Abstract
Purpose:
Informing adolescents of their own HIV infection is critical as the number of adolescents living with HIV increases. We assessed the association between HIV disclosure and retention in care and mortality among adolescents aged 10–14 years in Kenya’s national program.
Methods:
We abstracted routinely collected patient-level data for adolescents enrolled into HIV care in 50 health facilities from November 1, 2004, through March 31, 2010. We defined disclosure as any documentation that the adolescent had been fully or partially made aware of his or her HIV status. We compared weighted proportions for categorical variables using χ2 and weighted logistic regression to identify predictors of HIV disclosure; we estimated the probability of LTFU using Kaplan–Meier methods and dying using Cox regression-based test for equality of survival curves.
Results:
Of the 710 adolescents aged 10–14 years analyzed; 51.3% had severe immunosuppression, 60.3% were in WHO stage 3 or 4, and 36.6% were aware of their HIV status. Adolescents with HIV-infected parents, histories of opportunistic infections (OIs), and enrolled in support groups were more likely to be disclosed to. At 36 months, disclosure was associated with lower mortality [1.5% (95% CI.6%–4.1%) versus 5.4% (95% CI 3.6.6%–8.0%, p <.001)] and lower LTFU [6.2% (95% CI 3.0%–12.6%) versus 33.9% (95% CI 27.3%–41.1%) p <.001].
Conclusions:
Only one third of HIV-infected Kenyan adolescents in treatment programs had been told they were infected, and knowing their HIV status was associated with reduced LTFU and mortality. The disclosure process should be systematically encouraged and organized for HIV-infected adolescents.
Keywords: Adolescents, HIV, Disclosure, Kenya
In 2015, an estimated 1.8 million adolescents aged 10–19 years old were living with HIV globally, and more than 60% were living in East and Southern Africa [1]. An estimated 250,000 15-to-19-year olds were newly infected with HIV in 2015, of whom 65% were girls [1]. Adolescents and young people represent a rapidly growing subpopulation of people living with HIV worldwide. The expanded access to pediatric antiretroviral therapy (ART) has resulted in increased survival of perinatally infected children and contributed to an increasing population of HIV-infected adolescents [2]. This is in addition to the population of adolescents who were infected with HIV later in life either through sexual or parenteral transmission [3].
Kenya has a generalized HIV epidemic with an adult HIV prevalence of 5.6% and an estimated 1.4 million people living with HIV as of 2012. HIV prevalence was estimated to be.6% among 10–14 year-olds and 1.0% among 15–19-year-olds [4,5]. In 2012, there were roughly 150,000 HIV-infected adolescents aged 10–19 years living with HIV in Kenya [6].
Globally, there is a renewed commitment to the health of adolescents, as adolescence is increasingly being recognized as a unique period requiring age-appropriate quality health care [7,8]. The growing population of HIV-infected adolescents brings new challenges to HIV programs in terms of addressing psychosocial support and sexual and reproductive health (SRH) needs specific to adolescents, adherence to treatment, retention, preventing secondary HIV transmission and promoting overall physical and mental health [9]. As more perinatally infected children reach adolescence and as more adolescents acquire HIV infection, one challenge is determining how and when to inform them of their HIV status. Disclosure of HIV status can have various meanings in the HIV setting including (1) an adolescent’s gaining knowledge of his/her HIV status [28], (2) disclosure of caregivers’ HIV status to an adolescent [10] or (3) an adolescent’s disclosure of his or her own HIV status to others [11]. In this paper, we discuss disclosure in the context of the adolescent gaining knowledge of his/her HIV status.
For adolescents, learning of their HIV diagnosis is an important step towards the long-term disease management necessary for the transition from pediatric care into adolescent and adult care settings [12]. There are well-documented medical benefits of disclosing HIV status to children and adolescents including enabling them to understand HIV infection and make sense of disease-related experiences [13,14]. Furthermore, disclosure has been associated with better adherence to ART, higher self-esteem, improved participation in healthcare decision-making and higher CD4 counts [9,15,16]. Disclosure is also a key component of HIV prevention and can result in a reduction of high-risk sexual behaviors among adolescents [15]. Despite the known benefits, studies evaluating rates of disclosure in developing countries suggest that many adolescents living with HIV do not know they are infected; disclosure rates vary from 11%–40% depending on the adolescent’s age among other factors [16–20]. When, how to disclose positive HIV serostatus to an adolescent is a shared responsibility by parents, medical staff and other caregivers and represents a challenge for all involved [21,22]. Parents and other caregivers require adequate support by healthcare workers to disclose to HIV status. Since disclosure is a complex and critical clinical issue in the care of the HIV-infected adolescent, reasons for nondisclosure for the involved family members, caregivers, and health care workers must be addressed. Some reasons for nondisclosure include fear of discrimination and stigma toward both the adolescent and the family [13,15,23].
The World Health Organization (WHO) strongly encourages disclosing HIV infection status to school-aged children 6 to 12 years old and recommends that younger children be informed incrementally to accommodate their cognitive skills and emotional maturity [24]. Despite the importance of HIV disclosure, there have been few studies addressing adolescent disclosure and its effect on clinical outcomes in developing countries, particularly in sub–Saharan Africa [16]. We sought to determine the prevalence of HIV disclosure and to explore factors associated with disclosure and the association of disclosure with clinical outcomes (death and loss-to-follow-up) in a large cohort of HIV-infected adolescents attending the national pediatric HIV program in Kenya.
