Abstract
Many neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis are characterized by the progressive appearance of abnormal proteinaceous assemblies in the nervous system. Studies in experimental systems indicate that the assemblies originate from the prion-like seeded aggregation of specific misfolded proteins that proliferate and amass to form the intracellular and/or extracellular lesions that are typical of each disorder. The host in which the proteopathic seeds arise provides the biochemical and physiological environment that either supports or restricts their emergence, proliferation, self-assembly and spread. Multiple mechanisms influence the spatiotemporal spread of seeds and the nature of the resulting lesions, one of which is the cellular uptake, release, and transport of seeds along neural pathways and networks. The characteristics of cells and regions in the affected network govern their vulnerability and thereby influence the neuropathological and clinical attributes of the disease. The propagation of pathogenic protein assemblies within the nervous system thus is determined by the interaction of the proteopathic agent and the host milieu.
In 1889, Stephen Paget coined the phrase ‘seed and soil’ to describe how the metastasis of cancer cells is governed by the nature of the cells (the seed) and the site of secondary growth (the soil) 1. The basic concept remains valid today, and furnishes useful insights into the selective spread of metastatic cancer to other organs. A wealth of research in recent years reveals that many of the most common age-associated neurodegenerative diseases – Alzheimer’s disease (AD), Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS) among them – result from the transformation and accumulation of specific proteins within the nervous system. Although mechanistically different, this disease process can be likened conceptually to metastatic cancer, except that the disease agents that proliferate in these degenerative brain disorders are transformed proteins rather than transformed cells. As in the case of cancer cells, the dissemination of abnormal proteins and the nature of the resulting disease depend on both the proteinaceous agent – the seed – and the host milieu – the soil. The archetypical proteopathic seed is the prion, which was first identified as an unconventional infectious agent in a family of uniformly fatal brain diseases of humans and nonhuman species 2.
Prions: paradigmatic proteinaceous disease agents
Prions were initially defined as ‘proteinaceous infectious particles’ 2, though the definition has since broadened to ‘proteins that acquire alternative conformations that become self-propagating’ 3. The prion diseases include Creutzfeldt-Jakob disease, Kuru, Gerstmann-Sträussler-Scheinker syndrome, fatal insomnias, and variably protease-sensitive prionopathy in humans 4, 5, as well as scrapie in sheep and goats, bovine spongiform encephalopathy in cattle, chronic wasting disease in cervids, and several other nonhuman prionoses 6.
Prion diseases are unorthodox in that they can be infectious, genetic, or idiopathic (sporadic) in origin 7. They arise when normal cellular prion protein molecules (PrP-Cellular, or PrPC) misfold, self-assemble, and spread through the nervous system 3. Once established in the living organism, the aberrant proteins propagate by the corruptive templating of like molecules, which are continually produced in the natural course of cellular metabolism. The misfolded, pathogenic PrP molecules (conventionally referred to as PrP-Scrapie, or PrPSc) bind to one another and, in a crystallization-like process 8, the assemblies grow, fragment, and proliferate, eventually occupying many regions of the nervous system. In some (but not all) cases, PrPSc polymerizes into distinctive fibrils that amass to form amyloid (fibrillar, proteinaceous, congophilic deposits that birefringe in cross-polarized light), although small, oligomeric assemblies of PrPSc, which are not in the canonical (fibrillar) amyloid state, can be particularly pathogenic 3, 8–10. Importantly, characteristics of both the seeds and the host influence the infectivity of PrPSc as well as the nature of the ensuing disease 7, 10.
PrPSc in the broad sense thus comprises a range of pathogenic structures that are referred to simply as prions. However, with the expansion of the prion concept to include other self-propagating protein assemblies 8, 11, 12, and to minimize concern that non-PrP cerebral proteopathies might be similarly infectious, we here refer to prototypical (PrP) prions as PrP-prions.
Progression of neurodegenerative diseases and the prion paradigm
Experimental evidence now supports the concept that certain proteins involved in multiple neurodegenerative diseases acquire their pathogenicity by a prion-like mechanism (Figure 1). Some of these proteins (and the lesions they form) include amyloid-β (Aβ) (amyloid plaques and cerebral amyloid angiopathy [CAA] in AD), tau (neuronal and/or glial tauopathies in AD, chronic traumatic encephalopathy and other neurodegenerative disorders), and α-synuclein (Lewy bodies and Lewy neurites in PD, Lewy body dementia, and glial cytoplasmic inclusions in multiple system atrophy) 3, 8, 11, 12. In addition, evidence is growing that huntingtin (inclusion bodies in Huntington’s disease) and several proteins associated with ALS-frontotemporal dementia spectrum disorders, including superoxide dismutase 1 (SOD1) and TAR DNA-binding protein-43 (TDP-43) also acquire pathogenicity by a prion-like molecular process.
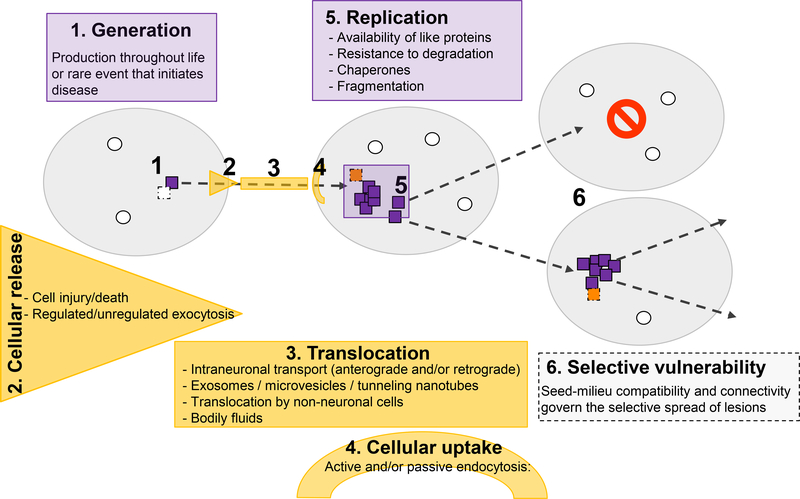
Figure 1.
Factors governing the genesis, replication, and spread of proteopathic seeds. Disease-specific seeds (orange) are generated when certain normally produced proteins (green) misfold, in which state they structurally corrupt like proteins and self-assemble into multimers. Whether seeds are produced throughout life and usually are actively removed or whether this is a rare event that inevitably marks the beginning of disease remains to be determined. The seeds move from one location to another by any of several potential mechanisms; in some instances, the affected site can be extracellular. All of these phenomena may contribute to selective local vulnerability; they can vary in different cell types and regions of the nervous system, where such factors as the presence of auxiliary agents for replication, transport and uptake mechanisms differ (dark blue, a cell in which different agents restrict further propagation). In addition, the expression level or isoform of the cognate proteins may differ among cells and compartments, thereby further supporting or restricting the spread of the seeds and/or defining the strain of seed that is propagated (see Figs. 2 and 3).
Although the formation and amplification of proteopathic seeds is fundamental to these disorders, the disease agents must also translocate among cells and to different regions (Figure 1). To this end, cross-sectional histopathologic analyses have consistently indicated that proteinaceous lesions do not appear and spread randomly; rather, they develop in disease-specific spatiotemporal patterns 13–16. More recently, in vivo imaging investigations have begun to confirm postmortem histopathological findings implicating the neural connectome as an important mediator of the region-to-region progression of proteopathic lesions 17,18. Ultimately, the development of pathology in recipient compartments is governed by host factors that render the local environment receptive or resistant to the propagation of the abnormal proteins (Figure 1).
