j.jfda.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Melo Pereira GV, Soccol VT, Pandey A, Medeiros AB, Andrade Lara JM, Gollo AL, et al. Isolation, selection and evaluation of yeasts for use in fermentation of coffee beans by the wet process. IntJFood Microbiol. 2014; 188:60–66. 10.1016/j.ijfoodmicro.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 19.Wolfe AD, Hahn FE. Mode of action of chloramphenicol IX. Effects of chloramphenicol upon a ribosomal amino acid polymerization system and its binding to bacterial ribosome. Biochim Biophys Acta.1965; 95(1):146–155. [DOI] [PubMed] [Google Scholar]
- 20.Barnett JA, Payne RW, Yarrow D. Yeasts: Characteristics and identification. Cambridge University Press; 1983. [Google Scholar]
- 21.Doyle JJ. Isolation of plant DNA from fresh tissue. Focus. 1990; 12:13–15 [Google Scholar]
- 22.Sutani A, Ueno M, Nakagawa S, Sawayama S. Melon aroma-producing yeast isolated from coastal marine sediment in Maizuru Bay, Japan. Fish Sci.2015; 81(5):929–936. 10.1007/s12562-015-0912-5 [DOI] [Google Scholar]
- 23.Liti G. The Natural History of Model Organisms: The fascinating and secret wild life of the budding yeast S. cerevisiae. Elife.2015; 4:e05835 10.7554/eLife.05835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan G, Sun B, Xu D, Teng C, Fu Z, Du Y, et al. Isolation and Identification of High-Yield Ethyl Acetate-Producing Yeast from Gujinggong Daqu and Its Fermentation Characteristics. J Am Soc Brew Chem. 2018; 76(2), 117–124. 10.1080/03610470.2017.1396849 [DOI] [Google Scholar]
- 25.Rezaei MN, Verstrepen KJ, Courtin CM. Metabolite analysis allows insight into the differences in functionality of 25 Saccharomyces cerevisiae strains in bread dough fermentation. Cereal Chem. 2015; 92(6):588–597. 10.1094/CCHEM-04-15-0061-R [DOI] [Google Scholar]
- 26.Nielsen J, Jewett MC. Impact of systems biology on metabolic engineering of Saccharomyces cerevisiae. FEMS Yeast Res. 2008; 8(1):122–31. 10.1111/j.1567-1364.2007.00302.x [DOI] [PubMed] [Google Scholar]
- 27.Detroy RW. Bioconversion of agricultural biomass to organic chemicals Organic chemicals from biomass. CRC press; 2018; pp 19–43 [Google Scholar]
- 28.Minervini F, Di Cagno R, Lattanzi A, De Angelis M, Antonielli L, Cardinali G, et al. Lactic acid bacterium and yeast microbiotas of 19 sourdoughs used for traditional/typical Italian breads: interactions between ingredients and microbial species diversity. ApplEnvironMicrobiol.2012; 78(4):1251–1264. 10.1128/AEM.07721-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandt MJ, Hammes WP, Gänzle MG. Effects of process parameters on growth and metabolism of Lactobacillus sanfranciscensis and Candida humilis during rye sourdough fermentation. Eur Food Res Technol.2004; 218(4):333–338. 10.1007/s00217-003-0867-0 [DOI] [Google Scholar]
- 30.Di Cagno R, Pontonio E, Buchin S, De Angelis M, Lattanzi A, Valerio F, et al. Diversity of the lactic acid bacteria and yeast microbiota switching from firm to liquid sourdough fermentation. Appl EnvironMicrobiol. 2014; AEM: 00309-14. 10.1128/AEM.00309-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tchango Tchango J, Njine T, Hornez J, Tailliez R. Modelling growth of the spoilage yeast Candida holmii in pineapple juice. Cahiers d'Etudes et de Recherches Francophones Agricultures (France).2000. [Google Scholar]
- 32.de Oliveira Leite AM, Miguel MA, Peixoto RS, Rosado AS, Silva JT, Paschoalin VM. Microbiological, technological and therapeutic properties of kefir: a natural probiotic beverage. Braz J Microbiol.2013; 44(2):341–349. 10.1590/S1517-83822013000200001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim D, Jang B, Kim J, Yu D, Kang K, Kang H, et al. Effect of dietary yeast (Saccharomyces exiguus) on growth performance, Cecal microflora and fecal ammonia gas in broiler chickens. Korean J Poult Sci.2007. a; 34(2):137–141. 10.5536/KJPS.2007.34.2.137 [DOI] [Google Scholar]