Methods
Study setting, design, and population
To assess the national Kenyan pediatric HIV care and treatment program using routinely collected programmatic and clinical indicators, we conducted a retrospective cohort study of children with confirmed HIV infection aged 2 to 14 years old at the time of enrollment into HIV care. Children presenting in the 50 largest HIV pediatric care and treatment facilities in Kenya from November 1, 2004, to March 31, 2010, were included. These enrollment end dates were selected to allow all patients to be in care for a minimum of 12 months before data abstraction. All health facilities offered a standard set of ART services, including HIV testing, pre-ART, and ART care, with counseling and disclosure of HIV (starting from 6 years of age taking into account the child’s maturity and the specific clinical and social context) and retention support as per Kenyan national guidelines. In this article, we present a secondary analysis of these data focusing on young adolescents who were between 10 and 14 years of age at the time of enrollment into HIV care.
Site selection and sampling procedures
The site-sampling frame included all the healthcare facilities that reported to National AIDS and Sexually Transmitted Infection (STI) Control Programme (NASCOP), provided ART for at least 40 pediatric patients < 15 years old as of October 31, 2008, and had recorded follow up of the children for ≥ 12 months as of March 31, 2008. We selected 50 clinics randomly using probability proportional to size.
Participants’ selection
In the selected facilities, the sampling frame included all registered HIV-infected children who were enrolled into HIV care between November 1, 2004, and March 31, 2010, and were 2–14 years of age at enrollment. We randomly selected patients aged ≥ 2 years but < 15 years old at enrollment. At each site, we compiled a sequential list of eligible patients from clinic enrollment registers. We used a random number generator to assign each eligible patient a study number, and from this list, we randomly selected 89 patients per clinic. Records from selected patients that could not be retrieved were replaced with the next randomly sampled patient.
Data abstraction and management
For each patient, trained research assistants abstracted clinical follow-up information from the routine care patient charts from the date of enrollment up to the date of the last visit. In addition to patient charts, other data sources including pharmacy and laboratory notes, social worker notes and community health worker log-books were reviewed. Scannable paper-based forms were used for data abstraction. The data collection tools were piloted and revised before the survey to assure that they were appropriate and that all research team members had been given consistent directions. A log for recording the quantity of missing records was kept at each site. At the end of each site visit, research team leads collected all data abstraction forms and sent them to the Kenya Medical Research Institute (KEMRI) for scanning into a database using Tele-form version 9.1 (HP Autonomy, Inc., Sunnyvale, California, USA).
Variables
We defined disclosure as any documentation that the adolescent had been made aware of his or her HIV status since enrollment into HIV care, either fully or partially regardless of whether they were informed by the health care worker or by the caregiver/guardian. We used the Centers for Disease Control and Prevention (CDC) classification for HIV immune status to categorize participants by age, CD4 count and CD4 percentage into three categories: no evidence of immunosuppression, moderate immunosuppression and severe immunosuppression [25]. If both CD4 counts and percentages were available for a participant, we classified him or her according to the CD4 percentage. Predictor variables included demographic, programmatic and clinical characteristics such as age, sex, orphanhood, WHO stage at ART initiation, the degree of immunosuppression at ART initiation, being on ART, history of hospitalization prior to ART initiation and ever having had tuberculosis (TB). For participants who transferred into the sampled clinics while on ART, we used the date of their initial enrollment into care at the current facility as their date of ART initiation. For these adolescents, their characteristics at enrollment in the study facility were used as the baseline characteristics at ART initiation. We defined retention as being in care at the facility at the time of chart abstraction. We defined being lost to follow up (LTFU) as patients not recorded as dead or transferred out who did not have a recorded clinical visit for more than 90 days from their last clinic appointment [26]. Mortality was all-cause deaths notified from any of the facility records. We calculated the observation time in the facility as the time from enrollment date to the date of chart abstraction, death, and transfer to another clinical center or LTFU.
Statistical analyses
We used descriptive analyses for summarizing characteristics and χ2 test to compare proportions for categorical variables; we considered a p <.05 as statistically significant. We included the factors that were significant in bivariate analyses in a logistic regression model to test for associations of demographic, clinical presentation, and treatment variables to disclosure. All significant factors were included in the logistic regression model. Variables in the final model included sex, WHO stage at enrollment, TB, family enrollment into HIV care, history of opportunistic infection (OI), ever having been hospitalized, and enrollment into a support group. We report unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CIs). For the analysis of outcomes after enrollment into HIV care, we used Kaplan–Meier estimators to estimate the probability of LTFU and death according to the HIV disclosure status. For analysis purposes, we assumed that any missing data were missing at random and did not impute values. Age was not systematically documented and therefore it was not possible to analyze associations between disclosure and age at HIV infection, disclosure, enrollment on ART, and age at last clinic visit or death.
All sample estimates were weighted to account for sampling design and for clustering by site. We used STATA/MP version 13.1 (STATA Corporation, College Station, Texas, USA) for analyses.
Ethical considerations
The protocol was approved by the University of Nairobi/Kenyatta National Hospital Ethical Review Committee and the U.S. Centers for Disease Control and Prevention Institutional Review Board. No informed consent was required since there was no contact with patients and no patient identifiers were collected.
Results
Between November 2004 and March 2010, 710 HIV-infected adolescents aged 10 to 14 years were enrolled in the national HIV treatment program, 383 (53%) were female, and 420 (60%) and 290 (40%) were 10–11 and 12–14 years old, respectively. The median age at HIV diagnosis was 11 years (interquartile range (IQR) 10–12), and the median age at enrollment into HIV care was 11 years (IQR 10–12). WHO staging was recorded for 395 (56%) adolescents, and of these, 232 (60%) were in WHO stage 3 or 4. Based on the immunological classification, 309 (67%) had advanced or severe HIV disease, 576 (79%) had had at least one OI during follow up, and 94 (13.6%) had ever been hospitalized during follow up. At any point during care, 217 (32%) had ever been diagnosed with TB. A total of 694 (98%) adolescents were enrolled in school at the time of data abstraction. Overall, 267 (37%) had at least one HIV-infected parent, 364 (51%) were orphaned (one or both parents dead), and 179 (25%) had their mother, father or both parents enrolled in HIV care. Over a median follow-up period of 2 years (IQR 0–4), 587 (82%) were still receiving HIV care in the initial healthcare facilities of enrollment. Overall, 280 adolescents had a documentation on psychosocial support status, but only 133 of these (49.0%) were enrolled in a psychosocial support group. Most of the adolescents, 545 (78%), had been started on ART (Table 1).