Postmortem histopathologic analyses and in vivo imaging modalities rely on the presence of distinctive deposits such as extracellular amyloid or intracellular inclusions to map the lesions in the human brain, but such studies only indirectly disclose the dynamic process by which the lesions spread. Moreover, it is important to recognize that these obvious lesions may not fully represent the distribution of the proteopathic seeds and their associated pathologic sequelae 10. Like PrPSc, many disease-related proteins have an enhanced tendency to form amyloid, but they may also comprise small, self-propagating oligomeric assemblies that can disrupt the function of cells and tissues, but which can be difficult to analyze unambiguously in biological samples. Hence, the amyloid state is indicative of a proteopathic process, but it is not always required for the manifestation of disease.
Experimental models have allowed researchers to methodically investigate the trafficking of seeds and the spatiotemporal emergence of anomalous proteinaceous lesions along neural pathways. In an in vivo exogenous seeding paradigm, both the characteristics of seeds and the site at which seeding originates can be carefully defined.
Propagation of proteopathic lesions
The prion-like propagation of proteopathic assemblies in neurodegenerative diseases other than PrP-prion disease was first established by the demonstration that Aβ seeds in brain extracts are necessary and sufficient to induce the aggregation of Aβ in transgenic mouse models (reviewed in 11). The prion concept has since expanded to include many of the aberrant proteins that characterize human neurodegenerative diseases (Figure 2; Table 1).
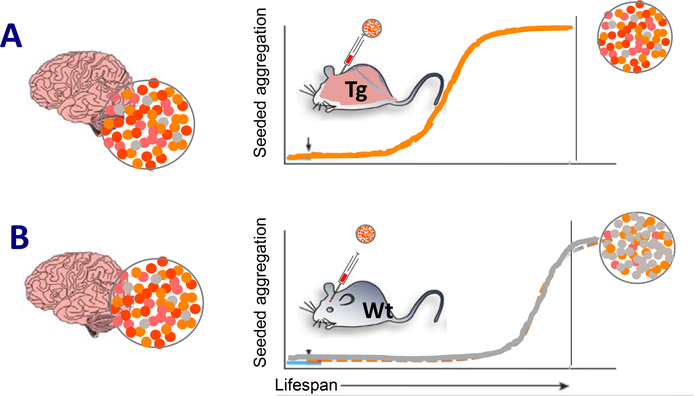
Figure 2.
Compatibility of seed and cognate host protein regulates the propagation of proteopathic seeds at the organismic level. A,B, Proteopathic seeds isolated from a human brain are conformationally heterogeneous (colored dots). In a murine host brain milieu that is permissive for amplification of the dominant conformation (orange), lesions with a molecular structure similar to that in the donor brain will be preferentially propagated following introduction of the exogenous seeds. A, To facilitate seeding, the host mice are often transgenic (Tg), and are engineered to express the human protein that forms specific lesions in the human brain. Seeding efficiency is augmented by high (transgenic) expression of the cognate protein in the host (orange mouse). B, Wild-type (WT) mice usually are more restrictive in propagating the human conformation (for instance, owing to different amino acid sequences in the proteins), but in some cases they may permit the propagation of a subconformation (gray dots). The more abundant the exogenous seeds and the closer their structural characteristics to the host protein, the more likely and efficient their propagation in the host. Hence, transmission of proteopathic lesions from a human donor to a WT mouse (gray) typically requires longer incubation times and sometimes may never occur during the lifetime of the mouse (see also Table 1).
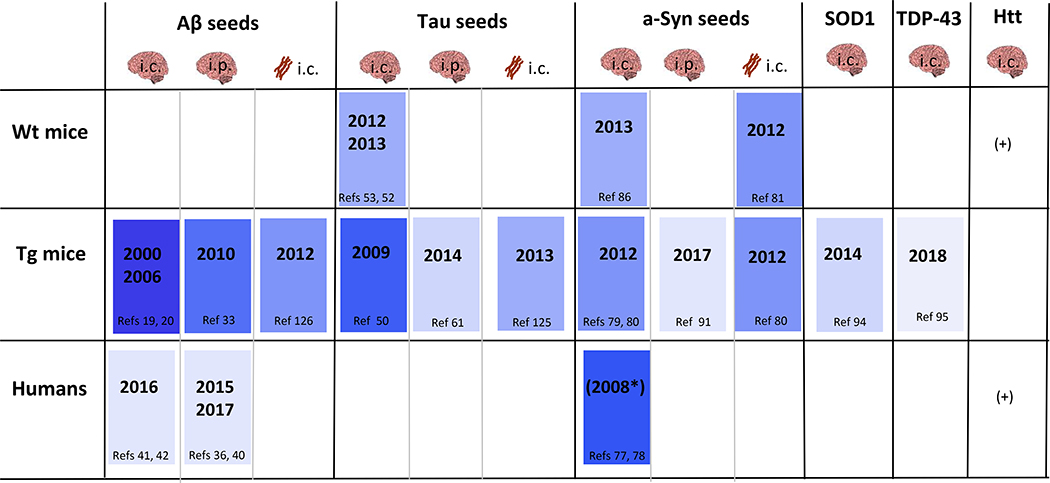
Table 1.
Framework for the transmission of proteopathic seeds to mice or humans either by intracerebral (i.c.) or peripheral (i.p.) inoculation. The year when the first transmission of brain- derived, synthetic, or recombinant seeds was reported is indicated; darker colors denote earlier proof of transmission. Brain symbols, brain-derived seeds; fibril schematic (brown), synthetic fibrils. *Conclusive evidence that synucleinopathy in human tissue grafts is induced by α -Syn seeds from the host is lacking, hence, supporting evidence comes from animal studies. +Huntingtin inclusions have been shown in WT grafts of Huntington’s disease patients151 or in mutant grafts in wild-type mice152. However, a prion-like proteopathic process in vivo, i.e., the sustained, seeded misfolding and accumulation of huntingtin initiated by minute quantities of inanimate seeds, has not yet been shown.
Propagation of Aβ-proteopathy
As in the case of PrP-prionopathies, Aβ deposition can be instigated in the brain by the introduction of minute amounts of brain-derived Aβ seeds into suitable hosts 19–26. Aβ seeds delivered to one brain area induce protein aggregation that spreads to interconnected regions, reminiscent of the neuronal transport and trans-synaptic spread of PrP-prions 27. For example, injection of Aβ-rich brain extract into the hippocampus induces local Aβ deposition, as expected. Once initiated, Aβ deposition then propagates non-randomly to axonally linked parts of the brain 28, 29. The involvement of neurons in the systematic emergence of lesions is supported by in vitro studies showing that Aβ aggregates are conveyed by axonal transport 30, 31, and by the occurrence of seeding-active intracellular Aβ assemblies 32. These studies do not rule out spread by passive diffusion (via the cerebrospinal fluid or interstitial fluid), but the early appearance of deposits at non-contiguous sites, their anatomically orderly proliferation, and the ability of neurons to transport Aβ in vitro argue for an important role of neurons themselves in the spread of disease within the brain.