- 34.Etienne-Mesmin L, Livrelli V, Privat M, Denis S, Cardot JM, Alric M, et al. Effect of a new probiotic Saccharomyces cerevisiae strain on survival of Escherichia coli O157: H7 in a dynamic gastrointestinal model. Appl EnvironMicrobiol. 2011; 77(3):1127–1131. 10.1128/AEM.02130-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fadel M, Keera AA, Mouafi FE, Kahil T. High level ethanol from sugar cane molasses by a new thermotolerant Saccharomyces cerevisiae strain in industrial scale. BiotechnolRes Int. 2013. 10.1155/2013/253286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parapouli M, Fragkos-Livanios L, Samiotaki M, Koukkou AI, Perisynakis A, Hatziloukas E, et al. Comparative proteomic analysis of alcoholic fermentation employing a new environmental strain of Saccharomyces cerevisiae. Process Biochem.2010; 45(7):1094–1102. 10.1016/j.procbio.2010.03.028 [DOI] [Google Scholar]
- 37.Verstrepen KJ, Van Laere SD, Vercammen J, Derdelinckx G, Dufour JP, Pretorius IS, et al. The Saccharomyces cerevisiae alcohol acetyl transferase Atf1p is localized in lipid particles. Yeast. 2004; 21(4):367–377. 10.1002/yea.1100 [DOI] [PubMed] [Google Scholar]
- 38.Lilly M, Lambrechts MG, Pretorius IS. Effect of increased yeast alcohol acetyltransferase activity on flavor profiles of wine and distillates. Appl EnvironMicrobiol.2000; 66(2):744–753. 10.1128/AEM.66.2.744-753.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao Z, Yu D, Niu Y, Ma N, Zhu J. Characterization of different aroma‐types of chinese liquors based on their aroma profile by gas chromatography–mass spectrometry and sensory evaluation. Flavour Fragr J. 2016; 31(3):217–227. 10.1002/ffj.3304 [DOI] [Google Scholar]
- 40.Löser C, Urit T, Bley T. Perspectives for the biotechnological production of ethyl acetate by yeasts. ApplMicrobiol Biotechnol. 2014; 98(12):5397–5415. 10.1007/s00253-014-5765-9 [DOI] [PubMed] [Google Scholar]
- 41.Chang YH, Chang KS, Chen CY, Hsu CL, Chang TC, Jang HD. Enhancement of the Efficiency of Bioethanol Production by Saccharomyces cerevisiae via Gradually Batch-Wise and Fed-Batch Increasing the Glucose Concentration. Fermentation. 2018; 4(2), 45 10.3390/fermentation4020045 [DOI] [Google Scholar]
- 42.Löser C, Urit T, Stukert A, Bley T. Formation of ethyl acetate from whey by Kluyveromyces marxianus on a pilot scale. JBiotechnol. 2013; 163(1):17–23. 10.1016/j.jbiotec.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 43.Löser C, Urit T, Keil P, Bley T. Studies on the mechanism of synthesis of ethyl acetate in Kluyveromyces marxianus DSM 5422. ApplMicrobiolBiotechnol. 2015; 99(3):1131–1144. 10.1007/s00253-014-6098-4 [DOI] [PubMed] [Google Scholar]
- 44.Yue G, Yu J, Zhang X, Tan T. The influence of nitrogen sources on ethanol production by yeast from concentrated sweet sorghum juice. Biomass Bioenerg. 2012; 39, 48–52. 10.1016/j.biombioe.2010.08.041 [DOI] [Google Scholar]
- 45.Barros de Souza R, Silva RK, Ferreira DS, de Sá Leitão Paiva Junior S, de Barros Pita W, de Morais Junior MA. Magnesium ions in yeast: setting free the metabolism from glucose catabolite repression. Metallomics.2016; 8(11):1193–1203. 10.1039/c6mt00157b [DOI] [PubMed] [Google Scholar]
- 46.Costa GHG, Messias RC, do Valle Lozano E, Nogueira LC, Blanco LM. The Effect of calcium concentration on the physiology of Saccharomyces cerevisiae yeast in fermentation. Sugar Tech.2018; 20(3):371–374. 10.1007/s12355-018-0603-5 [DOI] [Google Scholar]