Table 1.
Characteristics of HIV-infected adolescents aged 210–14 years in HIV care, Kenya, 2004–2010.
| Characteristics | Total | Aware of infection | Unaware of infection | p-value | |||
|---|---|---|---|---|---|---|---|
| N | Percent/IQR[95%CI]a | n | Percent [95%CI]a | n | Percent [95%CI]a | ||
| Total | 710 | 100 | 251 | 36.6 [27.9,46.2] | 459 | 63.4 [53.8,72.1] | |
| Sex | .086 | ||||||
| Male | 327 | 46.9 [43.3,50.5] | 125 | 40.3 [29.6,51.9] | 202 | 59.7 [48.1,70.4] | |
| Female | 383 | 53.1 [49.5,56.7] | 126 | 33.3 [25.3,42.3] | 257 | 66.7 [57.7,74.7] | |
| Age at diagnosis, years | .385 | ||||||
| 10–11 | 420 | 59.6 [54.9,64.2] | 142 | 35 [25.8,45.4] | 278 | 65 [54.6,74.2] | |
| 12–14 | 290 | 40.4 [35.8,45.1] | 109 | 38.9 [28.9,50.0] | 181 | 61.1 [50.0,71.1] | |
| Median age at HIV diagnosis | 589 | 11 [10–12] | 230 | 11 [10–12] | 359 | 11 [10–12] | |
| Median age at enrollment into HIV care | 710 | 11 [10–12] | 251 | 11 [10–12] | 459 | 11 [10–12] | |
| WHO stage | .109 | ||||||
| I or II | 163 | 39.7 [33.2,46.6] | 69 | 45.4 [32.0,59.5] | 94 | 54.6 [40.5,68.0] | |
| III or IV | 232 | 60.3 [53.4,66.8] | 80 | 35.5 [25.2,47.4] | 152 | 64.5 [52.6,74.8] | |
| TB at enrollment/follow-up | .874 | ||||||
| Ever TB | 217 | 31.6 [26.9,36.7] | 73 | 37.1 [26.2,49.6] | 144 | 62.9 [50.4,73.8] | |
| NoTB | 493 | 68.4 [63.3,73.1] | 178 | 36.3 [27.2,46.4] | 315 | 63.7 [53.6,72.8] | |
| Orphan status | .661 | ||||||
| Both parents alive | 164 | 30.9 [26.5,35.6] | 62 | 40.4 [28.8,53.2] | 102 | 59.6 [46.8,71.2] | |
| Orphan | 364 | 69.1 [64.4,73.5] | 147 | 42.9 [32.3,54.2] | 217 | 57.1 [45.8,67.7] | |
| Caregiver | .426 | ||||||
| Parents | 319 | 45.3 [39.9,50.9] | 124 | 40.1 [29.1,52.3] | 195 | 59.9 [47.7,70.9] | |
| Other relatives | 294 | 41.4 [36.6,46.5] | 99 | 34.6 [26.3,44.0] | 195 | 65.4 [56.0,73.7] | |
| Parents’ HIV status at enrollment | .013 | ||||||
| Both mother and father HIV-infected | 66 | 9.5 [7.2,12.5] | 25 | 39.1 [25.2,55.0] | 41 | 60.9 [45.0,74.8] | |
| Only one parent HIV-infected | 201 | 27.7 [23.5,32.4] | 88 | 47.2 [36.0,58.8] | 113 | 52.8 [41.2,64.0] | |
| Mother’s & father’s HIV status unknown | 443 | 62.7 [56.9,68.3] | 138 | 31.5 [23.1,41.3] | 305 | 68.5 [58.7,76.9] | |
| Immune status | .450 | ||||||
| No significant immunosuppression | 147 | 33.4 [29.0,38.1] | 57 | 38.8 [28.2,50.5] | 90 | 61.2 [49.5,71.8] | |
| Moderate | 69 | 15.0 [10.9,20.3] | 30 | 47.6 [31.2,64.5] | 39 | 52.4 [35.5,68.8] | |
| Severe | 240 | 51.6 [44.7,58.5] | 90 | 39.7 [30.1,50.2] | 150 | 60.3 [49.8,69.9] | |
| Current schooling status | .385 | ||||||
| In school | 694 | 98.1 [90.8,99.6] | 245 | 36.6 [27.8,46.3] | 449 | 63.4 [53.7,72.2] | |
| Dropped out | 9 | 1.9 [.4,9.2] | 2 | 21.8 [9.8,41.7] | 7 | 78.2 [58.3,90.2] | |
| Parent(s) enrollment into care | .004 | ||||||
| None/unknown | 531 | 75.1 [69.2,80.3] | 186 | 31.5 [23.2,41.1] | 365 | 68.5 [58.9,76.8] | |
| Mother/father or both | 179 | 24.9 [19.7,30.8] | 82 | 51.9 [39.3,64.2] | 94 | 48.1 [35.8,60.7] | |