Aβ seeds also have been shown to traffic from the periphery to the brain 33–35. Aβ deposits seeded from the periphery (by delivery of seeds into the peritoneal cavity or directly into the circulatory system) are predominantly associated with cerebral blood vessels (Aβ-CAA), suggesting a vascular route of transport and the possible participation of innate immune cells that endocytose and translocate Aβ seeds, although the mode of neuroinvasion remains uncertain 33–35.
The trafficking of Aβ seeds from periphery to brain has been proposed to underlie the intracerebral deposition of Aβ in people many years after having been treated as children with human-derived growth hormone 36. Some of the recipients later succumbed to CJD, apparently because the growth hormone was extracted from large batches of cadaveric pituitary glands that included glands from donors who had died while incubating PrP-prion disease 37–39. In a post-mortem analysis of 8 of these iatrogenic CJD cases (ranging from 36–51 years of age), four of the subjects also had extensive Aβ plaques and Aβ-CAA in the brain, and two others had sparse Aβ deposits 36. Aβ deposition also has been reported in the brains of cadaveric growth hormone recipients who died of causes other than CJD 38, 40, and in CJD patients who had received dura mater transplants contaminated with PrP-prions 38, 41–43.
Evidence of tauopathy was minimal 36, 38, 40, 44 or absent 45 in these iatrogenic cases. Abnormal tau is present in pituitaries from Alzheimer patients 46, and tau was detected in some batches of cadaver-derived human growth hormone 44 (abnormal tau has not been reported in dura mater). Why tauopathy is sparse in the growth hormone recipients is uncertain, considering that tau can seed tauopathy directly in animal models (below).
Although other interpretations cannot be ruled out, the most likely explanation for Aβ-proteopathy in these subjects is that some lots of growth hormone and dura mater were contaminated with Aβ seeds from AD (or incipient AD) donors. In support of this possibility, aggregated Aβ was detected in batches of cadaver-derived growth hormone 44, in pituitary glands from AD patients 46, and in samples of the dura mater implicated in transmitting CJD 45. In addition, Aβ-CAA was abundant in many of these human cases, similar to the increased vascular Aβ deposition in APP-transgenic mice following peripheral administration of Aβ seeds 33–35 (above). The possibility that Aβ deposition is somehow actuated by prion disease is unlikely in light of the findings that some non-CJD patients developed Aβ-proteopathy 40 and that PrP-prions do not induce Aβ deposition in mouse models 47. Whether the surviving recipients of tainted biologics are at a higher risk of developing the full clinicopathologic phenotype of AD is not known. Given the long, clinically silent incubation period for AD 48, signs of dementia would not be expected for years or even decades following the initiation of Aβ-proteopathy.
Propagation of tauopathy
Tauopathy is associated with over 20 different disorders 49, and thus is one of the most common proteopathies of the nervous system. Similar to PrP-prionopathies and Aβ-proteopathy, there is clear evidence that tau can self-assemble and propagate in vivo by a prion-like molecular process. The aggregation of hyperphosphorylated tau is inducible by the intracerebral infusion of small amounts of tau seeds into transgenic mice expressing human tau 50, 51, and, to a lesser degree, into wild-type mice 52–54 (Figure 2). This exogenously induced form of tauopathy then spreads systematically from the site of injection to axonally linked brain regions 55–57, indicative of the neuronal endocytosis, templated amplification, transport, and release of tau seeds 58–60. In addition, like PrP-prionopathies, Aβ-proteopathy, and α-synucleinopathy (below), tauopathy can be induced in the brain by delivery of tau seeds into the peritoneal cavity 61.
To model the endogenous emergence and spread of cerebral tauopathy, expression of a human tau transgene was restricted principally to projection neurons of the entorhinal cortex in genetically modified mice 62, 63. The mice developed tauopathy first in the entorhinal cortex, and subsequently in axonally-coupled areas 62, 63. Later studies found that the tau transgene is weakly expressed in other brain regions, which could influence the spatiotemporal pattern of lesion progression 64. However, in light of the stereotypical localization of tauopathy in interconnected brain regions in AD, chronic traumatic encephalopathy, and FTLD-tau 13, 16, 65, 66, the experiments in mouse models support the view that neuronal trafficking mechanisms contribute to the connectomic distribution of tau seeds within the nervous system.
In AD, genetic and biomarker analyses indicate that tauopathy is downstream of Aβ aggregation 48, 67. Experimentally, aggregated forms of Aβ have been shown to induce tau lesions and to promote the spread of tauopathy in mice 68–72. How the two proteins interact is incompletely understood, but it may involve the formation of tau seeds within Aβ-induced dystrophic neurites 71, 72, heterotypic tau seeding by Aβ 73, or the stimulation of tau release from neurons by Aβ-mediated neuronal hyperexcitability 74, 75. Whether the presence of Aβ seeds is necessary to continually drive the spread of tau, or whether Aβ assemblies simply trigger the self-sustaining propagation of tauopathy, is an open question with implications for therapeutic strategies targeting AD.
Propagation of α-synucleinopathy
α-Synuclein misfolds and self-aggregates into characteristic inclusions known as Lewy bodies and Lewy neurites in α-synucleinopathies such as PD and dementia with Lewy bodies, and into glial cytoplasmic inclusions in multiple system atrophy 76. Interest in the seeding capacity of abnormal α-synuclein was piqued with reports that Lewy bodies materialize in fetal brain cells that had been transplanted intracerebrally into PD patients in an attempt to alleviate the behavioral manifestations of the disease 77, 78. Examination of the brains of subjects who died years later disclosed that some of the transplanted cells had developed Lewy-pathology, suggesting (but not proving) that α-synuclein seeds in the host brain induced the misfolding and aggregation of the protein in the transplanted cells.
Experimental studies in animals subsequently provided further support for the self-propagation of α-synuclein seeds (Figure 2; Table 1). Exogenous introduction of brain-derived or synthetic α-synuclein seeds instigates progressive neurodegenerative disorders in animals that recapitulate some characteristics of human PD 79–83 or multiple system atrophy 84, 85. In addition, α-synuclein pathology seeded in one region of the brain propagates along anatomically connected structures 80, 81, 86, 87, suggestive of selective neuronal transport of the seeding agent.
Similar to other cerebral proteopathies, α-synucleinopathy is inducible in the brains of experimental animals by the peripheral infusion of α-synuclein seeds 88–91. This finding has re-invigorated consideration of the mechanisms underlying one of James Parkinson’s (1817) seminal observations of the “shaking palsy” 92 (now known as PD). Parkinson noted that constipation is a frequent symptom of the disease, and he even considered how a gastrointestinal disorder and a brain disorder could be related 92. Since then, immunoreactive α-synuclein inclusions in the autonomic nervous system have been described, whence α-synuclein seeds are hypothesized to travel to the brain via neuronal connections 93.
Propagation of other neurodegeneration-associated protein assemblies
In addition to PrP, Aβ, tau, and α-synuclein, some proteins associated the ALS-frontotemporal dementia spectrum exhibit self-propagating properties and spread in mouse models, including SOD1 94 and TDP-43 95 (Figure 2; Table 1). Although in vitro studies indicate molecular prion-like processes for disease-associated proteins such as FUS and polyglutamine-containing proteins (above), as well as dipeptide repeat proteins 96, definitive evidence for a bona fide prion-like mechanism in vivo remains to be demonstrated in these instances.