- 47.Park CB, Ryu DD, Lee SB. Inhibitory effect of L-pyroglutamate on extremophiles: correlation with growth temperature and pH. FEMS microbiol Lett. 2003; 221(2):187–190. 10.1016/S0378-1097(03)00213-1 [DOI] [PubMed] [Google Scholar]
- 48.Alonso-del-Real J, Lairón-Peris M, Barrio E, Querol A. Effect of temperature on the prevalence of Saccharomyces non cerevisiae species against a S. cerevisiae wine strain in wine fermentation: competition, physiological fitness, and influence in final wine composition. FrontMicrobiol. 2017; 8:150 10.3389/fmicb.2017.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Souza CJ, Costa DA, Rodrigues MQ, dos Santos AF, Lopes MR, Abrantes AB, et al. The influence of presaccharification, fermentation temperature and yeast strain on ethanol production from sugarcane bagasse. Bioresour Technol. 2012; 109:63–69. 10.1016/j.biortech.2012.01.024 [DOI] [PubMed] [Google Scholar]
- 50.Chen R. Optimization of Finger Lakes Riesling's fermentation kinetics by variation of nutrient type, temperature and yeast strain. 2018. [Google Scholar]
- 51.Kruis AJ, Levisson M, Mars AE, van der Ploeg M, Daza FG, Ellena V, et al. Ethyl acetate production by the elusive alcohol acetyltransferase from yeast. Metab Eng. 2017; 41:92–101. 10.1016/j.ymben.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 52.Mateos JR, Pérez-Nevado F, Fernández MR. Influence of Saccharomyces cerevisiae yeast strain on the major volatile compounds of wine. EnzymeMicrob Technol. 2006; 40(1):151–157. 10.1016/j.enzmictec.2005.10.048 [DOI] [Google Scholar]
- 53.de la Roza C, Laca A, García LA, Díaz M. Ethanol and ethyl acetate production during the cider fermentation from laboratory to industrial scale. Process Biochem. 2003; 38(10):1451–1456. 10.1016/S0032-9592(03)00026-8 [DOI] [Google Scholar]
- 54.Rapp A, Pretorius P, Kugler D. Foreign and undesirable flavours in wine Developments in Food Science. vol 28 Elsevier. 1992; pp 485–522. 10.1016/B978-0-444-88558-6.50025-8 [DOI] [Google Scholar]
- 55.Christiaens JF, Franco LM, Cools TL, De Meester L, Michiels J, Wenseleers T, et al. The fungal aroma gene ATF1 promotes dispersal of yeast cells through insect vectors. Cell Rep. 2014; 9(2):425–432. 10.1016/j.celrep.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 56.An K, Wu J, Tang D, Wen J, Fu M, Xiao G, et al. Effect of carbonic maceration (CM) on mass transfer characteristics and quality attributes of Sanhua plum (Prunus Salicina Lindl.). LWT-Food Sci Technol. 2018; 87:537–545. 10.1016/j.lwt.2017.09.032 [DOI] [Google Scholar]
- 57.Plata C, Millan C, Mauricio J, Ortega J. Formation of ethyl acetate and isoamyl acetate by various species of wine yeasts. Food Microbiol. 2003; 20(2):217–224. 10.1016/S0740-0020(02)00101-6 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Both S. cerevisiae 05 and S. cerevisiae 21447 were identified as S. cerevisiae in 99.9% probability.
(TIF)
The highest EA concentration at 100.75 ppm in fermented broth was obtained on the 4th day (A).The correlation of carbon source concentration and EA concentration. The treatment of glucose 20% yielded the highest EA concentration 120.20 ppm in fermented broth (B). The correlation of nitrogen source concentration and EA concentration. The treatment nitrogen 12% yielded the highest EA concentration 125.96 ppm in fermented broth (C). The correlation of fermented temperature and EA concentration. The temperature 30°C yielded the highest EA concentration 90.80 ppm in fermented broth (D).
(TIF)
Data Availability Statement
All relevant data are within the manuscript.