| History of OIb at enrolment | .026 | ||||||
| Did not have OIs | 134 | 20.9 [14.8,28.5] | 14 | 10.7 [4.9,21.6] | 120 | 89.3 [78.4,95.1] | |
| Had OIs | 576 | 79.1 [71.5,85.2] | 237 | 42.7 [33.6,52.4] | 339 | 57.3 [47.6,66.4] | |
| Ever had an OI† during follow-up | < .001 | ||||||
| No OIs | 137 | 19.3 [13.3,27.1] | 38 | 24.5 [14.4,38.5] | 99 | 75.5 [61.5,85.6] | |
| Developed OIs | 573 | 80.7 [72.9,86.7] | 213 | 39.7 [31.1,49.0] | 360 | 60.3 [51.0,68.9] | |
| Ever hospitalized during follow-up | .494 | ||||||
| Hospitalized | 94 | 13.6 [10.5,17.5] | 31 | 34.7 [23.4,48.1] | 63 | 65.3 [51.9,76.6] | |
| Not hospitalized | 561 | 86.4 [82.5,89.5] | 211 | 38.8 [29.5,49.1] | 350 | 61.2 [50.9,70.5] | |
| Enrolled in support group | .001 | ||||||
| Yes, enrolled | 133 | 49.0 [35.8,62.4] | 95 | 73.9 [61.2,83.6] | 38 | 26.1 [16.4,38.8] | |
| No, not enrolled | 147 | 51.0 [37.6,64.2] | 54 | 37.8 [27.2,49.6] | 93 | 62.2 [50.4,72.8] | |
| ART status | < .001 | ||||||
| On ART | 545 | 78.0 [72.5,82.6] | 221 | 41.8 [32.0,52.2] | 324 | 58.2 [47.8,68.0] | |
| Not on ART | 165 | 22.0 [17.4,27.5] | 30 | 18.1 [10.7,29.0] | 135 | 81.9 [71.0,89.3] | |
| Transferred from another facility | .097 | ||||||
| Not transferred | 587 | 82.3 [77.1,86.4] | 201 | 34.9 [26.5,44.3] | 386 | 65.1 [55.7,73.5] | |
| Transfer-ins | 123 | 17.7[13.6,22.9] | 50 | 44.2[31.6,57.6] | 73 | 5.8[42.4,68.4] | |
ART, antiretroviral therapy; CI, confidence interval; IQR, interquartile range; OI, opportunistic infection; TB, tuberculosis
Weighted percentages.
Opportunistic infections.
Overall, 251 (36.6%) adolescents were aware of their HIV status. In bivariate analyses, disclosure varied by multiple indicators, including but not limited to, parental enrollment in HIV care compared with parents not being enrolled in HIV care or their enrollment status being unknown (51.9% vs. 31.5%, p = .004), having had a history of an OI during follow up compared to not having an OI during follow-up (39.7% vs. 24.5%, p = .026), being enrolled in a support group versus not being enrolled (79.3 % vs. 37.8%, p = .001) and being on ART compared to not being on ART (41.8% vs. 18.1%, p < .001) (Table 1). In multivariate analysis, adolescents who had an OIs during follow up (adjusted OR (aOR), 10.6, 95% CI 2.8–40.6) and those who were enrolled in a support group (aOR 6.8, 95% CI 2.5–18.3) had higher adjusted odds of having been informed of their HIV status (Table 2).
Table 2.
Odds of disclosure of HIV status among 10-t0–14-year-old adolescents enrolled in HIV care, Kenya, 2004–2010.
| Characteristics | Total | Disclosed to | Unadjusted OR [95%CI] | Adjusted OR [95%CI] | ||
|---|---|---|---|---|---|---|
| N | Percent [95%CI] | N | Percent [95%CI] | |||
| Total | 710 | 251 | 36.6 [27.9,46.2] | |||
| Sex | ||||||
| Male | 327 | 46.9 [43.3,50.5] | 125 | 40.3 [29.6,51.9] | ref | |
| Female | 383 | 53.1 [49.5,56.7] | 126 | 33.3 [25.3,42.3] | 1.4[1.0–1.9] | NI |
| Age at diagnosis | ||||||