Heterogeneity of proteopathic seeds
Strains and clouds
In PrP-prion diseases, conformation-sensitive assays and molecular probes indicate the existence of structurally heterogeneous assemblies of PrPSc within the brain. Such variants are referred to as PrP-prion strains, and their heterogeneity constitutes what are known as conformational clouds 10, 25, i.e., a group or ‘cloud’ of related conformations within the same brain. PrP-prion strains can change and undergo differential amplification under selection pressure 10, 97, 98. Strains and clouds have been linked to the species (transmission) barrier and to the variable phenotypic expression of PrP-prion disease 99. The occurrence of strains and clouds of distinct conformations is a predicted feature of all amyloidogenic proteins 9. Indeed, misfolded Aβ, tau, and α-synuclein share with PrPSc the properties of conformational strains and clouds, a phenomenon that can influence both the propagation and characteristics of the respective proteinaceous lesions (Figure 2, 3).
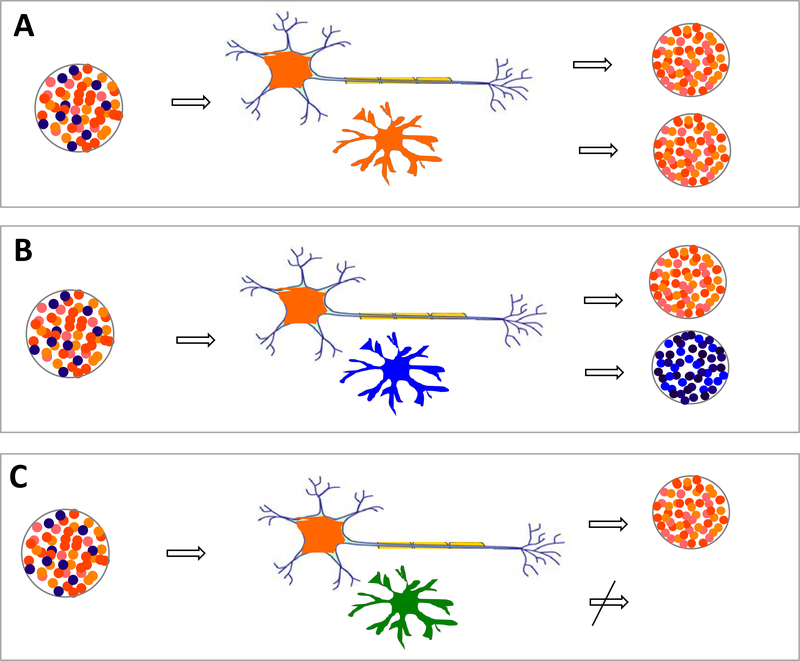
Figure 3.
Compatibility of seed and cognate cellular protein governs propagation at the cellular or compartmental level. For seeded propagation and spread, proteopathic seeds must translocate from cell-to-cell or compartment-to-compartment, and they must be replicated at each successive location (see also Fig. 1). Both steps are dependent on the host, and can vary in different cell types such as neurons and glia (upper and lower cells, respectively, in panels A–C), in which such factors as protein expression, isoforms, and auxiliary molecules influence cell tropism. As a result, some cells resist seeding, and others may select for particular proteopathic conformations (different colored dots). A, Neurons and glia both select for the same strains. B, Neurons and glia select for different strains; here the glia generate secondary seeds that differ from the initial seed. C, The glia are incapable of replicating any pathogenic form of the protein.
Multiple experimental approaches reveal a diversity of Aβ aggregates, 24, 25, 100–103 and tau aggregates 51, 104 in the human brain. Using solid-state nuclear magnetic resonance on AD-seeded, synthetic Aβ fibrils 100, or conformation-sensitive assays of brain samples 103, variant molecular structures have been detected that correspond to either typical AD or a rapidly progressing form of the disorder. In addition, conformation-sensitive amyloid-binding dyes confirm the variability of Aβ aggregates both within (clouds) and among AD brains 25, 101. These studies further show that the molecular attributes of aggregated Aβ deposits differ among familial and idiopathic AD patients 25, 101. High-resolution cryo-electron microscopy is becoming an important tool for defining the molecular architecture of aberrant Aβ 105 and other proteins within the brain. Recently, the variant structures of two different types of tau filament (paired helical and straight filaments) in an AD case were revealed by cryo-electron microscopy 106, and tau strains have been isolated from AD brains and propagated in clonal cells 51, although it remains to be determined whether the structures of the propagated strains have the same conformations as the those in the brain.
Several experiments indicate that it may be possible to replicate in animal models the strain-like features of aggregated proteins from human diseased brains. The intracerebral injection of brain extracts containing aggregated Aβ from different mice 107 or etiologically-different AD cases 24, 25 into susceptible host mice induces cerebral Aβ deposits with molecular traits that partially recapitulate those in the donor brains. Similarly, tau seeds extracted from the brains of humans who died of different tauopathies (AD, progressive supranuclear palsy (PSP), frontotemporal lobar degeneration-tau (FTLD-tau), or corticobasal degeneration (CBD)) induce tau inclusions in mice that are remarkably similar to the corresponding human lesions (including astroglial and oligodendroglial inclusions for PSP-tau and CBD-tau seeds, and neuronal inclusions for AD-tau seeds 52, 104, 108).
Aggregates of α-synuclein from PD brains exhibit differential proteinase-K cleavage patterns, indicative of variant molecular conformations of α-synuclein 109. Furthermore, brain extracts from patients with multiple system atrophy (MSA) or PD have been found to induce different phenotypes upon seeded transmission of α-synucleinopathy to mice 85. Indeed, oligodendrocytes are specifically able to convert α-synuclein into the MSA strain, which shows a much higher seeding capacity compared to neuronal α-synuclein seeds 84. Finally, recombinant α-synuclein fibrils can cross-seed tau fibrillization, and the efficacy of this cross-seeding is governed by strain-like variations in the α-synuclein seeds 109, although the in vivo relevance of synthetic α-synuclein strains remains uncertain (e.g. 110; see also below).
Durability and activity
Although the detailed molecular conformation of PrPSc and its variants is still tentative 111, certain structural and functional properties common to PrPSc and other proteopathic seeds contribute to their shared pathobiology. The enhanced ability to form amyloid renders some proteopathic seeds resistant to physicochemical degradation by harsh treatments such as heat, formaldehyde, or exposure to proteases. Similar to PrPSc, resistance to inactivation by formaldehyde has been shown for Aβ seeds 112 tau seeds 113 and α-synuclein seeds 114, 115. In addition, a subset of Aβ seeds, like PrPSc, are resistant to degradation by heat 20, 116 and proteinase-K 21. Notably, some Aβ seeds 117 and PrP-prions 118 can persist in the living brain for months following exogenous infusion.
Given the conformational variability of proteopathic seeds, it is not surprising that seed durability and bioactivity also vary, with some seeds being relatively fragile but exceptionally seeding-active. Oligomeric forms of PrPSc have a higher specific seeding activity than do larger multimers 119, 120. Similarly, potent seeding activity has been found for smaller aggregates of Aβ 21, 23, and tau 121. The smallest unit of infectivity/seeding capacity is not known, although estimates for PrP-prions are approximately 6 – 20 PrP molecules per particle 119, 120, and for tau, 3 - >10 molecules per particle 122, 123.
Assemblies of synthetic or recombinant Aβ, tau, and α-synuclein have been consistently shown to have comparatively weak seeding capacity compared to seeds derived from brain 20, 54, 81, 83, 124–126. For example, sub-attomolar amounts of brain-derived Aβ can induce Aβ deposition following intracerebral infusion into APP-transgenic mice 23, whereas aggregates of synthetic Aβ require 100 to 1000 times more Aβ and longer incubation times to induce histologically detectable seeded deposition 20, 126. Analysis of molecular structure could yield clues to the differential functionality of proteinaceous seeds.