| 10–11 years | 420 | 59.6 [54.9,64.2] | 142 | 35.0 [25.8,45.4] | ref | |
| 12–14years | 290 | 40.4 [35.8,45.1] | 109 | 38.9 [28.9,50.0] | 1.2 [.8–1.7] | NI |
| WHO stage | ||||||
| I or II | 163 | 39.7 [33.2,46.6] | 69 | 45.4 [32.0,59.5] | ref | |
| III or IV | 232 | 60.3 [53.4,66.8] | 80 | 35.5 [25.2,47.4] | .7 [.4–1.1] | .5 [.2–1.2] |
| TB at enrollment/follow-up | ||||||
| Ever TB | 217 | 31.6 [26.9,36.7] | 73 | 37.1 [26.2,49.6] | ref | |
| No TB | 493 | 68.4 [63.3,73.1] | 178 | 36.3 [27.2,46.4] | 1.0 [.6–1.5] | NI |
| Orphan status | ||||||
| Both parents alive | 164 | 30.9 [26.5,35.6] | 62 | 40.4 [28.8,53.2] | ref | |
| Orphan | 364 | 69.1 [64.4,73.5] | 147 | 42.9 [32.3,54.2] | 1.1 [.7–1.8] | NI |
| Caregiver | ||||||
| Parents | 319 | 52.2 [46.6,57.8] | 124 | 40.1 [29.1,52.3] | 1.3 [.8–2.0] | NI |
| Other relatives | 294 | 47.8 [42.2,53.4] | 99 | 34.6 [26.3,44.0] | ref | |
| Parents HIV status | ||||||
| Both known HIV+ | 66 | 25.6 [20.5,31.5] | 25 | 39.1 [25.2,55.0] | ref | |
| One known HIV+ | 201 | 74.4 [68.5,79.5] | 88 | 47.2 [36.0,58.8] | 1.4 [.8–2.4] | NI |
| Parent(s) enrollment into care | ||||||
| None/unknown | 531 | 75.1 [69.2,80.3] | 166 | 31.5 [23.2,41.1] | ref | |
| Mother/father or both | 179 | 24.9 [19.7,30.8] | 85 | 51.9 [39.3,64.2] | 2.3 [1.5–3.7] | 2.1 [.8–5.5] |
| History of OI during follow-up | ||||||
| No OIs | 134 | 19.3 [13.3,27.1] | 14 | 10.7 [4.9,21.6] | Ref | |
| Developed OIs | 576 | 80.7 [72.9,86.7] | 237 | 42.7 [33.6,52.4] | 6.3 [2.8–14.1] | 10.6 [2.8–40.6] |
| Ever hospitalized during follow-up | ||||||
| Yes | 94 | 13.6 [10.5,17.5] | 31 | 34.7 [23.4,48.1] | ref | NI |
| No | 561 | 86.4 [82.5,89.5] | 211 | 38.8 [29.5,49.1] | 1.2 [.7–2.0] | |
| Enrolled in support group | ||||||
| Not enrolled | 147 | 51.0 [37.6,64.2] | 54 | 37.8[27.2,49.6] | ref | |
| Yes, enrolled | 133 | 49.0 [35.8,62.4] | 96 | 73.9[61.2,83.6] | 4.7 [2.1–10.3] | 6.8 [2.5–18.3] |
| ART status | ||||||
| On ART | 545 | 78.0 [72.5,82.6] | 221 | 41.8[32.0,52.2] | 3.2 [1.7–6.2] | NI |
| Not on ART | 165 | 22.0 [17.4,27.5] | 30 | 18.1[10.7,29.0] | Ref | |
ART, antiretroviral therapy; NI, not included in multivariate model;‡OI, opportunistic infection; OR, odds ratio; TB, tuberculosis
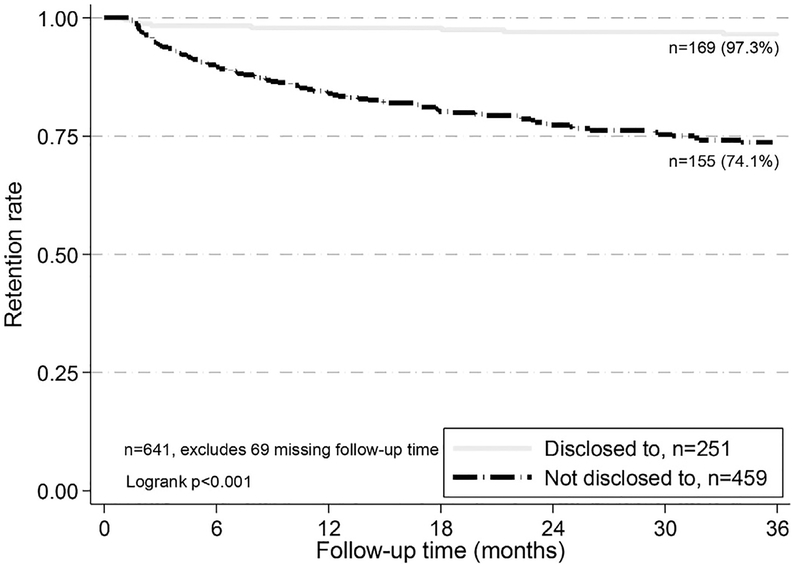
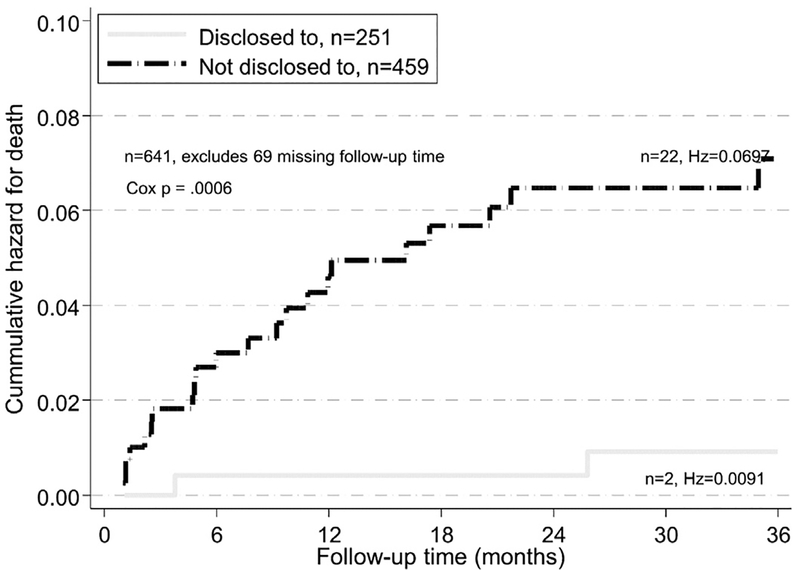
One hundred seventy-six (23.7%) adolescents were LTFU. At 36-months of follow-up; 97.3% of those who knew their HIV status were retained in care compared to 74.1% of those did not (p < .001) (Figure 1). Twenty-nine (4.0%) had died; the proportion dead was lower (1.5%, 95% CI.6–4.1) among those who had been told their HIV status compared to those to whom HIV status had not been disclosed (5.4%, 95% CI 3.6–8.0, p < .001) (Table 3). The cumulative hazard of death was significantly lower among those who knew their HIV status compared to those who did not (p = .003, Figure 2).
Figure. 1.
Retention rate after 36 months in HIV care by HIV disclosure status, 10-to-14-year-old adolescents, Kenya, 2004–2010 (n = 641).
Table 3.