Generating in vivo-active recombinant PrP seeds in vitro had been a longstanding challenge for the PrP-prion field, but infectivity of recombinant PrP can be capacitated by aggregation in the presence of particular cofactors 127. Whether other synthetic seeds might be similarly enabled by cofactors is not yet certain, but the in vivo seeding efficacy of synthetic Aβ seeds is enhanced if the Aβ is aggregated on living tissue slices in culture 128. The host milieu thus is a key element in the development of proteopathic seeds; host factors also control the susceptibility to disease as well as the resulting phenotype.
Host factors
As in PrP-prion diseases, the host plays a critical role in determining the formation and pathogenicity of other proteopathic seeds. Whether disease-specific seeds are produced throughout life and usually are actively removed, or whether the generation of seeds is a rare event that inevitably marks the beginning of the disease, remains to be determined. In both scenarios, however, the emergence and persistence of seeds is thought to be promoted by the age-related deterioration of the host proteostasis network 129. The host also provides the active and passive mechanisms by which seeds spread through the nervous system. Furthermore, as the source of auxiliary molecules such as chaperones along with the naïve protein molecules that serve as the substrate for templated conversion, the host regulates the self-propagation of seeds. Finally, a salient characteristic of neurodegenerative disorders is the selective vulnerability of different cell types and regions of the nervous system to disease. In virtually all neurodegenerative proteopathies, some cells are highly vulnerable whereas others are not, sometimes within the same local environment 130, 131. The topography of disease reflects in part the extended connectome of the afflicted areas, but also the temporal development of lesions and the intrinsic features of cells and tissues that render them selectively susceptible to disease.
Compatibility of host proteins and seeds
The transmission of PrP-prions and other seeds to new hosts follows a fundamentally similar molecular process. In exogenous seeding models, both the concentration of seeds and the structural compatibility with their proteinaceous substrate govern the subsequent self-propagation of aggregates 11. Thus, experimental transmission of human protein assemblies to mice is facilitated when the murine host overexpresses the corresponding human-sequence protein (Figure 2). An important determinant of seed-host compatibility thus is the amino acid sequence of the protein, which constrains the protein’s folding options, although post-translational modifications and other cellular factors also contribute. These, in turn, influence the complementarity of the molecular surfaces that interact to actuate the seeding cascade 9.
In addition, expression levels and isoforms of a protein may differ among cells and compartments, and thus the host can differentially select certain conformations for amplification in specific locations (Figure 3). For instance, the conformational features and cellular location of seeded α-synuclein lesions are dependent on the expression level and type of host α-synuclein 91, 132, and the same is true for the seeded induction of tau 52, 104 and Aβ lesions 34, 107, 133. Thus, the compatibility of host proteins and seeds is an important factor that regulates the selective propagation of proteinaceous assemblies in different cell types and brain regions 84, 130, 133, 134, 135. Another way in which the host mediates the non-random spread of proteopathic lesions is by the selective translocation of seeds from cell-to-cell or compartment-to-compartment.
Host mechanisms of seed spread
Several active and passive mechanisms may promote the dissemination of seeds (Figure 1). Of these, active axonal transport along defined neural pathways appears to play a major role. Axonal transport mechanisms enable the general translocation of diverse materials such as macromolecules, organelles and viruses to and from neuronal somata. Although fibrillar forms of Aβ, huntingtin and α-synuclein have been demonstrated to travel in both an anterograde and retrograde direction along axons, the rate of transport (at least in vitro) differs for the three proteins 31. Variant states of a protein also may influence how they move from place to place, as shown for PrP-prions; in mice expressing PrP with an intact glycosylphosphatidylinositol (GPI) anchor, infectivity traffics mainly along neuronal pathways, whereas in mice expressing PrP lacking the GPI anchor, infectivity is more likely to diffuse through the interstitial fluid 136.
Some means of translocation appear to be selective for certain proteopathic assemblies. The protein product of lymphocyte-activation gene 3 (LAG3) has been suggested to be a receptor for the endocytosis and spread of α-synuclein seeds (pre-formed fibrils) in neurons, but the same mechanism appears not to accommodate tau or Aβ seeds 137. Unconventional secretion pathways for the cellular release of both tau and α-synuclein have been described 138, 139, which, in one case (mediated by the ubiquitin-specific protease 19), appears to be specific for misfolded α-synuclein but not tau 138, although such specificity may vary among cell-types. These findings indicate that differential trafficking of seeds by the host may contribute to the selective vulnerability of different cells and brain areas in neurodegenerative diseases (Figure 3). However, it is also important to note that both the host milieu and the proteopathic seeds are likely to change throughout the long course of neurodegenerative diseases.
Host-seed interactions as disease progresses
The conditions that govern the interaction of host and seeds may evolve with advancing age and disease progression. Studies in transgenic mice show that the specific seeding activity of Aβ changes as Aβ deposition amplifies in the brain, and is highest in the earliest stages of Aβ-amyloidogenesis 140. The cellular release of Aβ, tau, and α-synuclein, and the spread of the resulting lesions, are promoted by neural activity 141–143. Neuronal connectivity contributes to the anatomic distribution of proteopathic lesions; as the disease process advances, however, the developing lesions can disrupt the connectome in ways that interfere with normal function and complicate the pathways by which the proteinaceous seeds spread further 18, 144. Finally, a role of microglia in the transport and processing of seeds has been described; hence, the activation and disease state of microglia influence the disease phenotype, probably in complex ways 145–147. Most importantly, similar to the prionoses, other neurodegenerative proteopathies have a long, quiet phase during which the abnormal proteins proliferate in the nervous system, even before the characteristic lesions can be detected with histological or imaging tools 140, 148, 149. This critical early phase of seed propagation is an important topic for future research.
Perspective: The seed and soil concept in neurodegenerative diseases
Compelling genetic, pathologic and experimental evidence now implicates the prion-like misfolding and corruptive templating of proteins in the pathogenesis of neurodegenerative diseases. In each disorder, specific proteins selectively aggregate in certain parts of the nervous system. The pattern of accumulation reflects the nature of the proteopathic seeds, the various pathways through which they can translocate, and the idiosyncratic features of the affected structures. Conceptually, the proliferation and selective spread of proteopathic seeds is reminiscent of the tissue tropism of malignant cells in metastatic cancer 150. Another similarity is the heterogeneity of the disease agents, which provides a varied substrate for the Darwinian selection of proteopathic strains or subclonal cancer cells in response to therapy 98, 150. A deeper understanding of the emergence, spread, and selective impact of pathogenic protein assemblies, particularly at early stages of disease, will yield useful insights into the pathobiology of a variety of human afflictions. Just as malignant cells and host factors interact to define the pathogenesis of cancer, the ‘seed and soil’ concept first proposed by Paget 1 could inform the coherent development of disease-modifying therapies for neurodegenerative disorders involving the seeded aggregation of proteins.
Acknowledgments:
We thank Jay Rasmussen, Mehtap Bacioglu and the members of our laboratories for critical discussions and comments. The help of Anja Apel and Gisela Rose with the manuscript and figures is gratefully acknowledged. Supported by the EC Joint Programme on Neurodegenerative Diseases under the Grants JPND-NewTargets and JPND-REfrAME (M.J.), Horizon 2020 IMPRiND (M.J.), National Institutes of Health (NIH) grants P50 AG025688, ORIP/OD P51OD011132, and by the Alexander von Humboldt Foundation (L.C.W.).
Footnotes
Competing interests: The authors declare no competing interests.
References
- 1.Paget S The distribution of secondary growths in cancer of the breast. The Lancet 133, 571–573 (1889). [PubMed] [Google Scholar]
- 2.Prusiner SB Novel proteinaceous infectious particles cause scrapie. Science 216, 136–144 (1982). [DOI] [PubMed] [Google Scholar]
- 3.Prusiner SB Biology and genetics of prions causing neurodegeneration. Annu. Rev. Genet 47, 601–623 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mead S & Reilly MM A new prion disease: relationship with central and peripheral amyloidoses. Nat. Rev. Neurol 11, 90–97 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Imran M & Mahmood S An overview of animal prion diseases. Virol J. 8, 493 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imran M & Mahmood S An overview of human prion diseases. Virol J. 8, 559 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeArmond SJ & Prusiner SB Etiology and pathogenesis of prion diseases. Am. J. Pathol 146, 785–811 (1995). [PMC free article] [PubMed] [Google Scholar]
- 8.Walker LC & Jucker M Neurodegenerative diseases: expanding the prion concept. Annu. Rev. Neurosci 38, 87–103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenberg D & Jucker M The amyloid state of proteins in human diseases. Cell 148, 1188–1203 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collinge J Mammalian prions and their wider relevance in neurodegenerative diseases. Nature 539, 217–226 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Jucker M & Walker LC Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501, 45–51 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goedert M NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science 349, 1255555 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Braak H & Braak E Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991). [DOI] [PubMed] [Google Scholar]
- 14.Brettschneider J, Del Tredici K, Lee VM & Trojanowski JQ Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat. Rev. Neurosci 16, 109–120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Tredici K & Braak H Review: Sporadic Parkinson’s disease: development and distribution of alpha-synuclein pathology. Neuropathol. Appl. Neurobiol 42, 33–50 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Saper CB, Wainer BH & German DC Axonal and transneuronal transport in the transmission of neurological disease: potential role in system degenerations, including Alzheimer’s disease. Neuroscience 23, 389–398 (1987). [DOI] [PubMed] [Google Scholar]
- 17.Iturria-Medina Y & Evans AC On the central role of brain connectivity in neurodegenerative disease progression. Front. Aging Neurosci 7, 90 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmqvist S, et al. Earliest accumulation of beta-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nature communications 8, 1214 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kane MD, et al. Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J. Neurosci 20, 3606–3611 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer-Luehmann M, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science 313, 1781–1784 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Langer F, et al. Soluble Abeta seeds are potent inducers of cerebral beta-amyloid deposition. J. Neurosci 31, 14488–14495 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morales R, Duran-Aniotz C, Castilla J, Estrada LD & Soto C De novo induction of amyloid-beta deposition in vivo. Mol. Psychiatry 17, 1347–1353 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Fritschi SK, et al. Highly potent soluble amyloid-beta seeds in human Alzheimer brain but not cerebrospinal fluid. Brain 137, 2909–2915 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watts JC, et al. Serial propagation of distinct strains of Abeta prions from Alzheimer’s disease patients. Proc. Natl. Acad. Sci. USA 111, 10323–10328 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen J, et al. Amyloid polymorphisms constitute distinct clouds of conformational variants in different etiological subtypes of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 114, 13018–13023 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruiz-Riquelme A, et al. Prion-like propagation of beta-amyloid aggregates in the absence of APP overexpression. Acta Neuropathol. Commun 6, 26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguzzi A & Calella AM Prions: protein aggregation and infectious diseases. Physiol. Rev 89, 1105–1152 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Hamaguchi T, et al. The presence of Abeta seeds, and not age per se, is critical to the initiation of Abeta deposition in the brain. Acta Neuropathol. 123, 31–37 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye L, et al. Progression of Seed-Induced Abeta Deposition within the Limbic Connectome. Brain Pathol. 25, 743–752 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domert J, et al. Spreading of amyloid-beta peptides via neuritic cell-to-cell transfer is dependent on insufficient cellular clearance. Neurobiol. Dis 65, 82–92 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Brahic M, Bousset L, Bieri G, Melki R & Gitler AD Axonal transport and secretion of fibrillar forms of alpha-synuclein, Abeta42 peptide and HTTExon 1. Acta Neuropathol. 131, 539–548 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzesco AM, et al. Highly potent intracellular membrane-associated Abeta seeds. Sci. Rep 6, 28125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisele YS, et al. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science 330, 980–982 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisele YS, et al. Multiple Factors Contribute to the Peripheral Induction of Cerebral beta-Amyloidosis. J. Neurosci 34, 10264–10273 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burwinkel M, Lutzenberger M, Heppner FL, Schulz-Schaeffer W & Baier M Intravenous injection of beta-amyloid seeds promotes cerebral amyloid angiopathy (CAA). Acta Neuropathol. Commun 6, 23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaunmuktane Z, et al. Evidence for human transmission of amyloid-beta pathology and cerebral amyloid angiopathy. Nature 525, 247–250 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Brown P, et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg. Infect. Dis 18, 901–907 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cali I, et al. Iatrogenic Creutzfeldt-Jakob disease with Amyloid-beta pathology: an international study. Acta Neuropathol. Commun. 6, 5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Will RG Acquired prion disease: iatrogenic CJD, variant CJD, kuru. Br. Med. Bull. 66, 255–265 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Ritchie DL, et al. Amyloid-beta accumulation in the CNS in human growth hormone recipients in the UK. Acta Neuropathol. 134, 221–240 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frontzek K, Lutz MI, Aguzzi A, Kovacs GG & Budka H Amyloid-beta pathology and cerebral amyloid angiopathy are frequent in iatrogenic Creutzfeldt-Jakob disease after dural grafting. Swiss Med. Wkly 146, w14287 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Hamaguchi T, et al. Significant association of cadaveric dura mater grafting with subpial Abeta deposition and meningeal amyloid angiopathy. Acta Neuropathol. 132, 313–315 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Herve D, et al. Fatal Abeta cerebral amyloid angiopathy 4 decades after a dural graft at the age of 2 years. Acta Neuropathol. 135, 801–803 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Duyckaerts C, et al. Neuropathology of iatrogenic Creutzfeldt-Jakob disease and immunoassay of French cadaver-sourced growth hormone batches suggest possible transmission of tauopathy and long incubation periods for the transmission of Abeta pathology. Acta Neuropathol. 135, 201–212 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Kovacs GG, et al. Dura mater is a potential source of Abeta seeds. Acta Neuropathol. 131, 911–923 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irwin DJ, et al. Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA neurology 70, 462–468 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rasmussen J, et al. Infectious prions do not induce Abeta deposition in an in vivo seeding model. Acta Neuropathol. 135, 965–967 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Jack CR Jr., et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spillantini MG & Goedert M Tau pathology and neurodegeneration. Lancet Neurol. 12, 609–622 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Clavaguera F, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol 11, 909–913 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaufman SK, et al. Tau Prion Strains Dictate Patterns of Cell Pathology, Progression Rate, and Regional Vulnerability In Vivo. Neuron 92, 796–812 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clavaguera F, et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl. Acad. Sci. USA 110, 9535–9540 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lasagne-Reeves CA, et al. Alzheimer brain-derived tau oligomers propagate from endogenous tau. Sci. Rep 2, 700 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo JL, et al. Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J. Exp. Med 213, 2635–2654 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iba M, et al. Tau pathology spread in PS19 tau transgenic mice following locus coeruleus (LC) injections of synthetic tau fibrils is determined by the LC’s afferent and efferent connections. Acta Neuropathol. 130, 349–362 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmed Z, et al. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 127, 667–683 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stancu IC, et al. Templated misfolding of Tau by prion-like seeding along neuronal connections impairs neuronal network function and associated behavioral outcomes in Tau transgenic mice. Acta Neuropathol. 129, 875–894 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu JW, et al. Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J. Biol. Chem 288, 1856–1870 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holmes BB, et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. USA 110, E3138–3147 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanders DW, et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82, 1271–1288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clavaguera F, et al. Peripheral administration of tau aggregates triggers intracerebral tauopathy in transgenic mice. Acta Neuropathol. 127, 299–301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Calignon A, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73, 685–697 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu L, et al. Trans-synaptic spread of tau pathology in vivo. PLoS One 7, e31302 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yetman MJ, Lillehaug S, Bjaalie JG, Leergaard TB & Jankowsky JL Transgene expression in the Nop-tTA driver line is not inherently restricted to the entorhinal cortex. Brain structure & function 221, 2231–2249 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Irwin DJ, et al. Deep clinical and neuropathological phenotyping of Pick disease. Ann. Neurol 79, 272–287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McKee AC, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dubois B, et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 12, 292–323 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gotz J, Chen F, van Dorpe J & Nitsch RM Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science 293, 1491–1495 (2001). [DOI] [PubMed] [Google Scholar]
- 69.Bolmont T, et al. Induction of tau pathology by intracerebral infusion of amyloid-beta -containing brain extract and by amyloid-beta deposition in APP x Tau transgenic mice. Am. J. Pathol 171, 2012–2020 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pooler AM, et al. Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer’s disease. Acta Neuropathol. Commun 3, 14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li T, et al. The neuritic plaque facilitates pathological conversion of tau in an Alzheimer’s disease mouse model. Nature communications 7, 12082 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He Z, et al. Amyloid-beta plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med 24, 29–38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vasconcelos B, et al. Heterotypic seeding of Tau fibrillization by pre-aggregated Abeta provides potent seeds for prion-like seeding and propagation of Tau-pathology in vivo. Acta Neuropathol. 131, 549–569 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Busche MA, et al. Critical role of soluble amyloid-beta for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 109, 8740–8745 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu JW, et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat. Neurosci 19, 1085–1092 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goedert M, Spillantini MG, Del Tredici K & Braak H 100 years of Lewy pathology. Nat. Rev. Neurol 9, 13–24 (2013). [DOI] [PubMed] [Google Scholar]
- 77.Kordower JH, Chu Y, Hauser RA, Freeman TB & Olanow CW Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med 14, 504–506 (2008). [DOI] [PubMed] [Google Scholar]
- 78.Li JY, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med 14, 501–503 (2008). [DOI] [PubMed] [Google Scholar]
- 79.Mougenot AL, et al. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol. Aging 33, 2225–2228 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Luk KC, et al. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J. Exp. Med 209, 975–986 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luk KC, et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Masuda-Suzukake M, et al. Pathological alpha-synuclein propagates through neural networks. Acta Neuropathol. Commun 2, 88 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Recasens A, et al. Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann. Neurol 75, 351–362 (2014). [DOI] [PubMed] [Google Scholar]
- 84.Peng C, et al. Cellular milieu imparts distinct pathological alpha-synuclein strains in alpha-synucleinopathies. Nature 557, 558–563 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prusiner SB, et al. Evidence for alpha-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc. Natl. Acad. Sci. USA 112, E5308–5317 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Masuda-Suzukake M, et al. Prion-like spreading of pathological alpha-synuclein in brain. Brain 136, 1128–1138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rey NL, et al. Widespread transneuronal propagation of alpha-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J. Exp. Med 213, 1759–1778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sacino AN, et al. Intramuscular injection of alpha-synuclein induces CNS alpha-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc. Natl. Acad. Sci. USA 111, 10732–10737 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peelaerts W, et al. alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522, 340–344 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Ayers JI, et al. Robust Central Nervous System Pathology in Transgenic Mice following Peripheral Injection of alpha-Synuclein Fibrils. J. Virol 91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sargent D, et al. ‘Prion-like’ propagation of the synucleinopathy of M83 transgenic mice depends on the mouse genotype and type of inoculum. J. Neurochem 143, 126–135 (2017). [DOI] [PubMed] [Google Scholar]
- 92.Parkinson J An essay on the shaking palsy. 1817. J. Neuropsychiatry Clin. Neurosci 14, 223–236; discussion 222 (2002). [DOI] [PubMed] [Google Scholar]
- 93.Braak H, de Vos RA, Bohl J & Del Tredici K Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett 396, 67–72 (2006). [DOI] [PubMed] [Google Scholar]
- 94.Ayers JI, et al. Experimental transmissibility of mutant SOD1 motor neuron disease. Acta Neuropathol. 128, 791–803 (2014). [DOI] [PubMed] [Google Scholar]
- 95.Porta YX, et al. Patient-derived frontotemporal lobar degeneration brain extracts induce formation and spreading of TDP-43 pathology in vivo. Nature Comm, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou Q, et al. Antibodies inhibit transmission and aggregation of C9orf72 poly-GA dipeptide repeat proteins. EMBO Mol. Med 9, 687–702 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Collinge J & Clarke AR A general model of prion strains and their pathogenicity. Science 318, 930–936 (2007). [DOI] [PubMed] [Google Scholar]
- 98.Li J, Browning S, Mahal SP, Oelschlegel AM & Weissmann C Darwinian evolution of prions in cell culture. Science 327, 869–872 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tanaka M, Collins SR, Toyama BH & Weissman JS The physical basis of how prion conformations determine strain phenotypes. Nature 442, 585–589 (2006). [DOI] [PubMed] [Google Scholar]