Outcomes by disclosure of HIV status among 10-to-14-year-old adolescents enrolled in HIV care, Kenya, 2004–2010.
| Outcomes | Aware of infection | Unaware of infection | Total | |||
|---|---|---|---|---|---|---|
| n | Percent [95% CI]a | n | Percent [95% CI]a | Nb | Percen t[95% CI]a | |
| Active | 214 | 86.7 [78.7,92.0] | 201 | 45.3 [37.9,52.9] | 415 | 60.4 [52.5,67.9] |
| Transferred out | 15 | 5.6 [2.9,10.5] | 74 | 15.5 [12.3,19.3] | 89 | 11.8 [9.3,14.9] |
| LTFU | 18 | 6.2 [3.0,12.6] | 158 | 33.9 [27.3,41.1] | 176 | 23.7 [17.9,30.8] |
| Dead p< .001 | 4 | 1.5 [.6,4.1] | 25 | 5.4 [3.6,8.0] | 29 | 4.0 [2.6,6.0] |
CI, confidence interval; LTFU, lost to follow-up
Weighted percentage.
Outcome for one adolescent was unknown.
Figure. 2.
Cumulative hazards of death after 36 months in HIV care by HIV disclosure status, 10-to-14-year-old adolescents, Kenya, 2004–2010.
Discussion
We found that about two-thirds of adolescents living with HIV who were enrolled in HIV care between 10 and 14 years of age and followed up for a median of two years were unaware of their HIV status. These findings are consistent with other studies in low-and middle-income countries where, generally, low rates of disclosure ranging from 14% to 30% have been described [16,22,23,27–29]. This variation in disclosure rates to a large extent depends on the age of the adolescents assessed and the context. One study in Ghana found a higher rate of disclosure (53%) among 13 to 22-year-olds, which is still suboptimal, onsidering that older adolescents and young adults were included [19]. In our study, the finding that only 73.9% of those adolescents in support groups were aware of their HIV status suggests that we may have underestimated disclosure by approximately 25% due to lack of documentation. This is because adolescents would have to be aware of their HIV status to participate in support groups. Moreover, only slightly more than 40% on ART had been told their HIV status; this is of concern because awareness of HIV status is critical in facilitating their understanding of their illness, which in turn promotes their participation in and responsibility for their treatment [30]. Furthermore, adolescents who are aware of their HIV infection status have better HIV treatment outcomes [31,32]. In the Kenyan, healthcare system where adolescents typically transition from pediatric to adult care starting from 12–15 years, it is crucial that they are aware of their HIV status for this transition to occur smoothly.
We identified some differences between adolescents who were aware of their HIV status compared to those who were not. First, we found an association between HIV disclosure and having suffered an OI. These findings may suggest that being sick may have necessitated the need for HIV disclosure to the adolescent. We also further found an association between disclosure and being enrolled in a peer support group. Even though we could not ascertain the temporal sequence of events, this finding is not surprising because for the adolescents to be enrolled in a support group, they would have to be aware of their HIV status. However, less than a half (49%) of the adolescents were enrolled in a support group despite the known benefits of better outcomes for those in support groups [31].
In this cohort, we observed a lower death rate of 4.1% compared to rates ranging from 4.9%–6.7% among Romanian 5–17 year olds [32], 6.1% among 10–21 year olds in Côte d’Ivoire, Mali and Senegal [16], and 7.8% among 0–15 year olds from nine West African countries [33]. However, we may have underestimated mortality due to possible misclassification of patients who had died as LTFU. In our study, about 28% of adolescents were LTFU, which is higher than the 11.2%–13% among 10–21-year-old adolescents and young adults from Cote d’Ivoire, Mali and Senegal and the 21.2% of LTFU reported in West Africa among children aged 0–15 years old [33]. Because we classified patients as LTFU if there was no record of a clinical visit for more than 90 days from their last clinic appointment [26], we may have overestimated LTFU compared to the studies from West Africa where patients were classified as LTFU if the interval between the last clinic visit registered in the database and the closing date of the database was ≥ 6 months [16,33]. Our results suggest the benefit of disclosure on retention in care of younger adolescents as evidenced by lower mortality and lower LTFU among those who were aware of their HIV status. It is likely that adolescents’ knowledge of HIV status may facilitate ongoing support from their families and promote their engagement in healthcare. Similar benefits of the disclosure have been described among 5-to-17-year-old Romanian children and adolescents [32]. However, despite these known benefits, the disclosure is generally low. Disclosure of HIV status to adolescents represents a challenge both for the family and for medical staff. Reasons for not disclosing HIV status have included fear of discrimination, stigma, and the perception that the child is not mature enough [13,34,35]. The low disclosure rates among this cohort of young adolescents who are in the health care system suggest that health service-related factors may have contributed to nondisclosure. The majority of studies that have assessed who is best positioned to disclose HIV status to adolescents status favored disclosure by caregivers with support from medical staff [36–39] while only one study found a preference for disclosure by health care staff [40]. Our findings demonstrate the urgent need to explore reasons for nondisclosure from the perspectives of caregivers’ and medical staff and to implement locally and culturally sensitive intervention programs to promote disclosure.
Strengths of our study include the larger sample size, which represents the majority of young adolescents receiving HIV care in Kenya’s national HIV program followed up over a long duration. The cohort includes patients receiving care throughout Kenya, and thus captures a wide range of differing patient experiences and allows for generalizability. Our study does have limitations. Due to the retrospective design and the use of routinely collected clinical information, some data were missing, which may have led to underreporting of disclosure due to lack of documentation. We also could not assess the timing of events including age at HIV infection, disclosure, and enrollment on ART or the person responsible for disclosing HIV status to the adolescent nor the reasons for nondisclosure. There was also a potential misclassification of deaths as LTFU. To mitigate these, we abstracted data from multiple patient records in the facility including social worker notes, community health care notes, and laboratory and pharmacy records.
This study provides insight into overall disclosure rates and the programmatic outcomes of the young adolescent population in this large public sector ART program in Kenya. The rate of disclosure of young adolescents’ HIV status was low in Kenya and likely reflects the lack of clear guidelines on disclosure in the earlier phases of Kenya’s public pediatric HIV program when these data were collected. Given that disclosure guidelines now exist, it is likely that more recent data may demonstrate improved disclosure rates due to improved clinical practices. Our findings underscore the need to review the reasons for non-disclosure and to implement locally and culturally sensitive interventions to promote disclosure.
IMPLICATIONS AND CONTRIBUTION.
This analysis provides an opportunity to understand the prevalence of HIV disclosure, the factors associated with disclosure and the impact of disclosure on clinical outcomes (death and loss-to-follow-up) in a large cohort of HIV-infected adolescents attending a national pediatric HIV program.
Acknowledgments
We acknowledge the technical contributions of Dr. Marta Ackers, Prof. Dorothy Mbori-Ngacha and Dr. James Kiarie during the conceptualization, protocol development, and the support during the implementation phase of this study. We wish to thank all the staff at participating health facilities who helped with the retrieval of patient records at the facilities and the study staff who abstracted data from the facility records. Dr. Ibrahim Mohammed, who was the head of the National AIDS and STI Control Programme, Ministry of Health, at the time of the survey provided both administrative and technical support for this study.
Funding Sources
This publication has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of cooperative agreements PS001814 and 5 U19 GH000069–05.
Abbreviations:
- OI
opportunistic infection
- LTFU
loss-to-follow-up
- WHO
World Health Organization
- ART
antiretroviral therapy
- SRH
sexual and reproductive health
- NASCOP
National AIDS/STI Control Program
- KEMRI
Kenya Medical Research Institute
- CDC
Centers for Disease Control and Prevention
- ALHIV
adolescents living with HIV
- TB
tuberculosis
- OR
odds ratios
- CI
confidence intervals
Footnotes
Conflicts of interest: The authors have declared that no competing interests exist.
Disclaimer: The findings and conclusions in this publication are those of the authors and do not necessarily represent the official position of the funding agencies.
References
- [1].United Nations Children’s Fund (UNICEF). UNICEF Analysis of UNAIDS 2012 HIV and AIDS Estimates New York, NY, USA: UNICEF; 2013. http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. [Google Scholar]
- [2].Patel K, Hernan MA, Williams PL, et al. Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study. Clin Infect Dis 2008;46(4):507–15. [DOI] [PubMed] [Google Scholar]
- [3].Ferrand RA, Munaiwa L, Matsekete J, et al. Undiagnosed HIV infection among adolescents seeking primary health care in Zimbabwe. Clin Infect Dis 2010;51 (7):844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ng’eno B, Mwangi A, Ng’ang’a L, et al. Burden of HIV infection among children aged 18 months to 14 years in Kenya: results from a nationally representative population-based cross-sectional survey. J Acquir Immune Defic Syndr 2014;66(Suppl 1):S82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kimanga DO, Ogola S, Umuro M, et al. Prevalence and incidence of HIV infection, trends, and risk factors among persons aged 15–64 years in Kenya: results from a nationally representative study. J Acquir Immune Defic Syndr 2014;66(Suppl 1):S13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].UNICEF. Towards an AIDS-free generation: children and AIDS, sixth stocktaking report New York, NY, USA: UNICEF; 2013. [Google Scholar]
- [7].Dick B, Ferguson BJ. Health for the world’s adolescents: a second chance in the second decade. J Adolesc Health 2015;56(1):3–6. [DOI] [PubMed] [Google Scholar]
- [8].Sawyer SM, Ambresin A E, Bennett KE, Patton GC. A measurement framework for quality health care for adolescents in hospital. J Adolesc Health 2014;55 (4):484–90. [DOI] [PubMed] [Google Scholar]
- [9].Bikaako-Kajura W, Luyirika E, Purcell DW, et al. Disclosure of HIV status and adherence to daily drug regimens among HIV-infected children in Uganda. AIDS Behav 2006;10(4 Suppl):S85–93. [DOI] [PubMed] [Google Scholar]
- [10].Armistead L, Forehand R. For whom the bell tolls: parenting decisions and challenges faced by mothers who are HIV seropositive. Clin Psychol: Sci Pract 1995;2(3):239–50. [Google Scholar]
- [11].Hogwood J, Campbell T, Butler S. I wish I could tell you but I can’t: adolescents with perinatally acquired HIV and their dilemmas around self-disclosure. Clin Child Psychol Psychiatry 2013;18(1):44–60. [DOI] [PubMed] [Google Scholar]
- [12].Wiener L, Mellins CA, Marhefka S, Battles HB. Disclosure of an HIV diagnosis to children: history, current research, and future directions. J Dev Behav Pediatr 2007;28(2):155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Waugh S Parental views on disclosure of diagnosis to their HIV-positive children. AIDS Care 2003;15(2):169–76. [DOI] [PubMed] [Google Scholar]
- [14].Blasini I, Chantry C, Cruz C, et al. Disclosure model for pediatric patients living with HIV in Puerto Rico: design, implementation, and evaluation. J Dev Behav Pediatr 2004;25(3):181–9. [DOI] [PubMed] [Google Scholar]
- [15].Gerson AC, Joyner M, Fosarelli P, et al. Disclosure of HIV diagnosis to children: when, where, why, and how. J Pediatr Health Care 2001;15(4):161–7. [DOI] [PubMed] [Google Scholar]