- 100.Qiang W, Yau WM, Lu JX, Collinge J & Tycko R Structural variation in amyloid-beta fibrils from Alzheimer’s disease clinical subtypes. Nature 541, 217–221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Condello C, et al. Structural heterogeneity and intersubject variability of Abeta in familial and sporadic Alzheimer’s disease. Proc. Natl. Acad. Sci. USA (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Di Fede G, et al. Molecular subtypes of Alzheimer’s disease. Sci. Rep 8, 3269 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cohen ML, et al. Rapidly progressive Alzheimer’s disease features distinct structures of amyloid-beta. Brain 138, 1009–1022 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Narasimhan S, et al. Pathological Tau Strains from Human Brains Recapitulate the Diversity of Tauopathies in Nontransgenic Mouse Brain. J. Neurosci 37, 11406–11423 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gremer L, et al. Fibril structure of amyloid-beta(1–42) by cryo-electron microscopy. Science 358, 116–119 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fitzpatrick AWP, et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 547, 185–190 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heilbronner G, et al. Seeded strain-like transmission of beta-amyloid morphotypes in APP transgenic mice. EMBO Rep. 14, 1017–1022 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boluda S, et al. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol. 129, 221–237 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo JL, et al. Distinct alpha-Synuclein Strains Differentially Promote Tau Inclusions in Neurons. Cell 154, 103–117 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Woerman AL, et al. Familial Parkinson’s point mutation abolishes multiple system atrophy prion replication. Proceedings of the National Academy of Sciences 115, 409 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wille H & Requena JR The Structure of PrP(Sc) Prions. Pathogens (Basel, Switzerland) 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fritschi SK, et al. Abeta seeds resist inactivation by formaldehyde. Acta Neuropathol. 128, 477–484 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaufman SK, Thomas TL, Del Tredici K, Braak H & Diamond MI Characterization of tau prion seeding activity and strains from formaldehyde-fixed tissue. Acta Neuropathol. Commun 5, 41 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schweighauser M, et al. Formaldehyde-fixed brain tissue from spontaneously ill alpha-synuclein transgenic mice induces fatal alpha-synucleinopathy in transgenic hosts. Acta Neuropathol. 129, 157–159 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Woerman AL, et al. MSA prions exhibit remarkable stability and resistance to inactivation. Acta Neuropathol. 135, 49–63 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Eisele YS, et al. Induction of cerebral beta-amyloidosis: intracerebral versus systemic Abeta inoculation. Proc. Natl. Acad. Sci. USA 106, 12926–12931 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ye L, et al. Persistence of Abeta seeds in APP null mouse brain. Nat. Neurosci 18, 1559–1561 (2015). [DOI] [PubMed] [Google Scholar]
- 118.Diack AB, et al. Insights into Mechanisms of Chronic Neurodegeneration. International journal of molecular sciences 17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim C, et al. Small protease sensitive oligomers of PrPSc in distinct human prions determine conversion rate of PrP(C). PLoS Pathog. 8, e1002835 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Silveira JR, et al. The most infectious prion protein particles. Nature 437, 257–261 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gerson J, et al. Tau Oligomers Derived from Traumatic Brain Injury Cause Cognitive Impairment and Accelerate Onset of Pathology in Htau Mice. J. Neurotrauma 33, 2034–2043 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mirbaha H, Holmes BB, Sanders DW, Bieschke J & Diamond MI Tau Trimers Are the Minimal Propagation Unit Spontaneously Internalized to Seed Intracellular Aggregation. J. Biol. Chem 290, 14893–14903 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jackson SJ, et al. Short Fibrils Constitute the Major Species of Seed-Competent Tau in the Brains of Mice Transgenic for Human P301S Tau. J. Neurosci 36, 762–772 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Falcon B, et al. Conformation determines the seeding potencies of native and recombinant Tau aggregates. J. Biol. Chem 290, 1049–1065 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iba M, et al. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J. Neurosci 33, 1024–1037 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stohr J, et al. Purified and synthetic Alzheimer’s amyloid beta (Abeta) prions. Proc. Natl. Acad. Sci. USA 109, 11025–11030 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Supattapone S Synthesis of high titer infectious prions with cofactor molecules. J. Biol. Chem 289, 19850–19854 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Novotny R, et al. Conversion of Synthetic Abeta to In Vivo Active Seeds and Amyloid Plaque Formation in a Hippocampal Slice Culture Model. J. Neurosci 36, 5084–5093 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Labbadia J & Morimoto RI The biology of proteostasis in aging and disease. Annu. Rev. Biochem 84, 435–464 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Surmeier DJ, Obeso JA & Halliday GM Parkinson’s Disease Is Not Simply a Prion Disorder. J. Neurosci 37, 9799–9807 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mattsson N, Schott JM, Hardy J, Turner MR & Zetterberg H Selective vulnerability in neurodegeneration: insights from clinical variants of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 87, 1000–1004 (2016). [DOI] [PubMed] [Google Scholar]
- 132.Luna E, et al. Differential alpha-synuclein expression contributes to selective vulnerability of hippocampal neuron subpopulations to fibril-induced toxicity. Acta Neuropathol. 135, 855–875 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mezias C & Raj A Analysis of Amyloid-beta Pathology Spread in Mouse Models Suggests Spread Is Driven by Spatial Proximity, Not Connectivity. Front. Neurol 8, 653 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hu PP, et al. Role of Prion Replication in the Strain-dependent Brain Regional Distribution of Prions. J. Biol. Chem 291, 12880–12887 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Freer R, et al. A protein homeostasis signature in healthy brains recapitulates tissue vulnerability to Alzheimer’s disease. Science advances 2, e1600947 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rangel A, et al. Distinct patterns of spread of prion infection in brains of mice expressing anchorless or anchored forms of prion protein. Acta Neuropathol. Commun 2, 8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mao X, et al. Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 353 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee JG, Takahama S, Zhang G, Tomarev SI & Ye Y Unconventional secretion of misfolded proteins promotes adaptation to proteasome dysfunction in mammalian cells. Nat. Cell Biol 18, 765–776 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Katsinelos T, et al. Unconventional Secretion Mediates the Trans-cellular Spreading of Tau. Cell reports 23, 2039–2055 (2018). [DOI] [PubMed] [Google Scholar]
- 140.Ye L, et al. Abeta seeding potency peaks in the early stages of cerebral beta-amyloidosis. EMBO Rep. 18, 1536–1544 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bero AW, et al. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat. Neurosci 14, 750–756 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yamada K, et al. Neuronal activity regulates extracellular tau in vivo. J. Exp. Med 211, 387–393 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yamada K & Iwatsubo T Extracellular alpha-synuclein levels are regulated by neuronal activity. Mol. Neurodegener 13, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Phinney AL, et al. Cerebral amyloid induces aberrant axonal sprouting and ectopic terminal formation in amyloid precursor protein transgenic mice. J. Neurosci 19, 8552–8559 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Asai H, et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci 18, 1584–1593 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Keren-Shaul H, et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 169, 1276–1290 e1217 (2017). [DOI] [PubMed] [Google Scholar]
- 147.Venegas C, et al. Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer’s disease. Nature 552, 355–361 (2017). [DOI] [PubMed] [Google Scholar]
- 148.DeVos SL, et al. Synaptic Tau Seeding Precedes Tau Pathology in Human Alzheimer’s Disease Brain. Front. Neurosci. 12, 267 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kaufman SK, Del Tredici K, Thomas TL, Braak H & Diamond MI Tau seeding activity begins in the transentorhinal/entorhinal regions and anticipates phospho-tau pathology in Alzheimer’s disease and PART. Acta Neuropathol. 136, 57–67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shen MM Cancer: The complex seeds of metastasis. Nature 520, 298–299 (2015). [DOI] [PubMed] [Google Scholar]
- 151.Cicchetti F, Lacroix S, Cisbani G, Vallières N, Saint-Pierre M, St-Amour I, Tolouei R, Skepper JN, Hauser RA, Mantovani D, Barker RA, Freeman TB Mutant huntingtin is present in neuronal grafts in Huntington disease patients. Ann. Neurol July;76(1):31–42 (2014). doi: 10.1002/ana.24174. Epub 2014 Jun 6. [DOI] [PubMed] [Google Scholar]
- 152.Jeon I, Cicchetti F, Cisbani G, Lee S, Li E, Bae J, Lee N, Li L, Im W, Kim M, Kim HS, Oh SH, Kim TA, Ko JJ, Aubé B, Oueslati A, Kim YJ, Song J Human-to-mouse prion-like propagation of mutant huntingtin protein. Acta Neuropathol. October;132(4):577–92 (2016). doi: 10.1007/s00401-016-1582-9. Epub 2016 May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]