- [16].Arrive E, Dicko F, Amghar H, et al. HIV status disclosure and retention in care in HIV-infected adolescents on antiretroviral therapy (ART) in West Africa. PLoS One 2012;7(3):e33690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vreeman RC, Scanlon ML, Mwangi A, et al. A cross-sectional study of disclosure of HIV status to children and adolescents in western Kenya. PLoS One 2014;9(1):e86616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].John-Stewart GC, Wariua G, Beima-Sofie KM, et al. Prevalence, perceptions, and correlates of pediatric HIV disclosure in an HIV treatment program in Kenya. AIDS Care 2013;25(9):1067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kenu E, Obo-Akwa A, Nuamah GB, Brefo A, Sam M, Lartey M. Knowledge and disclosure of HIV status among adolescents and young adults attending an adolescent HIV clinic in Accra, Ghana. BMC Res Notes 2014;7:844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pinzon-Iregui MC, Beck-Sague CM, Malow RM. Disclosure of their HIV status to infected children: A review of the literature. J Tropical Pediatr 2013;59 (2):84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Motshome P, Madiba S. Perceptions, reasons and experiences of disclosing HIV diagnosis to infected children in Kweneng District, Botswana. Int J Health Sci Res 2014;4(2):129–39. [Google Scholar]
- [22].Kallem S, Renner L, Ghebremichael M, Paintsil E. Prevalence and pattern of disclosure of HIV status in HIV-infected children in Ghana. AIDS Behav 2011;15(6):1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brown BJ, Oladokun RE, Osinusi K, Ochigbo S, Adewole IF, Kanki P. Disclosure of HIV status to infected children in a Nigerian HIV Care Programme. AIDS Care 2011;23(9):1053–8. [DOI] [PubMed] [Google Scholar]
- [24].World Health Organization: Guideline on HIV disclosure counselling for children up to 12 years of age. 2011. [PubMed]
- [25].Caldwell MB, Oxtoby MJ, Simonds RJ, Lou Lindegren M, Rogers MF. Morbidity and mortality weekly report: recommendations and reports. 1994: iii–10. [Google Scholar]
- [26].National AIDS and STI Control Program (NASCOP). Procedures Manual for HIV Data Management 2010.
- [27].Oberdorfer P, Puthanakit T, Louthrenoo O, Charnsil C, Sirisanthana V, Sirisanthana T. Disclosure of HIV/AIDS diagnosis to HIV–infected children in Thailand. J Paediatr Child Health 2006;42(5):283–8. [DOI] [PubMed] [Google Scholar]
- [28].Arun S, Singh AK, Lodha R, Kabra S. Disclosure of the HIV infection status in children. Indian J Pediatr 2009;76(8):805–8. [DOI] [PubMed] [Google Scholar]
- [29].Abebe W, Teferra S. Disclosure of diagnosis by parents and caregivers to children infected with HIV: prevalence associated factors and perceived barriers in Addis Ababa, Ethiopia. AIDS Care 2012;24(9):1097–102. [DOI] [PubMed] [Google Scholar]
- [30].Lester P, Chesney M, Cooke M, et al. When the time comes to talk about HIV: factors associated with diagnostic disclosure and emotional distress in HIV-infected children. J Acquir Immune Defic Syndr 2002;31(3):309–17. [DOI] [PubMed] [Google Scholar]
- [31].Menon A, Glazebrook C, Campain N, Ngoma M. Mental health and disclosure of HIV status in Zambian adolescents with HIV infection: implications for peer-support programs. J Acquir Immune Defic Syndr 2007;46(3):349–54. [DOI] [PubMed] [Google Scholar]
- [32].Ferris M, Burau K, Schweitzer A, et al. The influence of disclosure of HIV diagnosis on time to disease progression in a cohort of Romanian children and teens. AIDS Care 2007;19(9):1088–94. [DOI] [PubMed] [Google Scholar]
- [33].Ekouevi DK, Azondekon A, Dicko F, et al. 12-month mortality and loss-to-program in antiretroviral-treated children: the IeDEA pediatric West African Database to evaluate AIDS (pWADA), 2000–2008. BMC Public Health 2011;11 (1):519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rujumba J, Mbasaalaki-Mwaka CL, Ndeezi G. Challenges faced by health workers in providing counselling services to HIV-positive children in Uganda: a descriptive study. J Int AIDS Soc 2010;13(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].De Baets AJ, Sifovo S, Parsons R, Pazvakavambwa IE. HIV disclosure and discussions about grief with Shona children: a comparison between health care workers and community members in Eastern Zimbabwe. Soc Sci Med 2008;66(2):479–91. [DOI] [PubMed] [Google Scholar]
- [36].Kidia KK, Mupambireyi Z, Cluver L, Ndhlovu CE, Borok M, Ferrand RA. HIV status disclosure to perinatally-infected adolescents in Zimbabwe: a qualitative study of adolescent and healthcare worker perspectives. PLoS One 2014;9(1):e87322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Moodley K, Myer L, Michaels D, Cotton M. Paediatric HIV disclosure in South Africa-caregivers’ perspectives on discussing HIV with infected children. S Afr Med J 2006;96(3):201–4. [PubMed] [Google Scholar]
- [38].Beima-Sofie K, John-Stewart G, Shah B, Wamalwa D, Maleche-Obimbo E, Kelley M. Using health provider insights to inform pediatric HIV disclosure: a qualitative study and practice framework from Kenya. AIDS Patient Care STDs 2014;28(10):555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Myer L, Moodley K, Hendricks F, Cotton M. Healthcare providers’ perspectives on discussing HIV status with infected children. J Tropical Pediatr 2006;52(4):293–5. [DOI] [PubMed] [Google Scholar]
- [40].Gyamfi E, Okyere P, Appiah-Brempong E, Adjei RO, Mensah KA. Benefits of disclosure of HIV status to infected children and adolescents: perceptions of care-givers and health care providers. J Assoc Nurses AIDS Care 2015;26(6):770–80. [DOI] [PubMed] [Google Scholar]